Похожие презентации:
Solutions. Acid–base equilibrium in biological systems
1. Solutions. Acid–base equilibrium in biological systems
2. Plan
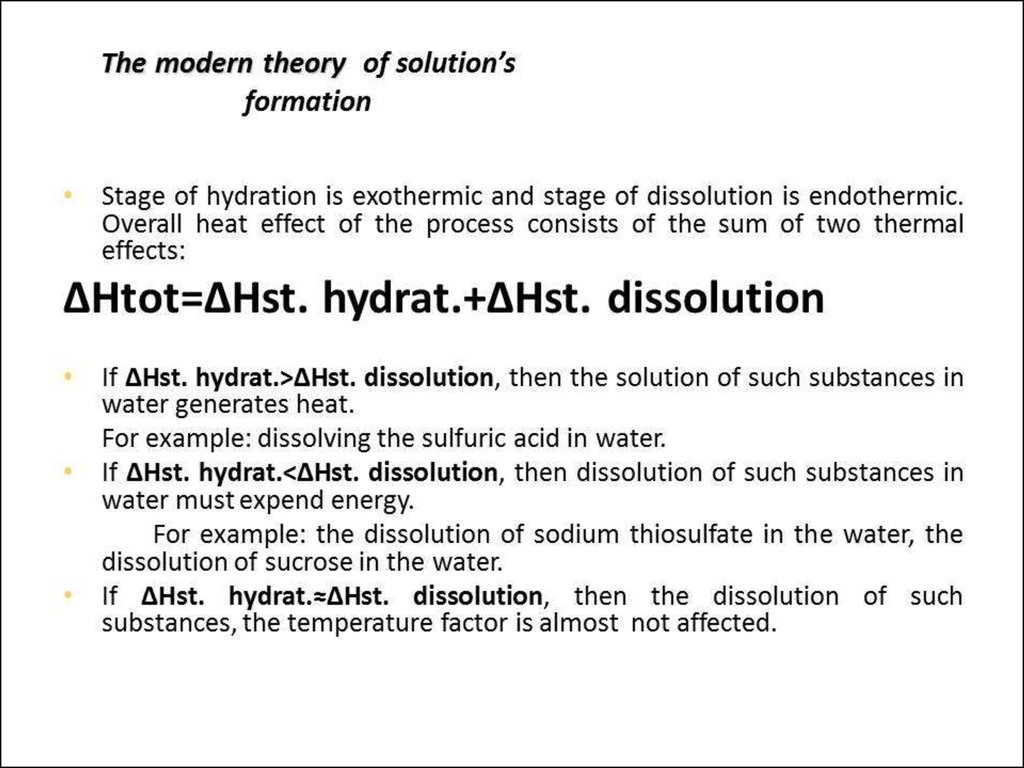
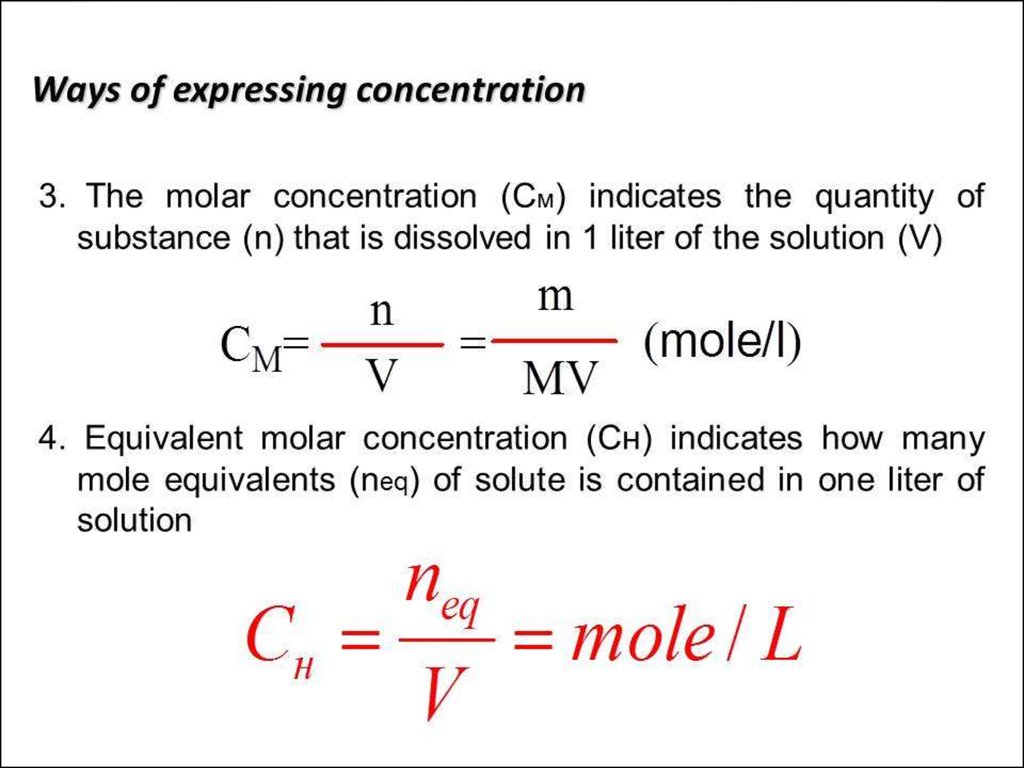
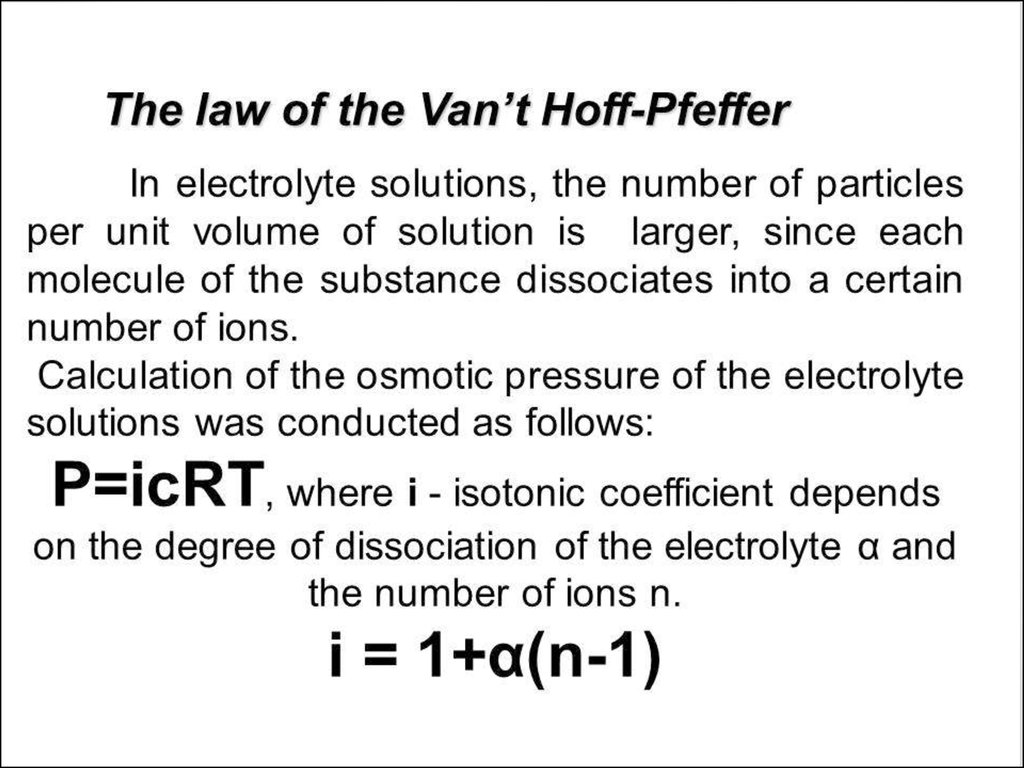
• 0. Solutions and their colligative properties• 1. The theory of electrolytic dissociation. Dissociation of
bases, acides and salts in water solutions.Strong and weak
electrolytes
• 2. Protolytic theory.
• 3. Dissociation of water. Hydrogen ion exponent.
• The homeostasis.
• 4. The importancy of pH maintenance in human body.
5. The concept of buffer solutions.
• 6. Hydrocarbonate buffer system
• 7. Phosphate buffer system
• 8. Protein buffer systems
• 9. Hemoglobin buffer system
• 10. Acidosis and alkalosis. Treatment of acidosis and
alkalosis.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
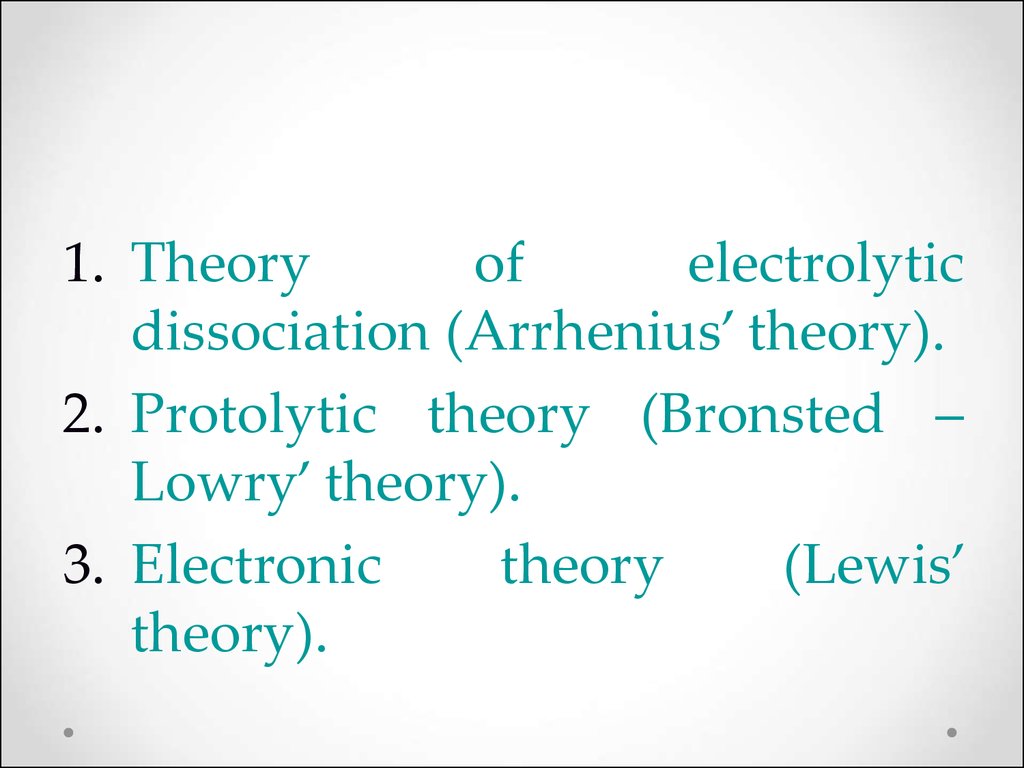
1. Theoryof
electrolytic
dissociation (Arrhenius’ theory).
2. Protolytic theory (Bronsted –
Lowry’ theory).
3. Electronic
theory
(Lewis’
theory).
20. The theory of electrolytic dissociation
21.
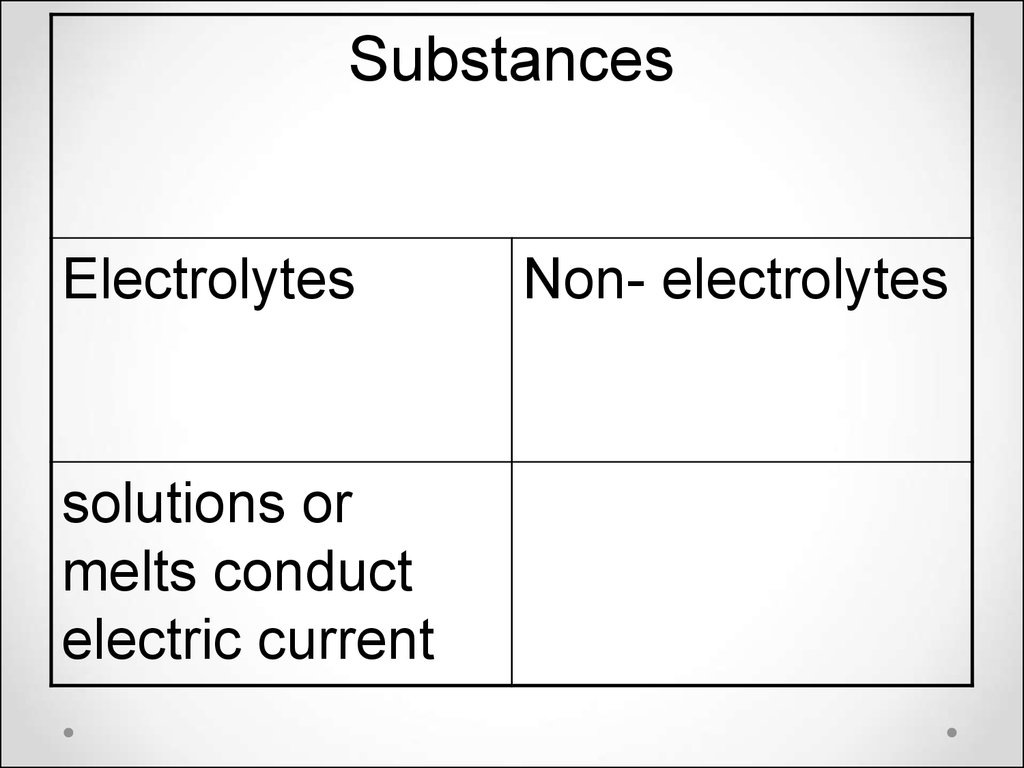
SubstancesElectrolytes
solutions or
melts conduct
electric current
Non- electrolytes
22.
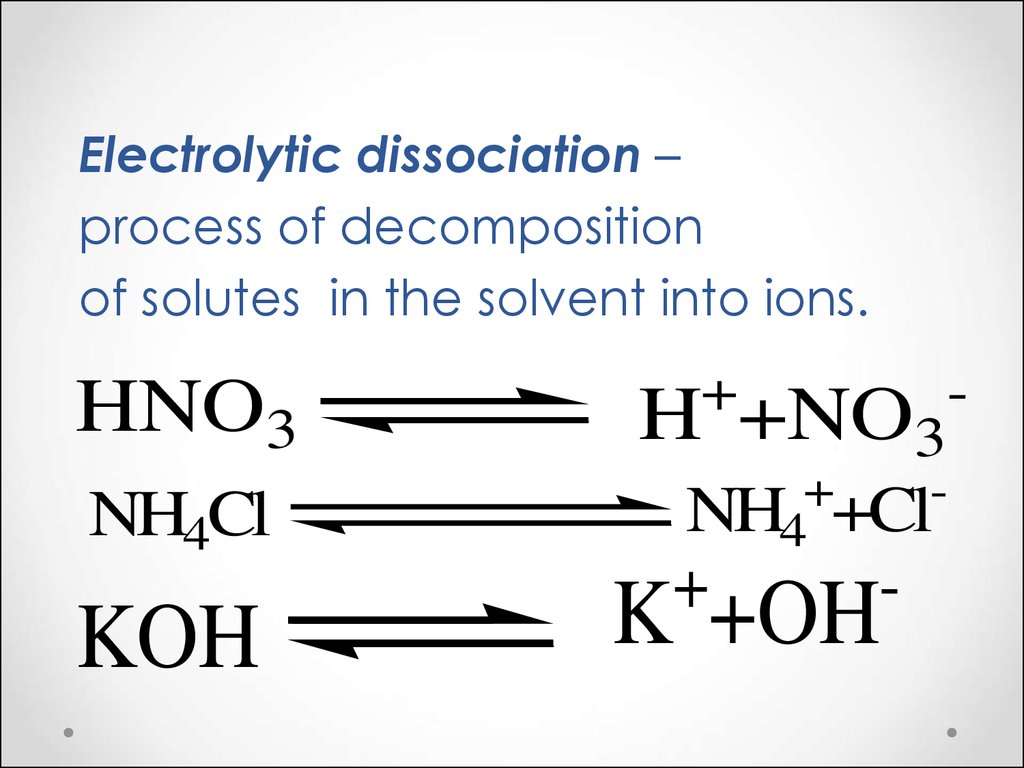
Electrolytic dissociation –process of decomposition
of solutes in the solvent into ions.
HNO3
NH4Cl
KOH
+
H
+NO3
+
NH4 +Cl
+
-
K +OH
23.
• 1)Substances dissociating in solutions or melts
into positively charged Cat+(cations) and negatively
charged An- (anions). The latter include acids, bases
and salts.
2)
In electric field Cat+ move to cathode, Anmove to anode.
3)
Electrolytes decompose into ions in different
degree.
4)
Dissociation depend of:
a) nature of electrolyte;
b) nature of solvent;
c) concentration;
d) temperature.
24. Dissociation of bases, acides and salts in water solutions
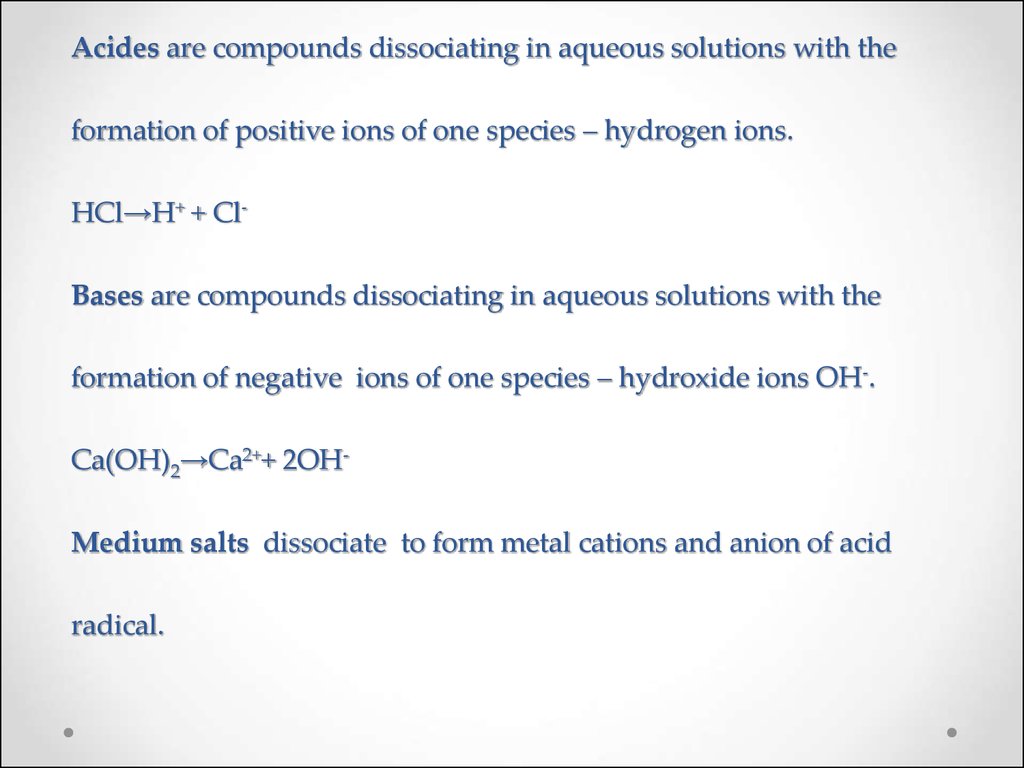
25. Acides are compounds dissociating in aqueous solutions with the formation of positive ions of one species – hydrogen ions. HCl→H+ + Cl- Bases are compounds dissociating in aqueous solutions with the formation of negative ions of one species – hydrox
Acides are compounds dissociating in aqueous solutions with theformation of positive ions of one species – hydrogen ions.
HCl→H+ + ClBases are compounds dissociating in aqueous solutions with the
formation of negative ions of one species – hydroxide ions OH-.
Ca(OH)2→Ca2++ 2OH-
Medium salts dissociate to form metal cations and anion of acid
radical.
26.
Strong and weak electrolytes27. Degree of dissociation α
NiNi
100%
Ntot
Ntot
Ni - the number of molecules, dissociating into ions;
Ntot – the total number of dissolved molecules.
28.
Classification of electrolytesweak
medium
strong
α<3%
3%< α<30%
α>30%
29. Strong electrolytes
Majority of salts.Some acids (HCl, HBr, HI, HNO3,
HClO4, H2SO4).
Alkalis (LiOH, NaOH, KOH,
RbOH, CsOH, Ca(OH)2 ,
Sr(OH)2, Ba(OH)2)
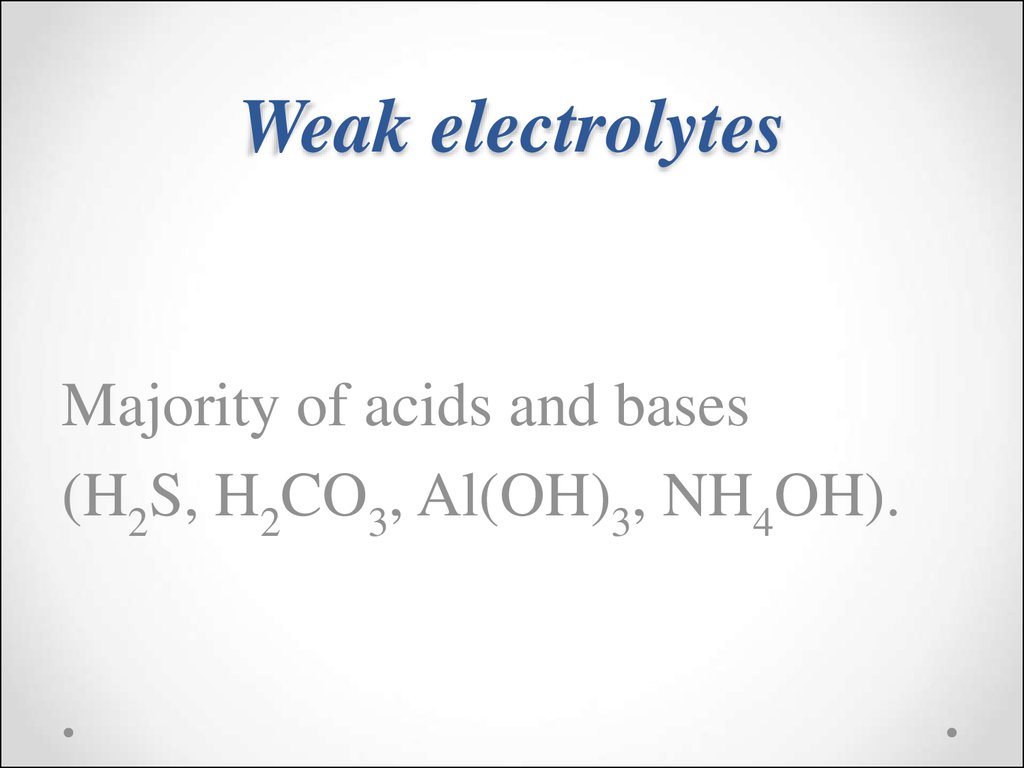
30. Weak electrolytes
Majority of acids and bases(H2S, H2CO3, Al(OH)3, NH4OH).
31. The dissociation of weak electrolytes is a reversible process
CatAnCat+ + An-
32. The equilibrium constant K is called the dissociation (ionization) constant
CC
Cat An
K
C
CatAn
33. Ostwald dilution law
α 2 СмКd =
1 α
Because in solutions of weak electrolytes, degree of dissociation of a very
small quantity, 1-α = 1, then
Кd = α См
2
Dissociation constant, Kd, and the degree of dissociation,
M is the molar concentration of the solution. Very often,
instead of the dissociation constants are in their common logarithms:
рК = lg Кd
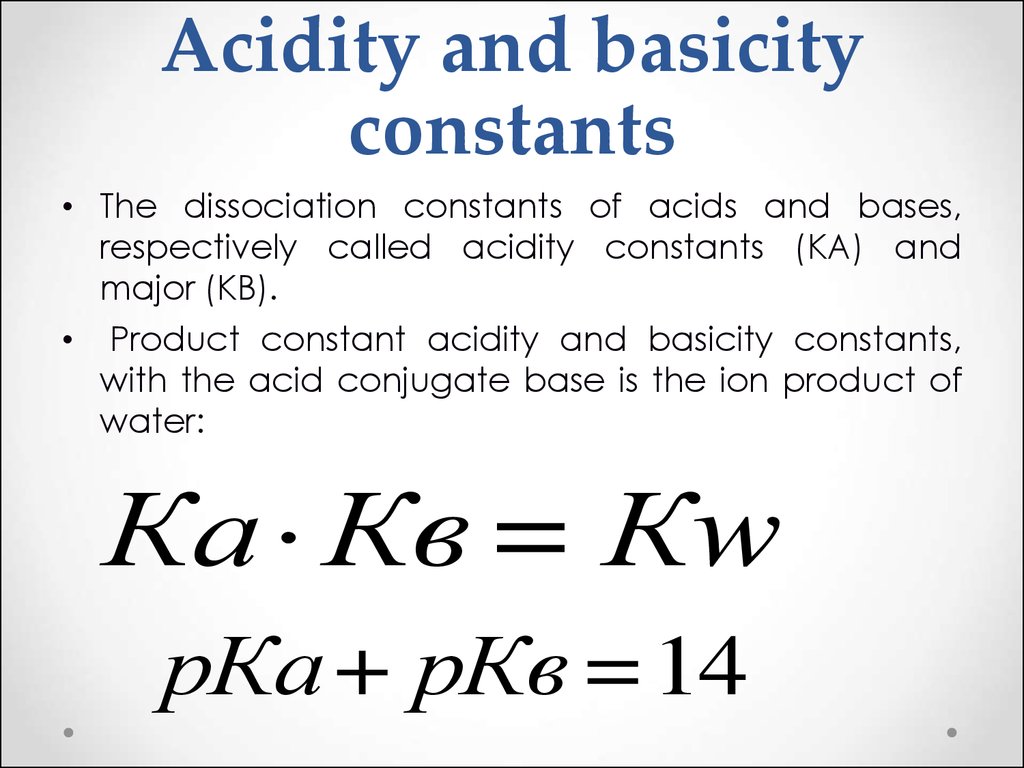
34. Acidity and basicity constants
• The dissociation constants of acids and bases,respectively called acidity constants (KA) and
major (KB).
• Product constant acidity and basicity constants,
with the acid conjugate base is the ion product of
water:
Ка Кв = Кw
рКа + рКв = 14
35.
36. Dissociation of water
H2OH+ + OH-
C
C
H
OH
K
C
H
2O
37.
14H
O H
OH
2
K
K
C
C
C
10
w
Kw is constant, ion product of water.
38. Hydrogen ion exponent
pH= -lg [H+]39.
pH Measurement• indicators
• pH - meters
40.
41. Protolytic theory
• Danish physicist and chemist Johannes Brønstedand the English chemist Thomas Lowry in 1928-1929
was offered Protolytic (protonic) theory of acids
and bases, according to which:
42.
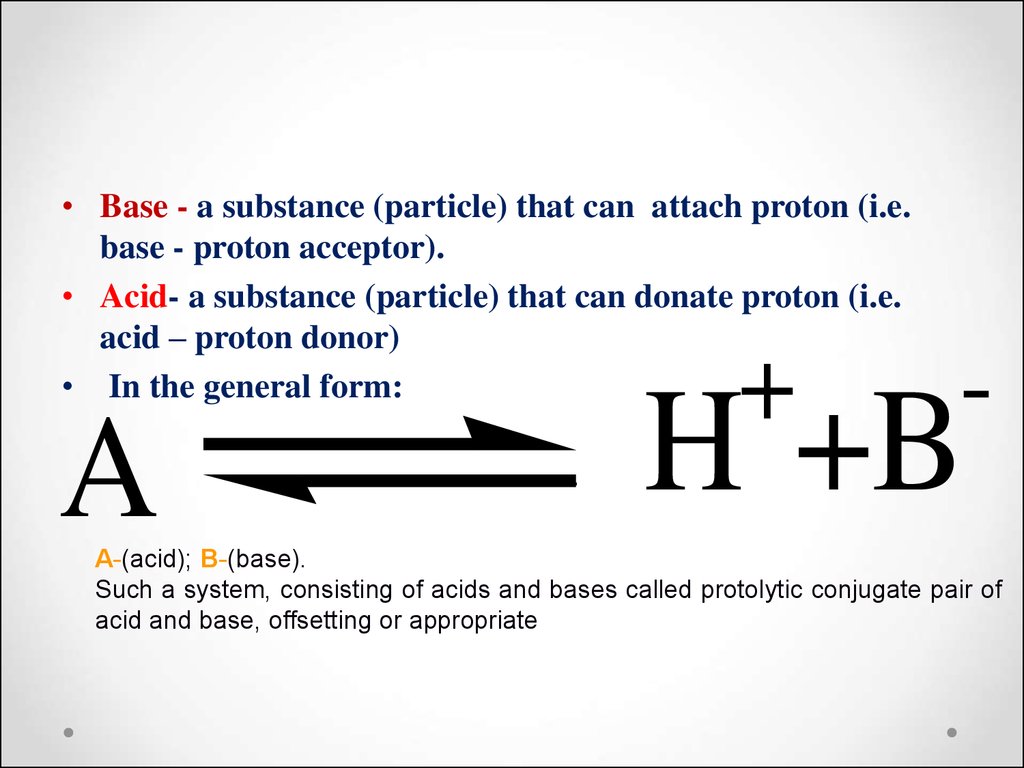
• Base - a substance (particle) that can attach proton (i.e.base - proton acceptor).
• Acid- a substance (particle) that can donate proton (i.e.
acid – proton donor)
• In the general form:
A
+
-
H +B
А-(acid); B-(base).
Such a system, consisting of acids and bases called protolytic conjugate pair of
acid and base, offsetting or appropriate
43. Salt - the reaction product of acid and base
• Example:HClO 4
Conjugated
acid
NH3+H+
Conjugated base
+
H
+ClO 4
Conjugated
base
+
NH4
Conjugated
acid
By this theory, acids and bases may be both neutral molecules
and ions (cations and anions).
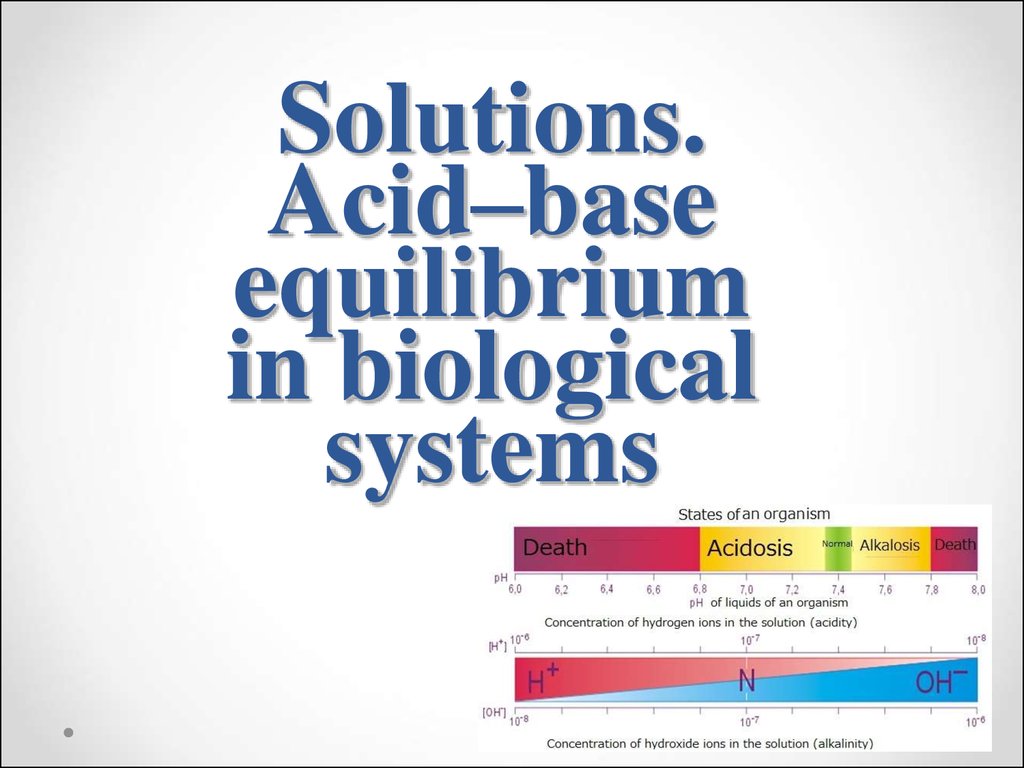
44. The homeostasis. The importancy of pH maintenance in human body
The human body has mechanisms ofcoordination of physiological and
biochemical processes proceeding inside
it and maintenance constancy of internal
medium (optimal value of pH, levels of
different substances, temperature, blood
preassure). This coordination and
mantanance are called homeostasis.
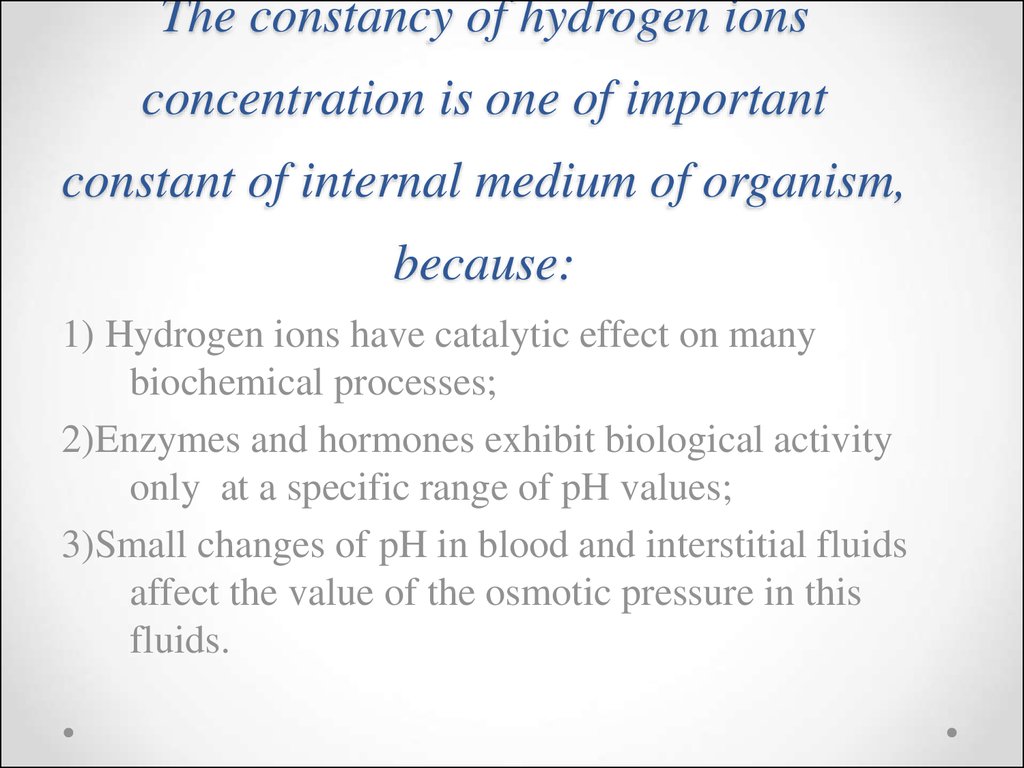
45. The constancy of hydrogen ions concentration is one of important constant of internal medium of organism, because:
1) Hydrogen ions have catalytic effect on manybiochemical processes;
2)Enzymes and hormones exhibit biological activity
only at a specific range of pH values;
3)Small changes of pH in blood and interstitial fluids
affect the value of the osmotic pressure in this
fluids.
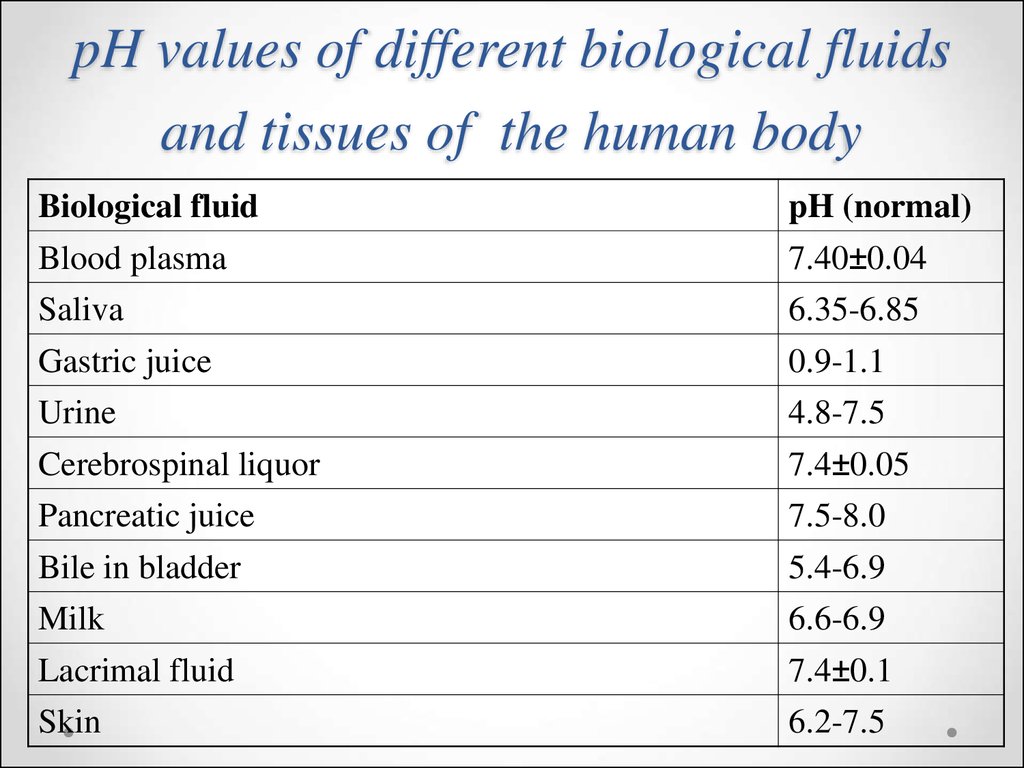
46. pH values of different biological fluids and tissues of the human body
Biological fluidpH (normal)
Blood plasma
7.40±0.04
Saliva
6.35-6.85
Gastric juice
0.9-1.1
Urine
4.8-7.5
Cerebrospinal liquor
7.4±0.05
Pancreatic juice
7.5-8.0
Bile in bladder
5.4-6.9
Milk
6.6-6.9
Lacrimal fluid
7.4±0.1
Skin
6.2-7.5
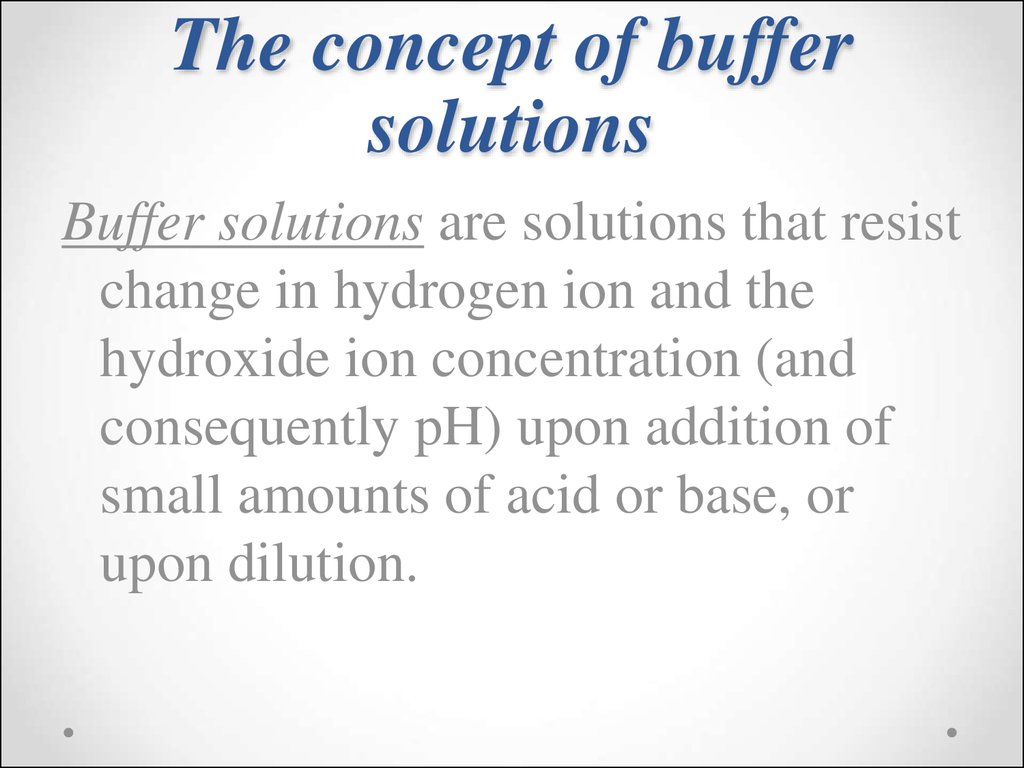
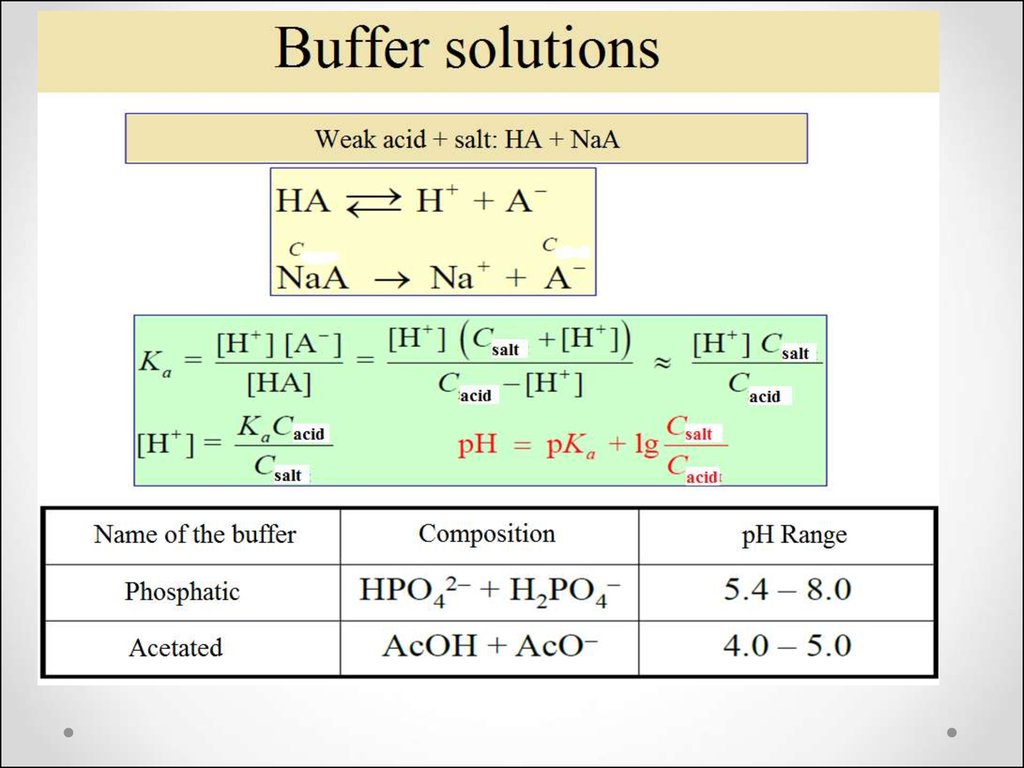
47. The concept of buffer solutions
Buffer solutions are solutions that resistchange in hydrogen ion and the
hydroxide ion concentration (and
consequently pH) upon addition of
small amounts of acid or base, or
upon dilution.
48.
49. The resistive action is the result of the equilibrium between the weak acid (HA) and its conjugate base (A−):
H+(aq) + A−(aq) → HA(aq)OH-(aq) + HA(aq) → A−(aq) +H2O(l)
50. Henderson-Hasselbah equation
Cb
pH
pK
lg
a
C
a
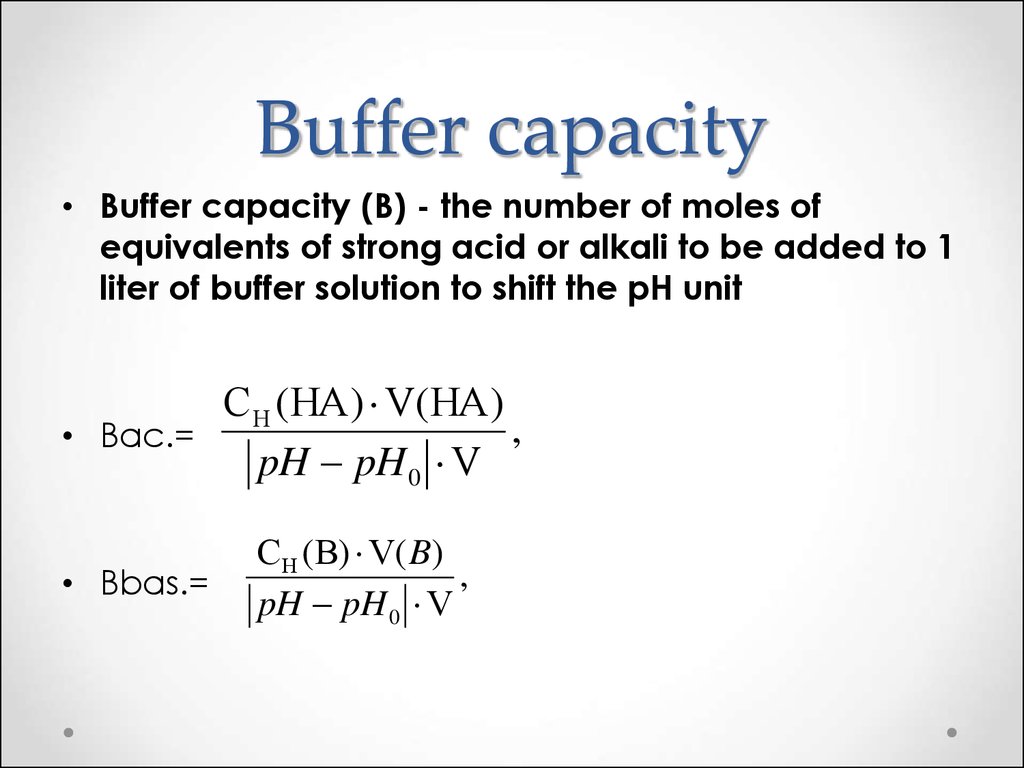
51. Buffer capacity
• Buffer capacity (B) - the number of moles ofequivalents of strong acid or alkali to be added to 1
liter of buffer solution to shift the pH unit
CH (HA) V(HA)
,
• Вac.=
pH pH 0 V
• Вbas.=
CH (B) V( B)
,
pH pH 0 V
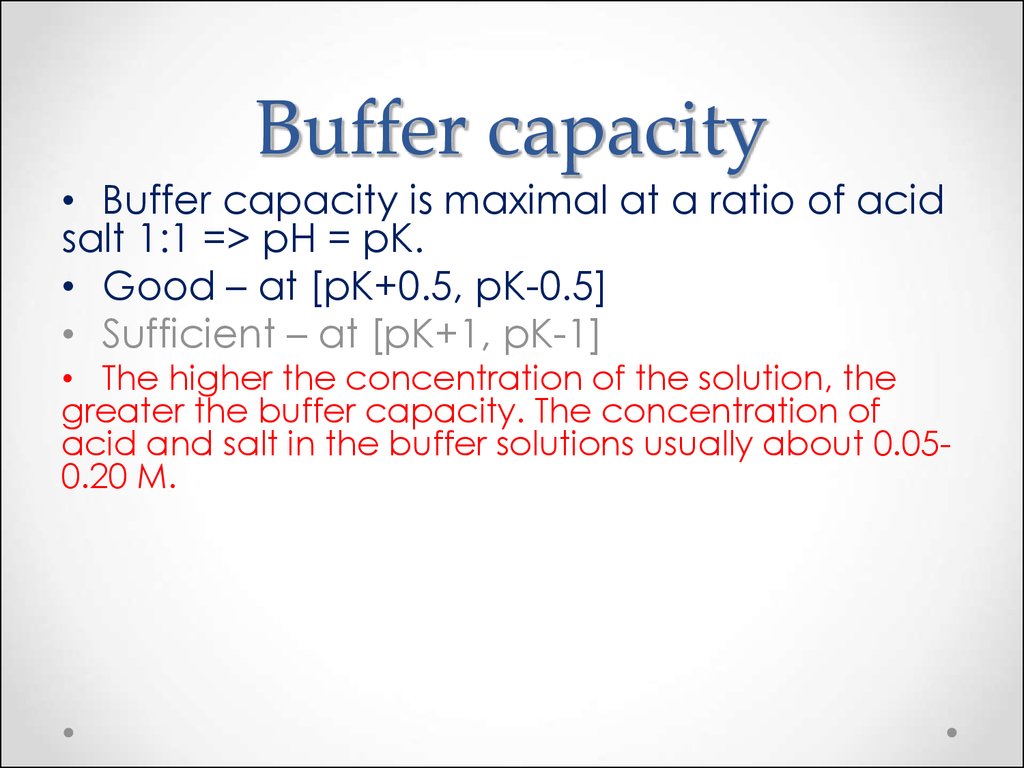
52. Buffer capacity
• Buffer capacity is maximal at a ratio of acidsalt 1:1 => pH = pK.
• Good – at [pK+0.5, pK-0.5]
• Sufficient – at [pK+1, pK-1]
• The higher the concentration of the solution, the
greater the buffer capacity. The concentration of
acid and salt in the buffer solutions usually about 0.050.20 M.
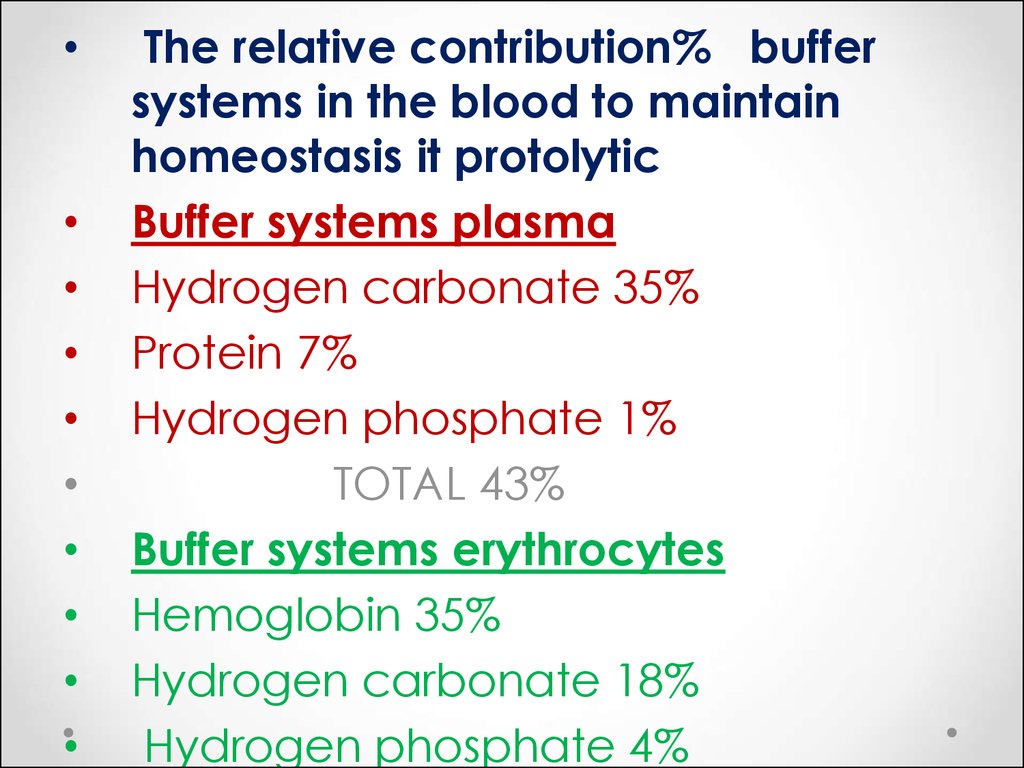
53.
The relative contribution% buffer
systems in the blood to maintain
homeostasis it protolytic
Buffer systems plasma
Hydrogen carbonate 35%
Protein 7%
Hydrogen phosphate 1%
TOTAL 43%
Buffer systems erythrocytes
Hemoglobin 35%
Hydrogen carbonate 18%
Hydrogen phosphate 4%
54. Hydrocarbonate buffer system
HCO3- +H+H2CO3+OHCO2+ H2O
H2CO3
HCO3-+ H2O
H2CO3
3
[
HCO
]
pH
pK
lg
a
[
H
CO
]
2
3
55.
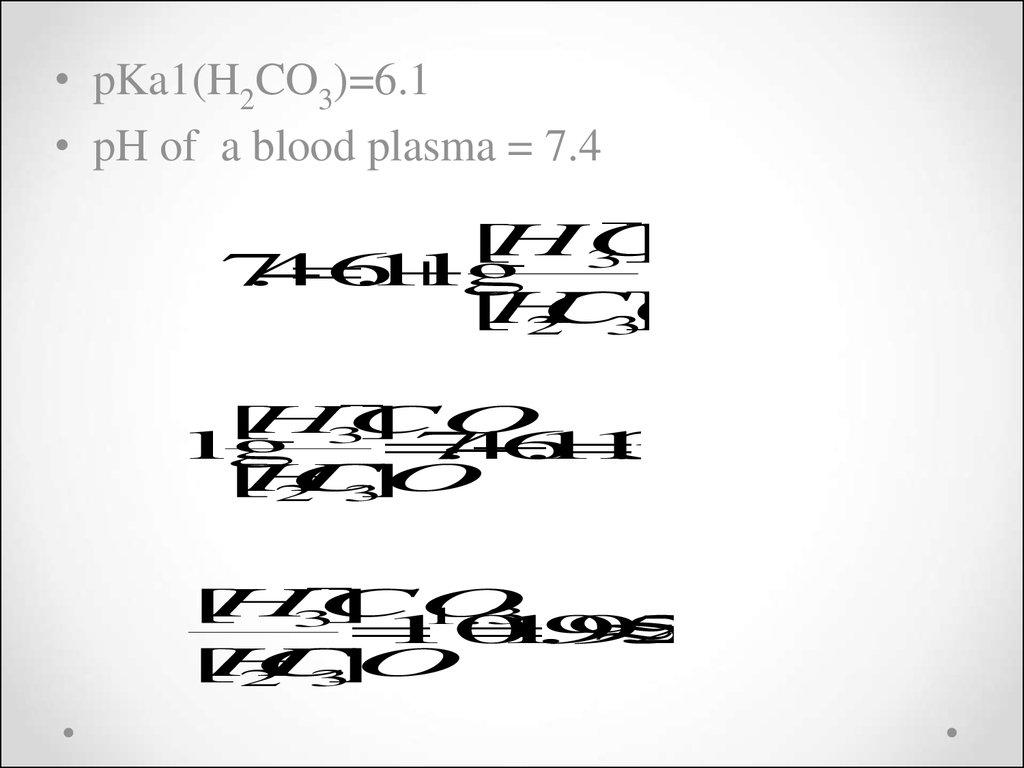
• pKa1(H2CO3)=6.1• pH of a blood plasma = 7.4
3
[
HCO
]
7
.4
6
.1
lg
[
H
CO
]
2
3
3
[
HCO
]
lg
7
.
4
6
.
1
1
.
3
[
H
CO
]
2
3
3
[
HCO
] 1.3
10
19
.
95
20
[
H
CO
]
2
3
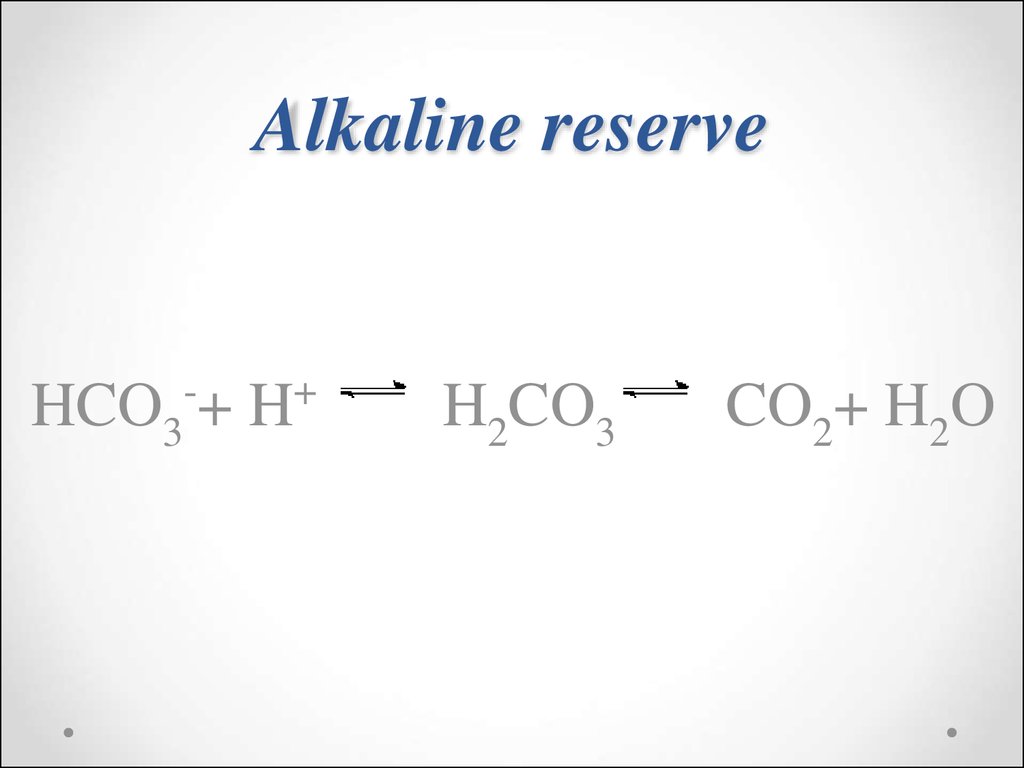
56. Alkaline reserve
HCO3-+ H+H2CO3
CO2+ H2O
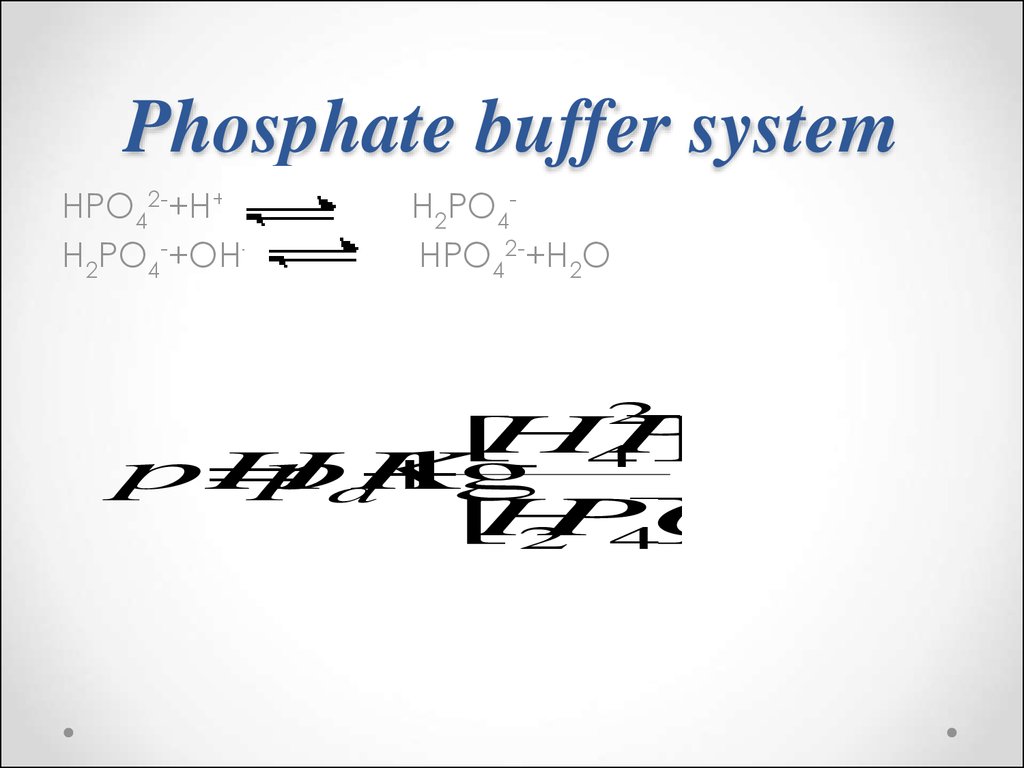
57. Phosphate buffer system
HPO42-+H+H2PO4-+OH-
H2PO4HPO42-+H2O
2
4
2 4
[
HPO
]
pH
pK
lg
a
[
H
PO
]
58.
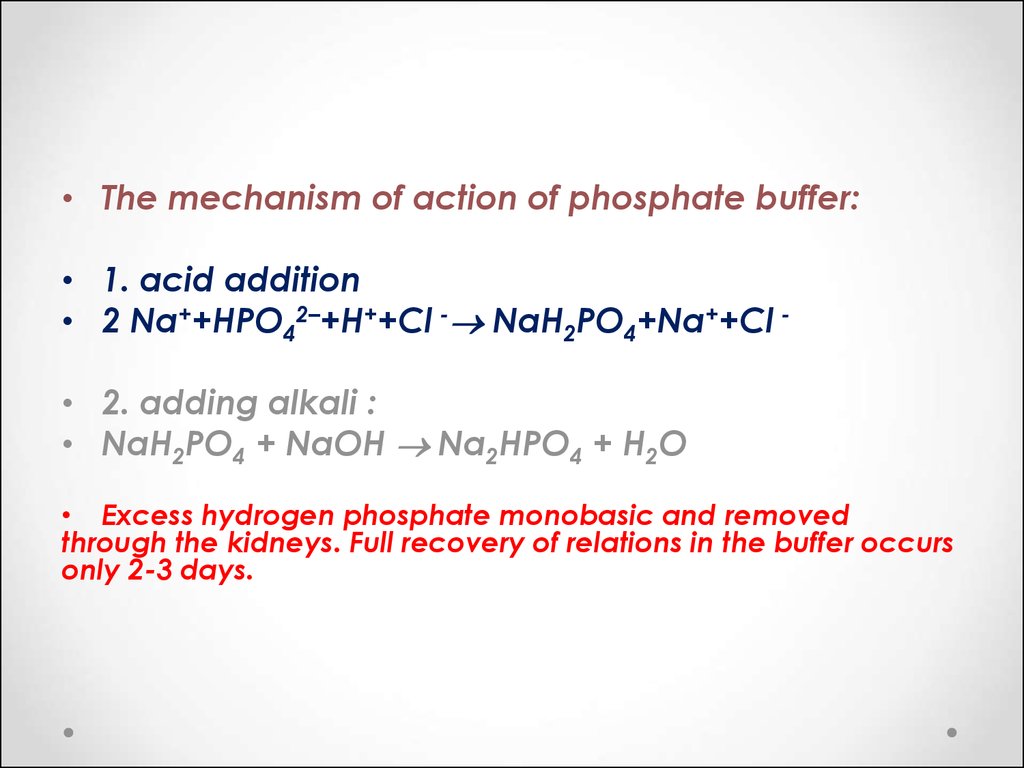
• The mechanism of action of phosphate buffer:• 1. acid addition
• 2 Na++HPO42–+H++Cl - NaH2PO4+Na++Cl • 2. adding alkali :
• NaH2PO4 + NaOH Na2HPO4 + H2O
• Excess hydrogen phosphate monobasic and removed
through the kidneys. Full recovery of relations in the buffer occurs
only 2-3 days.
59.
pKa(H2PO4-)=6.8pH of a blood plasma = 7.4
2
4
2 4
[
HPO
]
7
.4
6
.8
lg
[
H
PO
]
2
4
2 4
2
4
2 4
[
HPO
]
lg
7
.
4
6
.
8
0
.
6
[
H
PO
]
[
HPO
] 0
.
6
10
3
.
98
4
[
H
PO
]
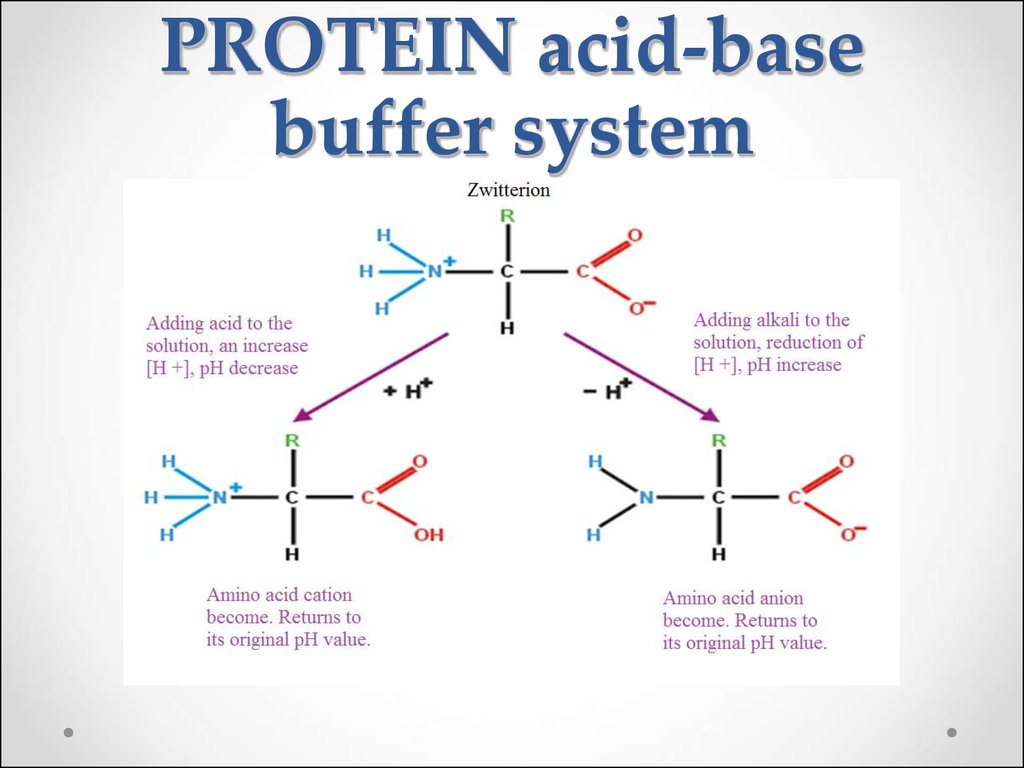
60. Protein buffer systems
The plasma proteins (albumins, globulins) are less important thanthe hemoglobin for maintenance of pH.
61. PROTEIN acid-base buffer system
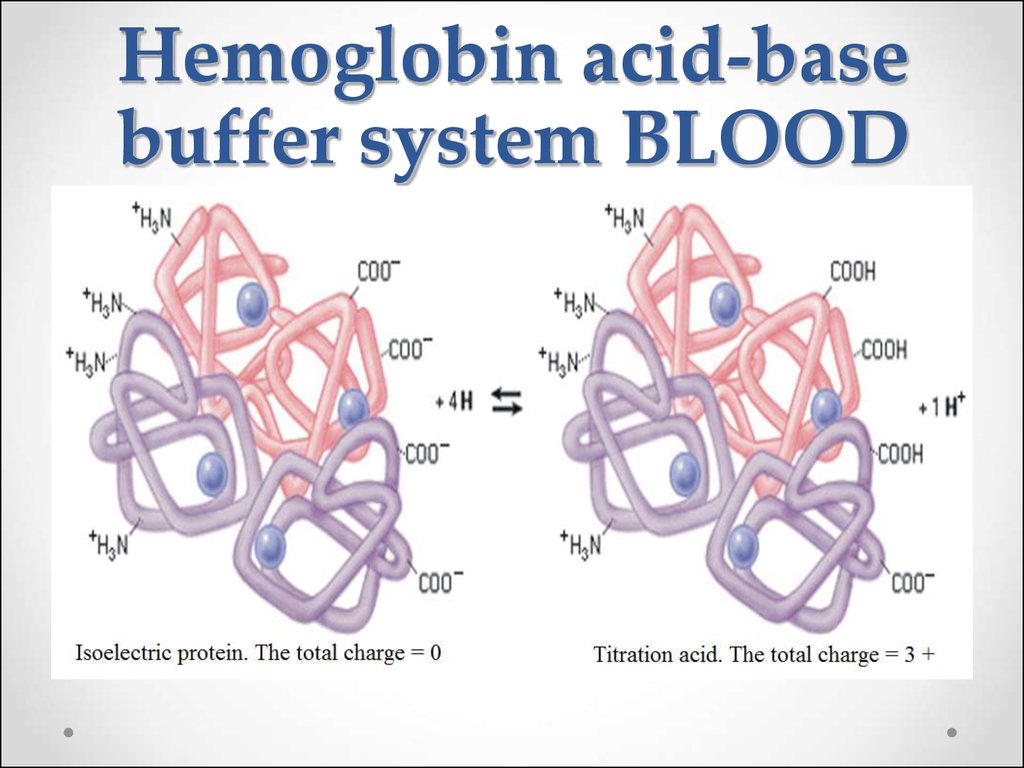
62. Hemoglobin buffer system
63.
64. Hemoglobin acid-base buffer system BLOOD
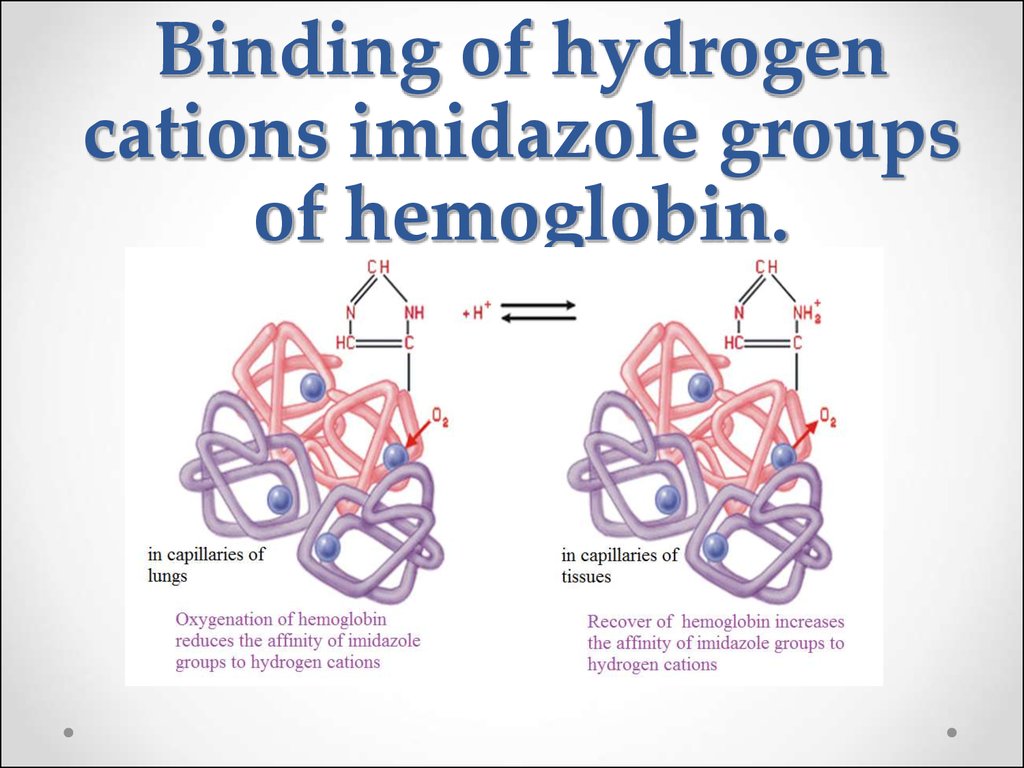
65. Binding of hydrogen cations imidazole groups of hemoglobin.
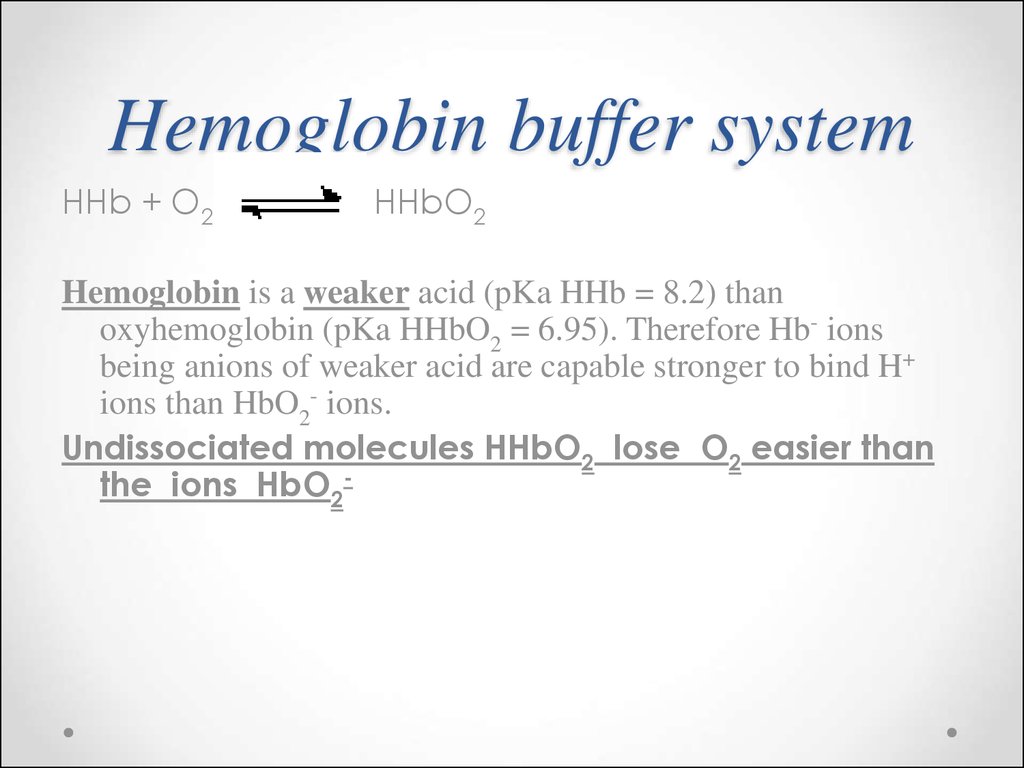
66. Hemoglobin buffer system
HHb + O2HHbO2
Hemoglobin is a weaker acid (pKa HHb = 8.2) than
oxyhemoglobin (pKa HHbO2 = 6.95). Therefore Hb- ions
being anions of weaker acid are capable stronger to bind H+
ions than HbO2- ions.
Undissociated molecules HHbO2 lose O2 easier than
the ions HbO2-
67.
a)the hemoglobin buffer system:HHb
H+ + Hb-;
b)the buffer system formed by
oxyhemoglobin:
HHbO2
H+ + HbO2-.
68.
In erythrocytes:HHbO2
HHb + O2 (1)
+
HHbO2
H + HbO2 (2)
HbO2
Hb + O2 (3)
69. In vessels of tissues
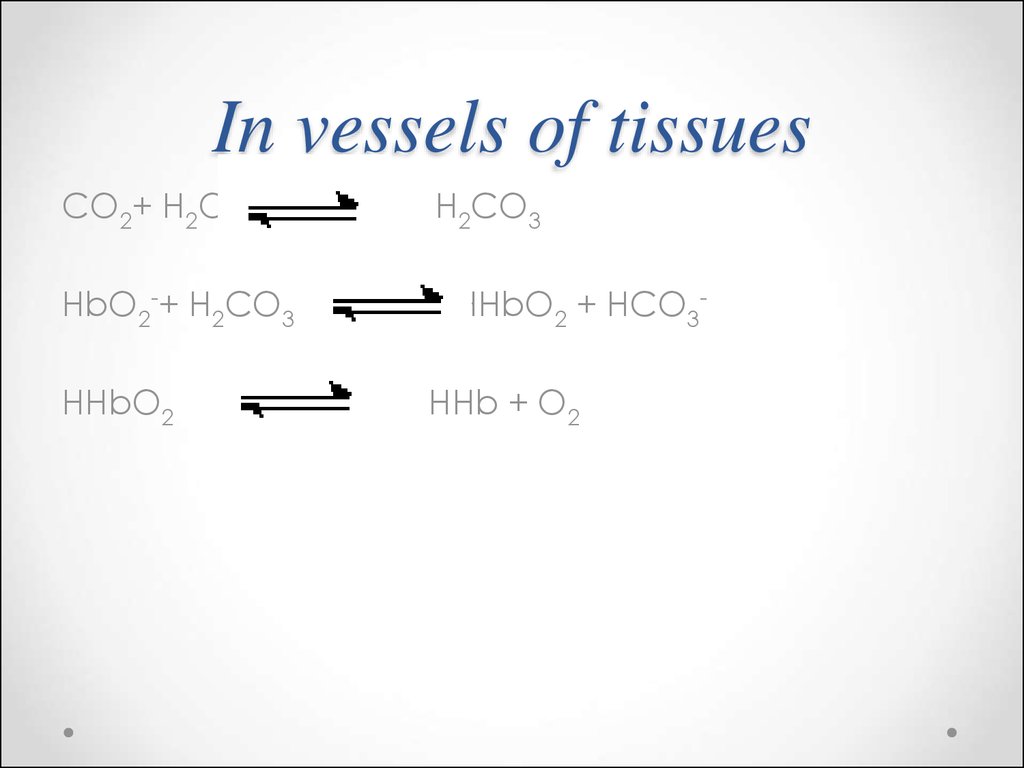
70.
71. In vessels of tissues
CO2+ H2OHbO2-+ H2CO3
HHbO2
H2CO3
HHbO2 + HCO3HHb + O2
72. In lungs
73.
74. In lungs
HHb + O2HHbO2+ HCO3-
H2CO3
HHbO2
HbO2-+ H2CO3
CO2+ H2O
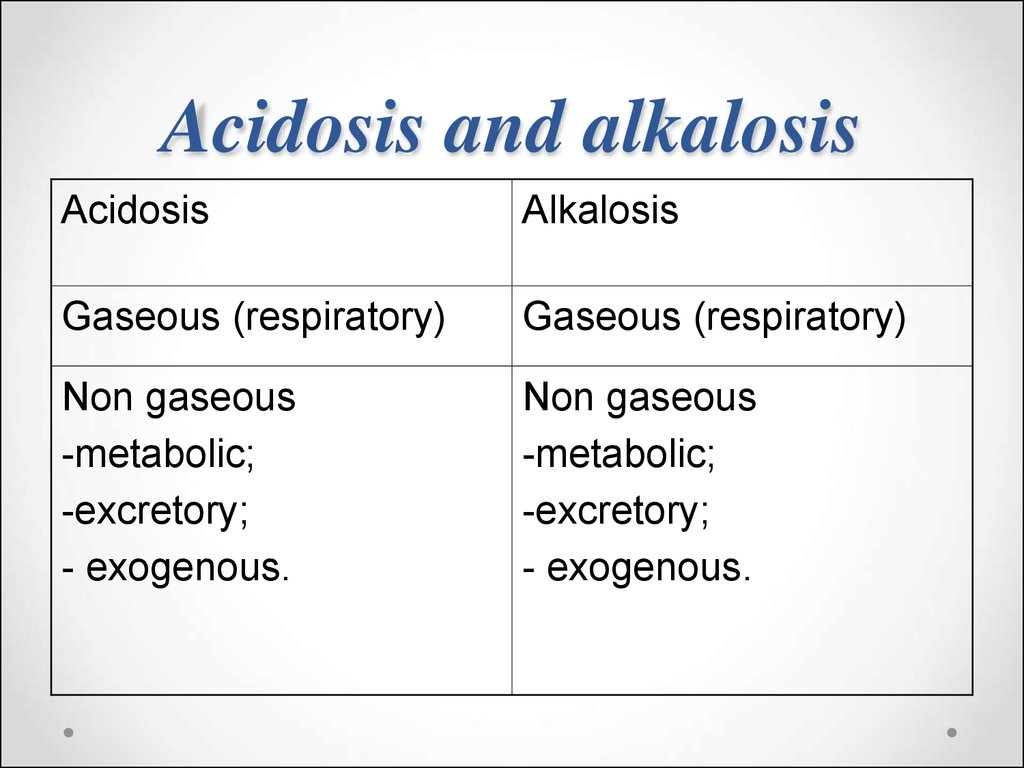
75. Acidosis and alkalosis
AcidosisAlkalosis
Gaseous (respiratory)
Gaseous (respiratory)
Non gaseous
-metabolic;
-excretory;
- exogenous.
Non gaseous
-metabolic;
-excretory;
- exogenous.
76. Literature
1. Medical Chemistry : textbook / V. A. Kalibabchuk [and al.] ;ed. by V. A. Kalibabchuk. - K. : Medicine, 2010.
2.http://www.chemeurope.com/en/encyclopedia/Buffer_solution.
html













































 Химия
Химия








