Похожие презентации:
Сarbonyl compounds. Carboxylic acids. Lipids
1. Lecture: carbonyl compounds. Carboxylic acids. Lipids.
MINISTRY OF PUBLIC HEALTHZAPOROZHYE STATE MEDICAL UNIVERSITY
DEPARTMENT OF ORGANIC AND BIOORGANIC CHEMISTRY
LECTURE:
CARBONYL COMPOUNDS.
CARBOXYLIC ACIDS.
LIPIDS.
Lecturer:
Assistant professor Antypenko Lyudmyla Mykolaivna
2.
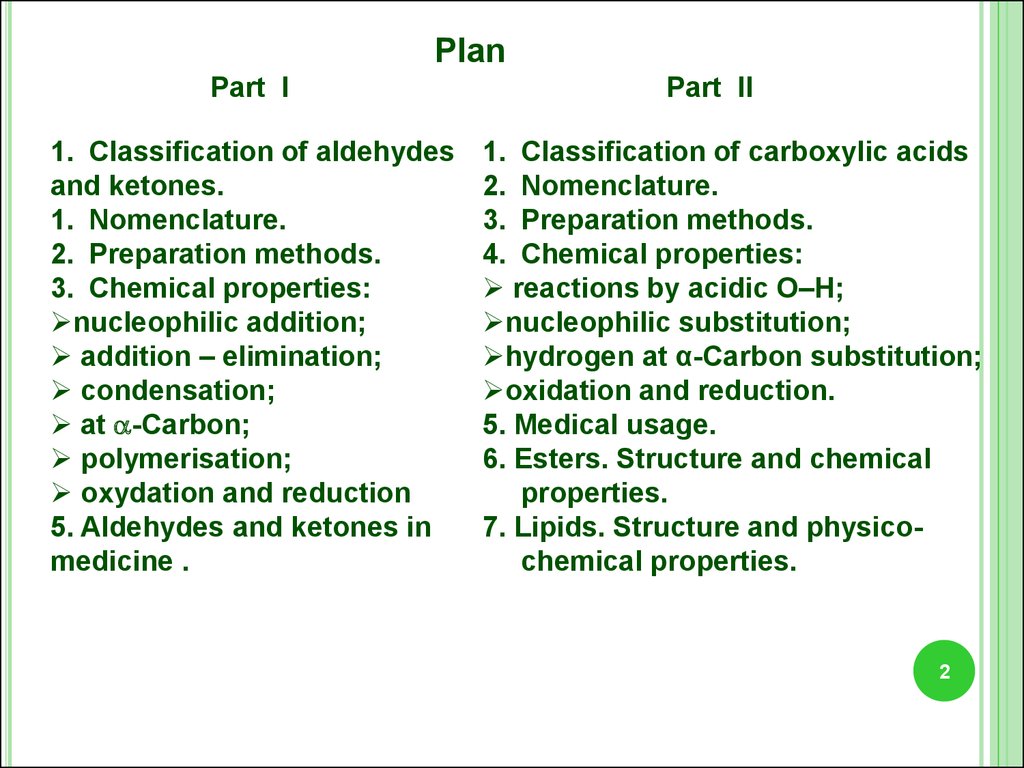
PlanPart I
1. Classification of aldehydes
and ketones.
1. Nomenclature.
2. Preparation methods.
3. Chemical properties:
nucleophilic addition;
addition – elimination;
condensation;
at -Carbon;
polymerisation;
oxydation and reduction
5. Aldehydes and ketones in
medicine .
Part II
1. Classification of carboxylic acids
2. Nomenclature.
3. Preparation methods.
4. Chemical properties:
reactions by acidic О–Н;
nucleophilic substitution;
hydrogen at α-Carbon substitution;
oxidation and reduction.
5. Medical usage.
6. Esters. Structure and chemical
properties.
7. Lipids. Structure and physicochemical properties.
2
3.
Aldehydes and ketonesA carbonyl group is a group of carbon atom double-bonded to an
oxygen atom.
O
R C
H
Aldehydes is organic compounds in which
carbon atom of the carbonyl group is bonded
to a hydrogen atom.
Ketones - organic substances, the molecules of
which contain a carbonyl group bonded with two
hydrocarbon radicals.
O
R
C
R'
3
4.
Aldehydes and ketonesClassification
Aldehydes, ketones
aliphatic
saturated
O
H3C
H
Acetaldehyde
O
unsaturated
H
Propenal
4
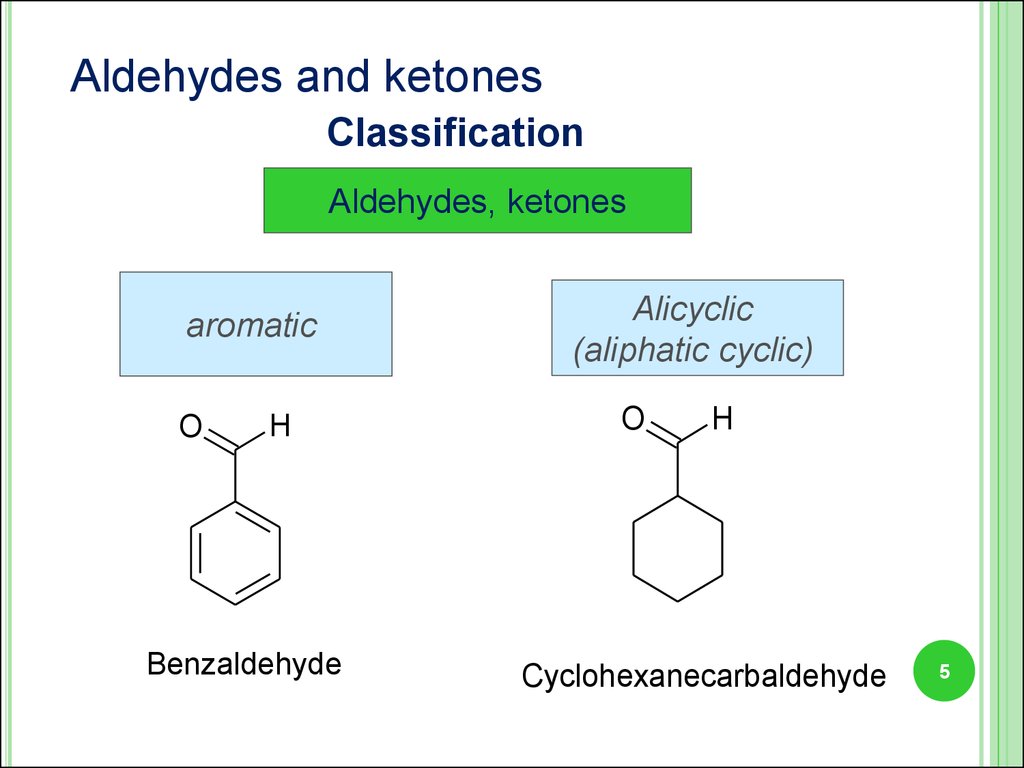
5.
Aldehydes and ketonesClassification
Aldehydes, ketones
aromatic
O
H
Benzaldehyde
Alicyclic
(aliphatic cyclic)
O
H
Cyclohexanecarbaldehyde
5
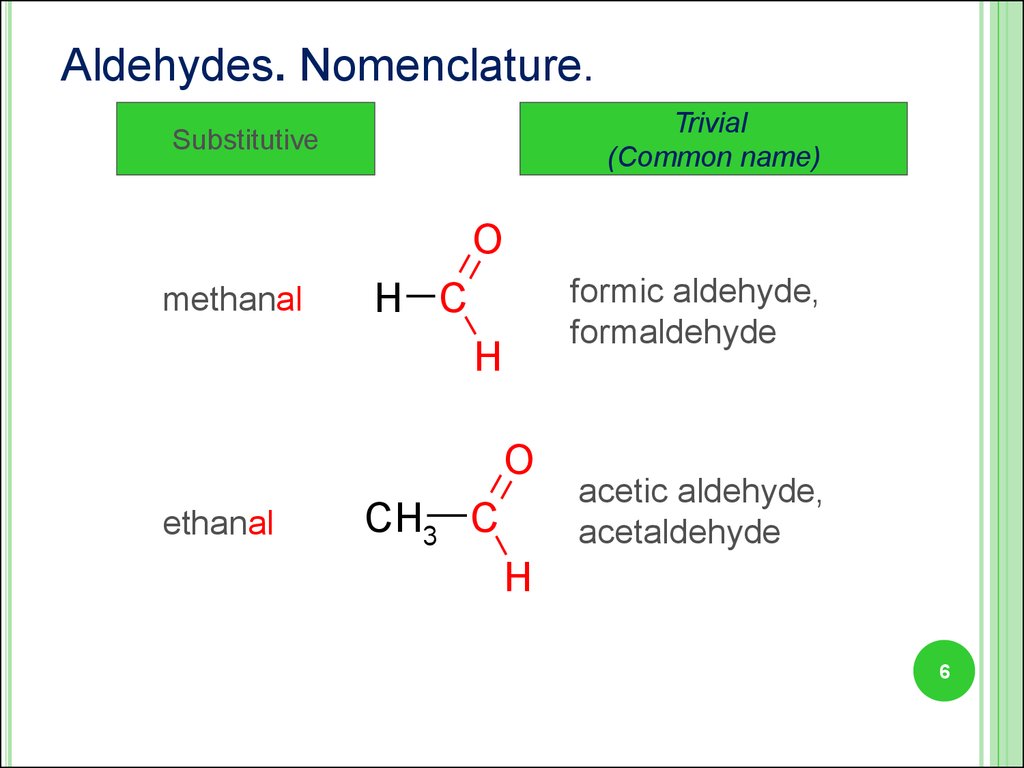
6.
Aldehydes. Nomenclature.Trivial
(Common name)
Substitutive
O
methanal
formic aldehyde,
formaldehyde
H C
H
O
ethanal
CH3 C
acetic aldehyde,
acetaldehyde
H
6
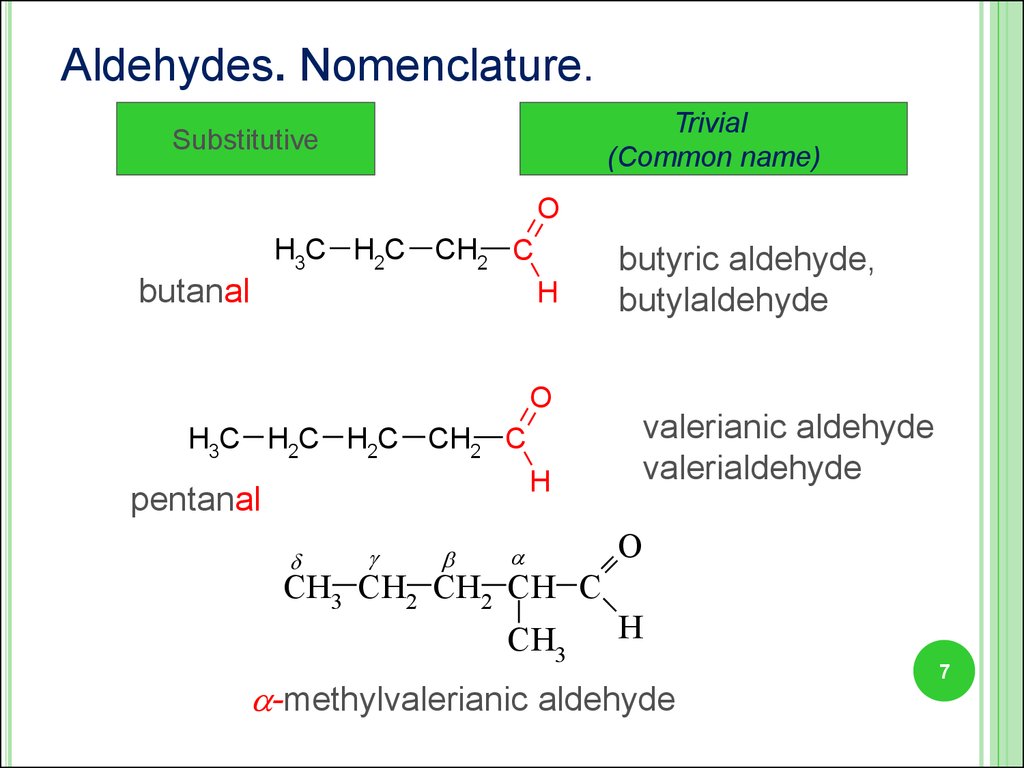
7.
Aldehydes. Nomenclature.Trivial
(Common name)
Substitutive
O
H3C H2C
CH2 C
butanal
H
butyric aldehyde,
butylaldehyde
O
H3C H2C H2C
CH2 C
H
pentanal
CH3 CH2 CH2 CH C
CH3
valerianic aldehyde
valerialdehyde
O
H
-methylvalerianic aldehyde
7
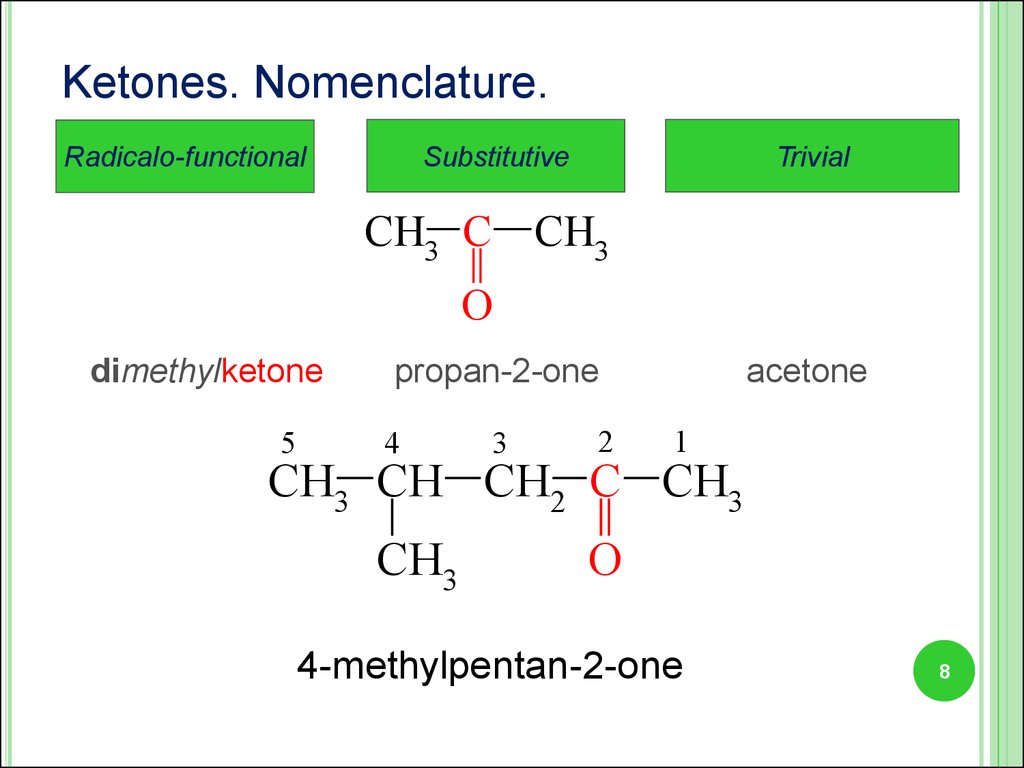
8.
Ketones. Nomenclature.Substitutive
Radicalo-functional
Trivial
CH3 C CH3
O
dimethylketone
5
propan-2-one
4
3
2
acetone
1
CH3 CH CH2 C CH3
CH3
O
4-methylpentan-2-one
8
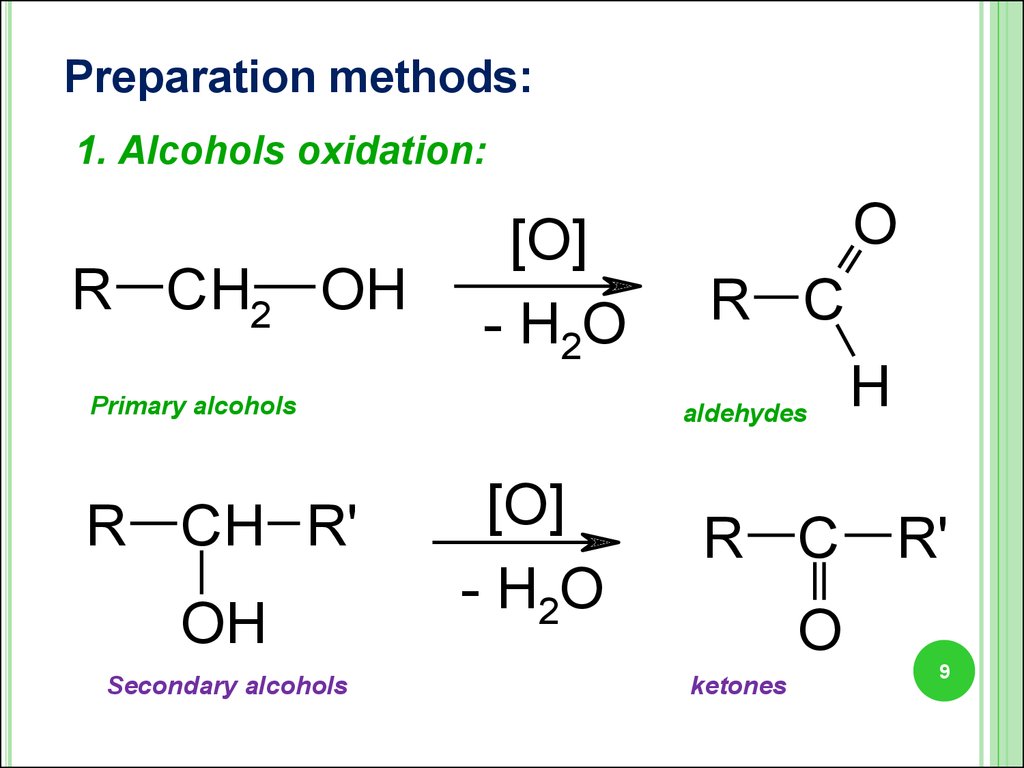
9.
Preparation methods:1. Alcohols oxidation:
R CH2 OH
[O]
- H2O
Primary alcohols
R CH R'
OH
Secondary alcohols
O
R C
aldehydes
[O]
- H2O
H
R C R'
O
ketones
9
10.
Preparation methods:2. Alkynes’s hydration (Kucherov’s reaction)
10
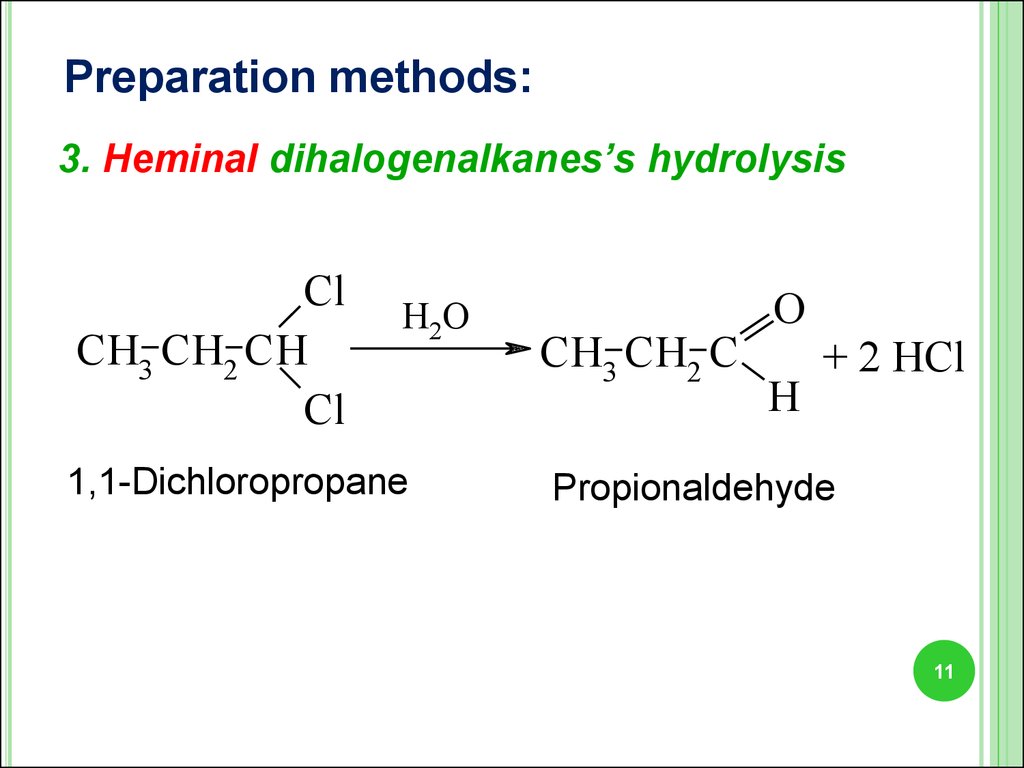
11.
Preparation methods:3. Heminal dihalogenalkanes’s hydrolysis
Cl
CH3 CH2 CH
Cl
H2O
1,1-Dichloropropane
CH3 CH2 C
O
H
+ 2 HCl
Propionaldehyde
11
12.
Preparation methods:4. Pyrolysis of carboxylic acids salts
CH3 C
H C
O
O
O
Ca
3000
C
O
CH3 C
H
+ CaCO3
O
12
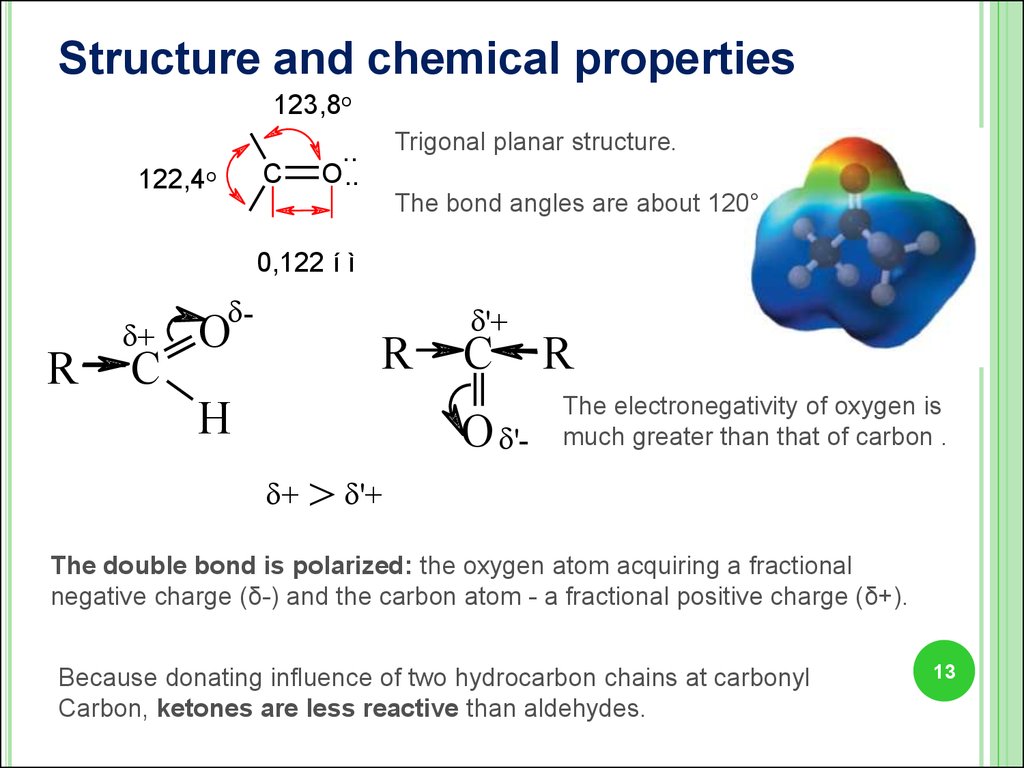
13.
Structure and chemical properties123,8o
C
122,4o
Trigonal planar structure.
..
O ..
The bond angles are about 120°
0,122 í ì
R
+
C
-
O
R
H
'+
C
O ' +
R
The electronegativity of oxygen is
much greater than that of carbon .
> '+
The double bond is polarized: the oxygen atom acquiring a fractional
negative charge (δ-) and the carbon atom - a fractional positive charge (δ+).
Because donating influence of two hydrocarbon chains at carbonyl
Carbon, ketones are less reactive than aldehydes.
13
14.
Chemical properties.Н
R
C
Н
+
C
-
O
H
Electrophilic
center
CH-acidic center
14
15.
Chemical properties.Characteristic reactions are:
nucleophilic addition;
addition – elimination;
condensation;
at -Carbon;
polymerisation;
oxydation and reduction
15
16.
Chemical properties.Tautomerism
Eltekov rule:
Н
R
C
+
C
Н
-
O
H
Enols (unsaturated aliphatic hydrocarbons containing a hydroxyl group at a
carbon-carbon double bond) are unstable and, at the time of formation, convert
into isomeric carbonyl compounds — aldehydes and ketones.
16
17.
Chemical properties.Nucleophilic addition (AN)
1. Addition of cyanic acid.
+
CH3 C
-
O
H
+
-
+ H CN
CN
-
CH3 CH CN
OH
2-hydroxypropanenitrile
17
18.
Chemical properties..Nucleophilic addition (AN)
2. Addition of alcohols
R C
O
H
OH
+ R' OH
R C OR'
+
H
H
hemiacetal
+
H
OR'
R C OR' + H2O
H
acetal
18
19.
Chemical properties.Addition – elimination reaction (AN-E)
1. Shiff bases formation
C O + H2N R
C N R + H2O
azomethins
19
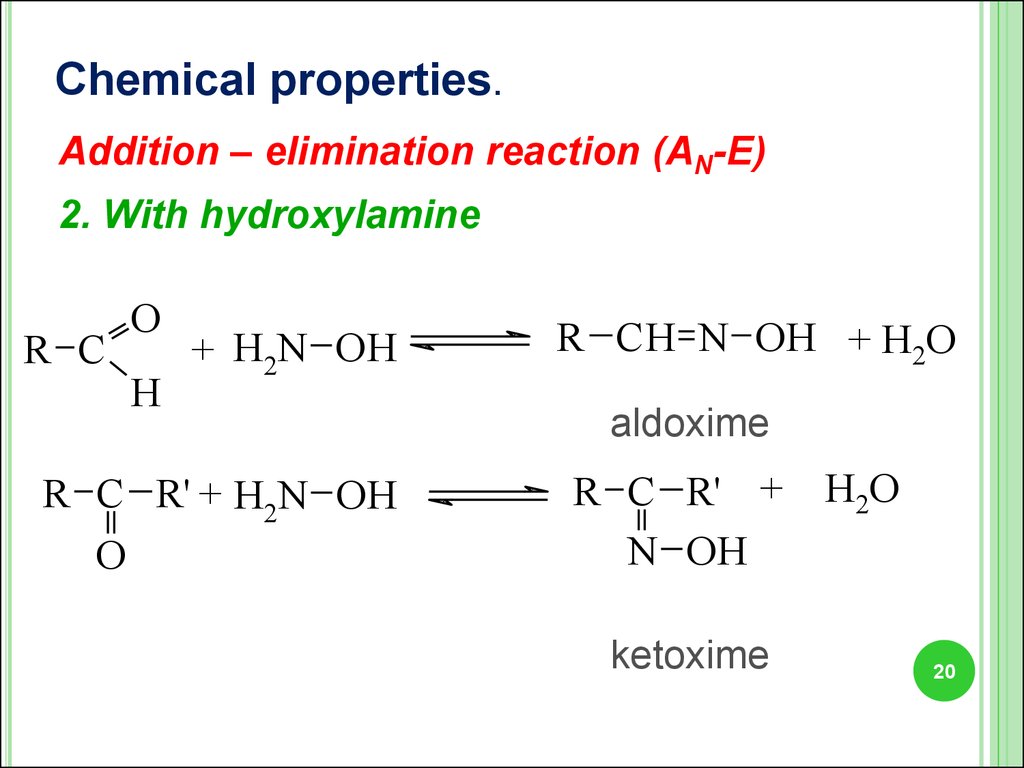
20.
Chemical properties.Addition – elimination reaction (AN-E)
2. With hydroxylamine
R C
O
H
+ H2N OH
R C R' + H2N OH
O
R CH N OH + H2O
aldoxime
R C R' +
N OH
ketoxime
H2O
20
21.
Chemical properties.Addition – elimination reaction (AN-E)
3. With hydrazine derivatives
R
C O + H2N NH
R'
phenylhydrazine
R
C N NH
R'
phenylhydrazone
+
H2O
21
22.
Chemical properties.Reactions at α–Carbon
1. Halogenation
O
CH3 CH2 C
H
+ Br2
+
H
O
CH3 CH C
Br
H
α-bromopropionic aldehyde
+ HBr
22
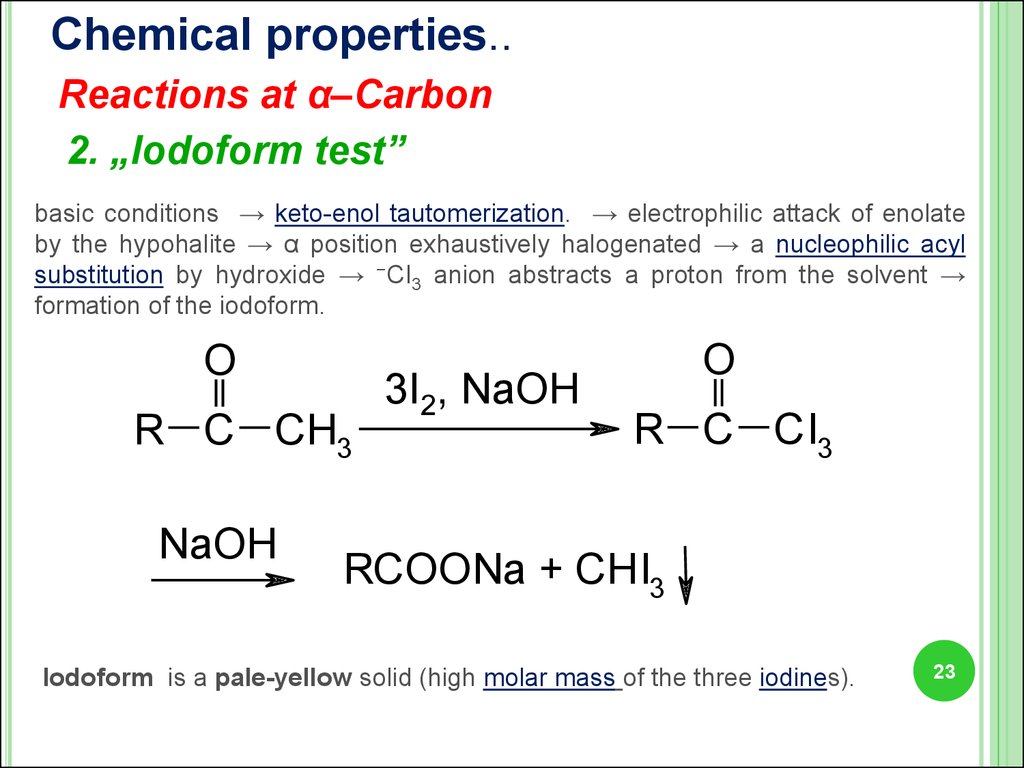
23.
Chemical properties..Reactions at α–Carbon
2. „Iodoform test”
basic conditions → keto-enol tautomerization. → electrophilic attack of enolate
by the hypohalite → α position exhaustively halogenated → a nucleophilic acyl
substitution by hydroxide → −CI3 anion abstracts a proton from the solvent →
formation of the iodoform.
O
R C CH3
NaOH
3I2, NaOH
O
R C CI3
RCOONa + CHI3
Iodoform is a pale-yellow solid (high molar mass of the three iodines).
23
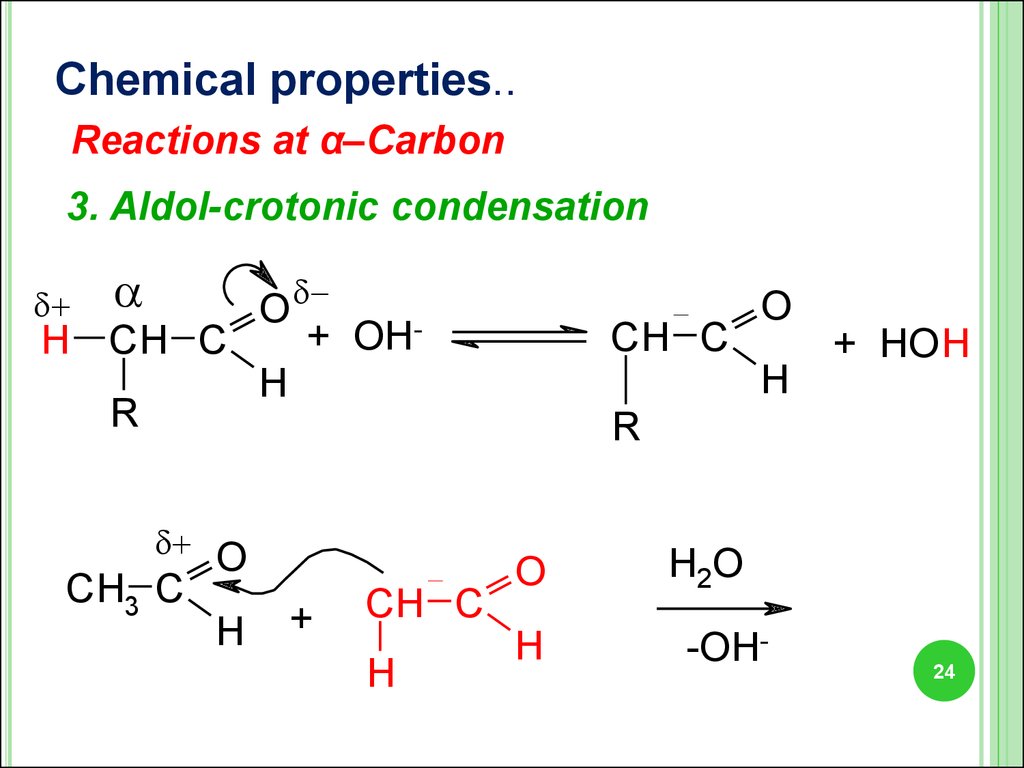
24.
Chemical properties..Reactions at α–Carbon
3. Aldol-crotonic condensation
O
+ OHH CH C
H
R
O
CH3 C
H
+
CH C
H
CH C
O
H
+ HO H
R
O
H
H2O
-OH-
24
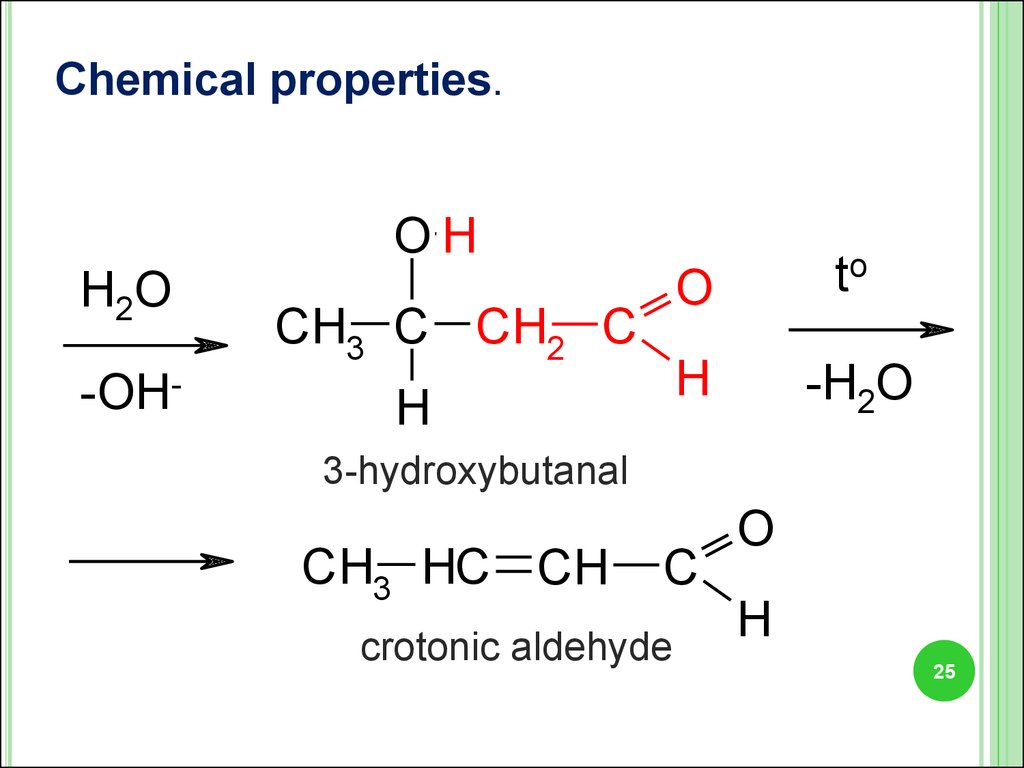
25.
Chemical properties.H2O
-OH-
OH
CH3 C CH2 C
H
O
to
H
-H2O
3-hydroxybutanal
CH3 HC CH
C
crotonic aldehyde
O
H
25
26.
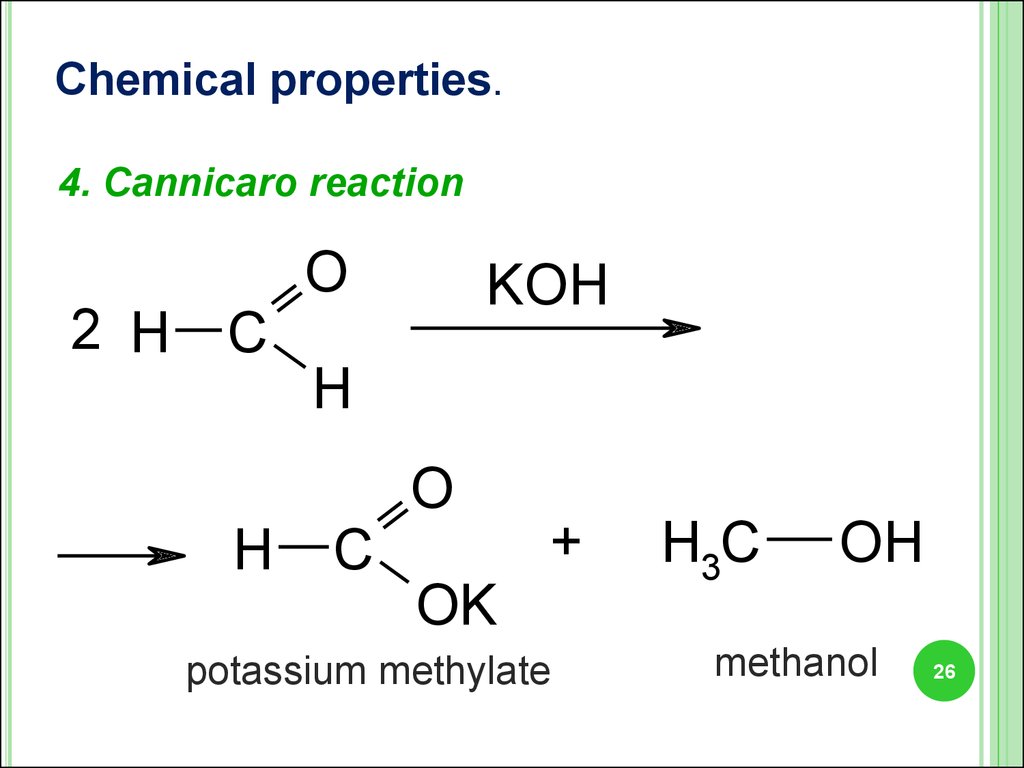
Chemical properties.4. Cannicaro reaction
2 H C
H
O
KOH
H
C
O
+
OK
potassium methylate
H3C
OH
methanol
26
27.
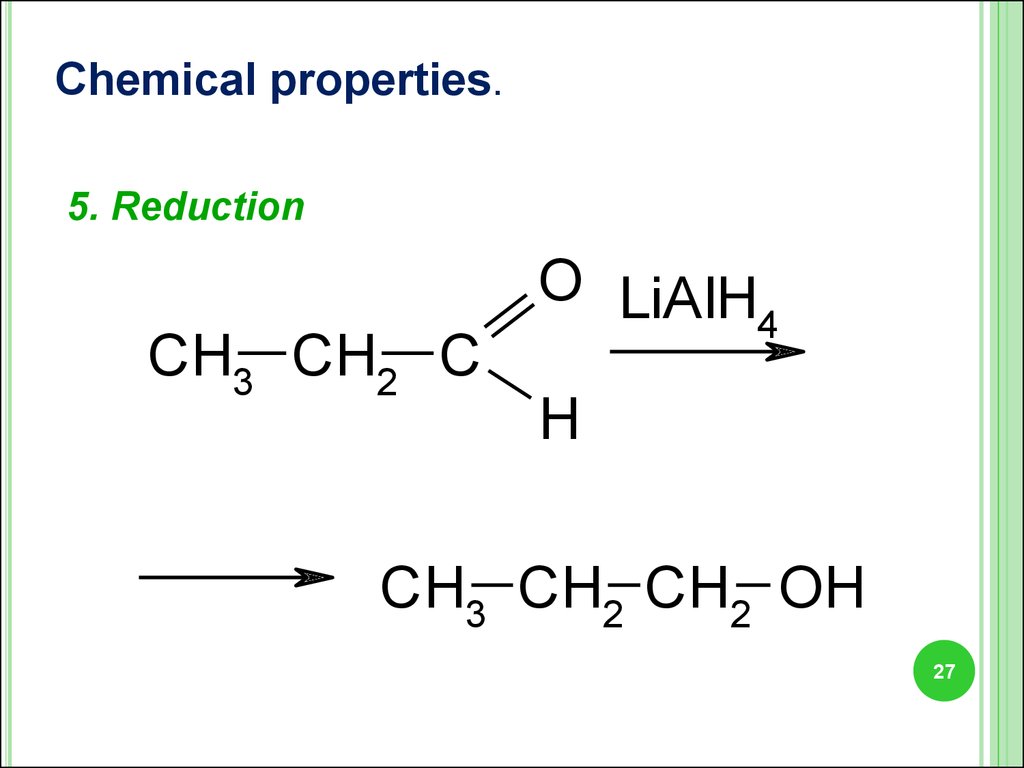
Chemical properties.5. Reduction
CH3 CH2 C
O LiAlH
4
H
CH3 CH2 CH2 OH
27
28.
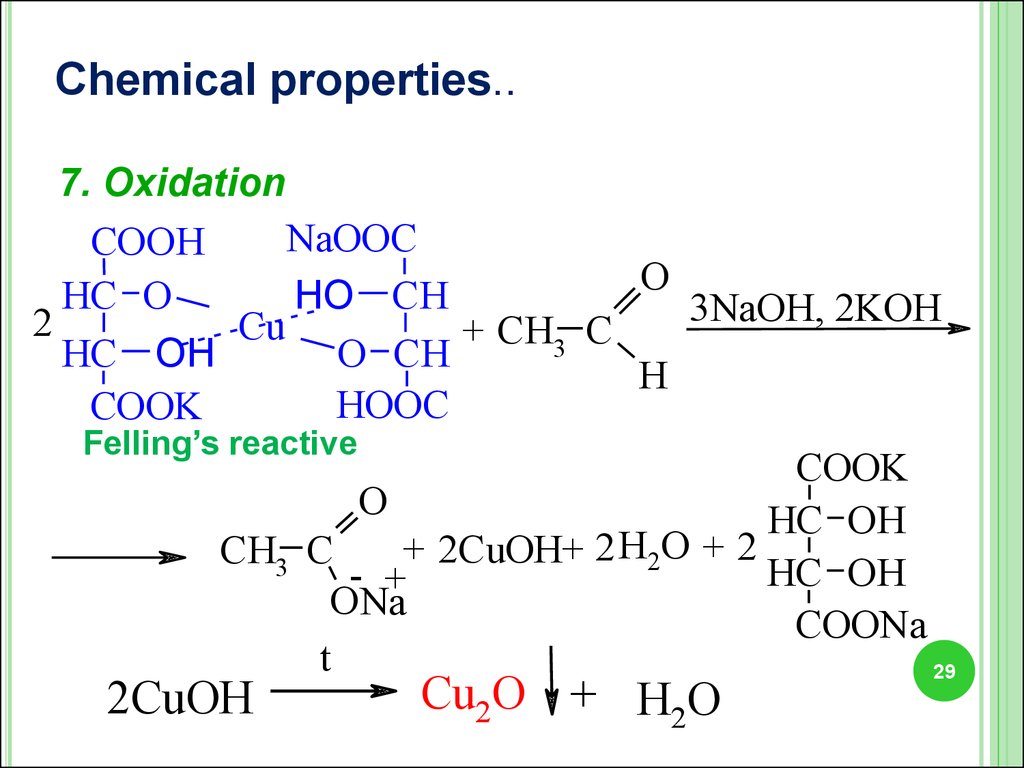
Chemical properties.6. Oxydation
O
R C
H
+ 2 [Ag(NH3 )2 ]OH
Tollen’s reagent
O
2Ag + R C
+
ONH4
+ 3NH3 + H2O
28
29.
Chemical properties..7. Oxidation
NaOOC
COOH
O
HC O
HO CH
3NaOH, 2KOH
2
Cu
+ CH3 C
HC OH
O CH
H
HООC
COOK
Felling’s reactive
COOK
O
HC OH
+ 2CuOH+ 2 H2O + 2
CH3 C
HC OH
- +
ONa
COONa
t
29
2CuOH
Cu2O + H2O
30.
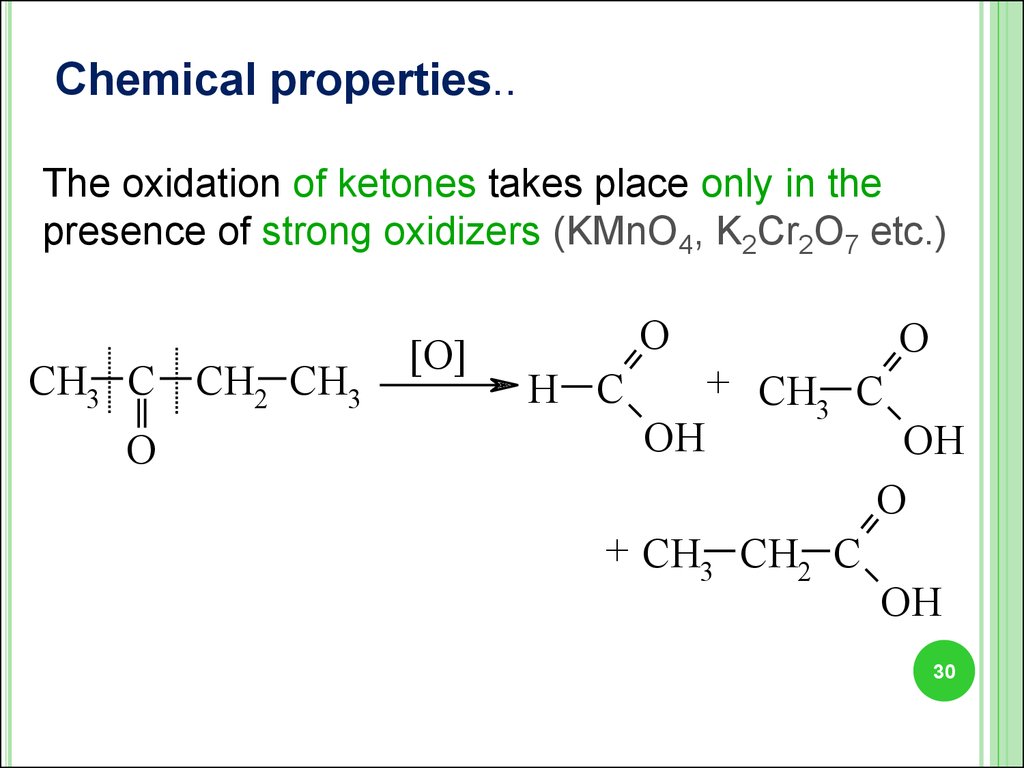
Chemical properties..The oxidation of ketones takes place only in the
presence of strong oxidizers (KMnO4, K2Сr2O7 etc.)
CH3 C CH2 CH3
O
[O]
O
H C
OH
+ CH C
3
+ CH3 CH2 C
O
OH
O
OH
30
31.
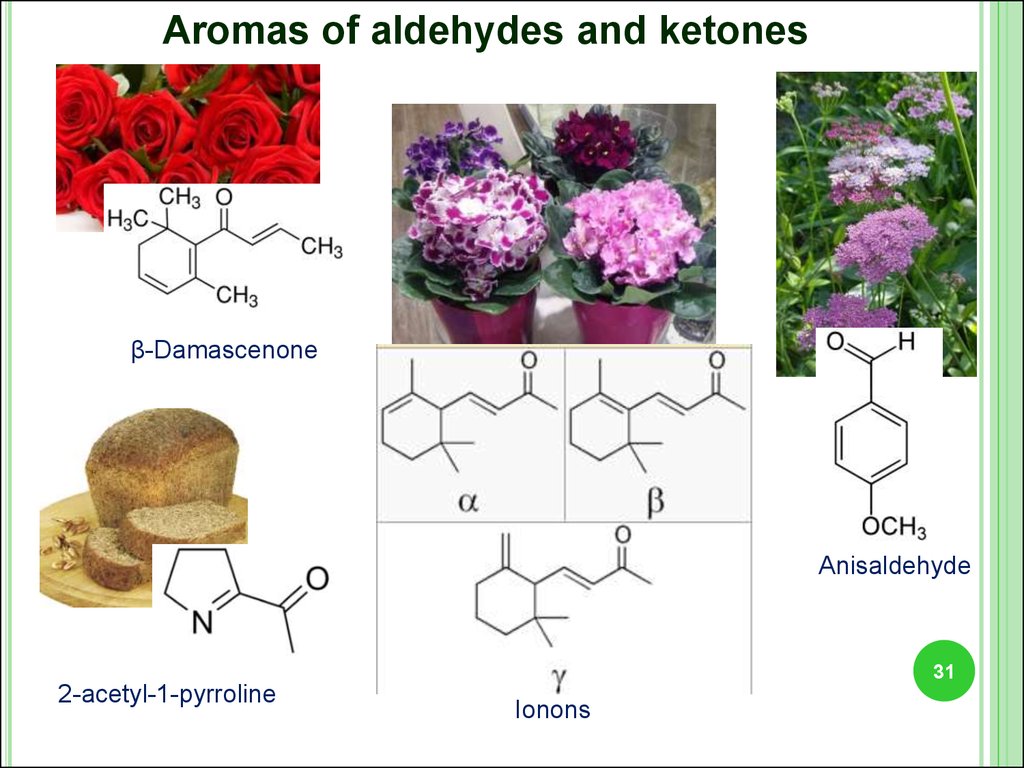
Aromas of aldehydes and ketonesβ-Damascenone
Anisaldehyde
31
2-acetyl-1-pyrroline
Ionons
32.
Aldehydes and ketonesTestosterone
Progesterone
Vanillin
Menton
32
Cinnamaldehyde
33.
Carboxylic acids - derivatives of hydrocarbons,containing in the structure the one or more carboxyl
groups – COOH.
Classification
• nature of hydrocarbon radical
aliphatic
saturated
aromatic
unsaturated
O
O
CH3 C
H2C CH
OH
C
alicyclic
O
O
C
C
OH
OH
OH
• number of groups – СООН
monocarboxylic
O
dicarboxylic
O
H C
O
C CH2 C
OH
HO
33
OH
34.
Carboxylic acid. Nomenclature.O
H C
CH3 C
OH
substitutive
methanoic
OH
ethanoic
methane
carboxylic acid
rational
trivial
O
formic acid
acetic
4
3
2
1
O
H3C H2C CH2 C
OH
butanoic
acids
1-propane
carboxylic acid
butyric
34
35.
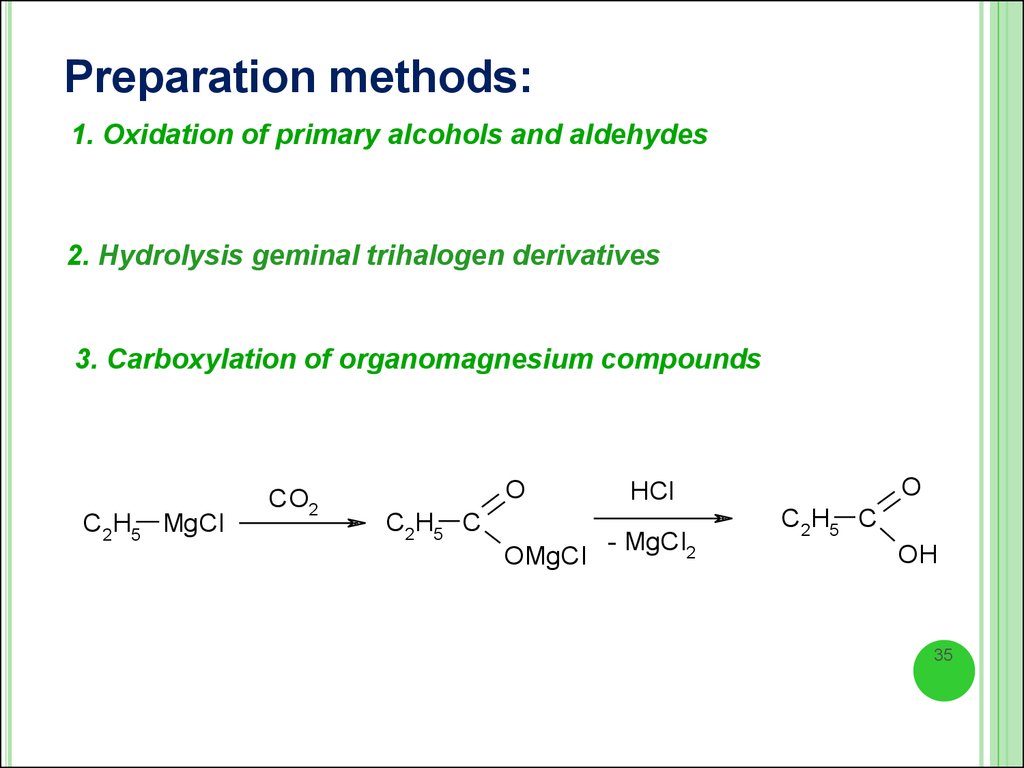
Preparation methods:1. Oxidation of primary alcohols and aldehydes
2. Hydrolysis geminal trihalogen derivatives
3. Carboxylation of organomagnesium compounds
C2H5 MgCl
CO2
O
C2H5 C
OMgCl
HCl
- MgCl2
O
C2H5 C
OH
35
36.
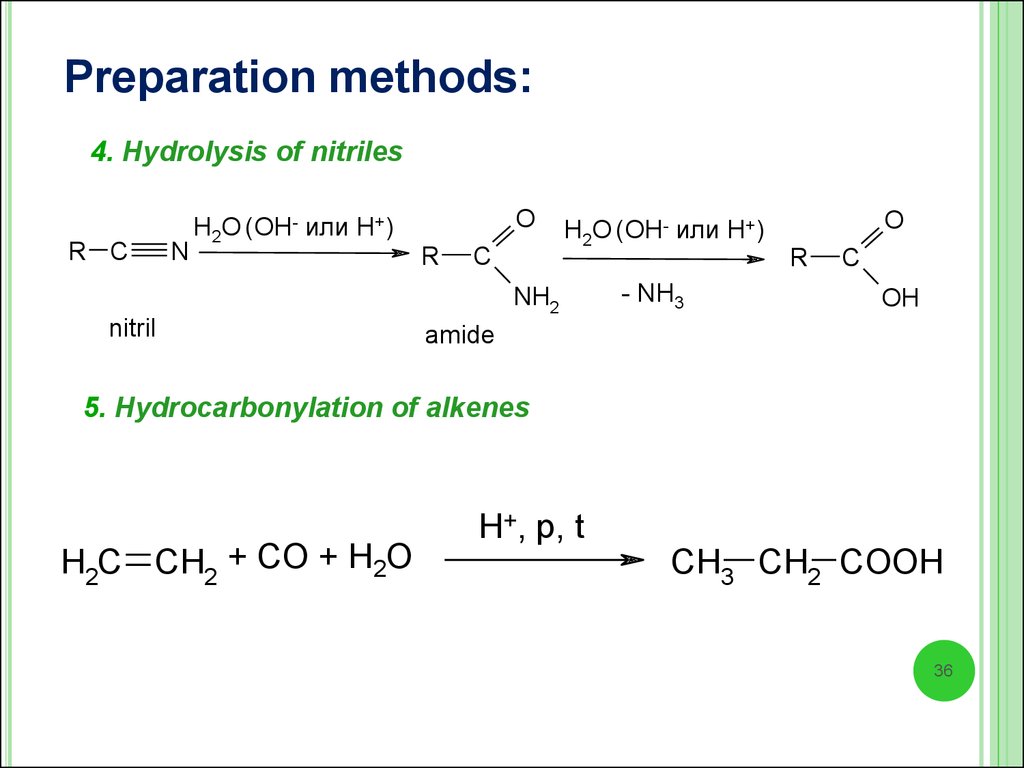
Preparation methods:4. Hydrolysis of nitriles
R C
N
H2O (OH- или H+)
O
R
C
H2O (OH- или H+)
NH2
nitril
- NH3
O
R
C
OH
amide
5. Hydrocarbonylation of alkenes
H2C CH2 + CO + H2O
H+, p, t
CH3 CH2 COOH
36
37.
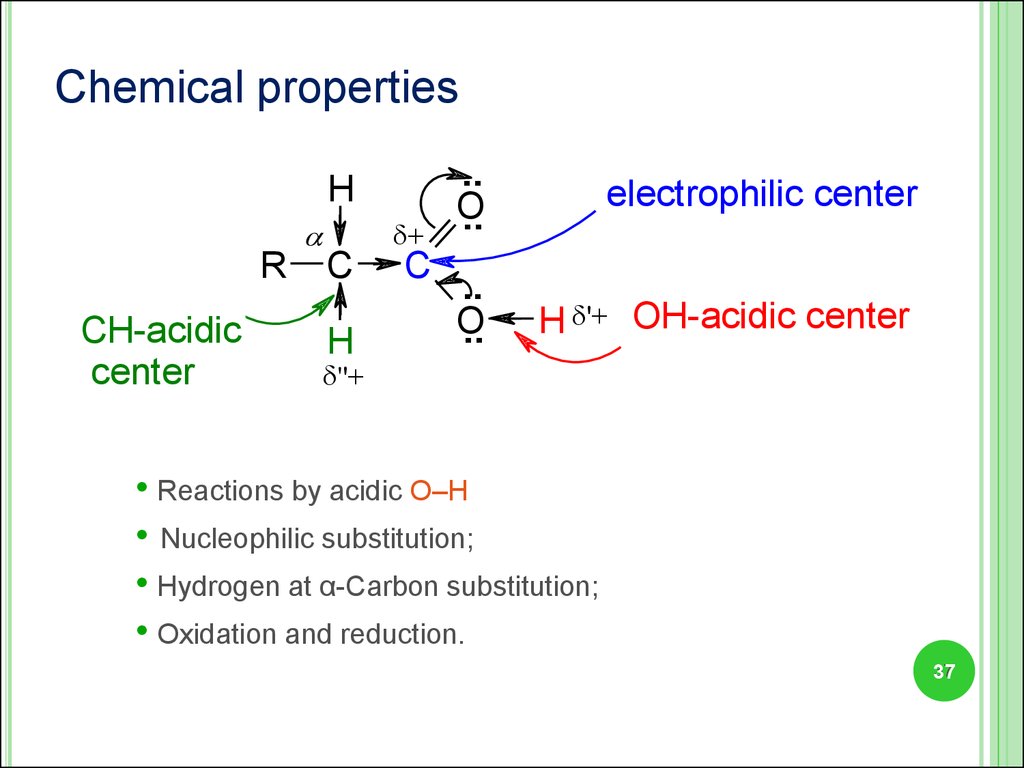
Chemical propertiesH
R
СН-acidic
center
C
H
+
C
..
O
..
electrophilic center
..
O
..
H '+ ОН-acidic center
''+
• Reactions by acidic О–Н
• Nucleophilic substitution;
• Hydrogen at α-Carbon substitution;
• Oxidation and reduction.
37
37
38.
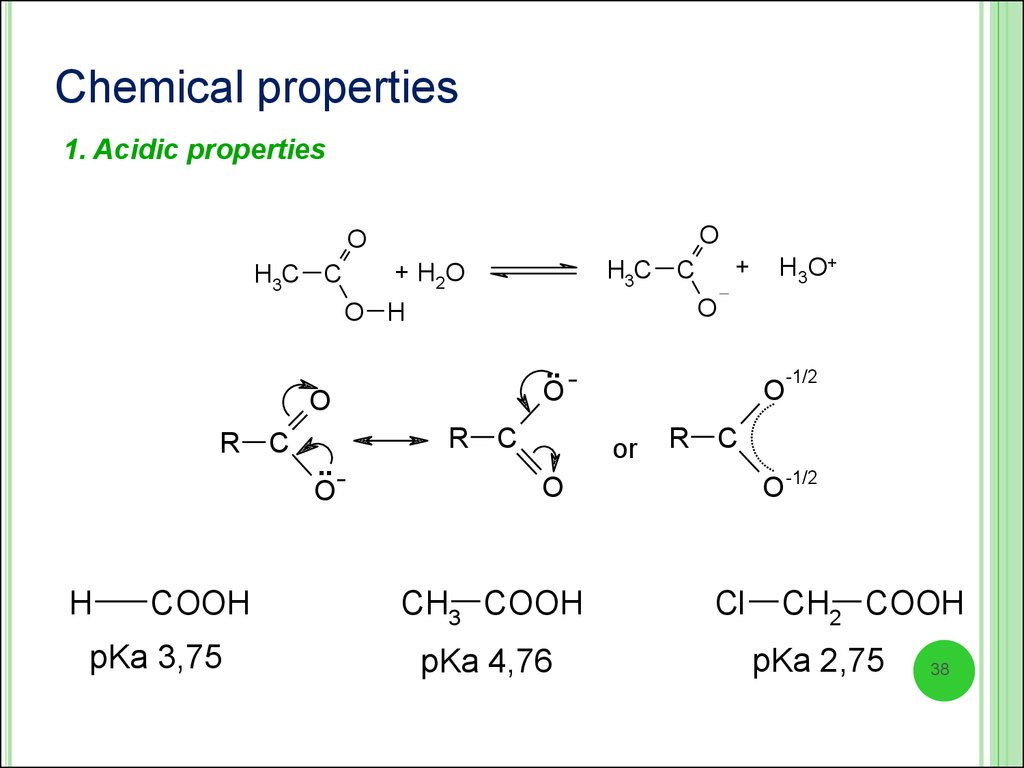
Chemical properties1. Acidic properties
O
O
+ H2O
H3C C
.. O
O
H
COOH
pKa 3,75
C
.. O
H3O+
O
O H
R
+
H3C C
R
C
O
or
R
C
O -1/2
O
CH3 COOH
pKa 4,76
-1/2
Cl
CH2 COOH
pKa 2,75
38
39.
Nucleophilic substitution reactions (SN)1. The esterification reaction
O
CH3 C
O
H2SO4
OH + H
CH3 C
OC2H5
+ H2O
OC2H5
2. Halogenation
CH3 COOH
SOCl2
O
+ SO2 + HCl
CH3 C
Cl
39
39
40.
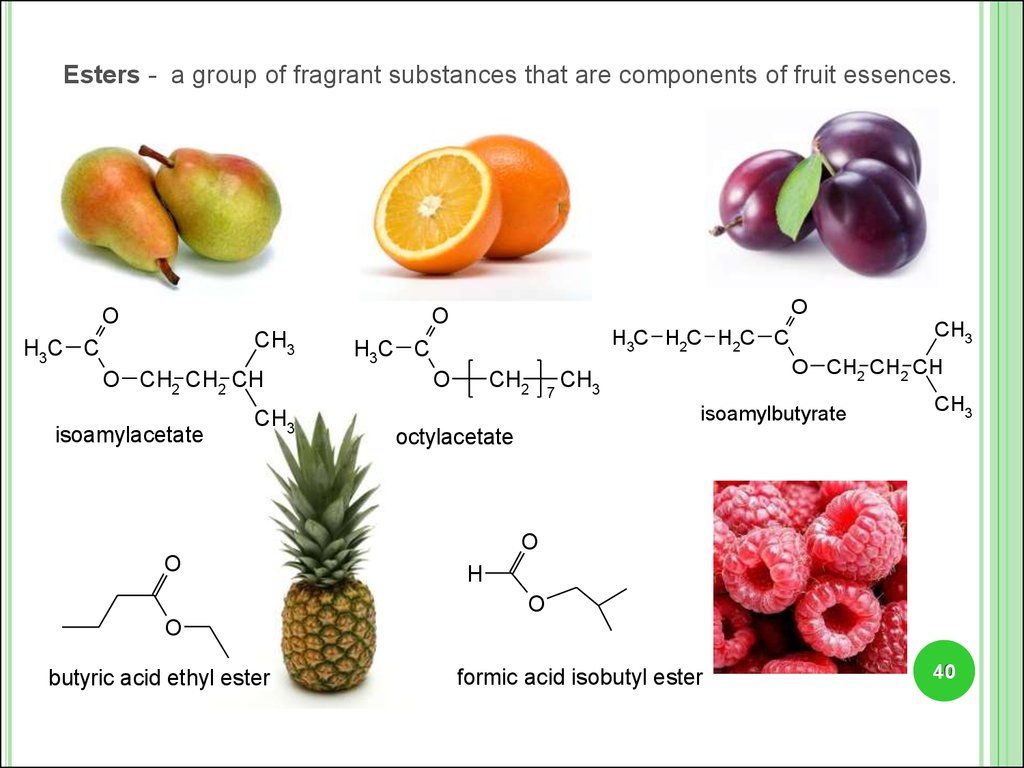
Esters - a group of fragrant substances that are components of fruit essences.O
O
O
CH3
H3C C
O CH2 CH2 CH
isoamylacetate
CH3
CH3
H3C H2C H2C C
H3C C
O
CH2
O CH2 CH2 CH
CH3
7
isoamylbutyrate
CH3
octylacetate
O
O
H
O
O
butyric acid ethyl ester
formic acid isobutyl ester
40
40
41.
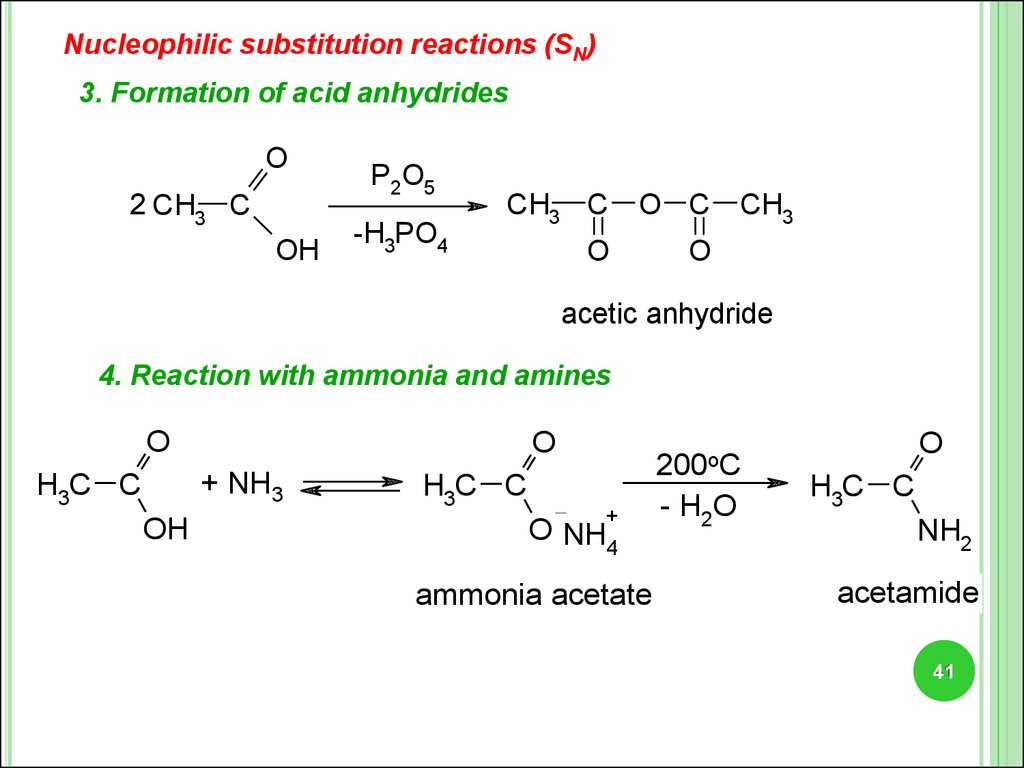
Nucleophilic substitution reactions (SN)3. Formation of acid anhydrides
O
2 CH3 C
OH
P2O5
-H3PO4
CH3 C
O C
O
O
CH3
acetic anhydride
4. Reaction with ammonia and amines
O
O
+ NH3
H3C C
OH
H3C C
200oC
+
O NH4
ammonia acetate
- H2O
O
H3C C
NH2
acetamide
41
41
42.
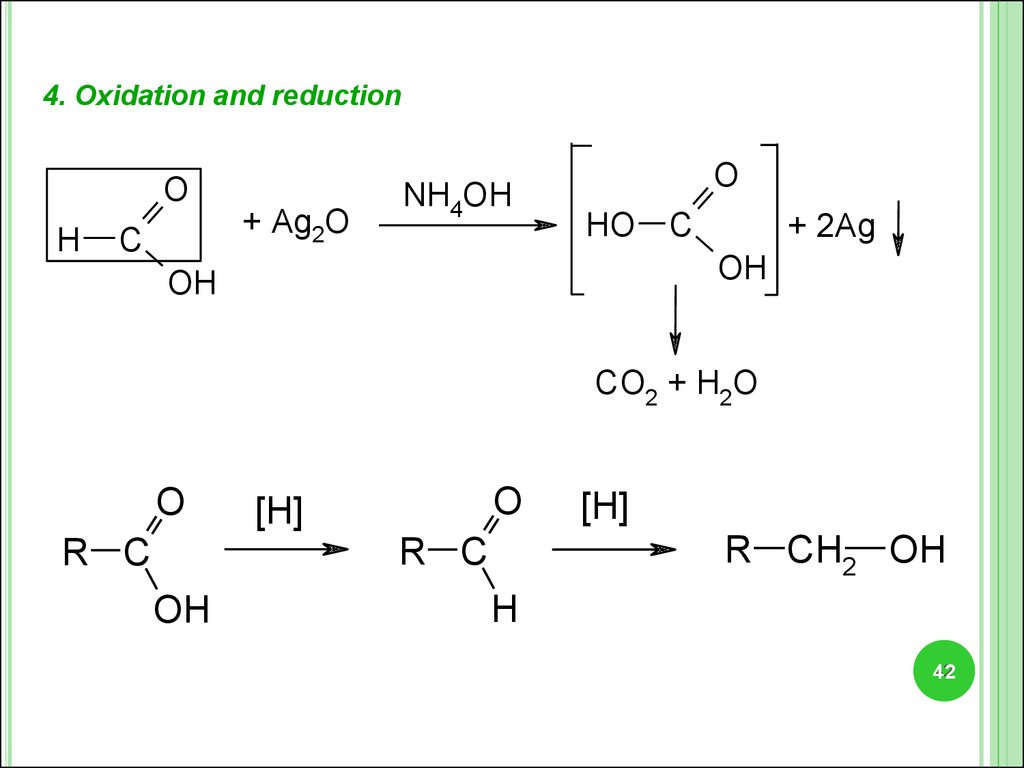
4. Oxidation and reductionO
H
C
+ Ag2O
NH4OH
O
HO C
+ 2Ag
OH
OH
CO2 + H2O
O
O
[H]
R CH2 OH
R C
R C
OH
[H]
H
42
42
43.
Dicarboxylic acidsNomenclature
O
O
O
C C
HO
Subsitutive
Trivial
C
OH
ethandioic
oxalic
O
O
CH2 C
HO
O
C
OH
propandioic
malonic
H2C CH2 C
HO
OH
butandioic
succinic
43
43
Rumex acetosa
Óxalis
Acidum malicum
Succinum
44.
Heating of the dicarboxilic acidsHOOC
COOH
200 oC
+ CO2
H COOH
formic acid
HOOC
CH2 COOH
150 oC
O
H2C
H2C
C
C
O H
OH
300 oС
- H2O
CH3 COOH + CO2
acetic acid
O
H2C
H2C
C
O
C
O
O
succinic anhydride
O
H2C C
O H
H2C
OH
H2C C
O
O
300 oС
- H2O
H2C C
H2C
O
H2C C
O
glutaric anhydride
44
44
45.
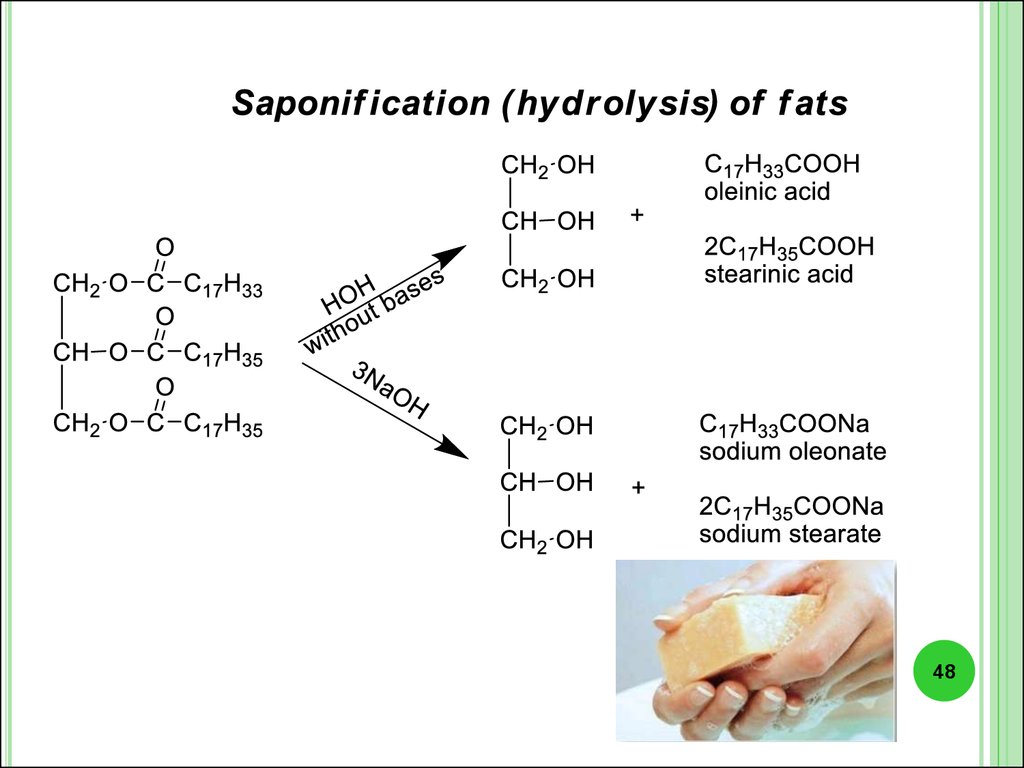
Lipids (fats) - are esters, that are formed of the trihydric alcohol glyceroland higher fatty acids.
45
46.
In triacylglycerol residues of animal origin predominate saturated acids.Usually they have a solid consistency.
Liquid vegetable oils contain mostly
unsaturated acids residues.
46
47.
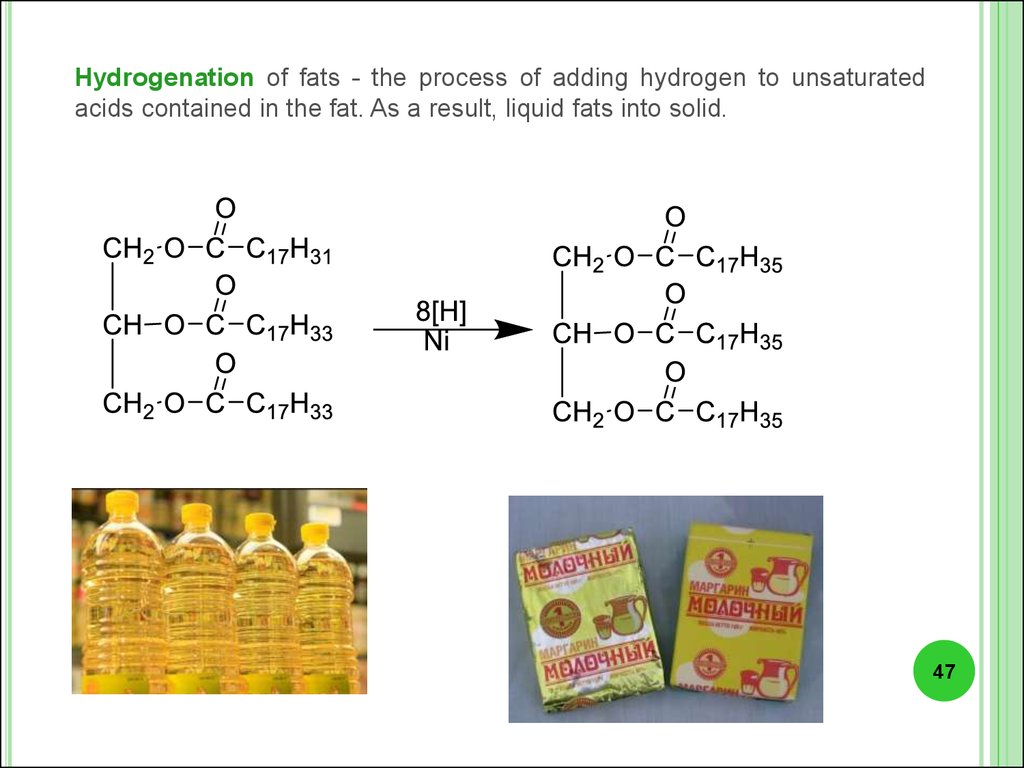
Hydrogenation of fats - the process of adding hydrogen to unsaturatedacids contained in the fat. As a result, liquid fats into solid.
47
48.
4849.
Iodine number - an indicator of unsaturated fat.49
50.
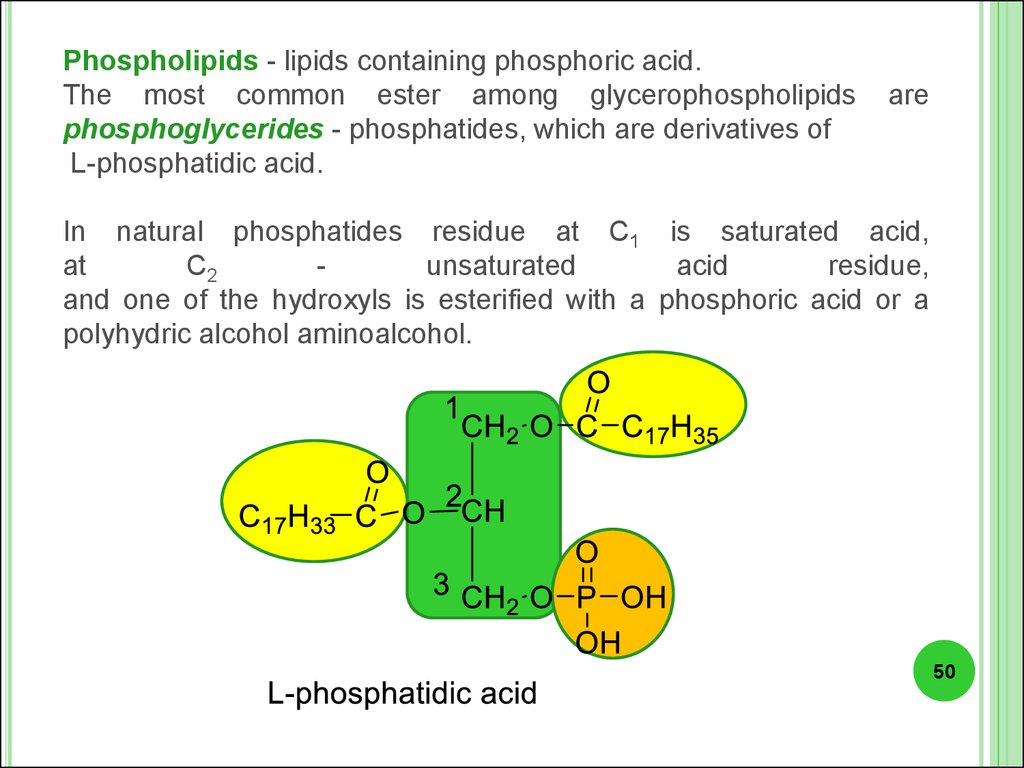
Phospholipids - lipids containing phosphoric acid.The most common ester among glycerophospholipids
phosphoglycerides - phosphatides, which are derivatives of
L-phosphatidic acid.
are
In natural phosphatides residue at C1 is saturated acid,
at
C2
unsaturated
acid
residue,
and one of the hydroxyls is esterified with a phosphoric acid or a
polyhydric alcohol aminoalcohol.
50
51.
Representatives of phosphatides are cephalins - containing amino alcoholcolamine.
The complete hydrolysis of the kephaline molecule gives glycerine, two
molecules of higher fatty acids, phosphoric acid and the molecule of
colamine.
51
52.
Lecithins - phosphatides containing aminoalcohol choline.52
53.
Thank Youfor Your
attention!
53

























 Химия
Химия








