Похожие презентации:
Oxygen
1. OXYGEN
2. Formula
O2
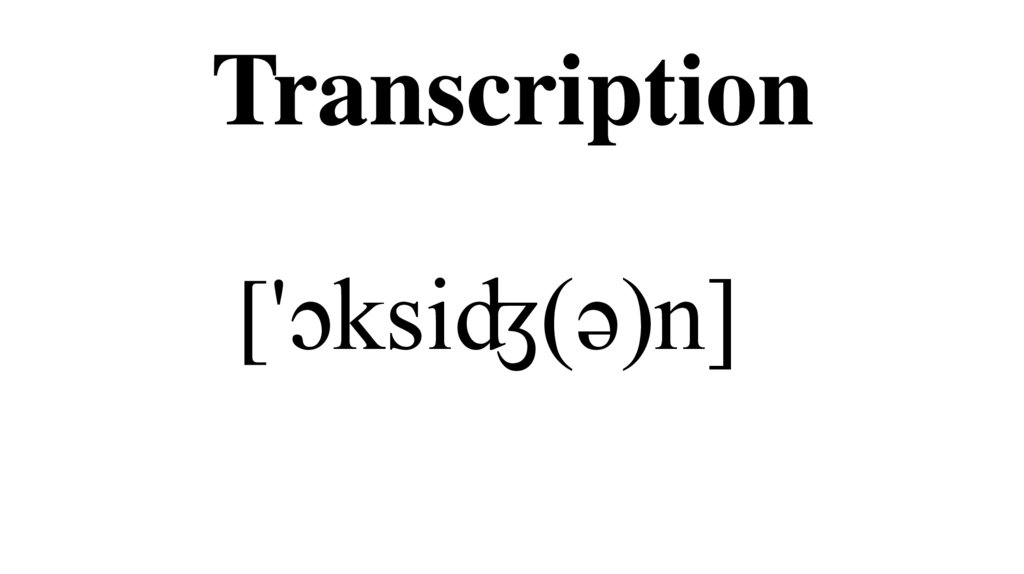
3. Transcription
['ɔksiʤ(ə)n]4. Etymology
It is derived from Greek and itmeans «acid former».
5. Position in the periodic table
Oxygen is in group 16 and period 2of the Periodic table.
6. Нistory of discovery
Oxygen was obtained by C. W. Scheele and J.Priestley independently.
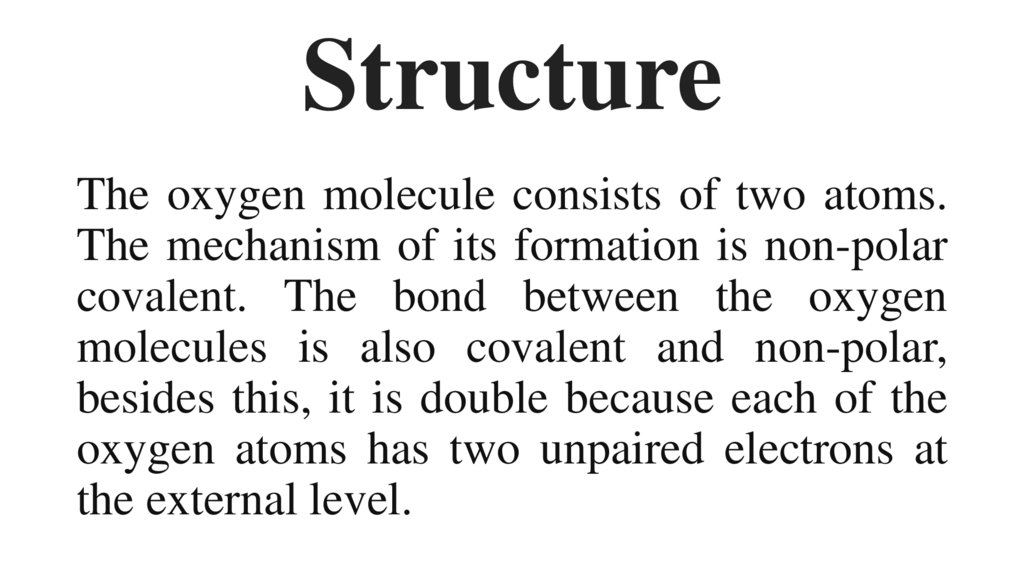
7. Structure
The oxygen molecule consists of two atoms.The mechanism of its formation is non-polar
covalent. The bond between the oxygen
molecules is also covalent and non-polar,
besides this, it is double because each of the
oxygen atoms has two unpaired electrons at
the external level.
8. Electronic configuration
22
4
1s 2s 2p
9. Atomic mass
16 grams per mole10. Radius of the oxygen atom
6011. Valence
–2, −1, –½, –⅓, 0, +½, +1, +212. Boiling/melting point
The melting point is 54.8 K (218.35 °C). The boiling point is90.19 K (-182.96 °C).
13. Isotopes
Oxygen-16Oxygen-17
Oxygen-18
14. Physical properties
Oxygen is the gas.Oxygen has no color, no taste, and no
smell.
Oxygen can be dissolved in organic
substances, absorbed by coal and metal
powders.
15. Prevalence in nature
It forms 21 % of the atmosphere,89 % 0f the water, 50% of the
earth’s crust.
16. Оxygen deposit
It occurs in the atmosphere, inwater, in the earth’s crust.
17. Мethods of obtaining oxygen
It is easily prepared in thelaboratory by heating potassium
chlorate.
It is made commercially mainly
by the distillation of liquid air.
18. Сhemical properties
Flammability - Does not burn;Combustion - Supports combustion but does not burn;
Compounds - Occurs in many compounds, including
water, carbon dioxide, and iron ore;
Oxidation - The common reaction in which it unites
with another substance is called oxidation;
Oxides of some metals form peroxides by the addition
of oxygen.
19. Application in industry
Oxygen is actively used in:• Metallurgy (during welding and cutting metals).
• The medicine.
• Agriculture.
• Like rocket fuel.
• For purification and disinfection of water.
• The synthesis of certain chemical compounds,
including explosives.
20. The biological role
The presence of oxygen (in combination withwater) has made life possible on our planet.
Oxygen in the upper atmosphere forms
the ozone ball, which protects all the
inhabitants of the Earth from harmful
ultraviolet solar radiation.
21. Toxicity
Oxygen toxicity is a condition resulting fromthe harmful effects of breathing molecular
oxygen (O2) at increased partial pressures.
Severe cases can result in cell damage and
death, with effects most often seen in the
central nervous system, lungs, and eyes.








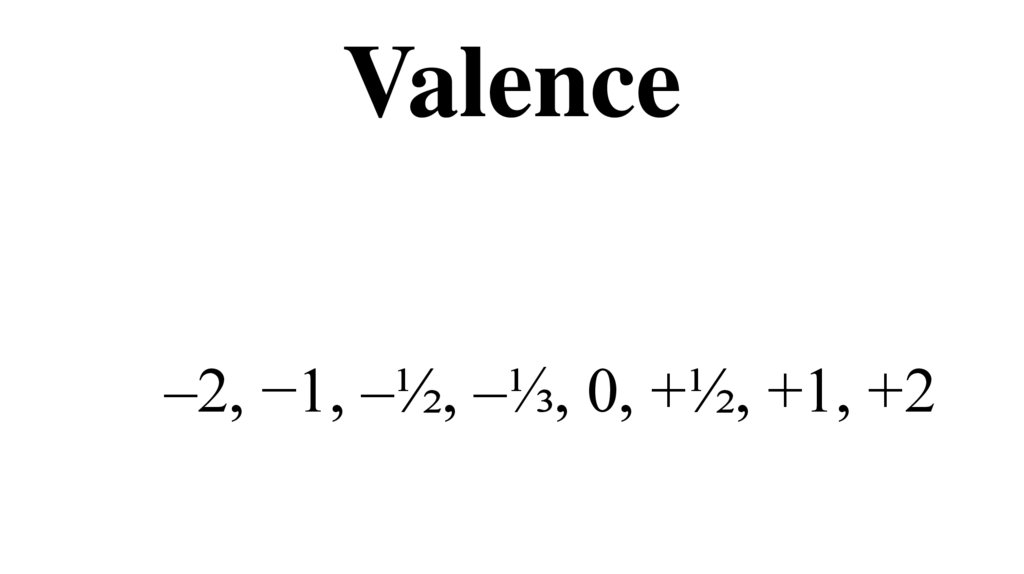

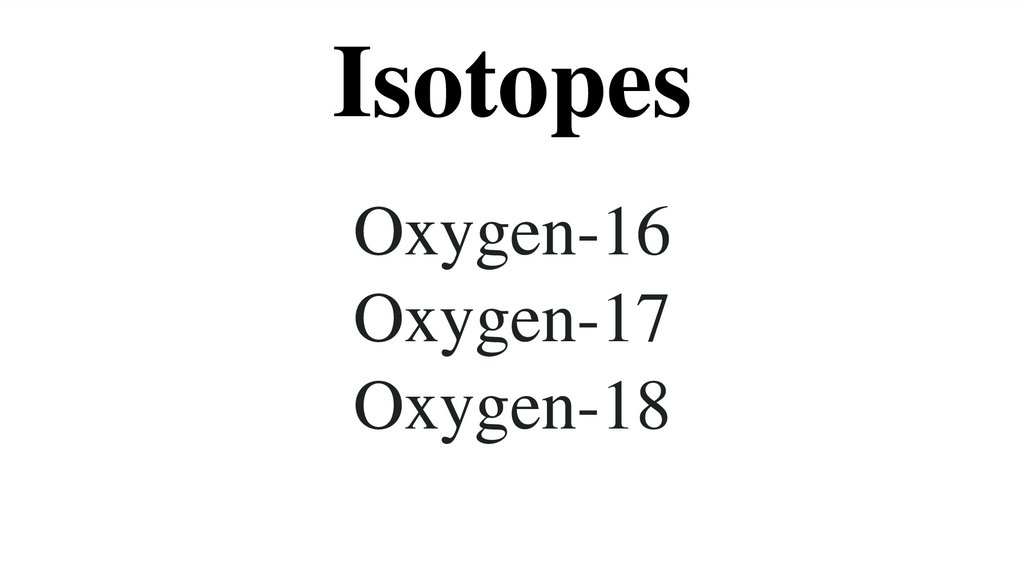








 Химия
Химия