Похожие презентации:
Clinical pathophysiology of the respiratory system
1.
Clinical pathophysiologyof the respiratory system
Lecturer, Kushkova N. E.
2.
Viral Respiratory TrackInfections
3.
EtiologyAcute respiratory infections are combined into the one group on
the basis of:
- A unified airborne transmission mechanisms
- Development of the main pathological process in the RT with
similar clinical symptoms
Viruses of acute respiratory diseases belong to different
taxonomic families and differ in structure and biological
characteristics. Human pathogenic respiratory viruses belong to
5 viral families, represented by 7 generations.
Acute respiratory viral diseases are uncontrollable infections,
which is due to the antigenic variability of influenza and the
absence of vaccine prevention for other ARIs.
4.
ARI pathogensRhinitis, pharyngitis - Phinoviruses (Picornaviridae)
Rhinopharyngitis – Enteroviruses - Coxsackie; ЕСНО (Picornaviridae)
Hemorrhagic conjunctivitis - Enterovirus 70, Coxsackie A24, ECHOvirus 7 (Picornaviridae)
Tracheitis, rhinopharyngitis - Influenza A, B, C (Orthomyxoviridae)
Hemorrhagic pulmonary edema – Influenza A, B; H5N1 avian
Influenza (Orthomyxoviridae)
Laryngitis – Parainfluenza 1-4 (Paramyxoviridae)
Bronchitis, bronchiolitis, pneumonia - Respiratory Syncytial.
Metapneumovirus. Bocavirus (Paramyxoviridae; Parvaviridae)
Pharyngitis, conjuctivis, keratitis epidemic - Adenoviruses
(Adenoviridae)
Pharyngitis, gastroenteritis, “atypical” coronavirus pneumonia
(Coronaviridae)
Mononucleosis, pharyngitis - Epstein-Barr virus (Herpesviridae)
Cardiopulmonary syndrome – Sin-Nombre (Bunyaviridae)
5.
InfluenzaFamily of Influenza - Orthomyxoviridae.
Influenza types – A & B
2 surface glycoproteins with hemagglutinating (HA) &
neurominidase (NA) activity,
There are presently known 17 subtypes of hemagglutinin
(human subtypes – H1, H2, H3) and 9 subtypes of
neuraminidase (human influenza viruses – N1, N2)
As a result of the disease acquires typospecific immunity,
recurrent diseases are due to infection with strains of
influenza virus with new antigenic properties.
6.
PathogenesisVirus replication in epithelial cells of the RT (trachea &
bronchi) → Virusemia (in 10-14 days)
The main element in the Pathogenesis is damage to the
vascular system and to the nervous system
Virus induces apoptosis in endothelial cells + releasing of free
oxygen radicals, lysosomal enzymes by neutrophils
→ Capillary walls destruction
→ increased vascular conductivity, fragility of it walls,
microcirculatory disorders.
→ Haemorrhagic manifestations from nasal bleeding to
haemorrhagic pulmonary edema and cerebral haemorrhages.
7.
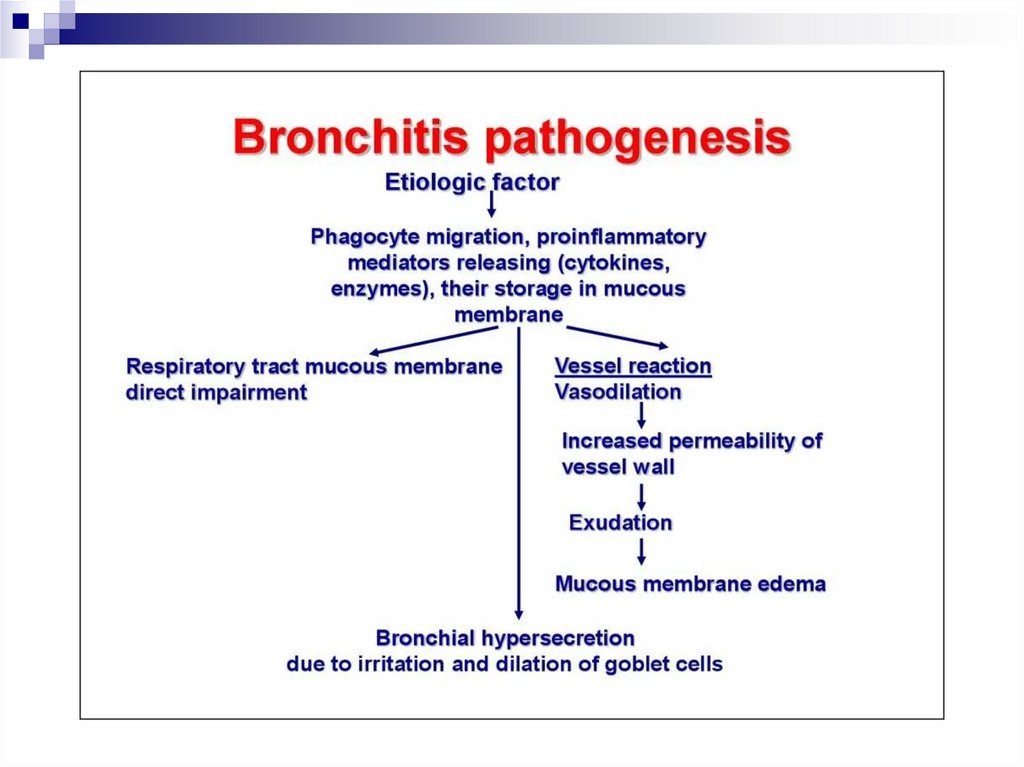
PathogenesisDystrophic changes in tracheal and bronchial epithelium with
involvement of submucosa and vasculature → catarrhal symptoms
(rhinitis, dry cough, dry wheezing)
Sharp drop in small vessel tone, increased endothelial permeability:
1. → scleral injection, mucosal petechiae
2. → damage to the alveolicapillary membrane → multiple
haemorrhages into the interstitium and development of Acute
Respiratory Distress Syndrome (ARDS) → Acute RF
3. → hypersecretion of cerebrospinal fluid → intracranial hypertension,
cerebral edema → headache, meningism
Immune response activation - interferons, T-lymphocytes, antibodies.
The severe acute on phase response - (fever, weakness, myalgia,
arthralgia)
Bacterial flora activation with the development of secondary infections
8.
TreatmentBasic (antiviral) therapy –
no later then 48 hs (standart – 24-36 hs)
- Oseltamivir (Tamiflu)
- zanamivir (Relenza)
- Inhavirin
- arbidol
- viferon
Respiratory support (oxygen inhalation, non-invasive
ventilation and Artificial lung Ventilation)
Pathogenetic and symptomatic therapy - paracetamol, ACC
(including antioxidant for ARDS), GCS (for shock and ARDS)
Antibiotics: Generation III-IV of cephalosporins,
carbapenems, generation IV of respiratory fluoroquinolones
are used as initial empirical therapy for pneumonia;
vancomycin or linezolid are used for staphylococcal aetiology.
9.
Коронавирусы (Coronaviridae)большое семейство РНК-содержащих
вирусов, способных инфицировать как
животных, так и человека.
У людей могут вызвать заболевания от
легких форм острой респираторной
инфекции (ОРВИ) до тяжелого острого
респираторного синдрома (ТОРС или
SARS).
10.
ПатогенезВходные ворота – эпителий ВДП и
эпителиоциты желудка и кишечника.
Начальный этап - проникновение SARS-CoV-2 в
клетки-мишени, имеющие рецепторы
ангиотензинпревращающего фермента II типа
(АПФ2).
АПФ2 располагается в ЦПМ в альвеолярных
клетках II типа, энтероцитах тонкого кишечника,
эндотелии артерий и вен, ГМК артерий,
макрофагах
Основная мишень – альвеолярные клетки →
диффузное альвеолярное повреждение
11.
«Цитокиновый шторм» прикритическом течении COVID-19
патологическая активация
врожденного и приобретенного иммунитета,
«дисрегуляция» синтеза «провоспалительных»,
иммунорегуляторных, «антивоспалительных»
цитокинов и хемокинов: ИЛ1, ИЛ2, ИЛ6…, КСФ, ФНОα,
ИФН, а также маркеров воспаления (СРБ, ферритин)
гиперактивация иммунного ответа
часто ограничивается легочной паренхимой,
прилегающей лимфоидной тканью и ассоциируется с
развитием ОРДС
развивается васкулярная эндотелиальная
дисфункция, коагулопатия, тромбозы с наличием
антител к фосфолипидам
12.
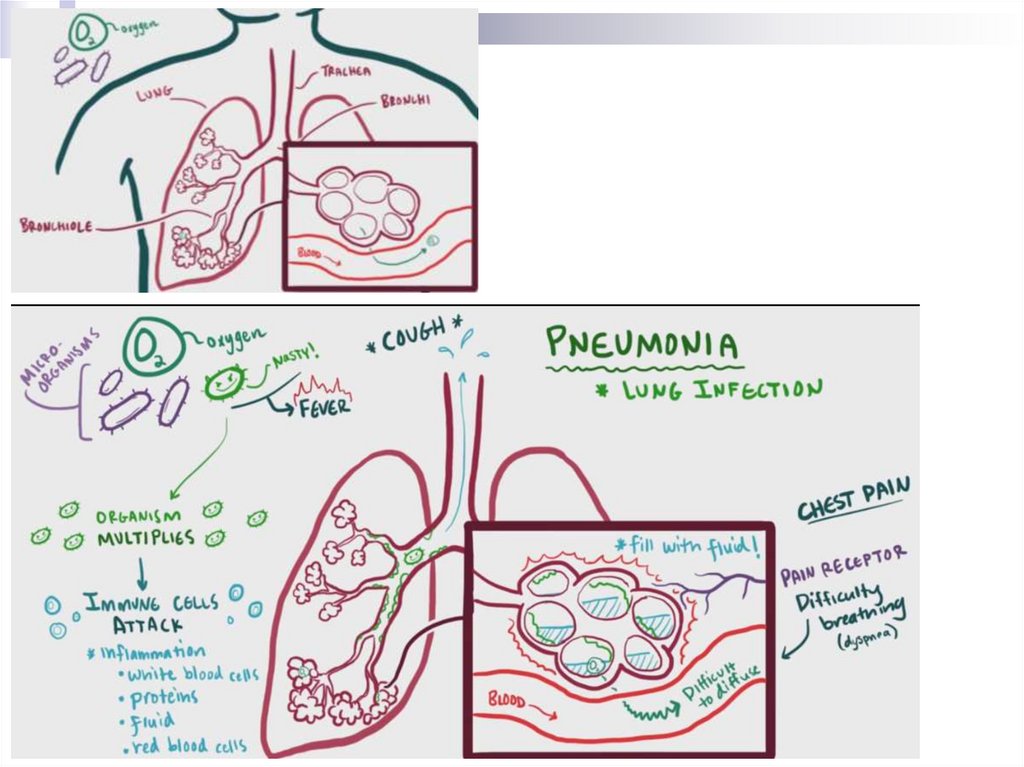
Pneumonia- Infection of the distal parts of the RT, included
by pathological process of the alveoli, bronchi
with small calibres and bronchioles
The result from the reproduction of pathogens
and the host’s response to it presence in the
respiratory parts of the lungs
13.
EtiologyStreptococcus pneumoniae - 30-50%
Haemophilus in uenzae, Staphylococcus aureus, Escherichia
coli, Klebsiella pneumoniae, Pseudomonas aeruginosa,
Acinetobacter spp – 3-5%
“Atypical” pathogens (Mycoplasma pneumoniae,
Chlamydophila pneumoniae, Legionella pneumophila,
Chlamydophila psittaci) – 8-30%
Viruses (avian (H5N1) and swine (H1N1) influenza,
coronavirus)
Pneumocystis jiroveci
The main route of infection is the aspiration of oropharyngeal
secretions containing colonising microorganisms; can be
reactivation of latent infection; haematogenous dissemination
14.
PathogenesisAspiration of oropharyngeal contents + failure "self-
cleansing" mechanisms of the tracheobronchial
tree
→ infection of bronchi, bronchioles, alveoli
→ activation of the immune response
→ leucocyte migration, mediator releasing,
hypersecretion, fluid releasing into the lumen of the
alveoli
→ cough, acute RF (breathlessness, hypoxia), pain
syndrome, acute phase of the response
manifestations
15.
16.
Therapy principlesRational anti-infective therapy
Mucolytics
Detoxification therapy
Immunocorrection
Oxygen therapy
Antipyretics – with indications!
17.
Chronic obstructive pulmonarydisease
18.
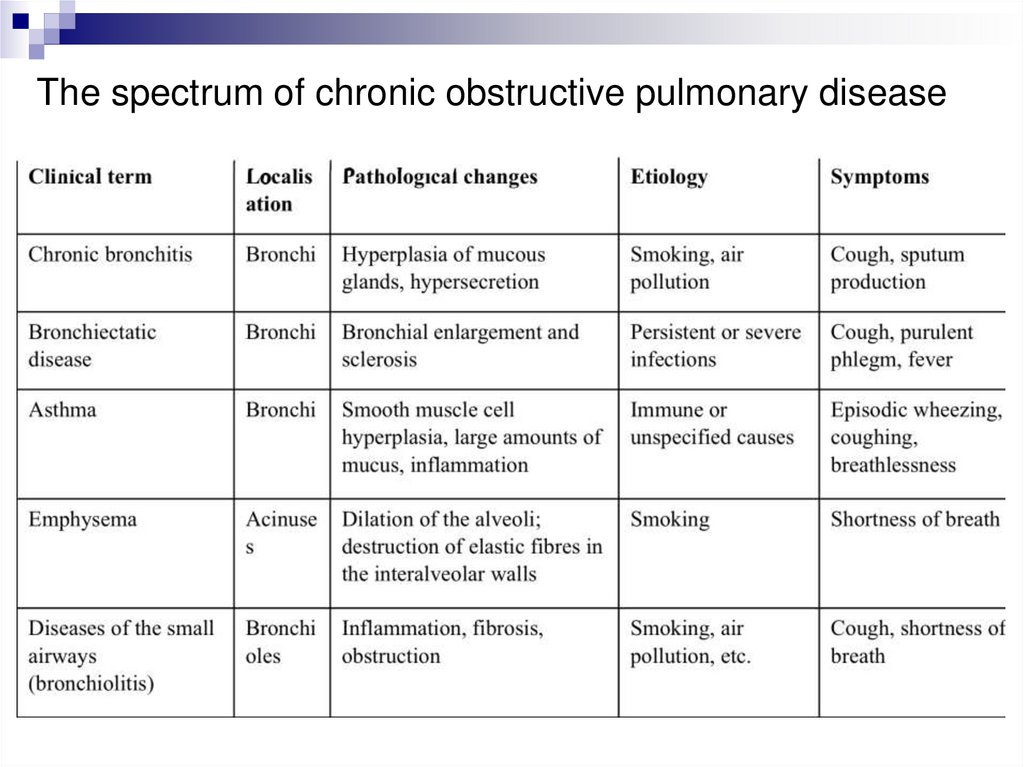
The spectrum of chronic obstructive pulmonary disease19.
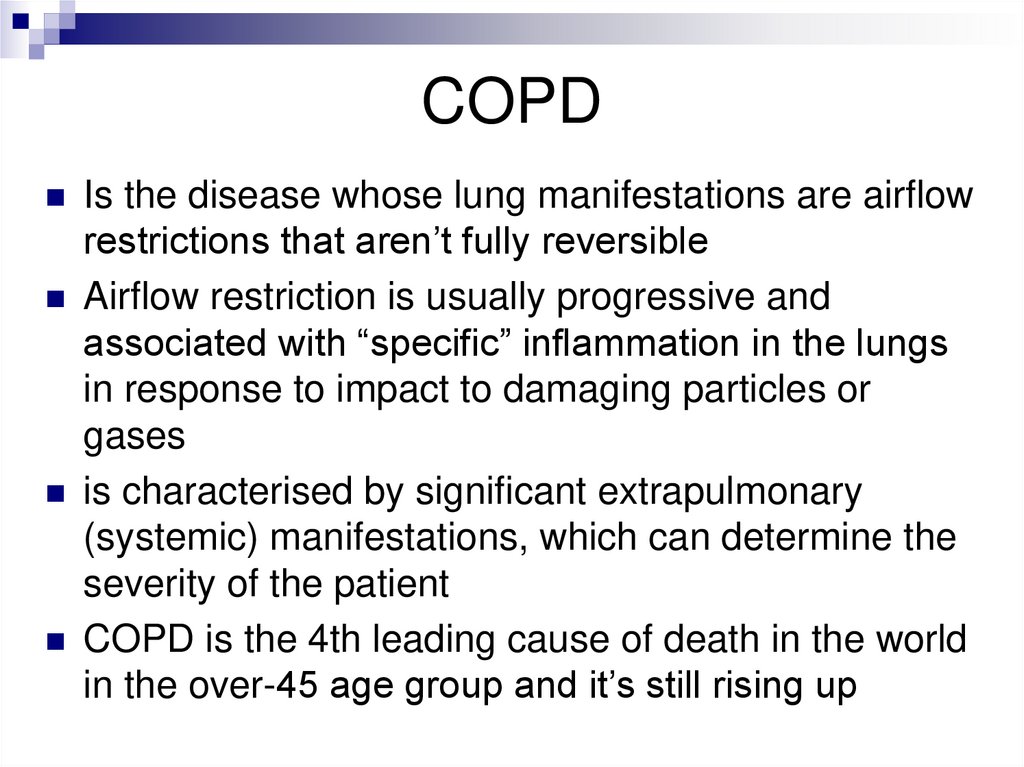
COPDIs the disease whose lung manifestations are airflow
restrictions that aren’t fully reversible
Airflow restriction is usually progressive and
associated with “specific” inflammation in the lungs
in response to impact to damaging particles or
gases
is characterised by significant extrapulmonary
(systemic) manifestations, which can determine the
severity of the patient
COPD is the 4th leading cause of death in the world
in the over-45 age group and it’s still rising up
20.
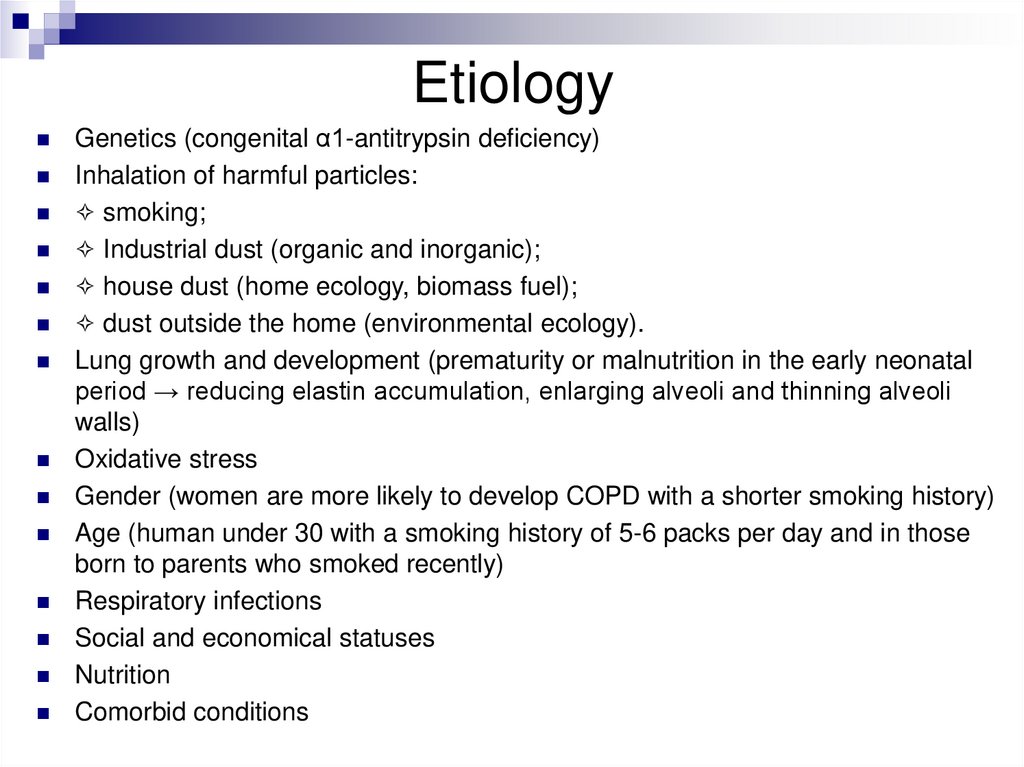
EtiologyGenetics (congenital α1-antitrypsin deficiency)
Inhalation of harmful particles:
✧ smoking;
✧ Industrial dust (organic and inorganic);
✧ house dust (home ecology, biomass fuel);
✧ dust outside the home (environmental ecology).
Lung growth and development (prematurity or malnutrition in the early neonatal
period → reducing elastin accumulation, enlarging alveoli and thinning alveoli
walls)
Oxidative stress
Gender (women are more likely to develop COPD with a shorter smoking history)
Age (human under 30 with a smoking history of 5-6 packs per day and in those
born to parents who smoked recently)
Respiratory infections
Social and economical statuses
Nutrition
Comorbid conditions
21.
PathogenesisEnvironmental risk factors + genetic predisposition = chronic
inflammatory process (affects all bronchial morphological
structures of different calibres, interstitial (peribronchial) tissue
and alveoli)
The main components of pathogenesis are oxidative stress,
proteolytic destruction of tissue, immune deficiency, and
microbial colonisation.
Main effector cells: neutrophils, their action enhanced by
lymphocytes, macrophages, eosinophils, mast cells, epithelial
cells, vascular endothelial cells.
Pro-inflammatory mediators form the main pathophysiological
components of COPD, primarily determining bronchial
obstruction.
Inflammation becomes a self-sustaining process
22.
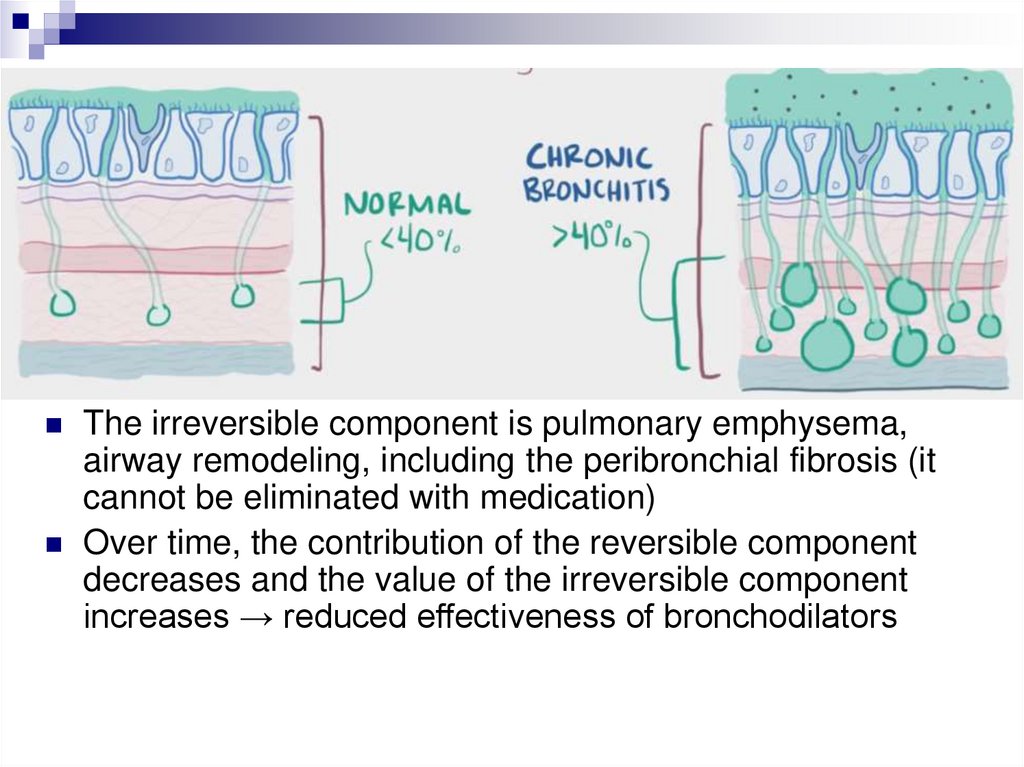
Бронхиальная обструкцияОбратимый компонент (отек, гиперсекреция,
бронхоспазм) формируется непосредственно
воспалительной реакцией, возникающей под влиянием
большого спектра провоспалительных медиаторов (IL8, ФНО-α, нейтрофильных протеаз и свободных
радикалов), может быть устранена под влиянием
соответствующих лечебных мероприятий
The irreversible component is pulmonary emphysema,
airway remodeling, including the peribronchial fibrosis (it
cannot be eliminated with medication)
Over time, the contribution of the reversible component
decreases and the value of the irreversible component
increases → reduced effectiveness of bronchodilators
23.
The main pathogenetic mechanisms of COPDInflammation
- increased in the number of inflammatory cells and cells
activation: CD8+ lymphocytes, monocytes/macrophages,
neutrophils
- increased production of inflammatory mediators: IL-8, TNF-a,
leukotrienes-B4, oxidants.
- protease/antiprotease imbalances.
- microorganisms colonisation
Mucociliary dysfunction
- bronchial mucus hypersecretion
- Reduction of mucociliary transport
- mucosal damage
24.
The main pathogenetic mechanisms of COPDStructural changes
- Hyperplasia/metaplasia of the bocalytic cells
- hypertrophy of mucous glands
- hypertrophy of smooth muscles
- Fibrosis of the airways
- alveolar destruction
Reduced exhaled airflow rate
- obstruction/disruption of the alveolar attachment to the bronchioles,
- spasm and hypertrophy of the smooth muscles,
- swelling of the mucous membrane.
- loss of elastic traction of the alveoli
Systemic (extrapulmonary) mechanisms
- Hypotrophy & reduced body mass index
- osteopenia & osteoporosis
- Skeletal muscle damage: weakness, hypotrophy
25.
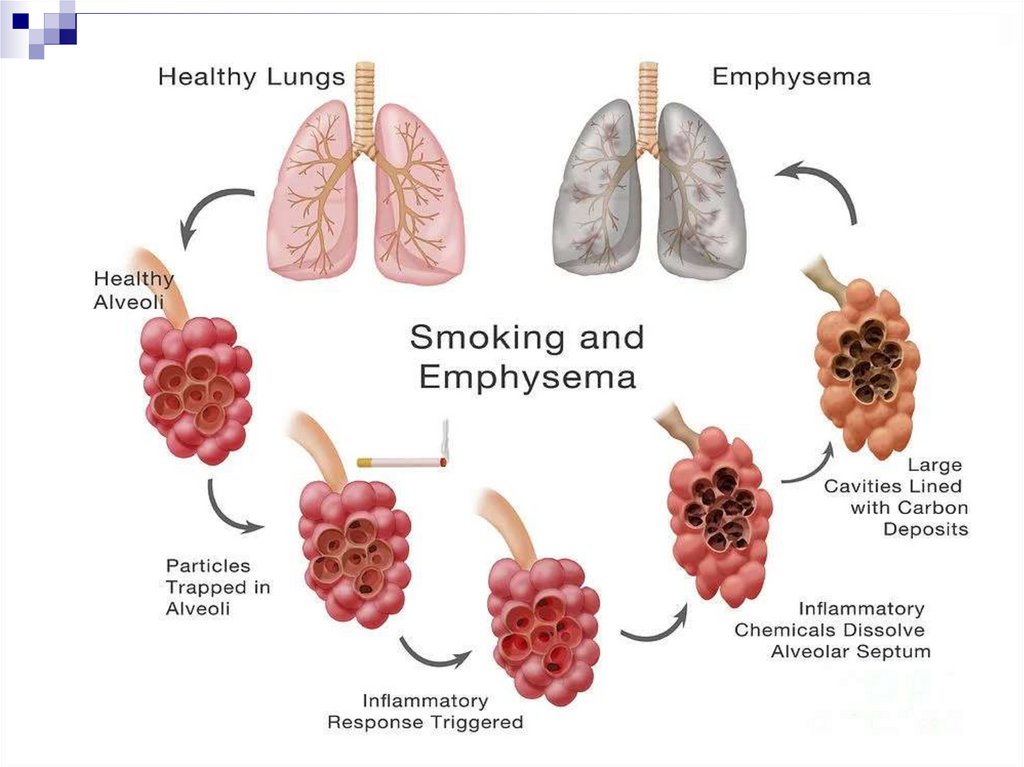
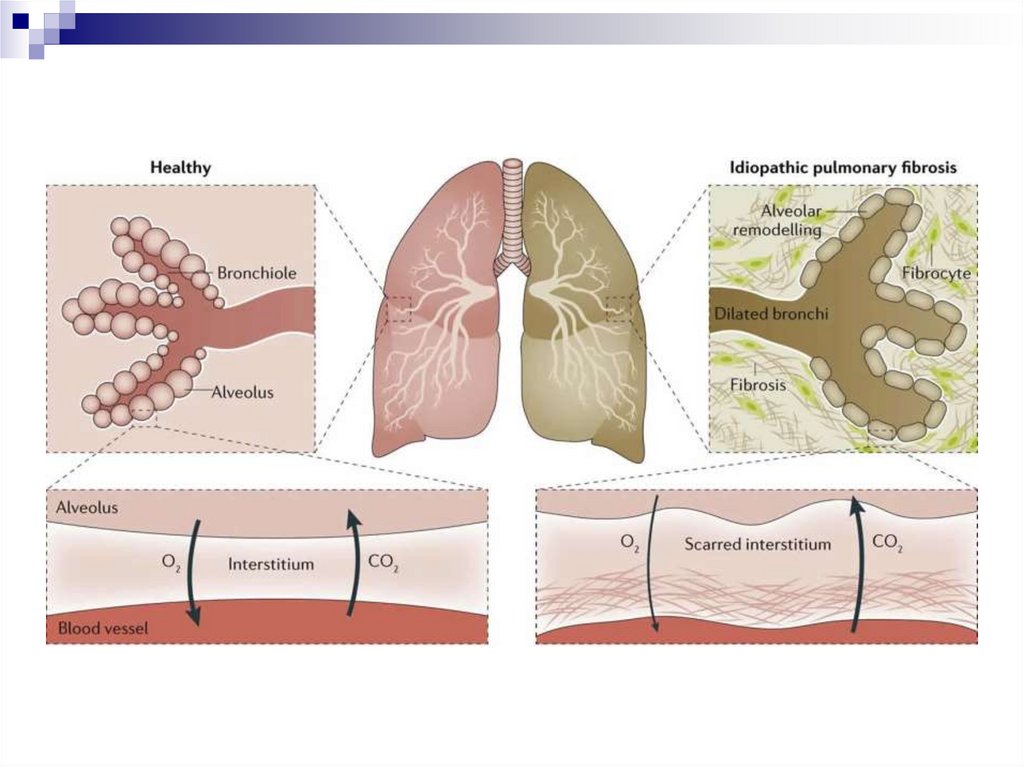
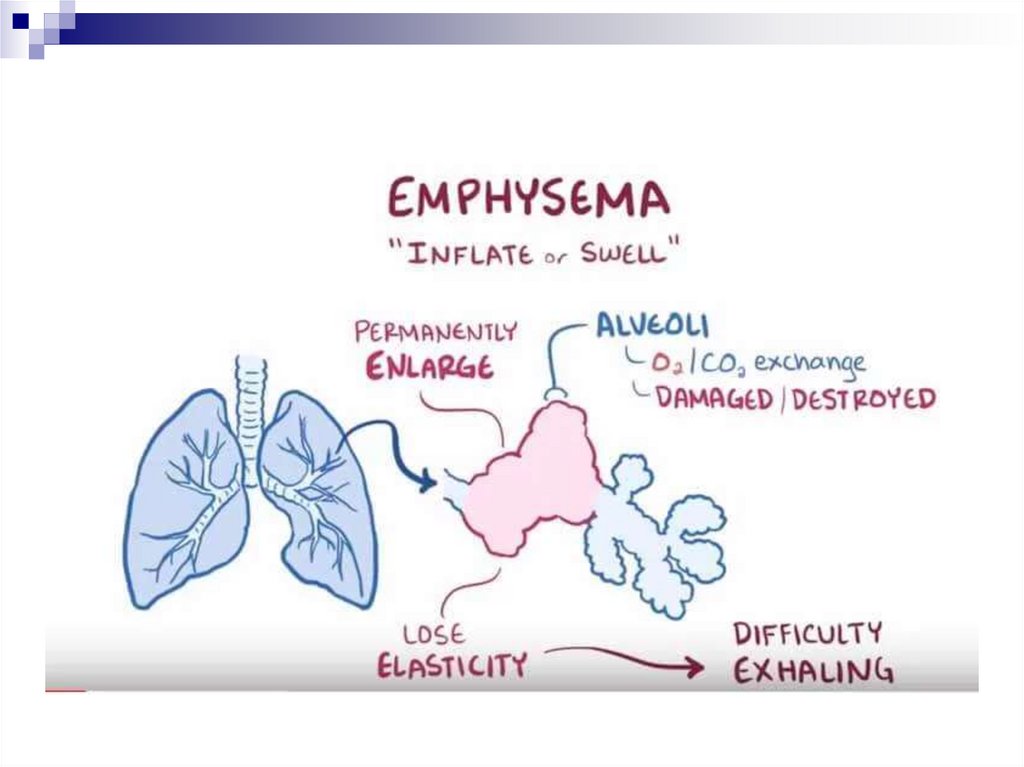
Pulmonary emphysemaDevelopment from the early stages of COPD
The defining position in the formation of the irreversible
component of bronchial obstruction
Deficiency of local protease inhibitors (gen. defect, inactivation
due to oxidative stress) + effect of neutrophil proteases =
destruction of the elastic alveolar stroma
→ lung elasticity is compromised → respiratory mechanics
change and expiratory collapse develops
→ delayed emptying of the lungs → dynamic hyperinflation
and overstretching
→ increase in functional residual volume → dysfunction,
fatigue of the respiratory musculature
26.
27.
Bronchitic form (type) of COPDCentriacinar emphysema
“Blue puffiness”
Persistent hypersecretion → increased inspiratory and expiratory
resistance → impaired ventilation → decreased O2 in alveoli,
impaired perfusion-diffusion relationships and blood bypass →
diffuse cyanosis
cough with profuse sputum are dominated on the clinical picture
Diffuse pneumosclerosis and obliteration of the blood vessel
lumen → rapid development of persistent pulmonary hypertension,
pulmonary heart diseases and decompensation
Significant hypoxaemia, erythrocytosis and persistent intoxication
due to severe inflammation of the bronchi contribute to the
progression
28.
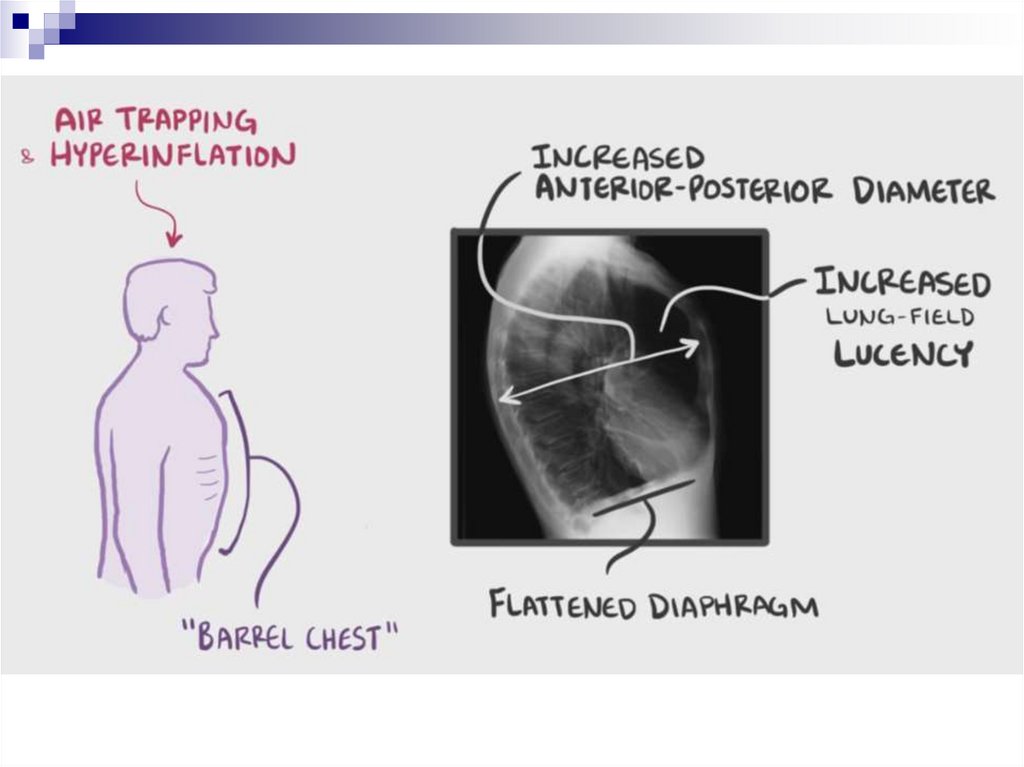
Emphysematous form (type) of COPDPanacinar emphysema.
“The Pink Puffers”
Primary damage to the alveoli → premature expiratory
collapse of the bronchi → exhalation is through folded lips,
accompanied by a puffing sound.
Dyspnoea at rest prevails due to decreased diffusing lung
surface area, dry cough or with small amounts of thick and
viscous sputum, very poor exercise tolerance
The complexion is pink as sufficient oxygenation of the blood
is maintained by increasing ventilation as much as possible
Pulmonary hypertension is moderate. The pulmonary heart is
the long-term compensated.
29.
30.
31.
32.
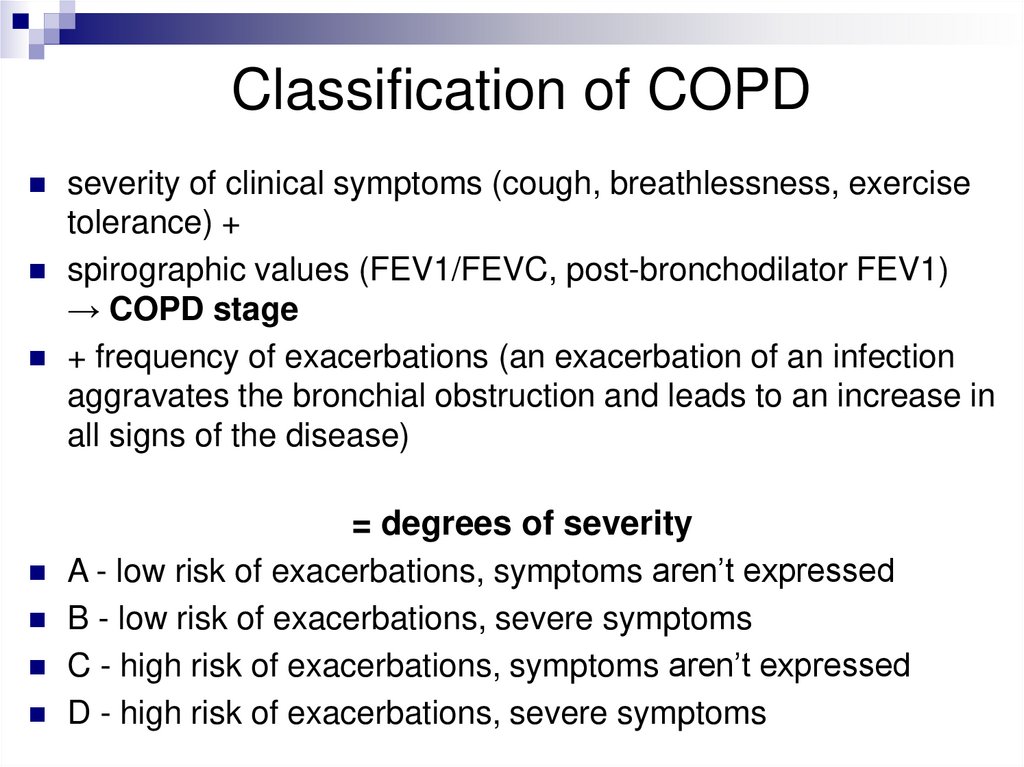
Classification of COPDseverity of clinical symptoms (cough, breathlessness, exercise
tolerance) +
spirographic values (FEV1/FEVC, post-bronchodilator FEV1)
→ COPD stage
+ frequency of exacerbations (an exacerbation of an infection
aggravates the bronchial obstruction and leads to an increase in
all signs of the disease)
= degrees of severity
A - low risk of exacerbations, symptoms aren’t expressed
B - low risk of exacerbations, severe symptoms
C - high risk of exacerbations, symptoms aren’t expressed
D - high risk of exacerbations, severe symptoms
33.
The main ways of treatmentNon-pharmalogical therapy
- reducing the impact of risk factors
- Influenza and pneumococcal vaccinations
Pharmalogical therapy
- bronchodilator therapy (prefer to long-acting using)
- mucolytics and antioxidants
- ? GKS
- antibiotic therapy - for acute exacerbations
Oxygen therapy - increases survival rate, reduces
haematocrit levels, reduces dyspnoea and increases
tolerance for physical exercises, reverses and prevents
progression of pulmonary hypertension, improves mental
status
34.
Bronchial asthmaIs a chronic inflammatory disease of the RT, in which
many cells and cellular elements are involved.
Chronic inflammation is associated with bronchial
hyperresponsiveness, leading to recurrent episodes
of wheezing, breathlessness, chest tightness and
coughing, particularly at night or early in the morning.
These episodes are usually associated with
widespread but variable bronchial obstruction, which
is often reversible spontaneously or with treatment
35.
EpidemiologyFrom the 1930s to the 1980s, there was a 7-10-fold increasing in
the prevalence of BA in children and adults in the USA and Europe
The prevalence of BA in Russia increased by 32.3% from 1991 to
1994 and by a further 28.2% from 1998 to 2002
There has been an increase in its severe forms (more hospital
admissions and deaths due to BA)
The prevalence of the main symptom of current BA is wheezing, is
on average 11.3% in the younger age group and 13.8% in
teenagers
Hypodiagnosis of BA by general practitioners is widespread. The
main reason is the underestimation of mild and rare episodes of the
disease
About 70% of children with BA are mild, 25% of them have a
moderate to severe course, 5% - have a severe course
36.
Genetic factorsA family history of atopy increases the risk of developing both allergic rhinitis
and BA by a factor of 3 to 5
More than 100 genes associated with BA have been identified
Genes associated with asthma formation: production of allergen-specific IgE
(atopy), development of bronchial hyperresponsiveness, synthesis of
inflammatory mediators (cytokines, chemokines, growth factors), relationship
between types of immune response involving Th1 and Th2 lymphocytes
Examples: genes encoding cytokines IL-3, IL-4, IL-5, IL-9 and IL-13 as well as
IL-4 receptors, LPS receptor gene (CD 14), HLA type II, etc.
Genetic predisposition to atopic asthma is manifested by an active response of
Tn2 cells to environmental antigens (allergens), to which most people respond
poorly or not to respond at all
Genes that determine the effectiveness of anti-asthmatic drugs: polymorphism
of genes encoding β2-adrenoreceptor synthesis (on chromosome 5q). There
are also genes that regulate patient response to GCS and antileukotriene
drugs
37.
AllergensThe prevalence of atopy in the general teenagers population was
35.7%, where’s among children with BA allergies occurred in 91.3%.
In adults, the atopic variant of BA was diagnosed in 68.3-72.8% of
cases
Allergens are predominant:
- house dust mites (46-76% in children and 53-84% in adults),
- cockroaches (19-63.7% of cases),
- mould fungi,
- plant pollen (ragweed, grass pollen, birch pollen and wormwood,
frequency 1-16%),
- epidermal allergens (5-37% for dogs and 15-67% for cats, 22-58%
in Russia)
Monovalent allergy is rare (20-25%). Most cases are polysensitised,
which contributes to a more severe course of BA and makes it more
difficult to treat
38.
AaeropollutantsThe prevalence of AD among the urban population (both children
and adults) is 1.6-1.8 times higher than in villages
Sulphur dioxide, ozone and nitrogen oxides, in concentrations
found in heavily polluted cities, can cause damage to the
respiratory epithelium, bronchoconstriction and influence the
allergic response.
The local defence system against viral and bacterial agents is
suppressed and acute and chronic inflammation develops.
Nitrogen oxides, by damaging the respiratory epithelium,
contribute to the penetration of allergens into the mucous
membranes.
The interaction of sulphur and nitrogen oxides with the allergens
increases their immunogenic properties, which lowers the
threshold dose of the allergens causing sensitisation and results in
higher levels of allergen-specific IgE.
39.
40.
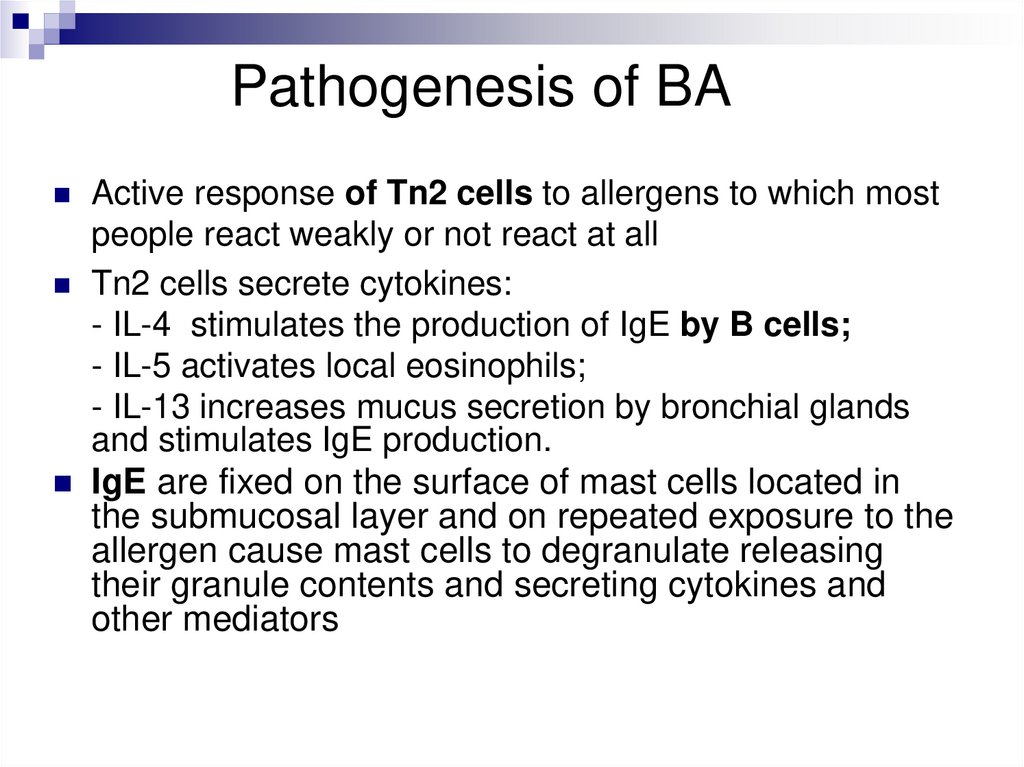
Pathogenesis of BAActive response of Tn2 cells to allergens to which most
people react weakly or not react at all
Tn2 cells secrete cytokines:
- IL-4 stimulates the production of IgE by B cells;
- IL-5 activates local eosinophils;
- IL-13 increases mucus secretion by bronchial glands
and stimulates IgE production.
IgE are fixed on the surface of mast cells located in
the submucosal layer and on repeated exposure to the
allergen cause mast cells to degranulate releasing
their granule contents and secreting cytokines and
other mediators
41.
42.
43.
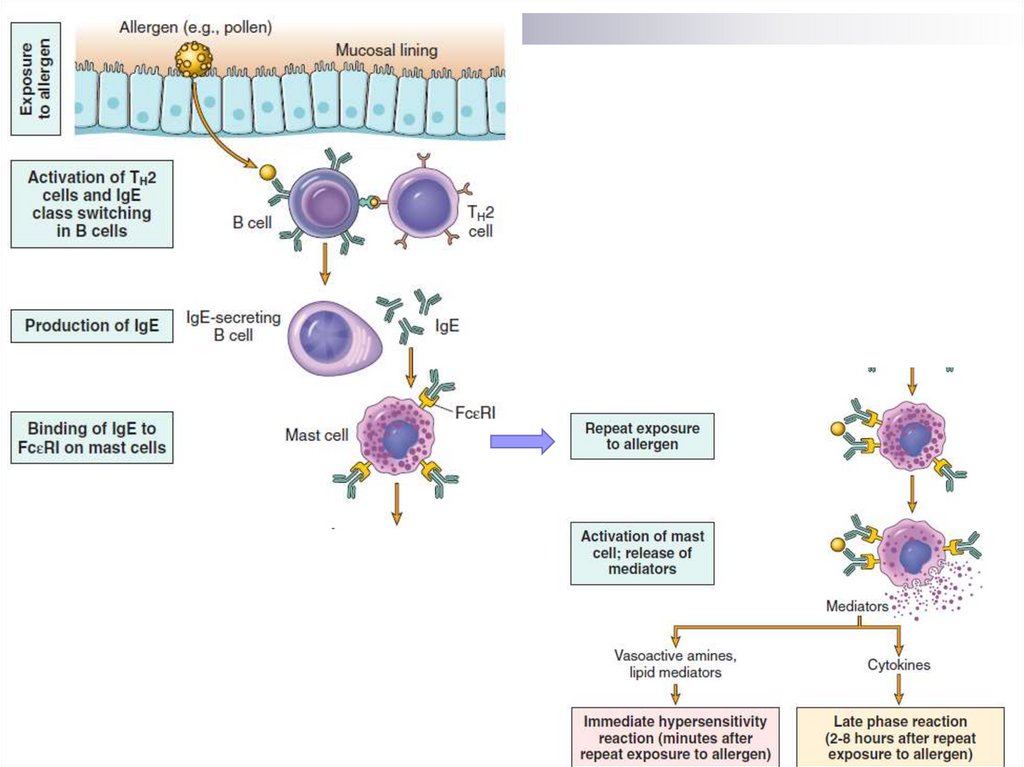
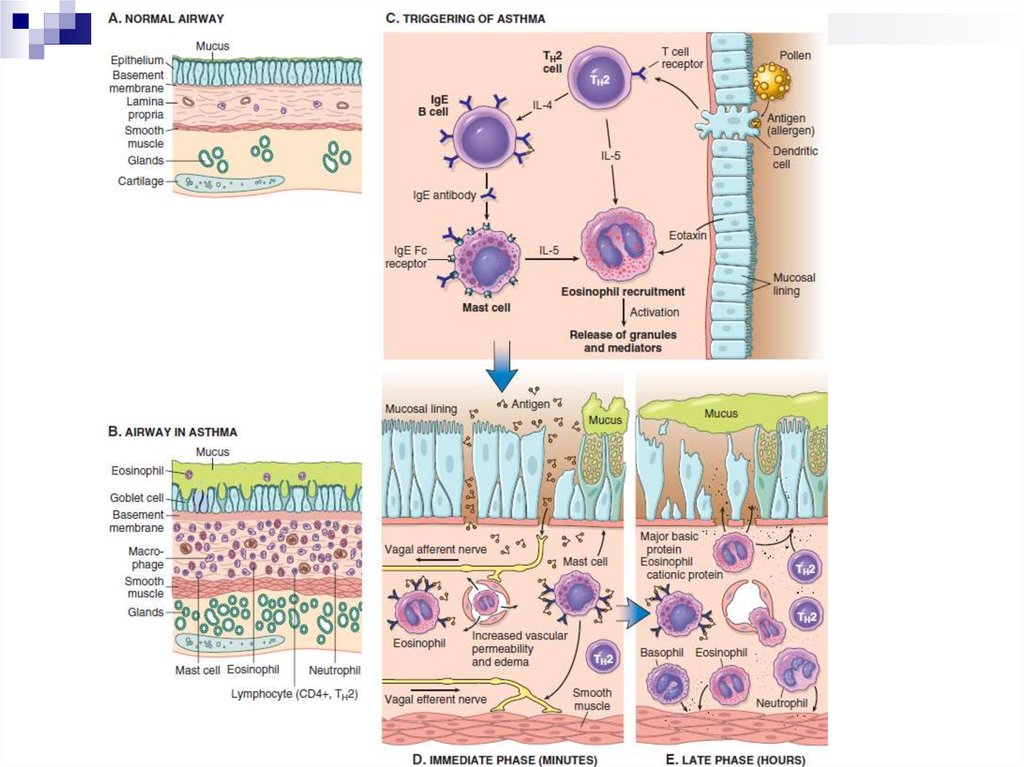
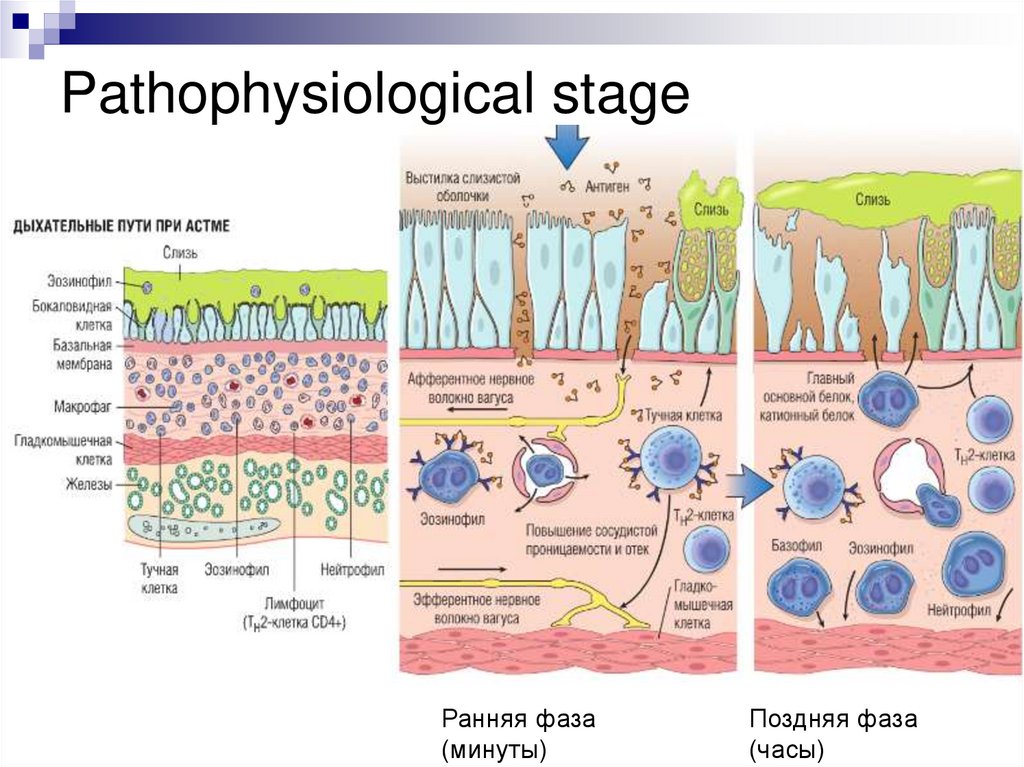
Pathogenesis of BAEarly phase of the allergic reaction:
bronchospasm, increased mucus production, vasodilation and
increased vascular permeability.
Bronchospasm is initiated by direct stimulation of the
subepithelial vagus nerve receptors (parasympathetic stimulation)
Late phase of the allergic reaction:
inflammation with a leucocytic infiltration consisting of eosinophils,
neutrophils and large numbers of T-cells.
Leukocyte accumulation in tissues is stimulated by cytokines and
chemokines secreted by mast cells, epithelial cells and T-cells.
For example, eotoxin, produced by airway epithelial cells, is a potent
chemoattractant and activator of eosinophils. In turn, the main
basic protein of eosinophils damages the epithelium and causes
even greater bronchial constriction
44.
Pathophysiological stageРанняя фаза
(минуты)
Поздняя фаза
(часы)
45.
Therapy principlesDrugs to relieving symptoms:
- bronchodilators - Short-acting β2-AM (salbutamol,
fenoterol), M-HB (ipratropium bromide);
- Long-acting β2-AM (salmeterol and formoterol),
phosphodiesterase inhibitors (theophylline).
Drugs for long-term disease control (basal medications)
- GCS (beclomethasone, budesonide, fluticasone propionate,
mometasone furoate)
- Cysteinyl leukotrienes blockers (zafirlukast and
montelukast)
- Humanized anti-IgE antibodies (omalizumab)
Prospects - anti-IL-5, anti-IL-13
46.
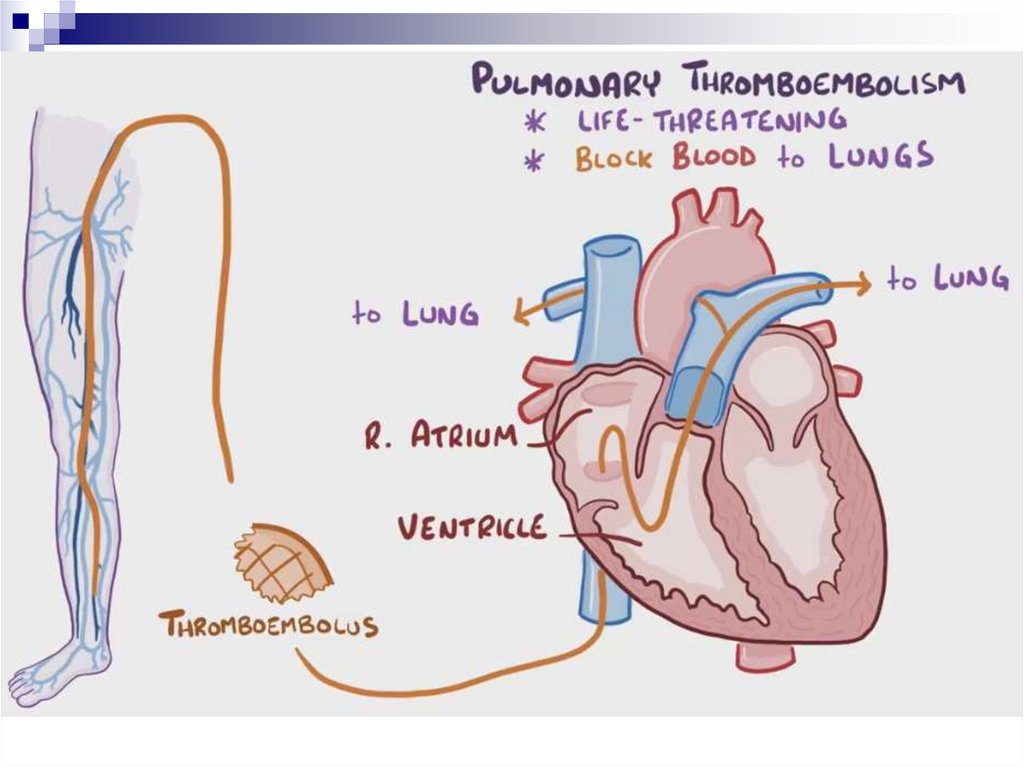
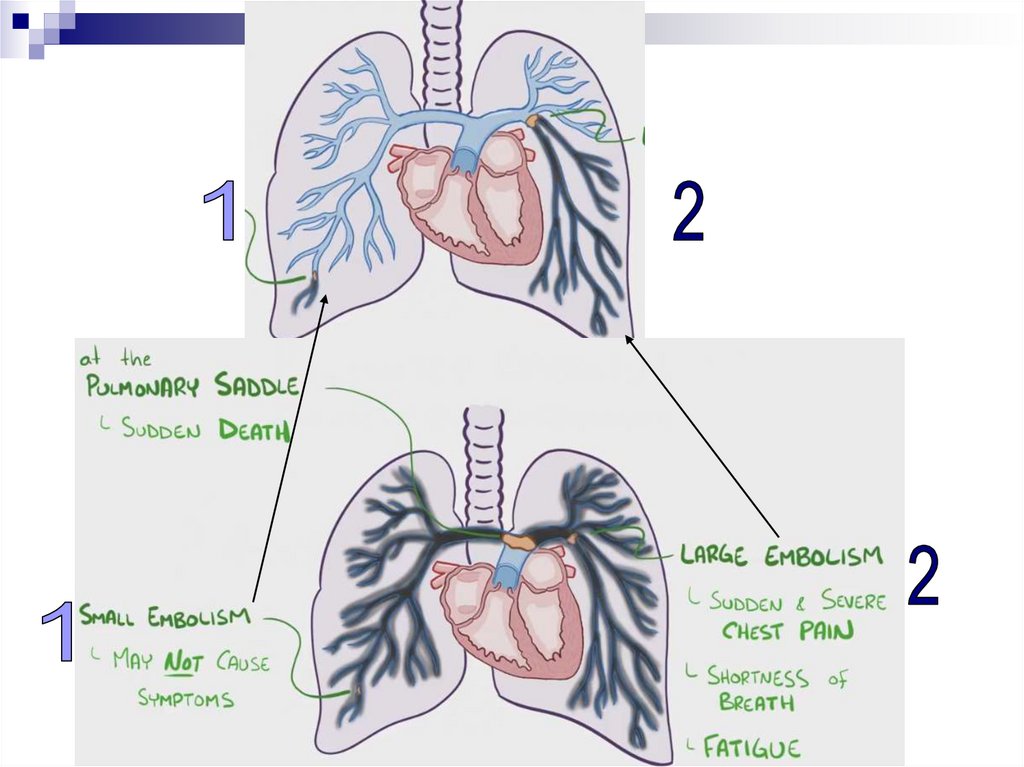
Pulmonary Thromboembolism (PTE)- is a syndrome characterised by blockage of pulmonary
artery branches by a thrombus and a series of
pathophysiological reactions, the severity of which is
directly related to the size of the thrombus
PTE becoming the third most common cause of death
in highly developed countries, behind only
cardiovascular disease and malignant neoplasm
In 50-80% of cases, PTE isn’t diagnosed at all, and in
many cases only a presumptive diagnosis is made
Lethality among untreated patients reaches 40%,
whereas with timely treatment it doesn’t exceed 10%
47.
EtiologyDeep vein thrombosis (DVT) is the most common cause of
PTE. The annual incidence is 100 per 100 000 population
Thrombosis in the right heart cavity and superior vena cava
system is much less common then DVT
48.
49.
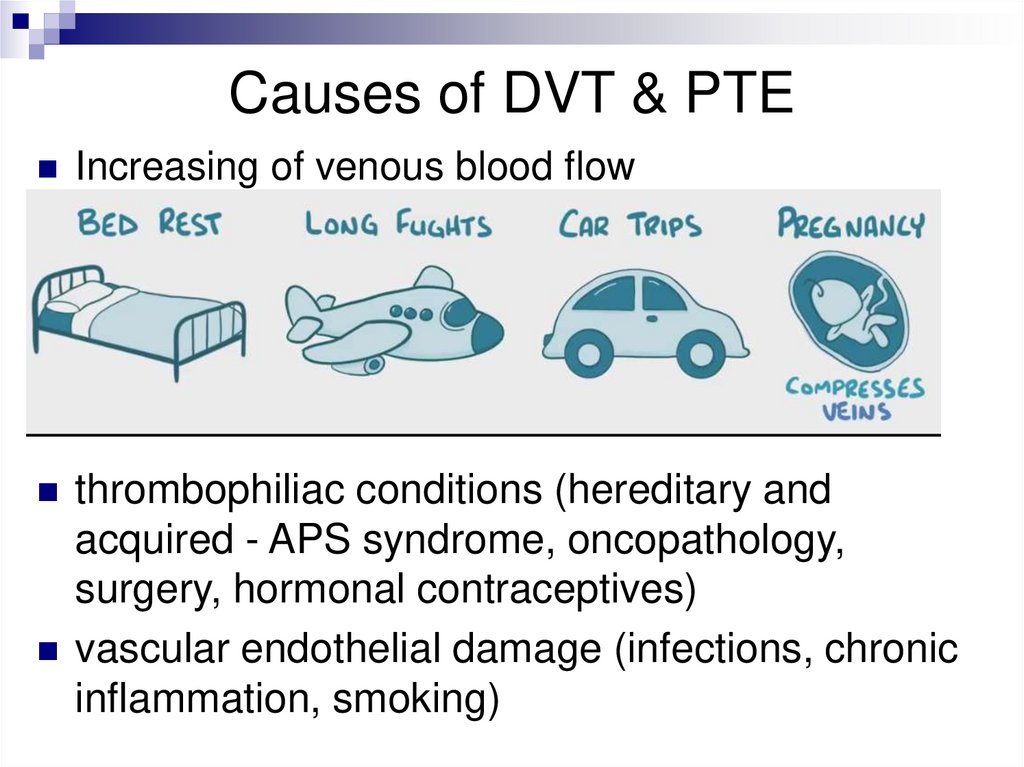
Causes of DVT & PTEIncreasing of venous blood flow
thrombophiliac conditions (hereditary and
acquired - APS syndrome, oncopathology,
surgery, hormonal contraceptives)
vascular endothelial damage (infections, chronic
inflammation, smoking)
50.
51.
52.
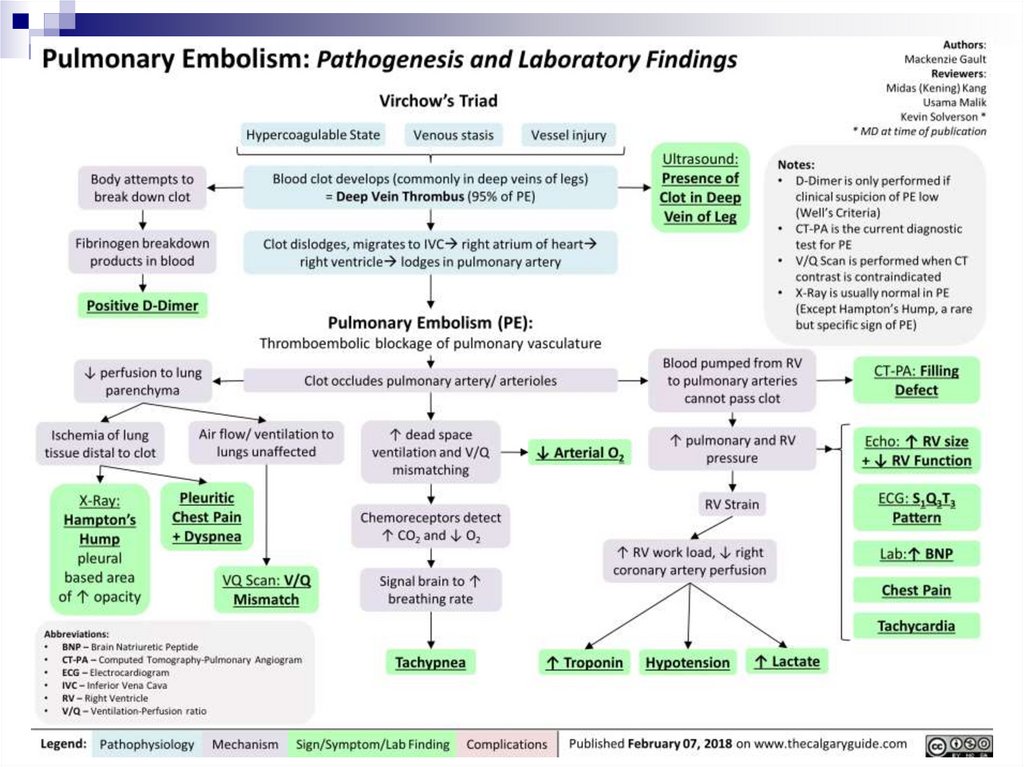
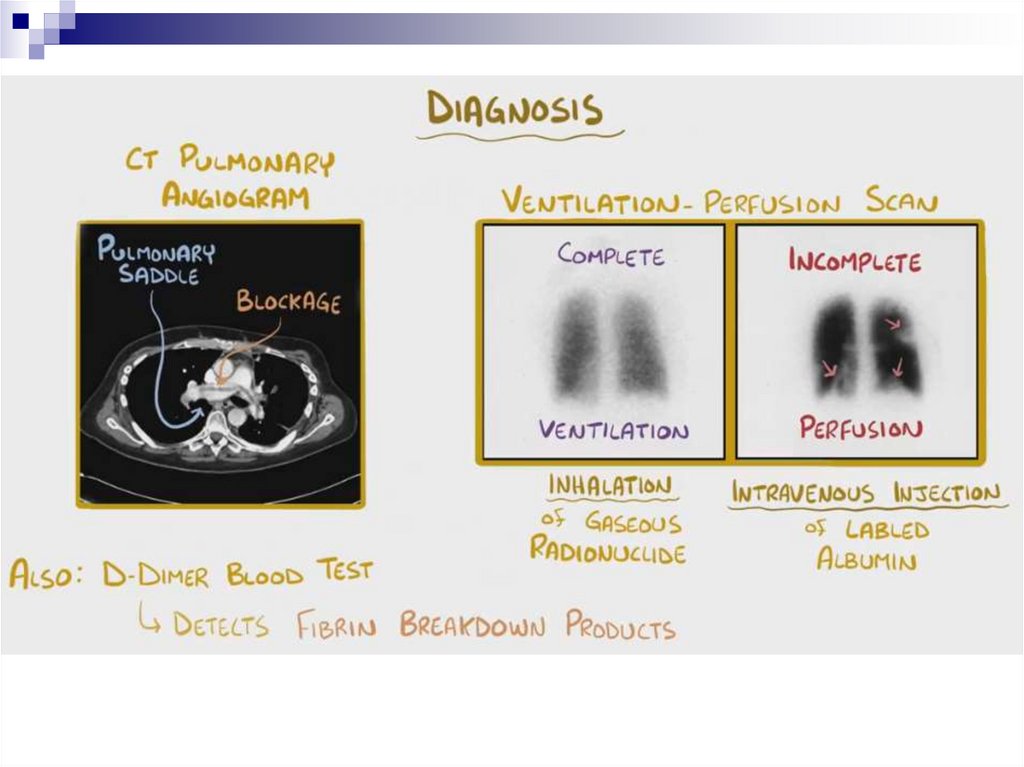
PTE DiagnosticsD-dimer
Low value with high probability (85-97%) excludes PTE
High levels have a small positive predictive value (around 30-50%)
Determination of blood gas composition
Troponin (VD overload)
Electrocardiography – overloading of VD, AD
Chest X-RAY
Echocardiography – signs of pulmonary hypertension, dilatation and
ventricular dexter overload. Transesophageal EchoCG can visualise
thrombus in the trunk and main branches of the pulmonary artery
Spiral CT scan with pulmonary vascular contrast – this is “the gold standard”
in PTE diagnostics
Magnetic resonance angiography
Scintigraphy
Angiopulmonography
Ultrasound Doppler – condition of lower limb veins, PTE
53.
54.
Therapy principlesEmergency anticoagulant therapy (UFH, LMWH,
selective factor Xa inhibitors)
Throbolysis – for massive PTE
Surgical treatment of PTE (opened surgical
embolectomy, very rare; endovascular catheterbased thrombus fragmentation and extraction)
Prolonged use of oral anticoagulants
Inferior vena cava filters














 Медицина
Медицина