Похожие презентации:
The structure of the Periodic table
1.
GUESS THE TOPIC2.
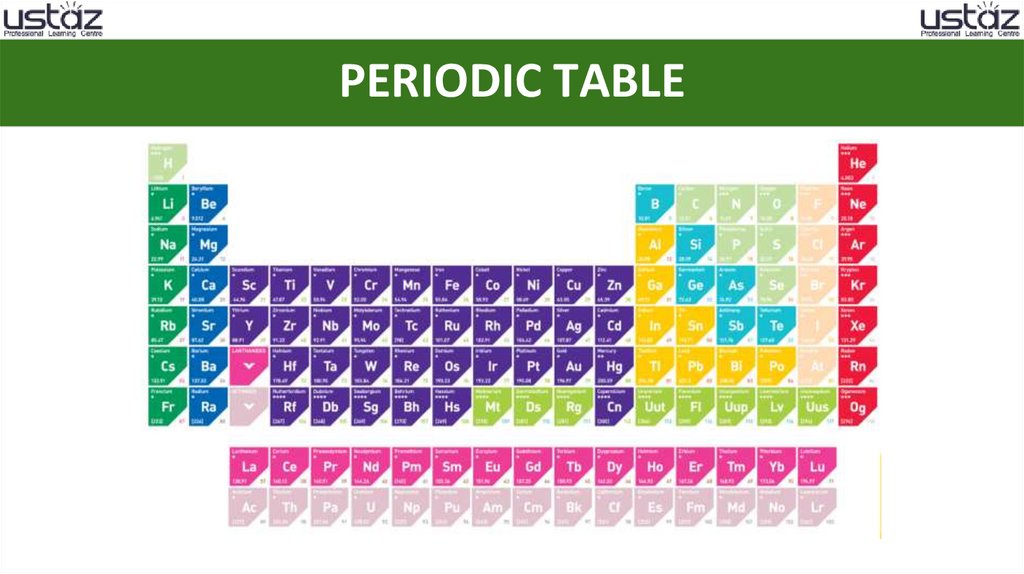
PERIODIC TABLE3.
MODERN PERIODIC TABLE4.
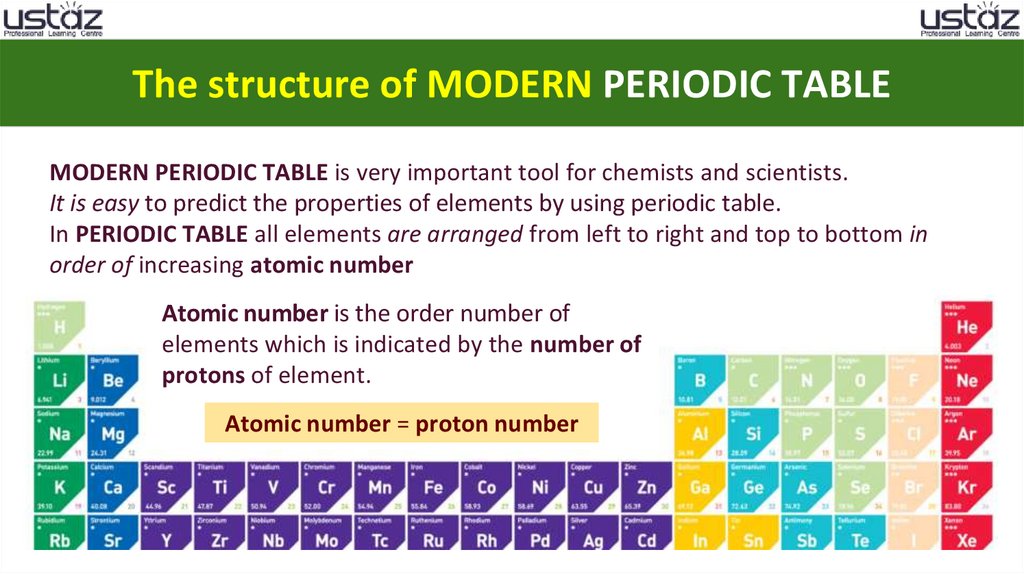
The structure of MODERN PERIODIC TABLEMODERN PERIODIC TABLE is very important tool for chemists and scientists.
It is easy to predict the properties of elements by using periodic table.
In PERIODIC TABLE all elements are arranged from left to right and top to bottom in
order of increasing atomic number
Atomic number is the order number of
elements which is indicated by the number of
protons of element.
Atomic number = proton number
5.
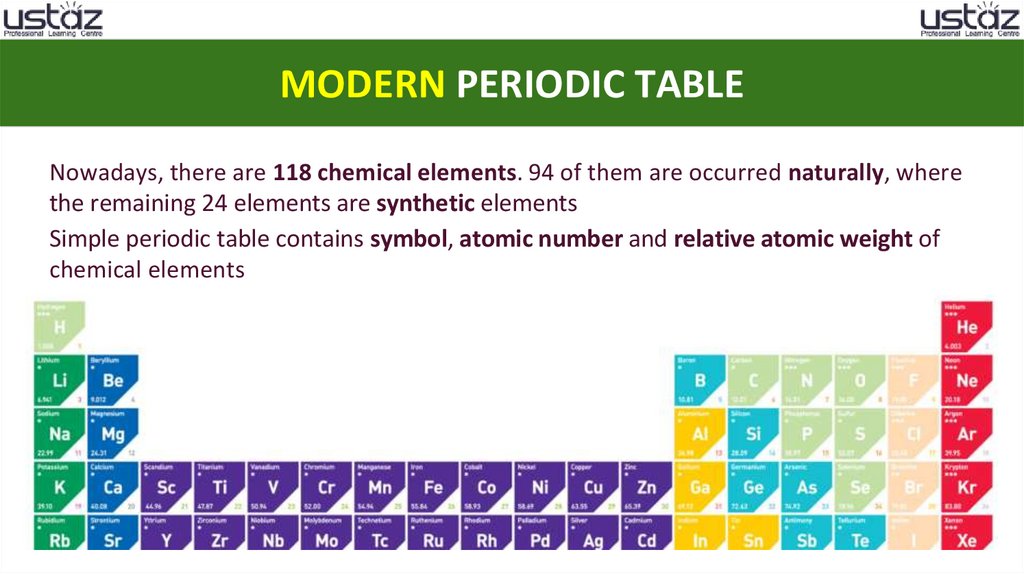
MODERN PERIODIC TABLENowadays, there are 118 chemical elements. 94 of them are occurred naturally, where
the remaining 24 elements are synthetic elements
Simple periodic table contains symbol, atomic number and relative atomic weight of
chemical elements
6.
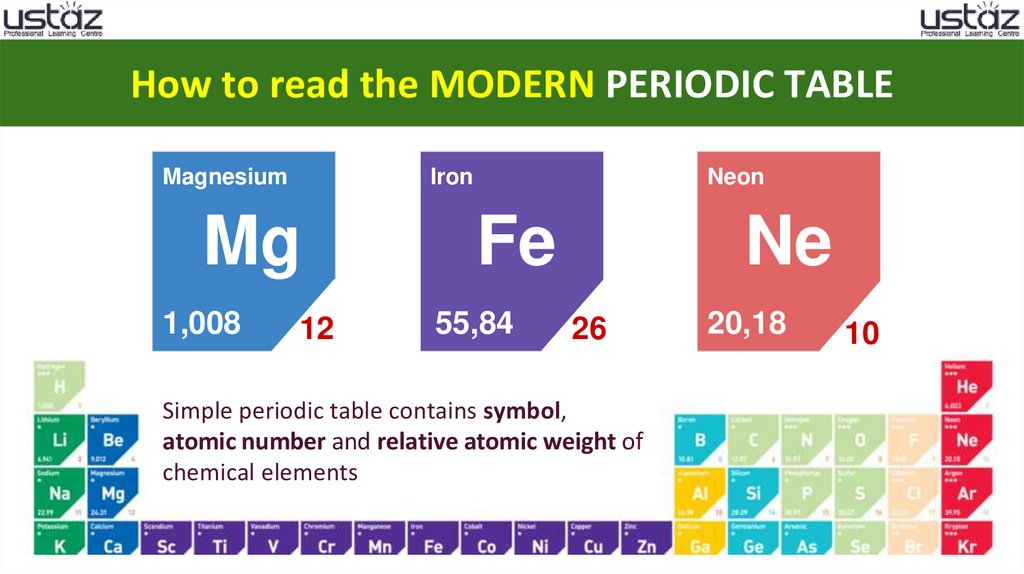
How to read the MODERN PERIODIC TABLEMagnesium
Iron
Mg
1,008
12
Neon
Fe
55,84
Ne
26
Simple periodic table contains symbol,
atomic number and relative atomic weight of
chemical elements
20,18
10
7.
The structure of MODERN PERIODIC TABLEWHAT IS THE PERIODS?
8.
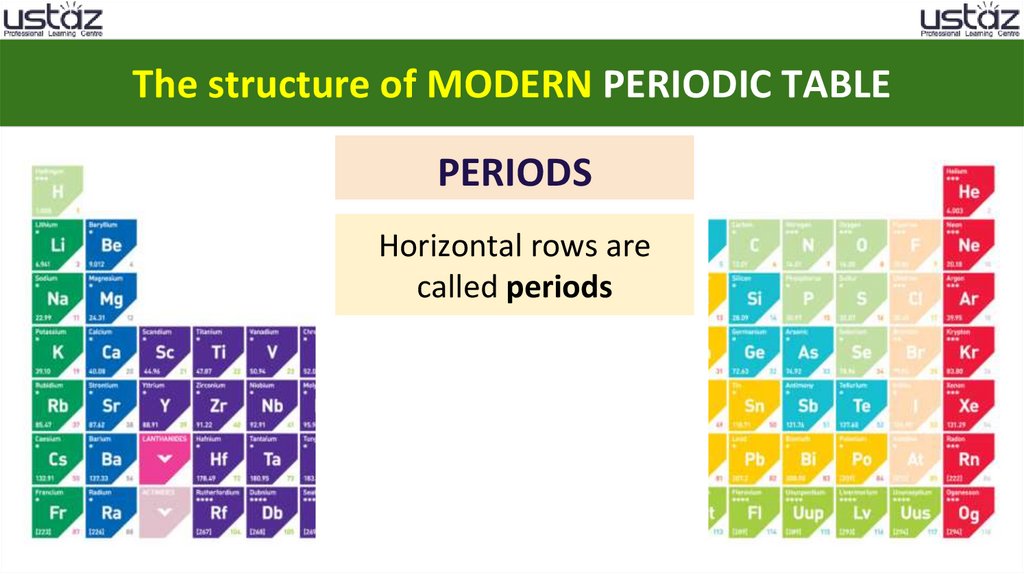
The structure of MODERN PERIODIC TABLEPERIODS
Horizontal rows are
called periods
9.
The structure of MODERN PERIODIC TABLEPERIODS
Horizontal rows are
called periods
10.
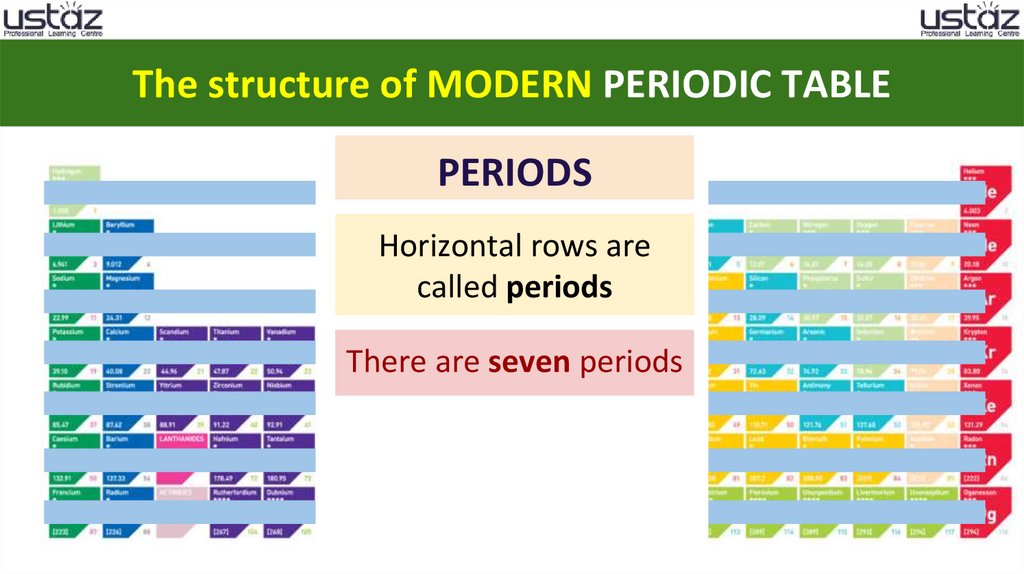
The structure of MODERN PERIODIC TABLEPERIODS
Horizontal rows are
called periods
There are seven periods
11.
The structure of MODERN PERIODIC TABLE1
2
3
4
5
6
7
PERIODS
Horizontal rows are
called periods
There are seven periods
12.
The structure of MODERN PERIODIC TABLE1
2
3
4
PERIODS
Horizontal rows are
called periods
There are seven periods
5
6
7
There are three short,
two medium and two
long periods
13.
MODERN PERIODIC TABLEShort periods
Medium periods
Long periods
14.
The structure of MODERN PERIODIC TABLEWHAT IS THE GROUPS?
15.
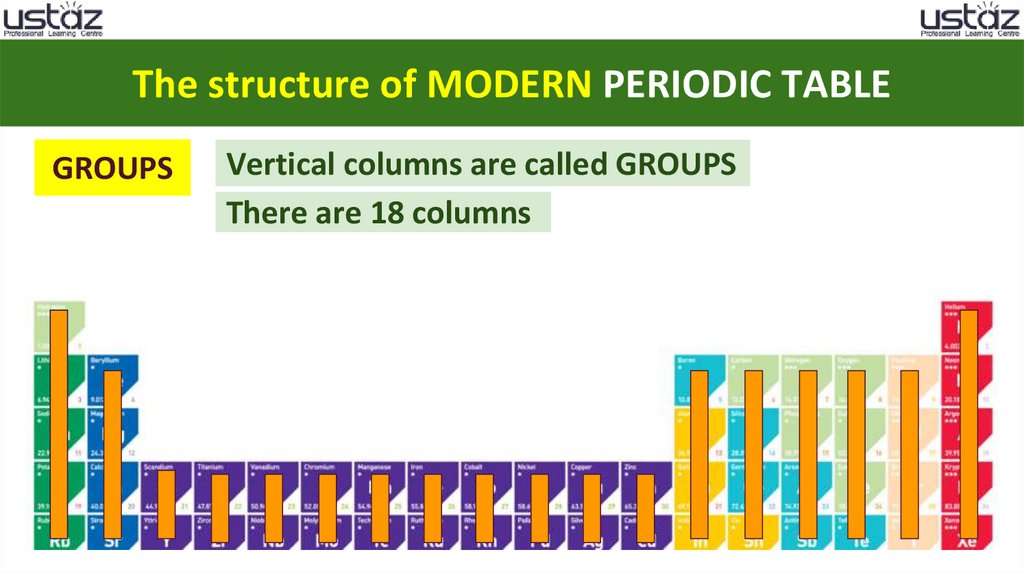
The structure of MODERN PERIODIC TABLEGROUPS
Vertical columns are called GROUPS
16.
The structure of MODERN PERIODIC TABLEGROUPS
Vertical columns are called GROUPS
17.
The structure of MODERN PERIODIC TABLEGROUPS
Vertical columns are called GROUPS
There are 18 columns
18.
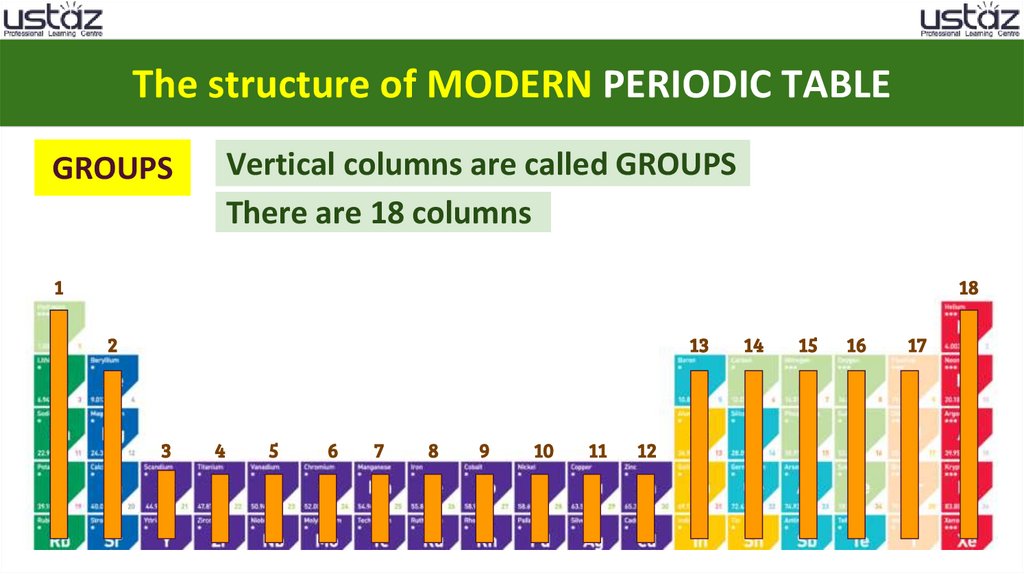
The structure of MODERN PERIODIC TABLEVertical columns are called GROUPS
There are 18 columns
GROUPS
1
18
2
13
3
4
5
6
7
8
9
10
11
12
14
15
16
17
19.
The structure of MODERN PERIODIC TABLEVertical columns are called GROUPS
There are 18 columns
GROUPS
Groups are classified as A and B
groups
1
18
2
13
3
4
5
6
7
8
9
10
11
12
14
15
16
17
20.
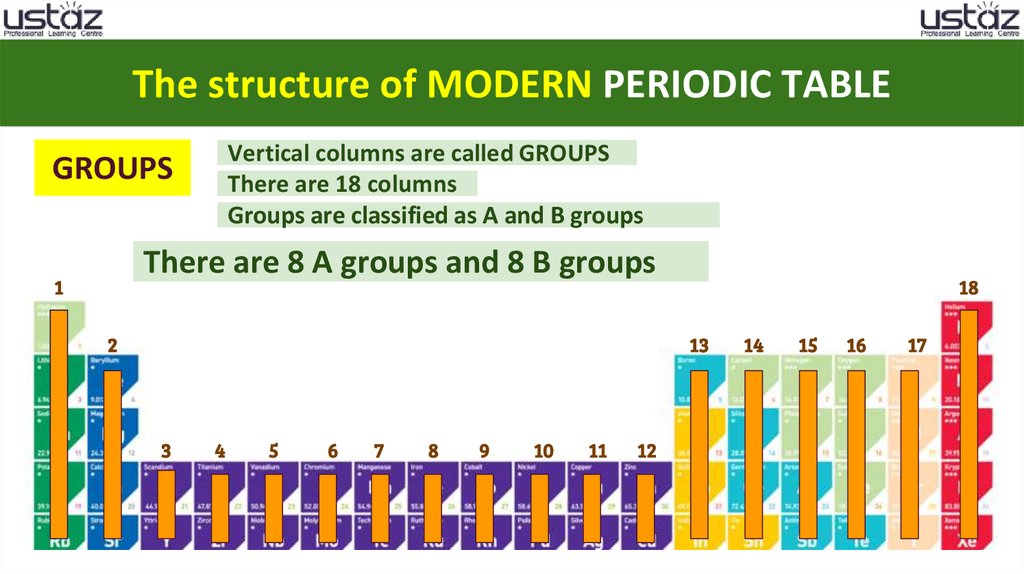
The structure of MODERN PERIODIC TABLEVertical columns are called GROUPS
There are 18 columns
Groups are classified as A and B groups
GROUPS
There are 8 A groups and 8 B groups
1
2
18
13
3
4
5
6
7
8
9
10
11
12
14
15
16
17
21.
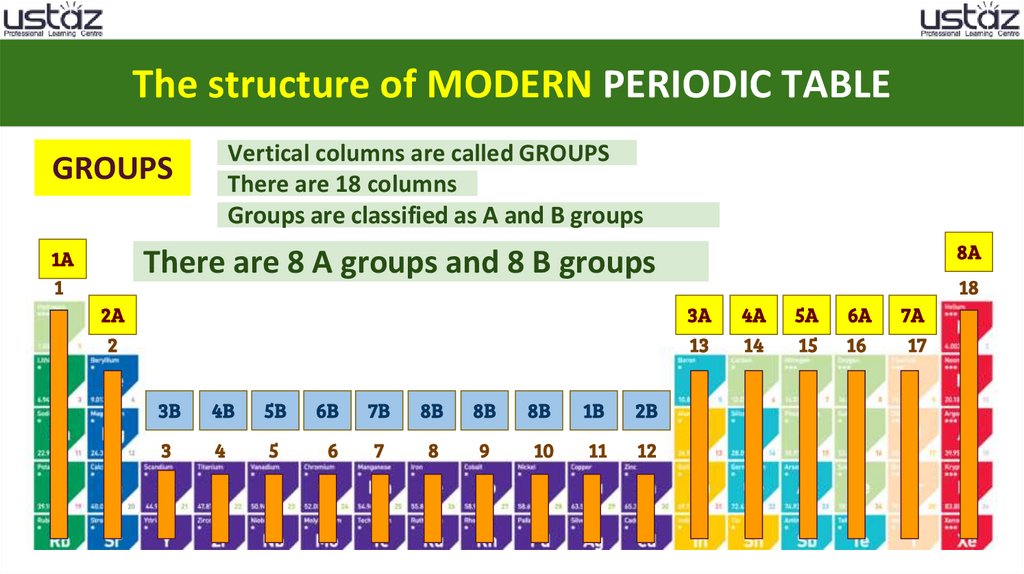
The structure of MODERN PERIODIC TABLEVertical columns are called GROUPS
There are 18 columns
Groups are classified as A and B groups
GROUPS
1A
1
8A
There are 8 A groups and 8 B groups
2A
2
18
3A
13
3B
4B
5B
6B
7B
8B
8B
8B
1B
2B
3
4
5
6
7
8
9
10
11
12
4A
14
5A
15
6A
16
7A
17
22.
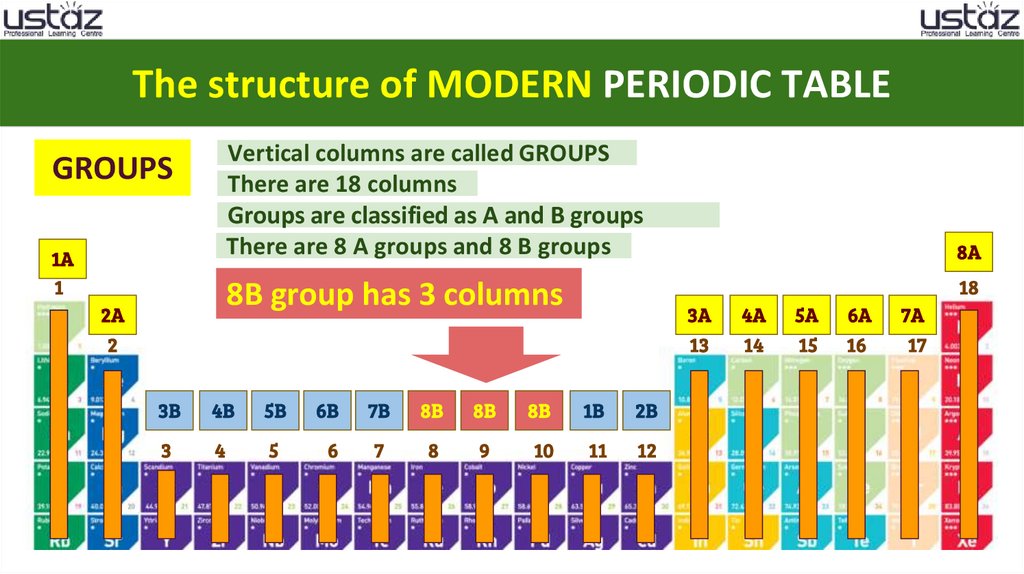
The structure of MODERN PERIODIC TABLEVertical columns are called GROUPS
There are 18 columns
Groups are classified as A and B groups
There are 8 A groups and 8 B groups
GROUPS
1A
1
18
8 B group has 3 columns
2A
2
8A
3A
13
3B
4B
5B
6B
7B
8B
8B
8B
1B
2B
3
4
5
6
7
8
9
10
11
12
4A
14
5A
15
6A
16
7A
17
23.
The structure of MODERN PERIODIC TABLEVertical columns are called GROUPS
There are 18 columns
Groups are classified as A and B groups
There are 8 A groups and 8 B groups
GROUPS
1A
1
18
8B group has 3 columns
2A
2
8A
3A
13
3B
4B
5B
6B
7B
8B
8B
8B
1B
2B
3
4
5
6
7
8
9
10
11
12
4A
14
5A
15
6A
16
7A
17
24.
The structure of MODERN PERIODIC TABLEGROUPS
sometimes called as
FAMILY
8A
1A
1
18
2A
2
3A
13
3B
4B
5B
6B
7B
8B
8B
8B
1B
2B
3
4
5
6
7
8
9
10
11
12
4A
14
5A
15
6A
16
7A
17
25.
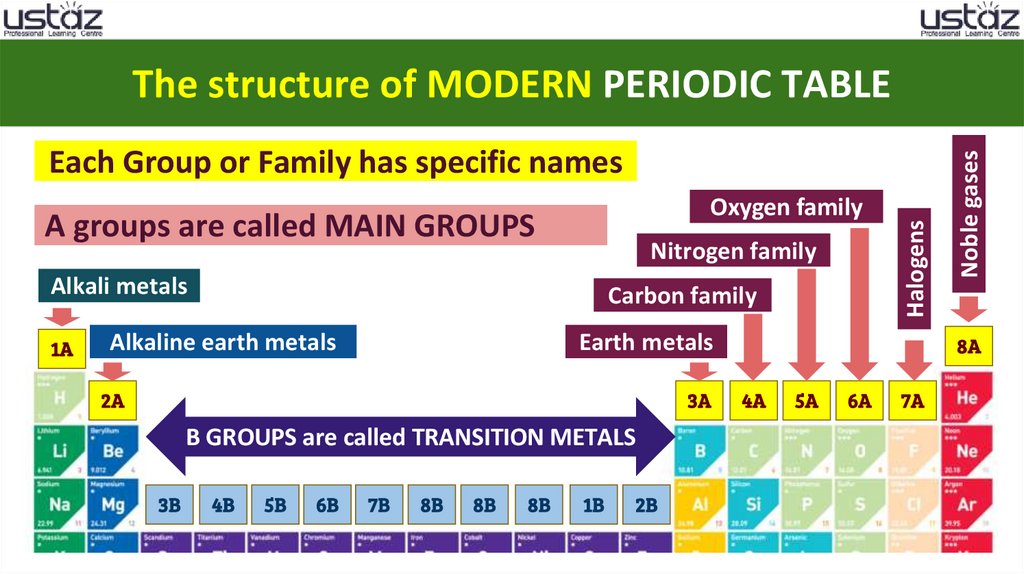
Oxygen familyA groups are called MAIN GROUPS
Nitrogen family
Alkali metals
1A
Carbon family
Alkaline earth metals
Earth metals
2A
3A
B GROUPS are called TRANSITION METALS
3B
4B
5B
6B
Halogens
Each Group or Family has specific names
7B
8B
8B
8B
1B
2B
Noble gases
The structure of MODERN PERIODIC TABLE
8A
4A
5A
6A
7A
26.
ALKALI METALS1A group of periodic table is called Alkali metals.
This group contains H (hydrogen), Li (Lithium), Na
(sodium), K (potassium), Rb (Rubidium), Cs
(Cesium), Fr (Francium)
But hydrogen is
nonmetals, it is
located in 1A group
because of its
electronic
configuration
Alkali metals are most active metals. They can
react even with air and water, so they are stored
under the kerosene
Cesium is most active
metal in 1A group
Alkali metals are very soft metals, so they can be
cut by knife
Francium is radioactive
metals
27.
Fill the tableElement
Carbon
Nitrogen
Oxygen
Fluorine
Neon
Sulfur
Chemical
Symbol
Total # of
electrons
# of valence
electrons
Dot diagram














 Химия
Химия








