Похожие презентации:
Drugs in pregnancy
1. Drugs in pregnancy
CSMUDepartment of O&G №1
Docent Kamilova I.K.
2.
• The use of drugs in pregnant andlactating women requires a thorough
understanding of the unique
interactions between the mother,
fetus/infant, and the pharmacologic
agents that are used in therapy. Any
agent that is consumed by a woman
may have adverse effects on the
fetus/infant.
3.
The use of drugs during pregnancyGraviora quadem
sunt remedia
persculis
(some drugs worse
than the disease Lat.)
4.
5.
Therapeutic drug use is commonand required in pregnancy for:
• Maternal condition
• Pregnancy related
condition
• Fetal condition
6.
Maternal condition commonly requiringtherapy in pregnancy:
• Asthma
• Hypertension
• Psychiatric condition
Diabetes
• Thyroid dyfhunction
• Autoimmune disorders
7.
Pregnancy-related conditionscommonly requiring therapy
• Gestational diabetes
• Gestational hypertension
• Preterm
• Posterm
• Hyperemesis (toxemia of
pregnancy)
8.
Fetal conditions commonly requiringdrug therapy:
• Cardiac conditions
• Impending preterm delivery
• IUGR
• Intrauterine distress
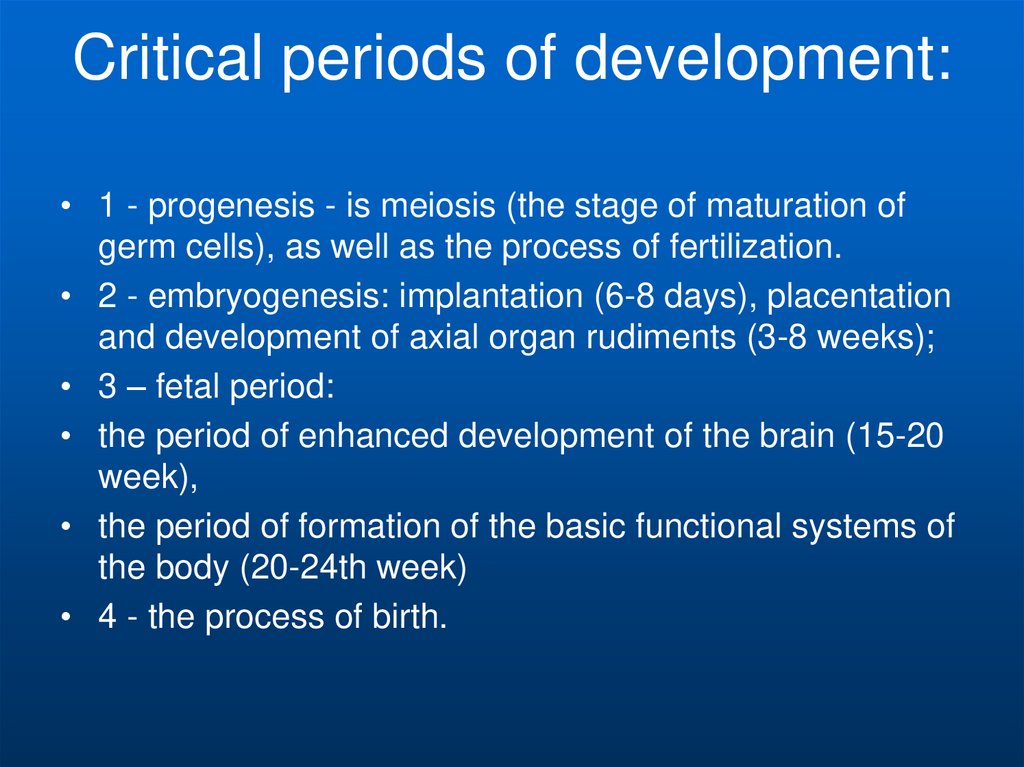
9. Critical periods of development:
• 1 - progenesis - is meiosis (the stage of maturation ofgerm cells), as well as the process of fertilization.
• 2 - embryogenesis: implantation (6-8 days), placentation
and development of axial organ rudiments (3-8 weeks);
• 3 – fetal period:
• the period of enhanced development of the brain (15-20
week),
• the period of formation of the basic functional systems of
the body (20-24th week)
• 4 - the process of birth.
10.
• First-trimester drugexposure has the largest
risk of malformations and
ideally all drug therapy
should be stopped before
attempting conception.
11. Drugs considered to be human teratogens (not exhaustive)
• ACE (adrenal cortical extract)inhibitors
• androgens
• antineoplastics (some)
• carbamazepine
• carbimazole
• danazol
• diethylstilboestrol
12. continuation
ethanol
lithium
misoprostil
penicillamine
phenytoin
tetracyclines
thalidomide
valproic acid
vitamin A & derivatives e.g. isotretinoin
warfarin
13.
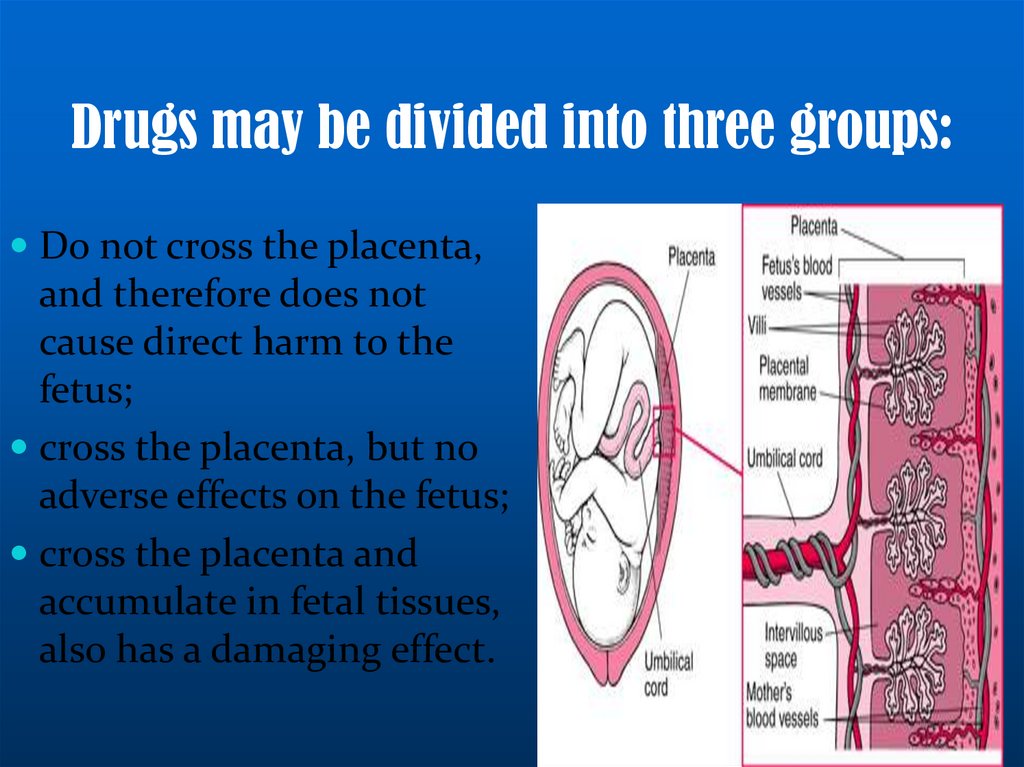
Drugs may be divided into three groups:Do not cross the placenta,
and therefore does not
cause direct harm to the
fetus;
cross the placenta, but no
adverse effects on the fetus;
cross the placenta and
accumulate in fetal tissues,
also has a damaging effect.
14. FDA Rating System for the Teratogenic Effects of Drugs
• The FDA, the government agency thatoversees the safety of drugs, provides the
most widely used system to grade the
teratogenic effects of medications. The
FDA assigns a safety category for
medications by using a 5-letter system: A,
B, C, D, and X. This safety category must
be displayed on the labels of all drugs.
15.
• A category• Summary: Fetal risk not revealed
in controlled studies in humans
Labeling: "Studies in pregnant women have not
shown that [the drug] increases the risk of fetal
abnormalities if administered during the first
[second, third, or all] trimester(s) of pregnancy. If
this drug is used during pregnancy, the
possibility of fetal harm appears remote.
Because studies cannot rule out the possibility of
harm, however, [the drug] should be used during
pregnancy only if clearly needed."
16.
• B category• Summary: Fetal risk not confirmed in studies in
humans but has been shown in some studies in
animals
Labeling: "Reproduction studies have been
performed in [animals] at doses up to [X] times
the human dose and have revealed no evidence
of impaired fertility or harm to the fetus due to
[the drug]. There are, however, no adequate and
well-controlled studies in pregnant women.
Because animal reproduction studies are not
always predictive of human response, this drug
should be used during pregnancy only if clearly
needed.
17.
• C category• Summary: Fetal risk revealed in studies in animals but
not established or not studied in humans; may use if
benefits outweigh risk to fetus
Labeling: "[The drug] has been shown to be
teratogenic (or to have an embryocidal effect or
other adverse effect) in [species] when given in
doses [X] times the human dose. There are no
adequate and well-controlled studies in pregnant
women. [The drug] should be used during
pregnancy only if the potential benefit justifies
the potential risk to the fetus."
18.
• D category• Summary: Fetal risk shown in humans;
use only if benefits outweigh risk to fetus
Labeling:
"The drug] can cause fetal harm when
administered to a pregnant woman.
If this drug is used during pregnancy, or if
the patient becomes pregnant while taking
this drug, the patient should be apprised of
the potential hazard to a fetus
19.
• X categorySummary: Contraindicated; benefit does
not outweigh risk
Labeling: "The drug may [or can] cause
fetal harm when administered to a
pregnant woman
The drug is contraindicated in women who
are or may become pregnant. If this drug
is used during pregnancy, or if the patient
becomes pregnant while taking this drug,
the patient should be apprised of the
potential hazard to a fetus."
20.
Oxytocin (Syntocinon)Octapeptide
Strong rhythmical contraction of
myometrium
Large doses- sustained contraction(↓
placental blood flow & fetal
hypoxia/death)
Clinical use:
- IOL (IVI 3U syntocinon+50 ml of saline)
- Augment slow labour (IVI same as
above)
-3rd stage of labour- 5 U IM for HTN
,cardiac disease
- IVI 40 U in 500ml
saline ( PPH)
-Surgical termination of preg./ERPC- 5U
slow IV
21.
ErgometrineSustained myometrial contraction &
vasoconstriction
Syntometrine IM:
5U syntocinon(rhythmic contraction in
2min) +
500µg ergometrine(sustained contraction
in 7 min)
Side effects – Nausea, vomiting,
abdominal pain, chest pain, palpitation,
severe HTN , Stroke & MI
Contraindication- HTN, Cardiac disease
Clinical use:
- Management of 3rd stage
- Management of PPH - 2nd dose give.
Alternatively IV ergometrine can be given
(works with in 40 sec)
22.
Dinoprostone( prostin E2)
Vaginal pessary/gel
Clinical use: IOL – 3mg 6hrs apart ( no more than 2 pessaries in
24hrs and max. 3 doses)
Side effect: Nausea ,vomiting, diarrhoea, fever,
Uterine hyperstimulation , HTN, bronchospasm
Advantages :
- Mobile patient
-Reduce need for syntocinon
23.
Carboprost ( Hemabate)Sustained myometrial contraction &
vasoconstriction
Syntometrine IM:
5U syntocinon(rhythmic contraction in
2min) +
500µg ergometrine(sustained contraction in
7 min)
Side effects – Nausea, vomiting, abdominal
pain, chest pain, palpitation, severe HTN ,
Stroke & MI
Contraindication- HTN, Cardiac disease
Clinical use:
- Management of 3rd stage
- Management of PPH - 2nd dose give.
Alternatively IV ergometrine can be given
(works with in 40 sec)
24.
Atosiban(Tractocile)Oxytocin receptor antagonist
Inhibition of uncomplicated preterm
labour between 24-33 weeks ( Tocolytic)
Contraindication: severe PET, eclampsia,
IUGR, IUD, placenta previa, placental
abruption, abnormal CTG, SROM after
30/40
Side effects: Nausea,vomiting,headache,
hot flushes, tachycardia, hypotension &
hyperglycemia
Dose- Stat IVI then continue infusion
until no contraction for 6 hrs.
25.
Other tocolyticsSalbutamol inhaler- 100 mcg x 2 puffs stat
Terbutaline- 250 mcg subcutaneous
Clinical use: both drugs are used for short term.
(i) relaxing uterus at C/S
(ii) ECV procedure
Side effects: Headache, palpitation, tachycardia, MI
,arrhythmias, hypotension & collapse
26.
NifedipineCalcium Channel blocker
Clinical use:
Mild to moderate- 5-20 mg TDS/PO
Severe HTN- 10 mg Retard/PO
Tocolytic- Incremental doses every 20 min until
contraction stop, then 20 mg TDS/PO
Side effects: Headache,dizziness,palpitation,
tachycardia, hypotension,sweating & syncope
27.
Mild /Moderate HPMethyldopa:
-Dose: 250mg BD/TDS ,
PO max dose 3g /day
-Side effects: Headache,dizziness,dry mouth , postural
hypotension,nightmares, mild psychosis, depression,hepatitis
& jaundice
- Important to stop drug in postnatal period
Labetolol 100-200mg BD/TDS PO max 2.4g/24hr
ACE inhibitors are contraindicated in pregnancy
28.
Severe Pre eclampsia / HPIV Labetolol (ß blocker):
- Side effects: headache,
nausea, vomiting,
postural hypotension & liver damage
- Contraindication: Asthma, marked bradycardia
IV hydralazine (vasodilator) :
- Side effects: headache,nausea, vomitting, dizziness, flushing,
tachycardia, palpitation & hypotension
- Because of hypotension preload with gelofusin adv.
- Contraindication- SLE, severe tachycardia & MI
29.
Magnesium SulphateClinical use: Prevention & treatment of
seizure in eclampsia / severe pre
eclampsia
Dose: 4g IV stat then 1g/hr to be
continued 24hr after last seizure
Side effects: nausea,vomiting,flushing,
drowsiness,confusion,loss of tendon
reflexes, hypotension, decrease U/O,
respiratory depression,
arrhythmias,cardiac arrest
Because of toxicity, Mg levels monitored
30.
Drugs in early pregnancyMifepristone- 200mg PO
Mechanism:
Antiprogestogenic steroid
Sensitizes myometrium to
prostaglandin-induced contractions
& ripens the cervix
Clinical use:
Medical termination of pregnancy
Medical management of miscarriage/IUD
Side effects: Gastro intestinal cramps, rash, urticaria,
headache,dizziness,
Contraindication: severe asthma
31.
MisoprostolSynthetic prostaglandin
PO/PV route
Clinical use:
- Medical TOP
- Medical management of miscarriage/ IUD
( For 1st trimester single dose of 400mcg
From 12- 34 weeks 400mcg 3hrly ,max 5
doses)
- Postpartum hemorrhage- 800mcg PR/PV
Side effects: nausea,vomiting, diarrhoea,
abdominal pain
32.
MethotrexateCinical use: Medical management of
ectopic pregnancy
Dose 50mg per kg/m2
Criteria- adenexal mass, non viable
pregnancy hCG< 3000U,
haemoperitonuem < 150ml
Side effects:
Disadvantage : repeated hCG levels,
emergency surgery
Advantage: Avoid surgery, tube preserved
33.
Menorrhagia / dysmenorrheaMefenamic acid:
- NSAID, reduces bleeding by 25%
- Dose: 250-500mgx TDS D1-3 of cycle or
PRN
- Side effects: Gastro-intestinal discomfort
nausea, diarrhoea, bleeding/ulceration
Tranexamic acid:
- Antifibrinolytic,reduces bleeding by 50%
- Dose: 1g TDS/QDS D1-4 of cycle
- Contraindication: thromboembolic
disease
- Side effects: nausea,vomiting,diarrhoea,
thrombo embolic event
34.
ProgestogensProgesterone is a hormone that naturally
occurs in the human body.
Vaginally dosed progesterone is being
investigated as potentially beneficial in
preventing preterm birth in women at
risk for preterm birth.
ART
Women with previous preterm labours cyclogest pessary 200mg PV/PR daily till
36 weeks
Following IVF/ICSI- Gestone inj +
cyclogest pessary
35.
Efficient, effective and safe use of drugs during pregnancyinvolves the following conditions:
• prescribe only established the security
of their applications, with well-known
pathways of metabolism in order to
avoid possible side effects;
due to the impossibility of determining
the period of final completion of
embryogenesis (in the absence of
urgent and uncontested evidence) it is
appropriate to postpone the use of
drugs to 22-24 weeks of pregnancy;
in the course of treatment requires
careful monitoring of the mother and
the fetus.
36.
37.
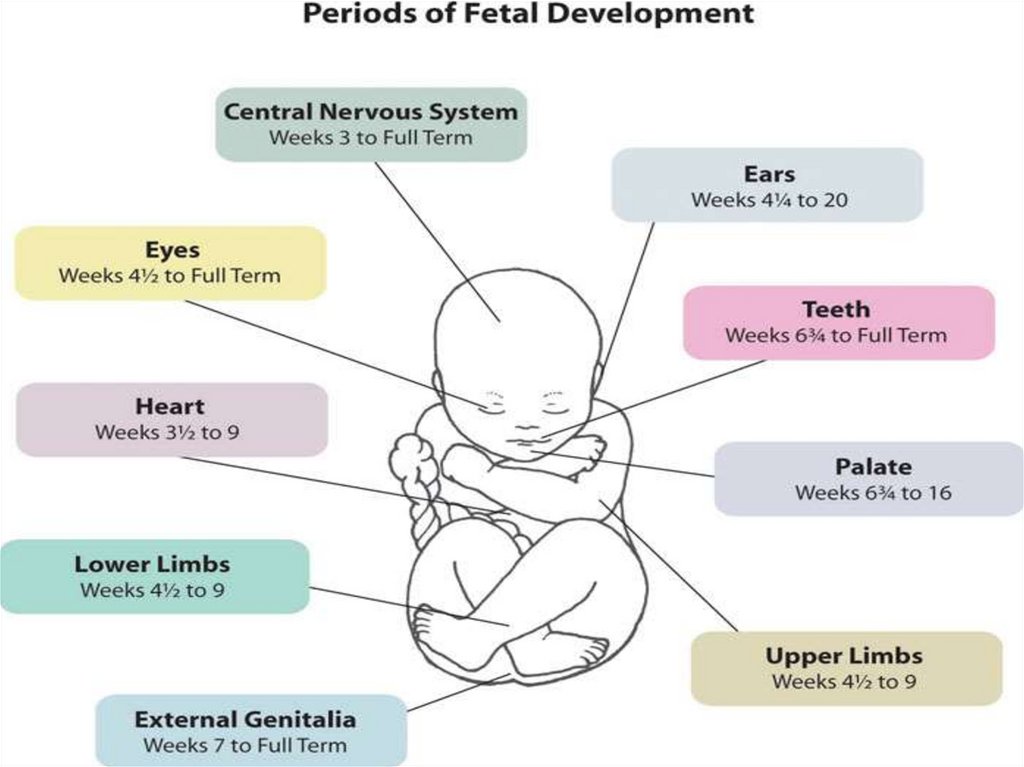
periods of pregnancy, when the fetus is mostsusceptible to the damaging effects of drugs:
1Up to 11 days from the moment of conception.
2. On the 11th day prior to the third week, when the fetus begins the
period of organogenesis.
3. Between 4 and 9 weeks of when the danger of fetal growth
retardation, but teratogenic practically does not occur.
4. The fetal period (9th week before birth). In this period, the
growth of structural defects usually do not occur, but may be in
breach of postnatal functions and various behavioral
abnormalities.
38. Critical periods of fetus development
• Pre-embryonic (days 0 – 17 postconception): drug exposure during thistime is not usually considered to pose
risk of malformations. An ‘all or nothing
response’ is said to occur ie. there is
early abortion or no adverse effect on
fetal development .
• However, the half-life of the drug must
be considered because many drugs
remain in the maternal circulation for a
long period after discontinuation.
39. continuation
• Embryonic (days 18 – 56post-conception): This is the
most important time in terms of
risk of fetal malformations.
40.
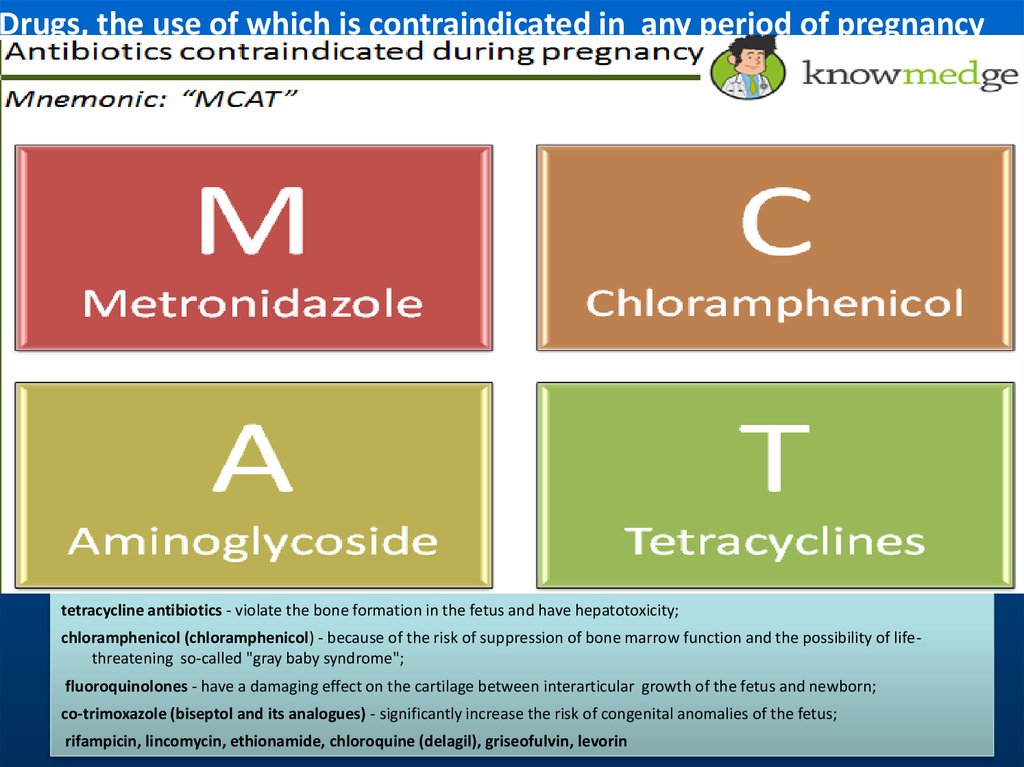
Drugs, the use of which is contraindicated in any period of pregnancytetracycline antibiotics - violate the bone formation in the fetus and have hepatotoxicity;
chloramphenicol (chloramphenicol) - because of the risk of suppression of bone marrow function and the possibility of lifethreatening so-called "gray baby syndrome";
fluoroquinolones - have a damaging effect on the cartilage between interarticular growth of the fetus and newborn;
co-trimoxazole (biseptol and its analogues) - significantly increase the risk of congenital anomalies of the fetus;
rifampicin, lincomycin, ethionamide, chloroquine (delagil), griseofulvin, levorin
41.
Drugs, the use of which is contraindicated in any period ofpregnancy
Other drugs:
All statins (lovastatin, simvastatin, Mevacor,
Zocor);
indirect anticoagulants (fenilin, pelentan);
Many antihistamines (diphenhydramine,
pipolfen, suprastin);
oral hypoglycemic agents;
antigonadotropnym drugs (danazol,
Clomid);
androgens;
Many antidepressants, barbiturates,
antipsychotics (haloperidol, teralen,
tizertsin);
benzodiazepines; antiparkinsonian agents
(parkopan, cyclodol, NAC);
Non-steroidal anti-inflammatory drugs
(meloxicam, phenylbutazone).
42. continuation
• Fetal period (days 56 – term): The risk ofmalformations is lower, but some
abnormalities may still occur because
development of organs/tissues such as the
central nervous system, teeth and
genitalia continues. For example, ethanol
exposure may affect central nervous
system development, and tetracyclines
may adversely discolor deciduous teeth
and suppress bone growth
43. Pharmacological risks
• Can be predicted based on themechanism of action of the drug
• The risk of some perinatal
complications may be reduced by
gradually reducing the maternal dose
towards the end of the third trimester
44. The effects of pregnancy on drug disposition (pharmacokinetics)
• The physiological changes thatoccur with pregnancy may affect
pharmacokinetics. These effects
vary with the drug and with the
individual, are generally difficult to
predict and frequently poorly
studied.
45. continuation
• Oral availability: Gastrointestinalmotility may be reduced during
pregnancy and this may result in
delayed absorption of orally
administered drugs. However, in the
vast majority of cases this is unlikely to
be of clinical significance as the total
amount of drug that is systemically
available will not change appreciably
46. continuation
• Distribution: Maternal water and fatcontent increases in pregnancy
and may increase the volume of
distribution of drugs. This should
only impact on those drugs that
are initiated with a loading dose,
when higher doses may be
required.
47. continuation
• Plasma albumin concentrationsdecrease in pregnancy and may result
in reduced protein binding of some
drugs .
48.
• For most drugs these changes should not impacton drug dosing because the unbound (active)
concentration should not change. However,
problems may arise when drug concentrations
are used to tailor drug therapy (eg.
anticonvulsants). Routine measured drug
concentrations are usually the total
concentrations ie. bound plus unbound (free).
Total drug concentrations may decline in
pregnancy so for drugs such as phenytoin it is
important to measure unbound concentrations
just prior to and during pregnancy.
49. Metabolism/elimination:
• Maternal drug clearance oftenincreases because of changes that
include increased renal and
hepatic blood flow and enzyme
induction. This generally means
that increased maintenance doses
of both metabolized and renally
eliminated drugs may be required
in pregnancy.
50.
• For example, drugs that are extensivelyrenally eliminated (eg. penicillins) will
have enhanced clearance in pregnancy
because of increased glomerular
filtration rates. Increased hepatic
metabolism of drugs is variable [2] but
can be expected to result in increased
dosage requirements in the third
trimester for agents such as phenytoin
and methadone.
51. General principles
• Avoid all drugs in pregnancy wherepossible, especially in the firsttrimester.
• Herbal and other complementary
therapies are often perceived by the lay
public as 'safe'. Unfortunately, data on
many of these products are very limited
and insufficient to determine their
safety in pregnancy. In general, herbal
remedies should be avoided during
pregnancy.
52. continuation
• Consider tapering and discontinuingunnecessary pharmacotherapy prior to
attempting conception. Remember that
some drugs or their metabolites may
have long half-life and persist for some
time after stopping therapy.
• Many conditions are self-limiting and
do not require drug treatment.
Reassurance or lifestyle measures (e.g.
avoidance of migraine triggers) may be
sufficient.
53. continuation
• Where possible, delay treatment untilafter results of laboratory testing (e.g.
swabs), or until after delivery.
• If drug therapy is needed, select drugs
with the most established safety
record.
54. continuation
• Use the lowest effective dose for theshortest possible time. Note: poor
control of some maternal disease
states may carry significant risk to the
development of the foetus. In addition,
the severity or frequency of some
maternal diseases (e.g. migraines) may
improve in pregnancy allowing a
reduction in the dosage of some drugs,
or cessation of treatment.
55. continuation
• It is also important to realise thatsome mothers may be very
anxious about the risks that their
drug therapy poses to their baby.
This may lead to noncompliance
with drug therapy, or unnecessary
pregnancy terminations.













































 Медицина
Медицина








