Похожие презентации:
How does knowledge of chemistry affect life?
1.
How doesknowledge of
chemistry affect
life?
Aikisheva A., Kazbek A., Kazhybai
A.
2.
AnnotationAs part of this project, students are looking for
possible ways for aluminum ions to enter the human
body through dishes and identify facts that
determine whether aluminum is harmful or not. For
this purpose, social surveys and demonstration
chemical experiments are conducted.
3.
Plan:1. Introduction
2. Theoretical part
3. Partical part
4. Results
5. Conclusion
6. Recommendations
7. Used literature
4.
Introduction
The purpose of this project is to
identify and establish the possibility of
aluminum ions entering the body
through metal dishes, to empirically
confirm that aluminum dishes are
unsuitable for cooking.
5.
Theoretical partAluminum, its physical and chemical properties, and its physiological role.
Aluminum is a light, silvery metal with high electrical conductivity. It is
chemically active, it is covered with an oxide film in the air, which
protects the metal from interaction with oxygen and water.
In terms
of prevalence in the world, aluminum ranks 3rd among chemical
elements (after oxygen and silicon), and first among metals. Several
hundred minerals containing aluminum are known, which primarily
include bauxites and aluminosilicates. Aluminum is obtained by
electrolysis of aluminum oxide (alumina).Aluminum metal alloys are
widely used as a base material in aircraft and shipbuilding. Especially
pure aluminum is used for the manufacture of conductors in electrical
engineering. Pure aluminum is used to make various kitchen utensils,
foil.
6.
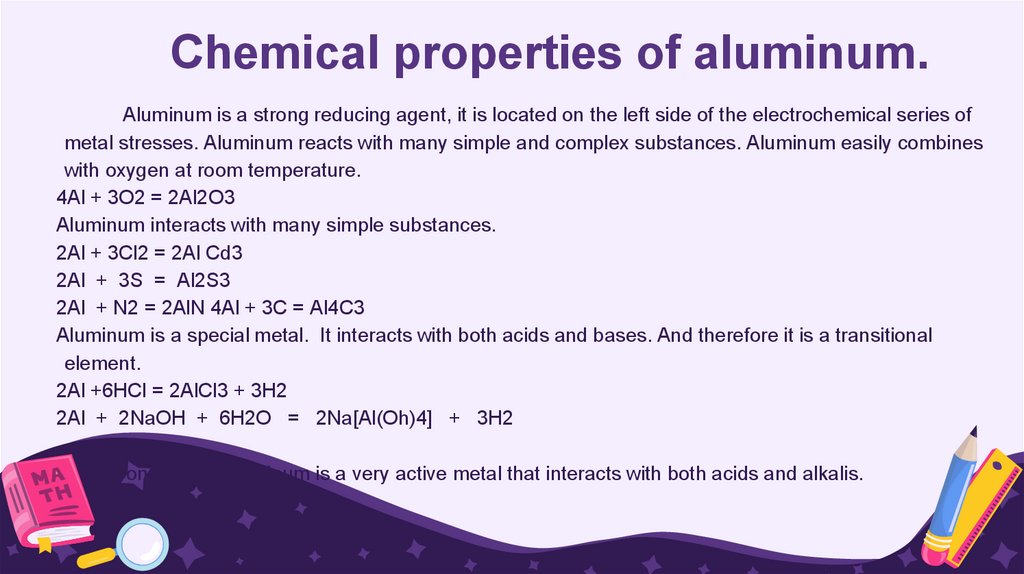
Chemical properties of aluminum.Aluminum is a strong reducing agent, it is located on the left side of the electrochemical series of
metal stresses. Aluminum reacts with many simple and complex substances. Aluminum easily combines
with oxygen at room temperature.
4Al + 3O2 = 2Al2O3
Aluminum interacts with many simple substances.
2Al + 3Cl2 = 2Al Cd3
2Al + 3S = Al2S3
2Al + N2 = 2AlN 4Al + 3C = Al4C3
Aluminum is a special metal. It interacts with both acids and bases. And therefore it is a transitional
element.
2Al +6HCl = 2AlCl3 + 3H2
2Al + 2NaOH + 6H2O = 2Na[Al(Oh)4] + 3H2
Conclusion: Aluminum is a very active metal that interacts with both acids and alkalis.
7.
The physiological role of aluminumFrom 5 to 50 mg of aluminum enters the human body every day, depending on
the region of residence. The optimal daily intake rate of aluminum is considered
to be a dose weighing from 20 to 100 micrograms. Vegetable products contain
50-100 times more aluminum than animal products. It is known that during the
hot processing of food products or baking bread, due to the use of aluminum
cookware, food is contaminated with this metal. The source of aluminum intake
into the body is also drinking water, which contains 3-4 mg / l. 2-4% of the
incoming aluminum is absorbed in the gastrointestinal tract, and soluble salts
such as AlCl3 are better absorbed. Aluminum also enters the body through the
lungs, which, with a high level of environmental pollution, leads to fibrosis.
Aluminum in small quantities is necessary for the body, especially for bone
tissue, but in case of excess, this metal can pose a great danger to the human
body. In general, aluminum is classified as a toxic element.
8.
The effects of excess aluminum in thebody:
1. Neurotoxicity and encephalopathy (memory disorders, nervousness,
tendency to depression, learning difficulties in childhood and progressive
senile dementia, etc.);
2. 2. Osteomalacia (softening of bones), as well as related fractures and
other diseases of the musculoskeletal system;
3. 3. Disorders of the gastrointestinal tract;
4. 4. Impaired renal function;
5. 5. The development of aluminosis (an occupational disease of
metallurgical workers) with characteristic changes in lung tissue;
6. 6. Metabolic disorders of iron, phosphorus, magnesium, calcium, zinc,
copper.
7. Aluminum cookware is prohibited for use in children's catering
establishments.
9.
Partical part10.
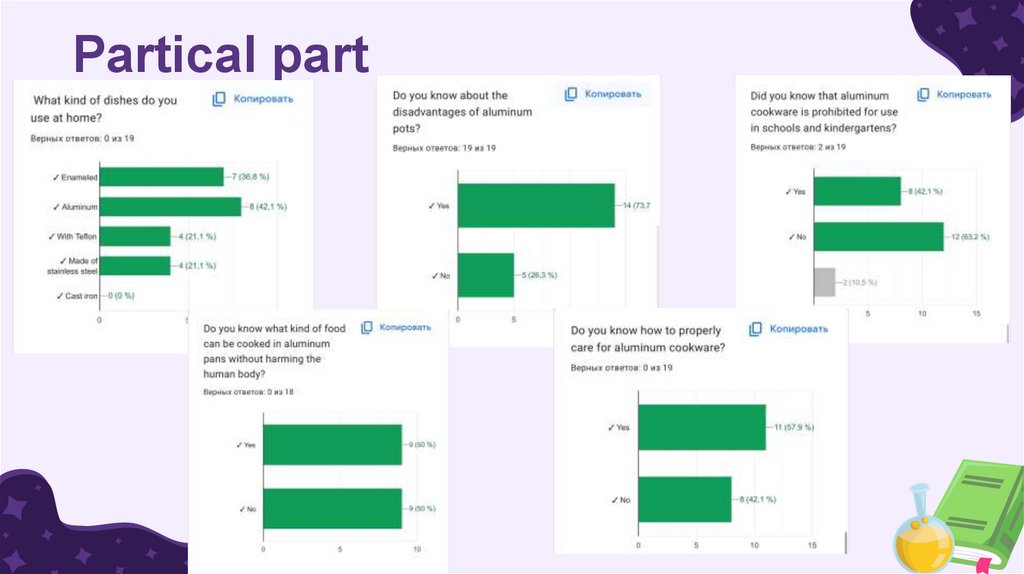
Conclusions of the sociologicalsurvey:
A sociological survey showed that the
population is very poorly aware that aluminum
cookware is dangerous, that it is not
recommended to use it and that this cookware
is in second place after Teflon-coated cookware
in terms of use!
11.
Laboratory experimentsExperience No. 1. The interaction of aluminum with a
solution of hydrochloric acid and dilute sulfuric acid.
2Al + 6HCl = 2AlCl3 + 3H2
2Al + 3H 2SO 4(разб) = Al 2(SO4) 3 + 3H2
12.
Experience No. 2. Interaction of aluminum with alkali2Al + 2NaOH + 6H 2O = 2Na[Al(OH) 4] + 3H2
2(NaOH•H 2O) + 2Al = 2NaAlO2 + 3H2
13.
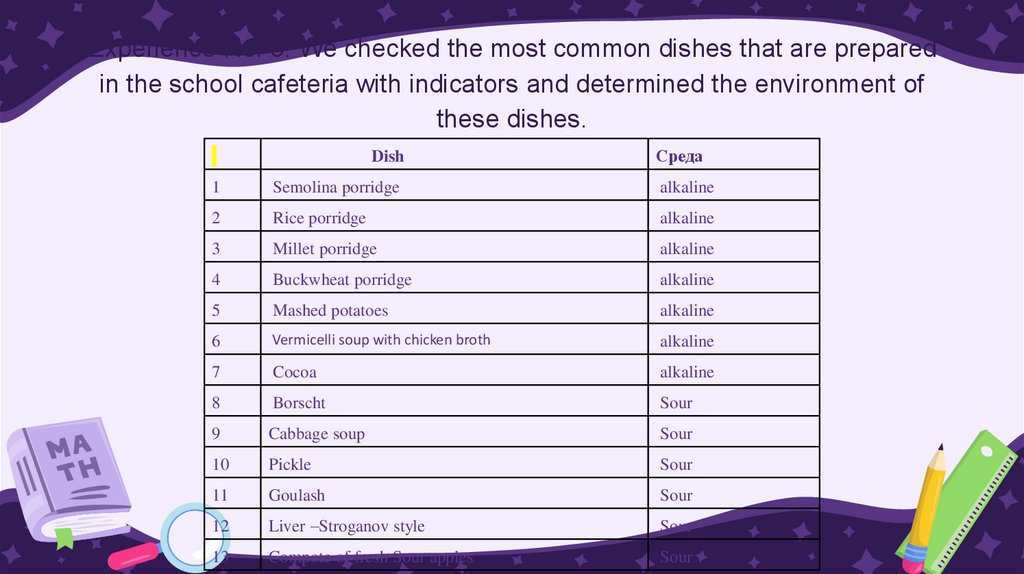
Experience No. 3. We checked the most common dishes that are preparedin the school cafeteria with indicators and determined the environment of
these dishes.
Dish
Среда
1
Semolina porridge
alkaline
2
Rice porridge
alkaline
3
Millet porridge
alkaline
4
Buckwheat porridge
alkaline
5
Mashed potatoes
alkaline
6
Vermicelli soup with chicken broth
alkaline
7
Cocoa
alkaline
8
Borscht
Sour
9
Cabbage soup
Sour
10
Pickle
Sour
11
Goulash
Sour
12
Liver –Stroganov style
Sour
13
Compote of fresh Sour apples
Sour
14.
Conclusion: We found that different dishes have differentsolution environments: milk porridges have an alkaline
environment, meat dishes cooked with tomato sauce have
an acidic environment, all compotes have an acidic
environment.
15.
16.
Conclusions on the work1.
2.
3.
4.
5.
6.
A sociological survey showed that more than half of the surveyed
population knows that aluminum cookware is unsafe, but they still continue
to use it.
2. Experimentally confirmed the fact that aluminum ions pass through the
dishes into the human body. The most intense transition is when cooking
food that has an acidic and alkaline environment. (Compotes, fruit drinks,
pickles, etc.)
3. The myth that it is best to cook porridge in aluminum dishes has been
destroyed! You can not cook porridge in aluminum dishes!
4. When boiling water, the transition of aluminum is almost not observed,
since the water is neutral.
5. Aluminum cookware is unsuitable for storing food and water in it,
because during long storage, aluminum ions begin to pass into food.
6. It is necessary to raise the issue of the harm of aluminum as often as
possible, so that the population understands the danger of using these
17.
Tips for housewives1.Only clean water can be boiled in aluminum dishes without harm to health.
2.It is impossible to use aluminum cookware all the time, since aluminum ions can
accumulate in the human body, which contributes to the deterioration of human health.
3. It is forbidden to cook dairy dishes and dishes from vegetables and fruits in
aluminum dishes.
4. Do not cook various marinades with the addition of acetic and citric acids in
aluminum dishes.
5.Do not store drinking water for a long time in an aluminum container.
6.Do not store various cereals in aluminum dishes.
7. In no case is it suitable for cooking dietary dishes and baby food.
8.Do not wash aluminum dishes with metal brushes and washcloths and abrasive
cleaning agents, as they destroy the oxide film.
9. If the pan is very dirty, then it can be washed with a soda solution (a spoon per liter
of water), the dirt will go away with the dissolved film, and a new and clean one will
form in their place
18.
List of used literature1. Chemical elements in Human physiology and
ecology (Skalny A.V.) Year of publication: 2004
Publisher: ONYX 21st Century Publishing
House
2. 2. The Country of Moms website The article
"Harmful dishes. Enameled, stainless steel,
aluminum". Harmful dishes. Enameled,
stainless steel, aluminum.
3. 3. The website of the "Center for healthy
nutrition. Know what you have!" http://eatinfo.ru/references/microelements/alyuminiy/
4. 4.http://zavodfoto.livejournal.com/638887.html
Website "Industry of Russia"
5. 5."New sanitary standards in kindergartens"














 Английский язык
Английский язык








