Похожие презентации:
Bacterial infections, sepsis
1. Neonatal sepsis
2. Background
• Neonatal sepsis may be categorized as earlyonset or late-onset. Of newborns with earlyonset sepsis, 85% present within 24 hours, 5%present at 24-48 hours, and a smaller
percentage present within 48-72 hours.
• Onset is most rapid in premature neonates.
• Early-onset sepsis is associated with acquisition
of microorganisms from the mother.
• Late-onset sepsis occurs at 4-90 days of life and
is acquired from the caregiving environment.
3. Pathophysiology
1. Specific microbial factors - The bacterial capsule polysaccharide ofStaphylococcus epidermidis adheres well to the plastic polymers of the
catheters; organism provides a barrier to the host defense
2. Host factors that predispose the newborn to sepsis
• cellular immunity
-neonatal PMNs are deficient in chemotaxis and killing capacity
-neutrophil reserves are easily depleted because of the diminished
response of the bone marrow, especially in the premature infant
-Formation of antigen-specific memory function after primary infection is
delayed
• humoral immunity
-lower levels of immunoglobulins are found with increasing prematurity
-Mature complement activity is not reached until infants are aged 6-10
months.
• barrier function.
-Skin and mucous membranes are broken down easily in the premature
infant.
-Neonates who are ill, premature, or both are at additional risk because of
the invasive procedures that breach their physical barriers to infection.
4. The microorganisms most commonly associated with early-onset infection
The microorganisms mostcommonly associated with earlyonset infection
Group B Streptococcus (GBS)
Escherichia coli
Coagulase-negative Staphylococcus
Haemophilus influenzae
Listeria monocytogenes
5.
Early onset sepsis in the United States6.
Early onset sepsis among very low birth weight infants in the United States7.
Early onset sepsis in developing nations.8. The most common risk factors associated with early-onset neonatal sepsis
• Maternal GBS colonization (especially ifuntreated during labor)
• Premature rupture of membranes (PROM)
• Preterm rupture of membranes
• Prolonged rupture of membranes
• Prematurity
• Maternal urinary tract infection
• Chorioamnionitis
9. Other factors that are associated with or predispose to early-onset neonatal sepsis
Low Apgar score (< 6 at 1 or 5 minutes)
Maternal fever greater than 38°C
Maternal urinary tract infection (UTI)
Poor prenatal care
Poor maternal nutrition
Low socioeconomic status
History of recurrent abortion
Maternal substance abuse
Low birth weight
Difficult delivery
Birth asphyxia
Meconium staining
Congenital anomalies
10. Organisms that have been implicated in causing late-onset sepsis
Coagulase-negative Staphylococcus
Staphylococcus aureus
E coli
Klebsiella
Pseudomonas
Enterobacter
Candida
GBS
Serratia
Acinetobacter
Anaerobes
11. Late-onset sepsis is associated with the following risk factors
• Prematurity• Central venous catheterization (duration
>10 days)
• Nasal cannula or continuous positive
airway pressure (CPAP) use
• H2 -receptor blocker or proton pump
inhibitor (PPI) use
• GI tract pathology
12.
Factors that confer a greater risk for LOS in the neonate.13. Meningitis
• The principal pathogens in neonatal meningitis are GBS (36% ofcases), E coli (31%), and Listeria species (5-10%). Other organisms
that may cause meningitis include the following:
• S pneumoniae
• S aureus
• S epidermidis
• H influenzae
• Pseudomonas species
• Klebsiella species
• Serratia species
• Enterobacter species
• Proteus species
14. Meningitis
Ventriculitis
Arachnoiditis
Vasculitis
Cerebral edema
Infarction
15. Epidemiology
• The incidence of culture-proven sepsis in theUnited States is approximately 2 per 1000 live
births.
• Of the 7-13% of neonates who are evaluated for
neonatal sepsis, only 3-8% have culture-proven
sepsis.
• Because early signs of sepsis in the newborn
are nonspecific, diagnostic studies are often
ordered and treatment initiated in neonates
before the presence of sepsis has been proved.
16.
Incidence of early-onset and late-onset invasive group B Streptococcus (GBS) disease.17. Age-, sex-, and race-related demographics
• Black infants have an increased incidence ofGBS disease and late-onset sepsis.
• In all races, the incidence of bacterial sepsis and
meningitis, especially with gram-negative enteric
bacilli, is higher in males than in females.
• Premature infants have an increased incidence
of sepsis.
• The risk of death or meningitis from sepsis is
higher in infants with low birth weight than in fullterm neonates.
18. History
• Maternal group B Streptococcus (GBS)status
• Premature rupture of membranes (PROM)
• Prematurity
• Chorioamnionitis
19. Physical Examination
• nonspecific clinical signs of early sepsis are alsoassociated with other neonatal diseases, such
as respiratory distress syndrome (RDS),
metabolic disorders, intracranial hemorrhage,
and a traumatic delivery.
• In view of the nonspecificity of these signs, it is
prudent to provide treatment for suspected
neonatal sepsis while excluding other disease
processes.
20. Signs and symptoms of neonatal infection (most are NONSPECIFIC):
• Apnea and dusky episodes for no clear reason.• Lethargy, poor color, hypoactivity, poor capillary
refill.
• Feeding intolerance (more than usual spit-up),
abdominal distention.
• Clinical appearance; doesn't look "good".
• Tachypnea, temperature instability, look of
distress.
NO GOLD STANDARD for the diagnosis of
neonatal infection
21. Congenital pneumonia and intrauterine infection
• Tachypnea, irregular respirations, moderate retracting, apnea,cyanosis, and grunting
The chest radiograph may depict bilateral consolidation or pleural
effusions.
– Klebsiella species and S aureus are especially likely to generate severe
lung damage, producing microabscesses and empyema.
– Early onset GBS pneumonia has a particularly fulminant course, with
significant mortality in the first 48 hours of life.
• Postnatally acquired pneumonia may occur at any age
• If the infant has remained hospitalized in an NICU environment,
especially with endotracheal intubation and mechanical ventilation,
the organisms may include Staphylococcus or Pseudomonas
species.
• , these hospital-acquired organisms frequently demonstrate multiple
antibiotic resistances.
22. Cardiac signs
• In overwhelming sepsis, an initial early phasecharacterized by pulmonary hypertension,
decreased cardiac output, and hypoxemia may
occur.
• This phase is followed by further progressive
decreases in cardiac output with bradycardia
and systemic hypotension. The infant manifests
overt shock with pallor, poor capillary perfusion,
and edema.
23. Metabolic signs
Hypoglycemia
Hyperglycemia
metabolic acidosis
jaundice
24. Neurologic signs
Impairment of consciousness (ie, stupor with or without irritability)
Coma
Seizures
Bulging anterior fontanelle
Extensor rigidity
Focal cerebral signs
Cranial nerve signs
Nuchal rigidity
Temperature instability
decreased tone
Lethargy
poor feeding
25. Differential Diagnoses
Bowel Obstruction in the Newborn
Congenital Diaphragmatic Hernia
Congenital Pneumonia
Heart Failure, Congestive
Hemolytic Disease of Newborn
Meconium Aspiration Syndrome
Necrotizing Enterocolitis
Pericarditis, Bacterial
Pulmonary Hypoplasia
Respiratory Distress Syndrome
26. Laboratory studies used to evaluate for sepsis
• complete blood count (CBC) and differential:thrombocytopenia or neutropenia, a left shift,
changes in the ratio of immature to total
neutrophils.
• blood and cerebrospinal fluid (CSF) cultures
• measurement of levels of C-reactive protein
(CRP) and other infection markers.
• Coagulation studies (DIC - abnormalities in the
prothrombin time (PT), the partial thromboplastin
time (PTT), and fibrinogen and D-dimer levels)
27. Hematologic signs
• Neutrophil ratios - the immature-to-total (I/T) ratio is themost sensitive.
-the maximum acceptable ratio for excluding sepsis during the
first 24 hours is 0.16.
-An I:T ratio of >0.2 has been considered abnormal
-Disseminated intravascular coagulation (DIC):abnormalities in
prothrombin time (PT), partial thromboplastin time (PTT),
and fibrinogen and D-dimer levels
If infants show signs consistent with impaired coagulation,
including gastric blood, bleeding from intravenous or
laboratory puncture sites, or other bleeding, evaluating
coagulation by checking these values is important.
28. CSF findings in infective neonatal meningitis
• Elevated WBC count (predominantlyPMNs)
• Elevated protein level
• Decreased glucose concentration
• Positive culture results
29. Approach Considerations
• When neonatal sepsis is suspected, treatmentshould be initiated immediately because of the
neonate’s relative immunosuppression.
• Begin antibiotics as soon as diagnostic tests are
performed
• Monitoring of blood pressure, vital signs,
hematocrit, platelets, and coagulation studies is
vital.
• An infant with temperature instability needs
thermoregulatory support with a radiant warmer
or incubator.
30. Antibiotic Therapy
• In the United States and Canada, the current approachto the treatment of early-onset neonatal sepsis includes
combined IV aminoglycoside and expanded-spectrum
penicillin antibiotic therapy.
• This provides coverage for gram-positive organisms,
especially group B Streptococcus (GBS), and gramnegative bacteria, such as Escherichia coli.
• If an infection appears to be nosocomial (late-onset
sepsis), antibiotic coverage should be directed at
organisms implicated in hospital-acquired infections,
including S aureus, S epidermidis, and Pseudomonas
species. Vancomycin and oxacillin has been favored for
this coverage
• Aminoglycosides and vancomycin both have the
potential to produce ototoxicity and nephrotoxicity and
should therefore be used with caution.
31.
Risk benefit uneven.32.
Risk benefit even.33. The need for continued therapy should be based not on the diagnostic data
Culture results
Maternal and intrapartum risk factors
CSF results
Complete blood cell (CBC) count and differential
C-reactive protein (CRP) trends
Radiographs
Clinical progress
34. Additional therapies that have been investigated for the treatment of neonatal sepsis
Granulocyte transfusion
IVIg infusion
Exchange transfusion
Recombinant cytokine administration
35. Prevention
• The Committee on Infectious Diseases ofthe AAP recommends that obstetric care
include a strategy for managing earlyonset GBS disease.
• Women with GBS bacteriuria should be
treated during pregnancy when the
condition is diagnosed and during the
intrapartum period.
36.
37.
38. Imaging studies in the workup of neonatal sepsis
• chest radiography to evaluate pulmonaryinvolvement
• computed tomography (CT)
• magnetic resonance imaging (MRI)
• ultrasonography of the head in cases of
meningitis.
39.
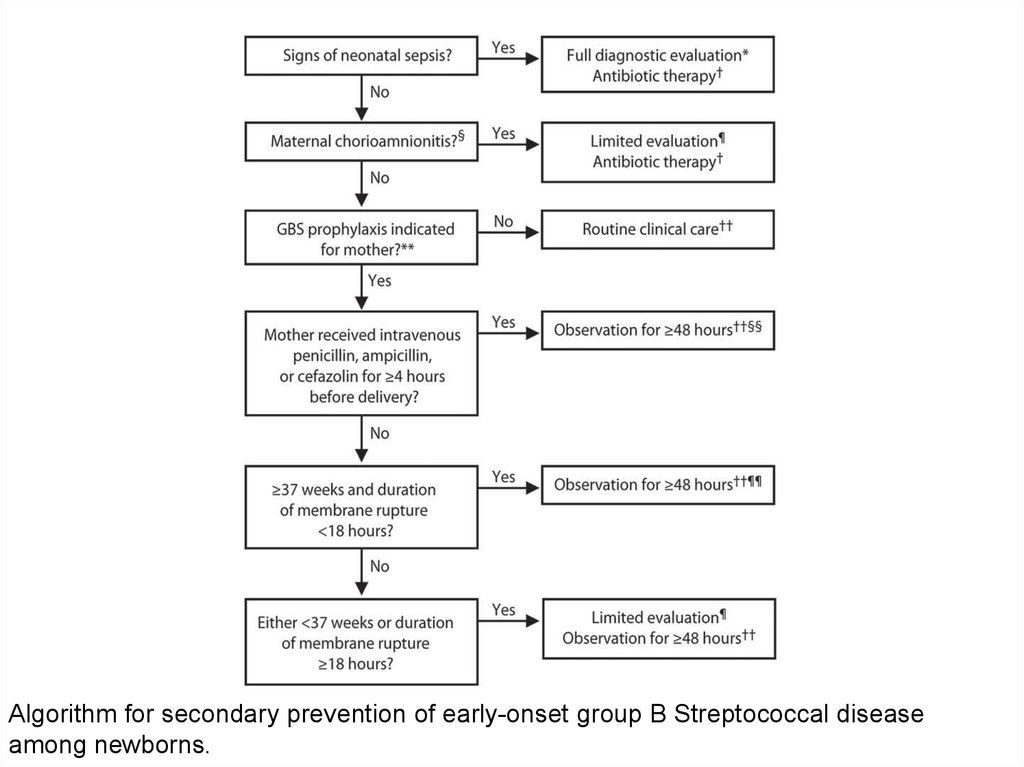
Algorithm for secondary prevention of early-onset group B Streptococcal diseaseamong newborns.
40. Antibiotics
• Ampicillin• Ampicillin is a beta-lactam antibiotic that is
bactericidal for susceptible organisms, such as
group B Streptococcus (GBS), Listeria, non–
penicillinase-producing Staphylococcus, some
strains of Haemophilus influenzae, and
meningococci. Some publications recommend
ampicillin (in combination with gentamicin) as
first-line therapy for suspected sepsis in the
newborn.
41. Antibiotics
• Gentamicin• Gentamicin is an aminoglycoside that is
bactericidal for susceptible gram-negative
organisms, such as Escherichia coli and
Pseudomonas, Proteus, and Serratia species. It
is effective in combination with ampicillin for
GBS and Enterococcus. Some publications
recommend gentamicin (in combination with
ampicillin) as first-line therapy for suspected
sepsis in the newborn.
42. Antibiotics
• Cefotaxime (Claforan)• Cefotaxime is a third-generation cephalosporin
with excellent in vitro activity against GBS and E
coli and other gram-negative enteric bacilli.
Good concentrations can be achieved in serum
and cerebrospinal fluid (CSF). Concern exists
that emergence of drug-resistant gram-negative
bacteria may occur more rapidly with cefotaxime
coverage than with traditional penicillin and
aminoglycoside coverage.
43. Antibiotics
• Vancomycin• Vancomycin is a bactericidal agent that is
effective against most aerobic and anaerobic
gram-positive cocci and bacilli. It is especially
important in the treatment of methicillin-resistant
Staphylococcus aureus (MRSA) and is
recommended when coagulase-negative
staphylococcal sepsis is suspected. However,
therapy with rifampin, gentamicin, or cephalothin
may be required in cases of endocarditis or CSF
shunt infection with coagulase-negative
staphylococci.
44. Antibiotics
• Chloramphenicol• Chloramphenicol has been shown to be effective in the
treatment of highly resistant bacterial meningitis. It
inhibits protein synthesis by binding reversibly to 50S
ribosomal subunits of susceptible organisms, which, in
turn, prevents amino acids from being transferred to
growing peptide chains.
• Oxacillin
• Oxacillin is a bactericidal antibiotic that inhibits cell wall
synthesis. It is used in the treatment of infections caused
by penicillinase-producing staphylococci. It may be given
as initial therapy when a staphylococcal infection is
suspected.
45. Antibiotics
• Metronidazole (Flagyl)• Metronidazole is an antimicrobial that has been shown to
be effective against anaerobic infections, especially
Bacteroides fragilis meningitis, ventriculitis, and
endocarditis. This agent is also useful in the treatment of
infections caused by Trichomonas vaginalis.
• Piperacillin
• Piperacillin is an acylampicillin with excellent activity
against Pseudomonas aeruginosa. It is also effective
against Klebsiella pneumoniae, Proteus mirabilis, B
fragilis, Serratia marcescens, and many strains of
Enterobacter. Administer it in combination with an
aminoglycoside.
46. Antibiotics
• Erythromycin base (Erythrocin, Ery-Tab, EryPed, E.E.S.)• Erythromycin is a macrolide antimicrobial agent that is
primarily bacteriostatic and is active against most grampositive bacteria, such as Neisseria species, Mycoplasma
pneumoniae, Ureaplasma urealyticum, and Chlamydia
trachomatis. It is not well concentrated in the CSF.
• Trimethoprim/sulfamethoxazole (Bactrim DS, Septra DS)
• Trimethoprim-sulfamethoxazole has been shown to be
effective in the treatment of highly resistant bacterial
meningitis. Trimethoprim-sulfamethoxazole inhibits bacterial
growth by inhibiting the synthesis of dihydrofolic acid.
Trimethoprim-sulfamethoxazole should not be used if
hyperbilirubinemia and kernicterus are of concern in the
newborn.
47. Antivirals
• Acyclovir (Zovirax)• Acyclovir is used for treatment of mucosal,
cutaneous, and systemic HSV-1 and HSV2 infections.
• Zidovudine (Retrovir)
• Zidovudine is a thymidine analogue that
inhibits viral replication. It is used to treat
patients with HIV infection.
48. Antifungals
Fluconazole (Diflucan)
Fluconazole is used to treat susceptible fungal infections, including
oropharyngeal, esophageal, and vaginal candidiasis. It is also used for
systemic candidal infections and cryptococcal meningitis. Fluconazole has
fungistatic activity. It is a synthetic oral antifungal (broad-spectrum
bistriazole) that selectively inhibits fungal CYP450 and sterol C-14 alphademethylation, which prevents conversion of lanosterol to ergosterol,
thereby disrupting cellular membranes.
Amphotericin B (AmBisome)
Amphotericin B is used to treat severe systemic infections and meningitis
caused by susceptible fungi, such as Candida and Aspergillus species,
Histoplasma capsulatum, and Cryptococcus neoformans. This agent is a
polyene produced by a strain of Streptomyces nodosus; it can be fungistatic
or fungicidal. Amphotericin B binds to sterols, such as ergosterol, in the
fungal cell membrane, causing intracellular components to leak and
subsequent fungal cell death.
Liposomal amphotericin B (AmBisome) may be considered for patients with
systemic fungal infections resistant to amphotericin B or for patients with
renal or hepatic failure. This product consists of amphotericin B within a
single-bilayer liposomal drug delivery system.














































 Медицина
Медицина Английский язык
Английский язык








