Похожие презентации:
Chemestry of scandiute compounids
1.
Performed By: Adilzhanov.DAccepted By: Shaimerdenova M
Group: 1504-19
2.
Scandium Sc, yttrium Y, lanthanum La andactinium Ac make up the scandium subgroup.
d-Elements of group III of the periodic system
of D.I. Mendeleev are complete electronic
analogues with the configuration of valence
electrons (n - 1)d1ns2.
3.
Dmitry Mendeleev, whose father is called the periodic table, predicted the existence of the elementekaboron, with atomic mass between 40 and 48 in 1869. Lars Fredrik Nilson and his team identified
this element in the minerals eusenite and gadolinite in 1879. He called the element scandium in Latin
Scandia meaning "Scandinavian". Nilsson did not seem to know Mendeleev's prediction, but
Theodore recognized the correspondence for Cleve and made a message to Mendeleev.The metal
scandium was first produced in 1937 by electrolysis of a eutectic mixture of potassium, lithium, and
scandium chloride, at 700-800 °C. the first pound of 99% pure scandium metal was produced in
1960. The production of aluminum alloys began in 1971 after a US Patent.aluminum-scandium alloys
were also developed in the USSR.Gadolinium-scandium-gallium Garnet (GSGG) laser crystals have
been used in strategic defense programs. Strategic Defense Initiative (SDI) 1980-90.
HISTORY
4.
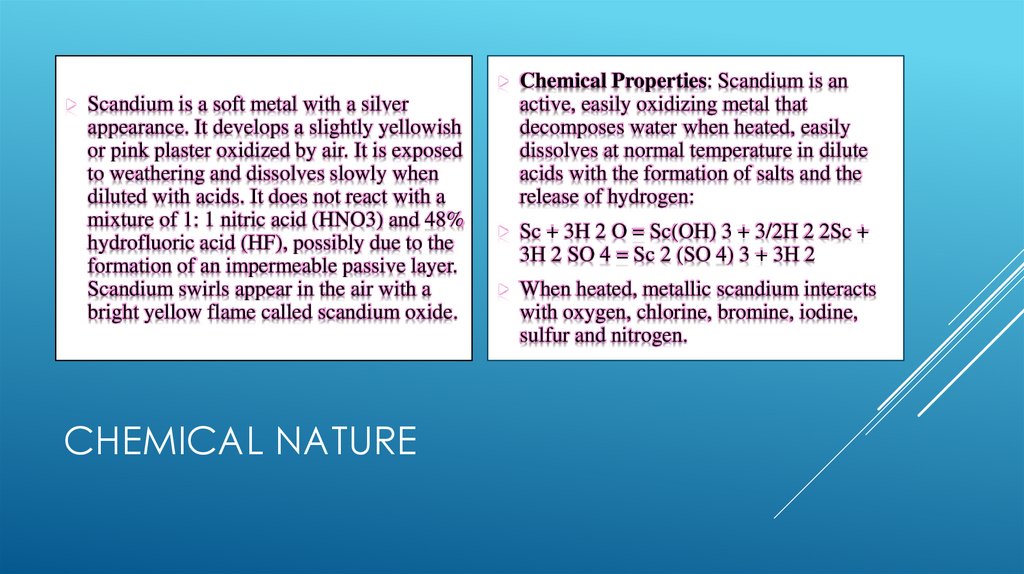
Chemical Properties: Scandium is anScandium is a soft metal with a silver
appearance. It develops a slightly yellowish
or pink plaster oxidized by air. It is exposed
to weathering and dissolves slowly when
diluted with acids. It does not react with a
mixture of 1: 1 nitric acid (HNO3) and 48%
hydrofluoric acid (HF), possibly due to the
formation of an impermeable passive layer.
Scandium swirls appear in the air with a
bright yellow flame called scandium oxide.
CHEMICAL NATURE
active, easily oxidizing metal that
decomposes water when heated, easily
dissolves at normal temperature in dilute
acids with the formation of salts and the
release of hydrogen:
Sc + 3H 2 O = Sc(OH) 3 + 3/2H 2 2Sc +
3H 2 SO 4 = Sc 2 (SO 4) 3 + 3H 2
When heated, metallic scandium interacts
with oxygen, chlorine, bromine, iodine,
sulfur and nitrogen.
5.
Scandium is obtained by reducing ScF3 orScCl3 with metallic calcium, followed by
distillation in vacuum.Physical properties:
Scandium is a light metal with a characteristic
yellow tint that appears when metal comes into
contact with air. Metallic scandium has a
specific gravity of 2.99 g/ cm3 at 20 ° C (light
metal), melts at 1539 ° C, boils at 2727 ° C, is
brittle, weakly paramagnetic.
PHYSICAL PROPERTIES
6.
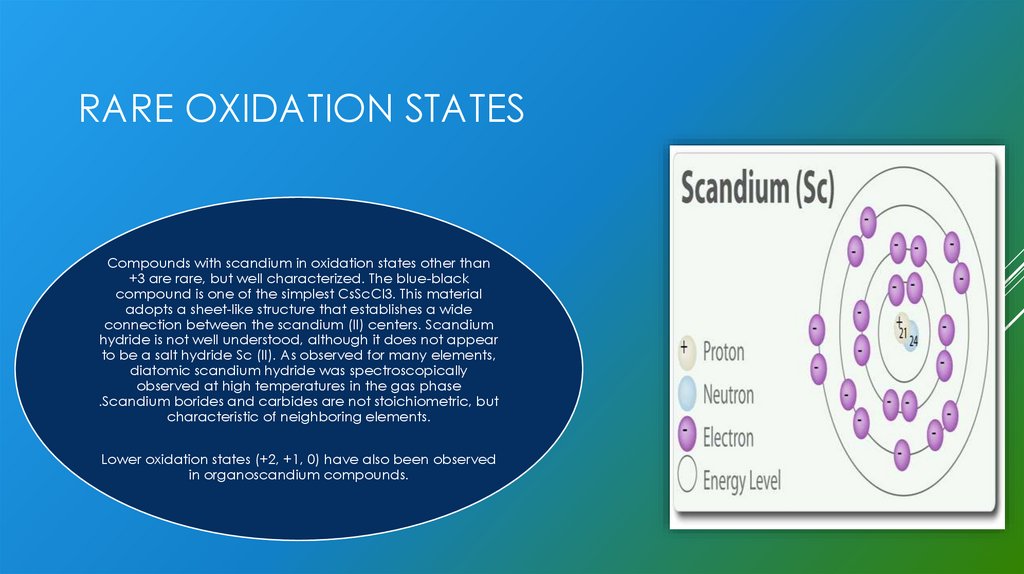
RARE OXIDATION STATESCompounds with scandium in oxidation states other than
+3 are rare, but well characterized. The blue-black
compound is one of the simplest CsScCl3. This material
adopts a sheet-like structure that establishes a wide
connection between the scandium (II) centers. Scandium
hydride is not well understood, although it does not appear
to be a salt hydride Sc (II). As observed for many elements,
diatomic scandium hydride was spectroscopically
observed at high temperatures in the gas phase
.Scandium borides and carbides are not stoichiometric, but
characteristic of neighboring elements.
Lower oxidation states (+2, +1, 0) have also been observed
in organoscandium compounds.
7.
The use of scandium as a micro-alloying impurity has a significant impact on a number ofpractically important alloys, for example, the addition of 0.4% scandium to aluminummagnesium alloys increases the temporary tear resistance by 35%, and the yield strength by 6584%, while the elongation remains at 20-27%. The addition of 0.3-0.67% to chromium increases
its resistance to oxidation up to a temperature of 1290 ° C, and has a similar but even more
pronounced effect on heat-resistant alloys of the "nichrome" type and in this area the use of
scandium is much more effective than yttrium. Scandium oxide has a number of advantages for
the production of high-temperature ceramics over other oxides, so the strength of scandium
oxide increases when heated and reaches a maximum at 1030 ° C, at the same time scandium
oxide has minimal thermal conductivity and the highest resistance to thermal shock. Yttrium
scandate is one of the best materials for structures operating at high temperatures. A certain
amount of scandium oxide is constantly consumed for the production of germanate glasses for
optoelectronics.
APPLICATION
8.
THANKS FOR YOURATTENTION!






 Химия
Химия