Похожие презентации:
Biochemestry of blood
1.
2. The aim
TO study :Blood as the liquid tissue, makes the internalenvironment of an organism of the person and carries out a
lot of biological functions.
Every possible infringements of a metabolism, structure and
functions of various cells lead to changes of structure of
blood.
Carrying out of the biochemical analysis of blood is used for
diagnostics of diseases.
3. Plan of lecture
1. The basic biological functions and biochemicalconstants of blood.
2. Structure and chemical composition of erythrocytes.
3. Types of hemoglobin. Hemoglobin derivates.
4. Antioxidant system.
5. Clinical significance of biochemical analysis of
blood. Enzymes of blood.
6. Clotting of the blood. Intrinsic and extrinsic
systems. Heritable hemophilia.
7. Functions of vitamin K.
8. Anticoagulant system. Inhibitions of clotting.
9. Fibrinolysis.
4.
The functions of blood:a) Regulatory function.
b) Transport function
c) Alimentary function
d) Thermoregulation
e) Protective function (fibrinogen of plasma and
immunog1obu1ins).
k) Respiratory function
The blood consists of plasma and from of formed
elements of blood. The volume of blood in the normal
condition constitutes (man) 4.9-5.3 l, (women) 3.8-4.5 l.
5.
1- Specific gravity – 1.02 -1-030;2- PH – 7.36-7.44;
3- Osmotic pressure about 7.6 atmospher
4- Oncotic pressure – 0.02 atmospher
Structure of the blood
/
\
Plasma 55’%
blood of cells-45%
6.
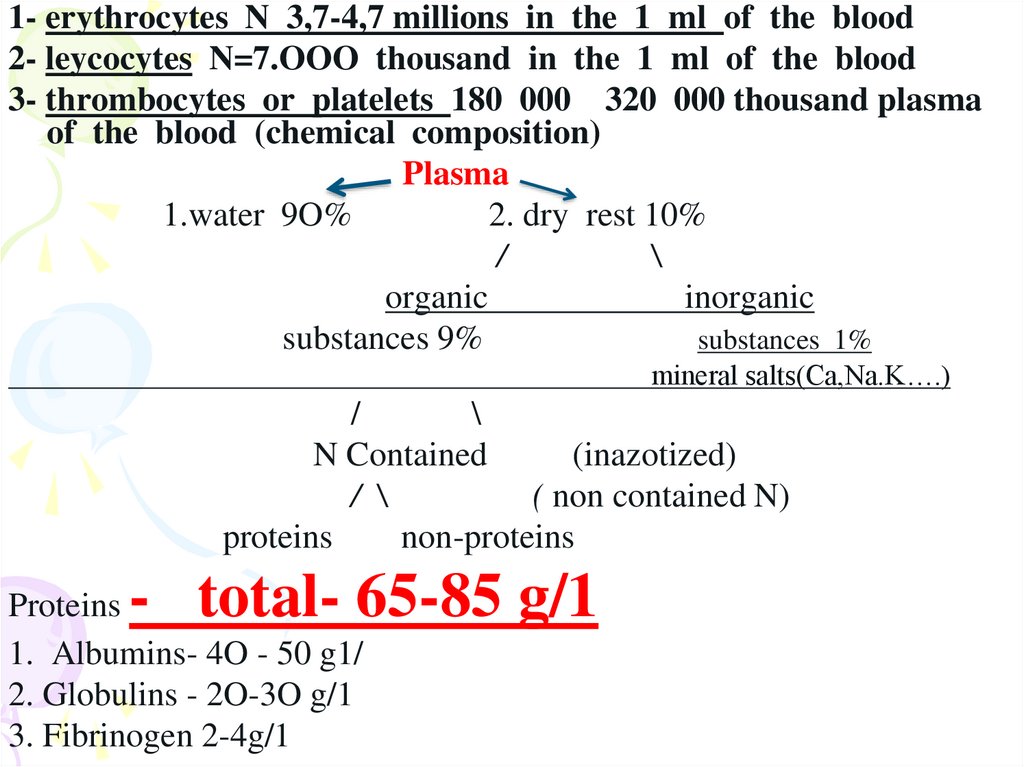
1- erythrocytes N 3,7-4,7 millions in the 1 ml of the blood2- leycocytes N=7.OOO thousand in the 1 ml of the blood
3- thrombocytes or platelets 180 000 320 000 thousand plasma
of the blood (chemical composition)
Plasma
1.water 9O%
2. dry rest 10%
/
\
organic
inorganic
substances 9%
substances 1%
mineral salts(Ca,Na.K….)
/
\
N Contained
(inazotized)
/ \
( non contained N)
proteins
non-proteins
- total- 65-85 g/1
Proteins
1. Albumins- 4O - 50 g1/
2. Globulins - 2O-3O g/1
3. Fibrinogen 2-4g/1
7. Function of plasma proteins:
1. Nutrition and tissue formation.2. Viscosity. The plasma proteins provide viscosity to the plasma. This helps in
providing the resistance to blood flow in the cardio vascular system.
3. Osmotic pressure. Plasma albumin is more important in this respect and
responsible for about 5O% of the total osmotic pressure exerted by plasma
proteins.
4. Transport function
Albumins transport bilirubin, certain hormones and drags, FFA.
Lipoproteins carry lipids; transkartin carries cortisol, ceruloplasmin and
transferrin carry copper and Iron respectively.
5. Protective.
Blood coagulation - fibrinogen.
6. Homeostesis.
Buffer action.
7. Immunity- resistance against infections.
8. Humoral - A large number of hormones are proteins in nature
9. Enzymes- Most plasma enzymes are very important for diagnosing diseases.
8.
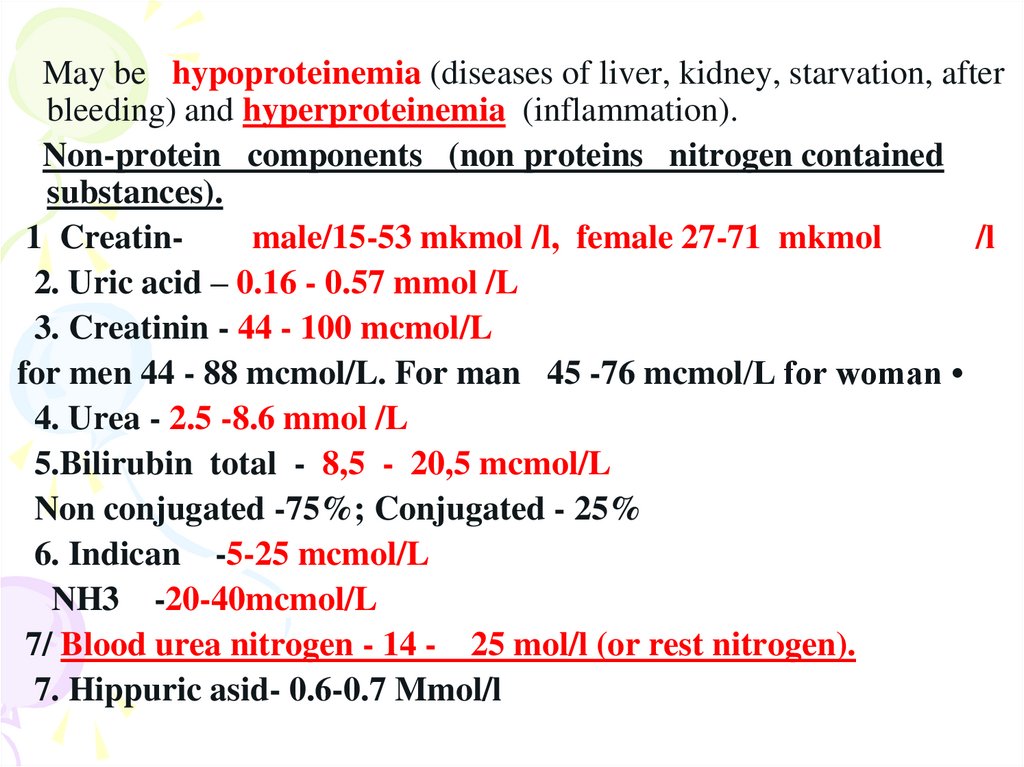
May be hypoproteinemia (diseases of liver, kidney, starvation, afterbleeding) and hyperproteinemia (inflammation).
Non-protein components (non proteins nitrogen contained
substances).
1 Creatinmale/15-53 mkmol /l, female 27-71 mkmol
/l
2. Uric acid – 0.16 - 0.57 mmol /L
3. Creatinin - 44 - 100 mcmol/L
for men 44 - 88 mcmol/L. For man 45 -76 mcmol/L for woman
4. Urea - 2.5 -8.6 mmol /L
5.Bilirubin total - 8,5 - 20,5 mcmol/L
Non conjugated -75%; Conjugated - 25%
6. Indican -5-25 mcmol/L
NH3 -20-40mcmol/L
7/ Blood urea nitrogen - 14 - 25 mol/l (or rest nitrogen).
7. Hippuric asid- 0.6-0.7 Mmol/l
9.
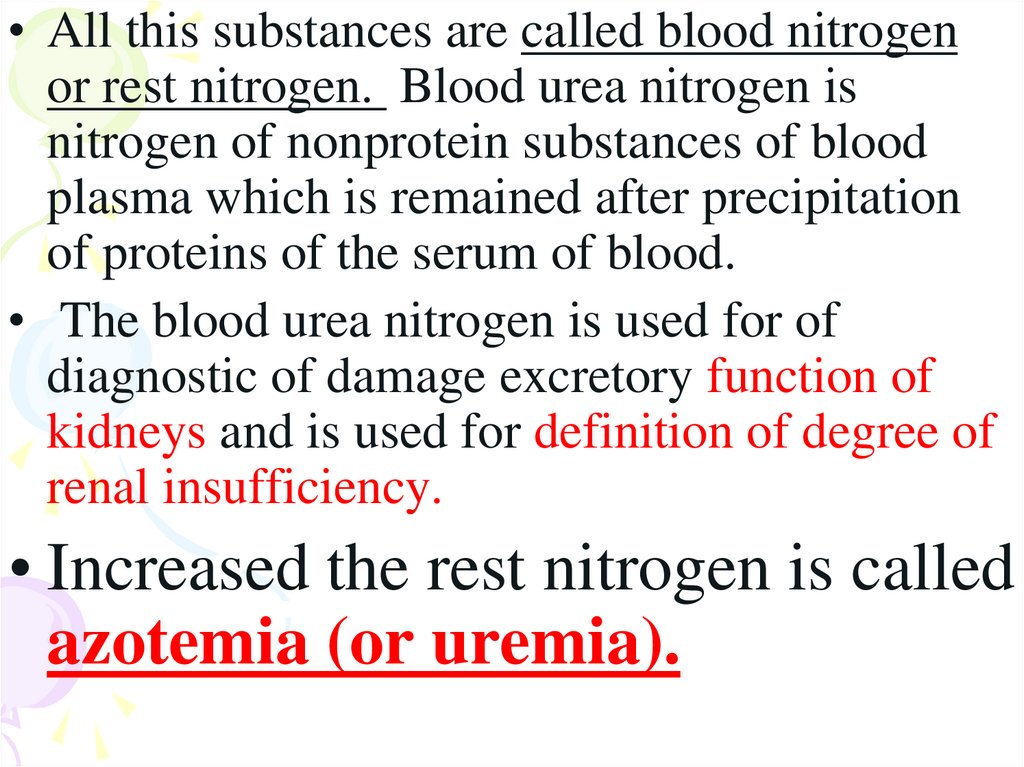
• All this substances are called blood nitrogenor rest nitrogen. Blood urea nitrogen is
nitrogen of nonprotein substances of blood
plasma which is remained after precipitation
of proteins of the serum of blood.
• The blood urea nitrogen is used for of
diagnostic of damage excretory function of
kidneys and is used for definition of degree of
renal insufficiency.
• Increased the rest nitrogen is called
azotemia (or uremia).
10.
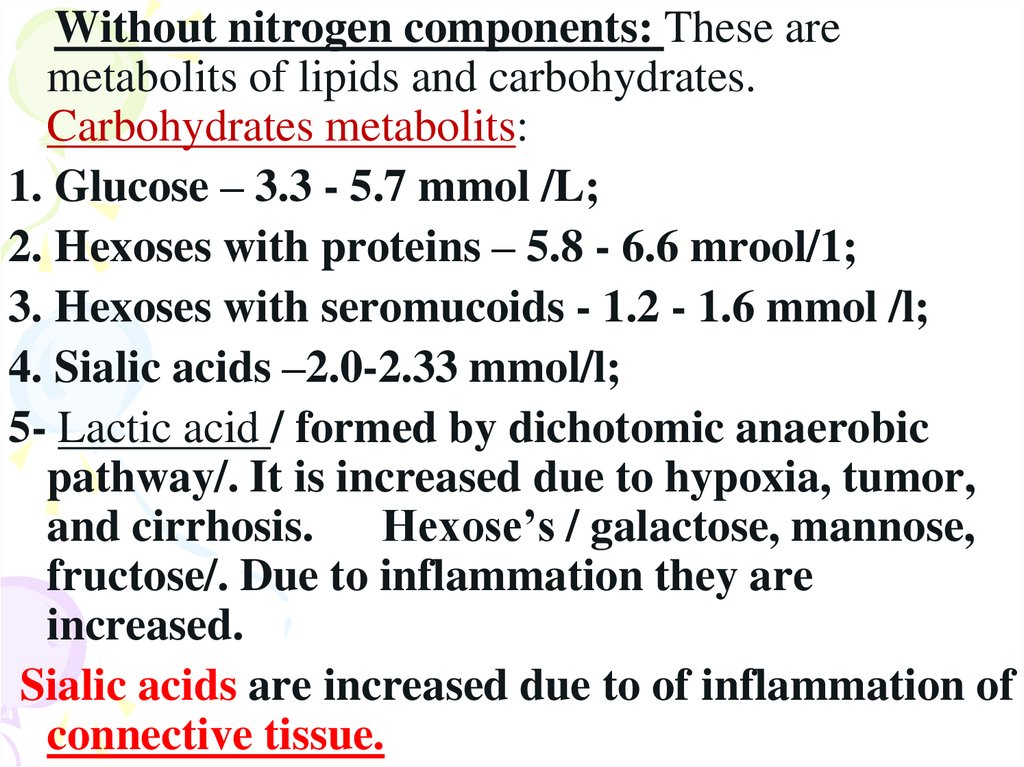
Without nitrogen components: These aremetabolits of lipids and carbohydrates.
Carbohydrates metabolits:
1. Glucose – 3.3 - 5.7 mmol /L;
2. Hexoses with proteins – 5.8 - 6.6 mrool/1;
3. Hexoses with seromucoids - 1.2 - 1.6 mmol /l;
4. Sialic acids –2.0-2.33 mmol/l;
5- Lactic acid / formed by dichotomic anaerobic
pathway/. It is increased due to hypoxia, tumor,
and cirrhosis. Hexose’s / galactose, mannose,
fructose/. Due to inflammation they are
increased.
Sialic acids are increased due to of inflammation of
connective tissue.
11.
Metabolits of lipids:1. NEFA - 400-800 mcmol/L;
2. Ketone bodies - 100-600 mcmol/L;
3. TAG- 0.55 – 1.64 mmol /l till 3.9 mMol/1/;
4. Phospholipids - 2 – 4.7 mmol /L;
5. Cholesterol – 3.9 – 6.5 mmol /L Conjugated
cholesterol - 70 %; 30%- FREE CHOL.
6. Total lipids - 4 - 7 g/L;
The increased of total lipids is called as hyperlipidemia.
May be physiological hyperlipidemia after eating and
pathologic hyperlipemia may be due to chronic
hepatitis, obstructive jaundice. The increase of TAG
may be due to nephrosis, leycosis.
NEFA - the increase may be due to diabetes mellitus,
nephrosis, atherosclerosis. Ketonuria due to diabetes,
mellitus, starvation.
12.
CholesterolHypercholesterolemia may be due to atherosclerosis.
May be alimentary hypercholesterolemia and congenital.
Congenital hypercholesterolemia may be due to
increased maintenance / contents/ of LDL, atherogenic
index-AI
Total cholesterol - cholesterol of HDL
AI = ---------------------------------------Cholesterol of HDL
1. For patient at the age of 2O-30 y it is 2.0-2.8.
2. For patient at the age of more than 3O y it is 3.0-3.5;
3. At patient with atherosclerosis atherogenic index is 4.0
HDL - high density lipoproteins are antiatherogenic
lipoproteins. AI - is the relation of summary maintenance
of cholesterol in the LDL and VLDL to cholesterol of HDL,
at newborn AI is 1.
13.
Hemoglobin derivates1.Physiological hemoglobin derivatives
• Oxy Hb - it is used for of transport of
O2 in the body (HbO2).
• Hb NH COOH- carbhemoglobin
2.Pathological derivatives of hemoglobin
• /corboxyhemoglobin/ -Hb - CO
• Met-Hb Fe 3+
14.
Physiological -carbhemoglobinHbC02HbNHCOOH is used for transfer
of C02 from peripheral tissues to
the lungs /carbhemoglobin,
HbC02/.
15.
• Abnormal hemoglobinAbnormal hemoglobin are the resultant of mutations in the
genes that code for α or β chains of globin.
• Hemoglobinopathias
Sickle-cell anemia (HbC )and hemoglobin C disease (HbC)
are the classical examples of abnormal hemoglobin. The
structure of hemoglobin contains two α- and two β-globin
chains.
• Molecular basis of HbC
In case of sickle-cell anemia, the hemoglobin (HbC) has
two normal α-globin and two abnormal β-globin chains.
This is due to a difference in a single amino acid. In HbC,
glutamate at sixth position of β-chain is replaced by
valine.
Thalassemias, on the other hand, are caused by decreased
synthesis of normal hemoglobin
16.
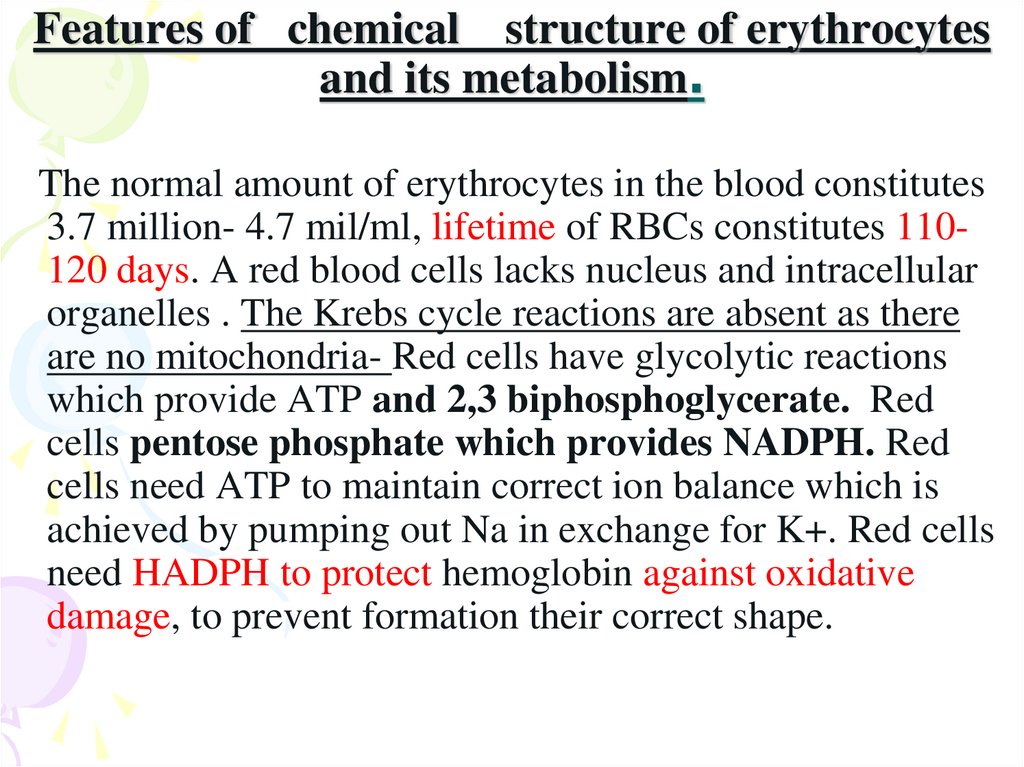
17. Features of chemical structure of erythrocytes and its metabolism.
The normal amount of erythrocytes in the blood constitutes3.7 million- 4.7 mil/ml, lifetime of RBCs constitutes 110120 days. A red blood cells lacks nucleus and intracellular
organelles . The Krebs cycle reactions are absent as there
are no mitochondria- Red cells have glycolytic reactions
which provide ATP and 2,3 biphosphoglycerate. Red
cells pentose phosphate which provides NADPH. Red
cells need ATP to maintain correct ion balance which is
achieved by pumping out Na in exchange for K+. Red cells
need HADPH to protect hemoglobin against oxidative
damage, to prevent formation their correct shape.
18.
In erythrocytes it occurs as supplement toglycolysis. In this pathway 1,2 biphosphoglycerate is changed into 2,3 biphosphoglycerate ( 2,3 DPG) by
phosphoglycerate mutase. In the presence of
2.3 DPG, hemoglobin has reduced affinity for
02.
This change is required in conditions of O2
deficiency-Thus under such conditions / for
example at high altitudes/ metabolism of red
cells sweetness over to produce more 2,3
DPG /old name was 2,3 diphosphoglycerate/.
19.
O2 is both essential to human life and toxic. We aredependent on O2 for oxidation reactions in the pathways
of ATP generation, detoxification and biosynthesis.
However, when O2 accepts single electron, it is
transformed highly reactive oxygen radicals that damage
cellular lipids, proteins and DNA. Damage by reactive
oxygen radicals contributes to cellular death and
degeneration in a wide range of diseases.
20.
Antioxidant system/ protective system/
These are
4 enzymes:.
21.
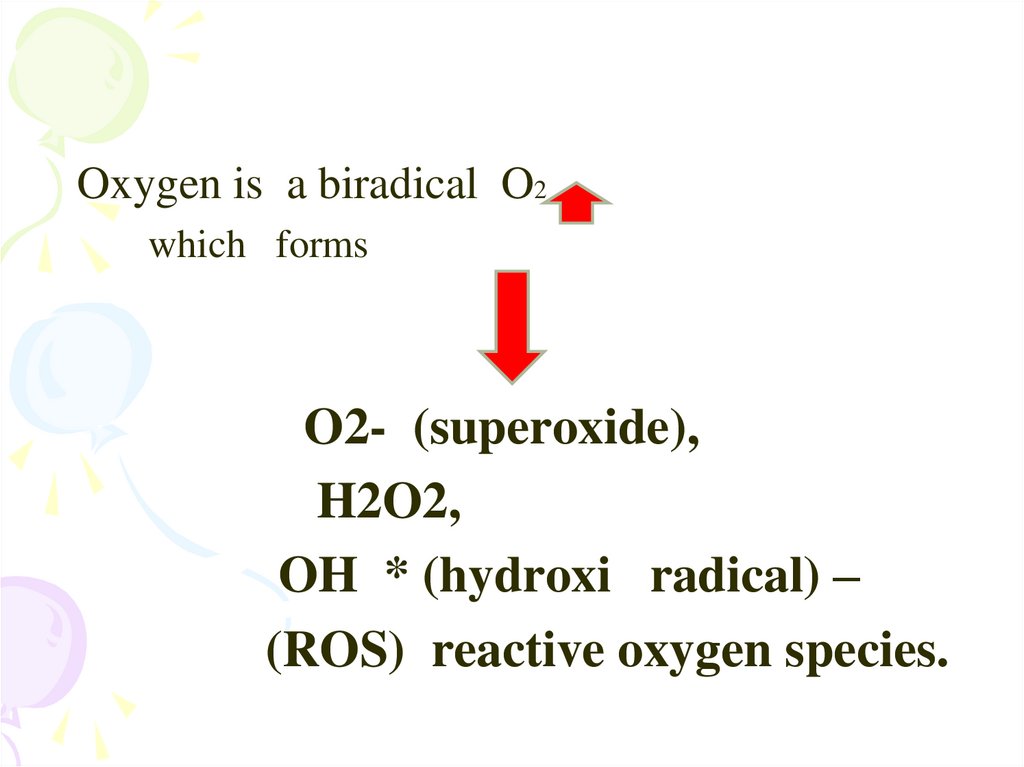
Oxygen is a biradical O2which forms
O2- (superoxide),
H2O2,
OH * (hydroxi radical) –
(ROS) reactive oxygen species.
22.
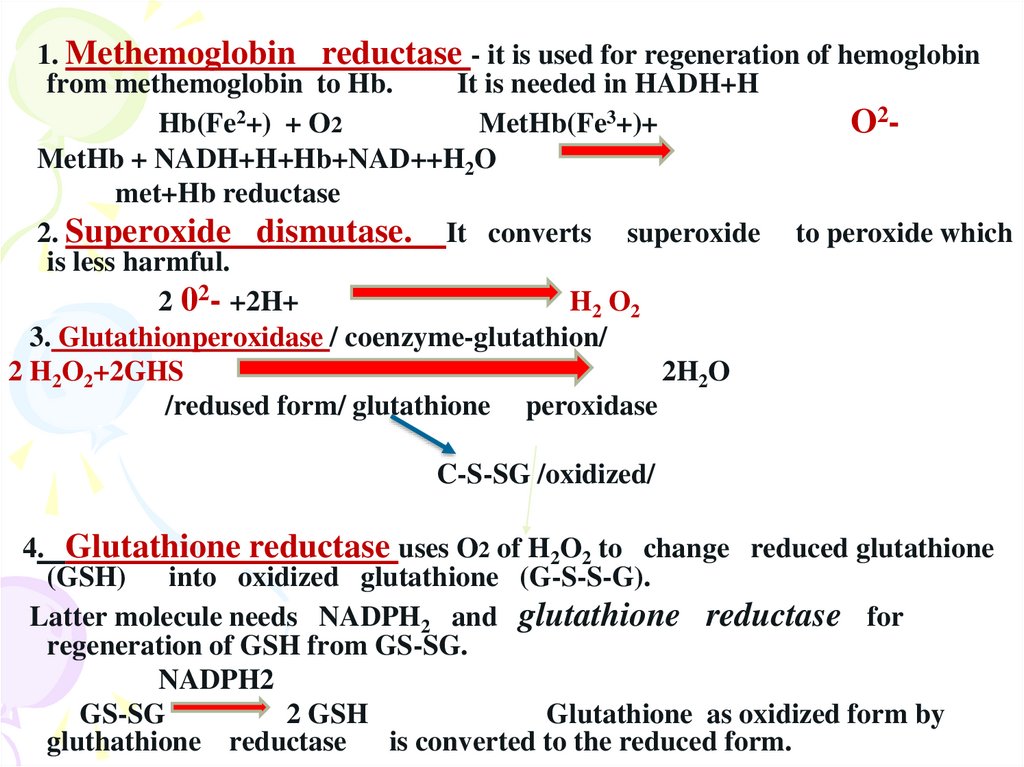
1. Methemoglobin reductase - it is used for regeneration of hemoglobinfrom methemoglobin to Hb.
It is needed in HADH+H
Hb(Fe2+) + O2
MetHb(Fe3+)+
O2MetHb + NADH+H+Hb+NAD++H2O
met+Hb reductase
2. Superoxide dismutase. It converts superoxide to peroxide which
is less harmful.
2 02- +2H+
H2 O2
3. Glutathionperoxidase / coenzyme-glutathion/
2 H2O2+2GHS
2H2O
/redused form/ glutathione peroxidase
C-S-SG /oxidized/
4. Glutathione reductase uses O2 of H2O2 to change reduced glutathione
(GSH) into oxidized glutathione (G-S-S-G).
Latter molecule needs NADPH2 and glutathione reductase for
regeneration of GSH from GS-SG.
NADPH2
GS-SG
2 GSH
Glutathione as oxidized form by
gluthathione reductase is converted to the reduced form.
23.
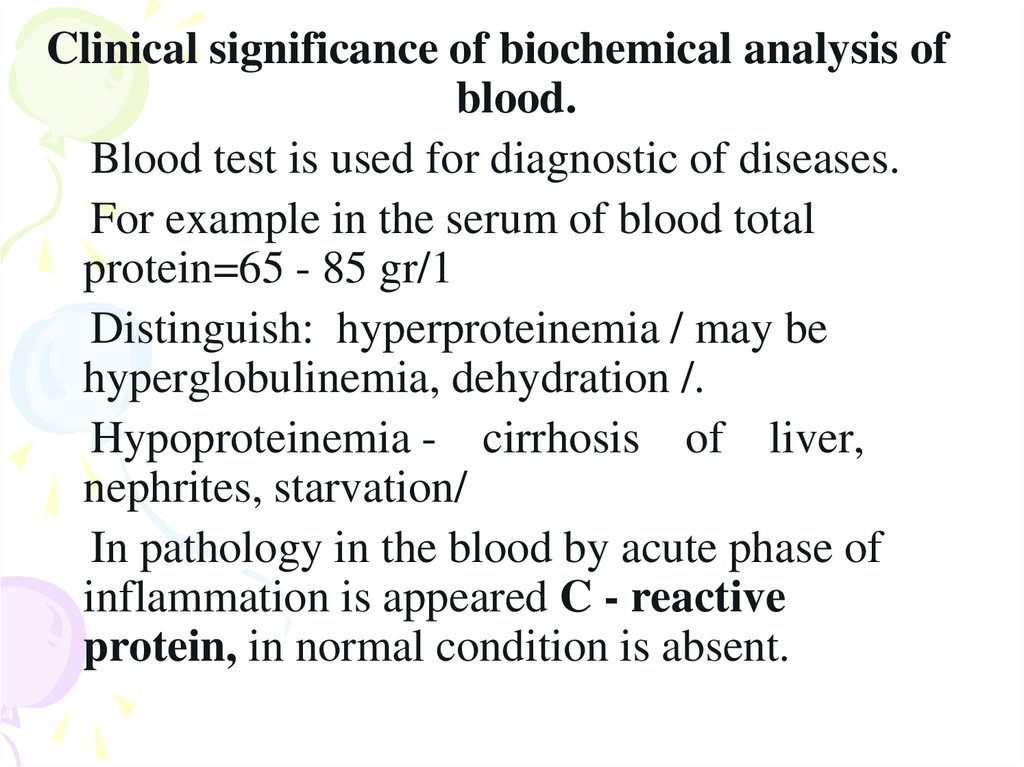
Clinical significance of biochemical analysis ofblood.
Blood test is used for diagnostic of diseases.
For example in the serum of blood total
protein=65 - 85 gr/1
Distinguish: hyperproteinemia / may be
hyperglobulinemia, dehydration /.
Hypoproteinemia - cirrhosis of liver,
nephrites, starvation/
In pathology in the blood by acute phase of
inflammation is appeared C - reactive
protein, in normal condition is absent.
24.
Changes of amounts severalproteins of plasma of blood by
definite pathological conditions.
25.
26.
PROTEINSConditions by which change amounts of
proteins
decrease
increase
Acidic alpha-1 glicoprotein of cancer
tumors
Chronic inflammations, (rheumatoid
arthritis)
Prothrombin
in diseases of the liver
Ceruloplasmin
By disease Vilson's Konovalow's
in pregnancy
alpha antythrobin
infringement of functions of liver
by in inflammations
alpha antythrombin
in infringement of
liver
functions of the
alpha 2- macroglobulin
cholesterase
in diabetes, disease of 1iver,nephrotic
syndrome
in disease of liver,
hypothyroidism, burns traumatic shock,
operation, heart attack, bronchial asthma
Haptoglabin.
Transferrin
inflammation, infections, fiver, cancer
Cancer of the liver
C reactive protein
Acute infections / acute phase of
inflammations
Beta lipoproteins or LDL
'obstucative jaundice, 'hypothyroidism,
atherosclerosis,
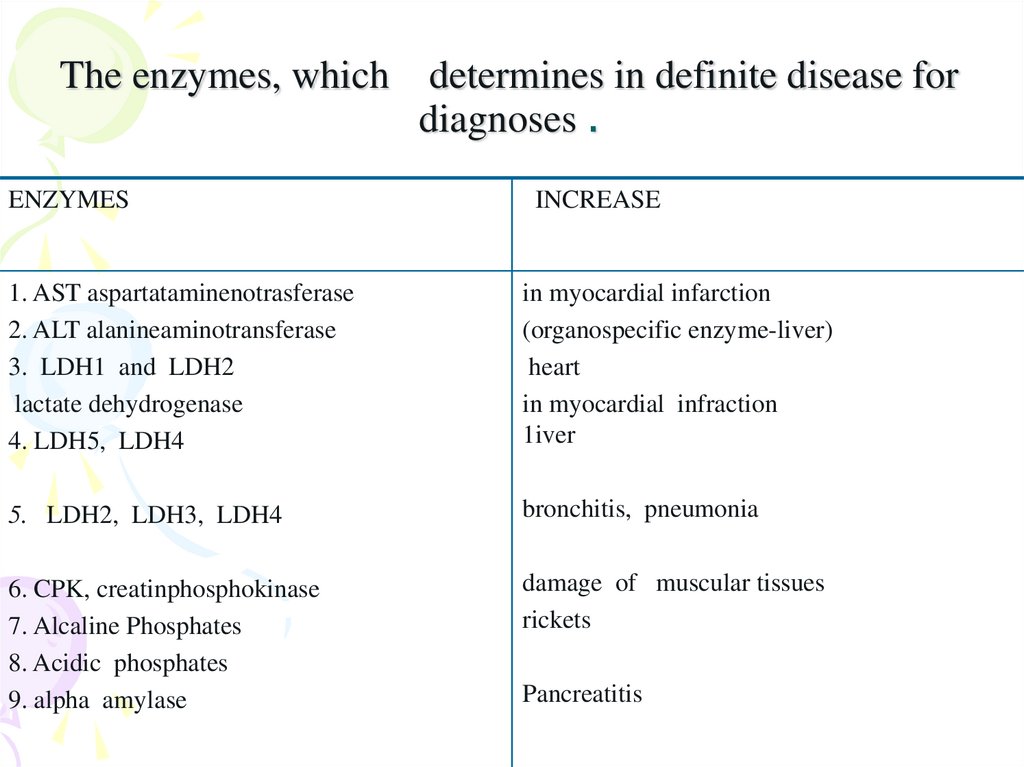
27. The enzymes, which determines in definite disease for diagnoses .
ENZYMESINCREASE
1. AST aspartataminenotrasferase
2. ALT alanineaminotransferase
3. LDH1 and LDH2
lactate dehydrogenase
4. LDH5, LDH4
in myocardial infarction
(organospecific enzyme-liver)
heart
in myocardial infraction
1iver
5. LDH2, LDH3, LDH4
bronchitis, pneumonia
6. CPK, creatinphosphokinase
7. Alcaline Phosphates
8. Acidic phosphates
9. alpha amylase
damage of muscular tissues
rickets
Pancreatitis
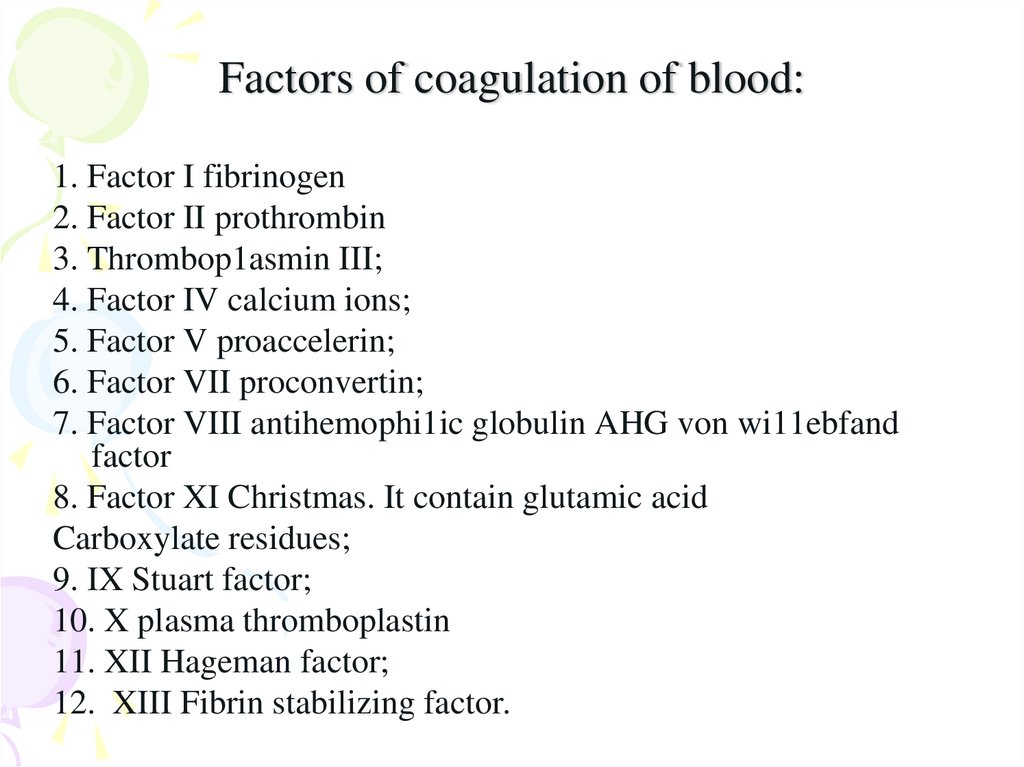
28. Factors of coagulation of blood:
1. Factor I fibrinogen2. Factor II prothrombin
3. Thrombop1asmin III;
4. Factor IV calcium ions;
5. Factor V proaccelerin;
6. Factor VII proconvertin;
7. Factor VIII antihemophi1ic globulin AHG von wi11ebfand
factor
8. Factor XI Christmas. It contain glutamic acid
Carboxylate residues;
9. IX Stuart factor;
10. X plasma thromboplastin
11. XII Hageman factor;
12. XIII Fibrin stabilizing factor.
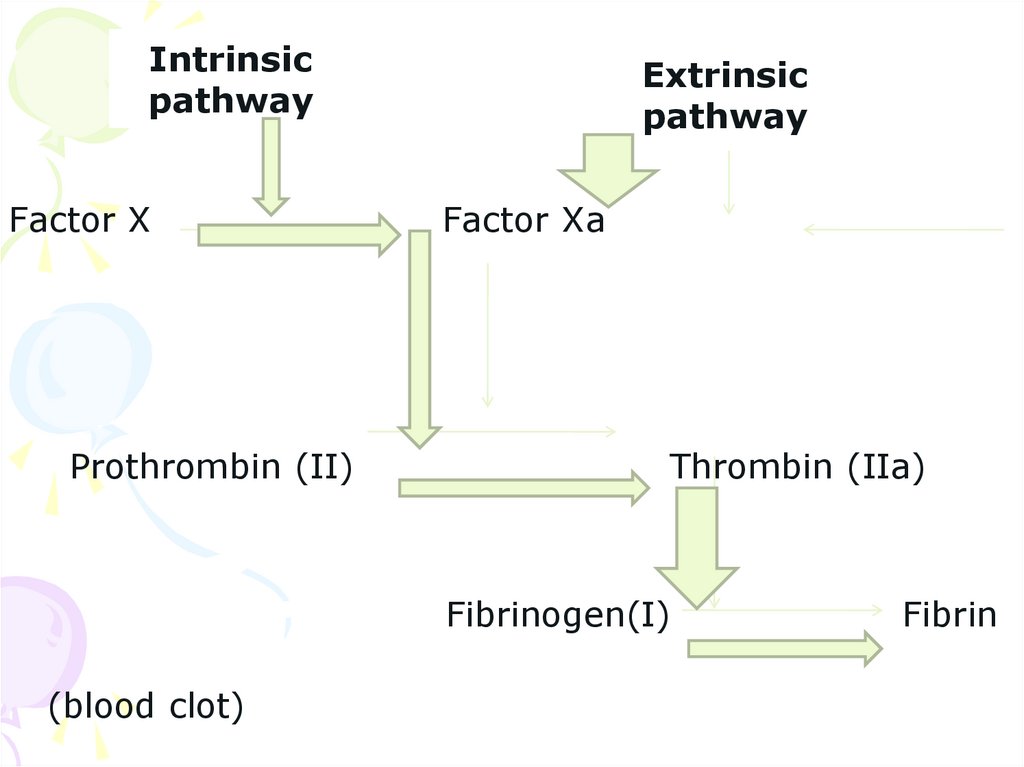
29. The extrinsic pathway
The extrinsic pathway is very rapid andoccurs in response to tissue injury . This
pathway essentially involves the conversion
of proconvertin (VII) to its active form
(VIIa) and the generation factor Xa. The
tissiu factor (III), found to be necessary to
accelerate the action VIIa on a factor X, is
present in lung and brain.
30.
31.
Intrinsicpathway
Factor X
Prothrombin (II)
Extrinsic
pathway
Factor Xa
Thrombin (IIa)
Fibrinogen(I)
(blood clot)
Fibrin
32.
33. Conversion of fibrinogen to fibrin
Fibrinogen (factor I) is a soluble glycoprotein that constitutes 2-3 % ofplasma proteins ( plasma constitutes 0.3 g/dl). Fibrinogen consists of
6 poly peptide chains-two A α, two B β and two γ making the
structure (A α)₂ (B β) ₂ γ ₂. Fibrinogen undergoes proteolytic cleavage
catalysed by thrombin to release small fibrinopeptides (A and B).
This is results in the formation of fibrin monomers which can stick
together to form hard clots. Clot formation is further stabilized by
covalent cross-linking between glutamine and lysine residues . This
reaction cross-links fibrin stabilizing factor (XIII). The red color of
the clot is due to the presence of red cells entangled in the fibrin
cross-links.
34. Conversion of prothrombin to thrombin
• Prothrombin (II) is the inactive zymogen form of thrombin(IIa). The activation of prothrombin occurs on the platelets and
requires the presence of factors Va and Xa, besides
phoshholypids and Ca²⁺.
• The extrinsic pathway
The extrinsic pathway is very rapid and occurs in response to
tissue injury . This pathway essentially involves the
conversion of proconvertin (VII) to its active form (VIIa) and
the generation factor Xa. The tissiu factor (III), found to be
necessary to accelerate the action VIIa on a factor X, is
present in lung and brain.
35.
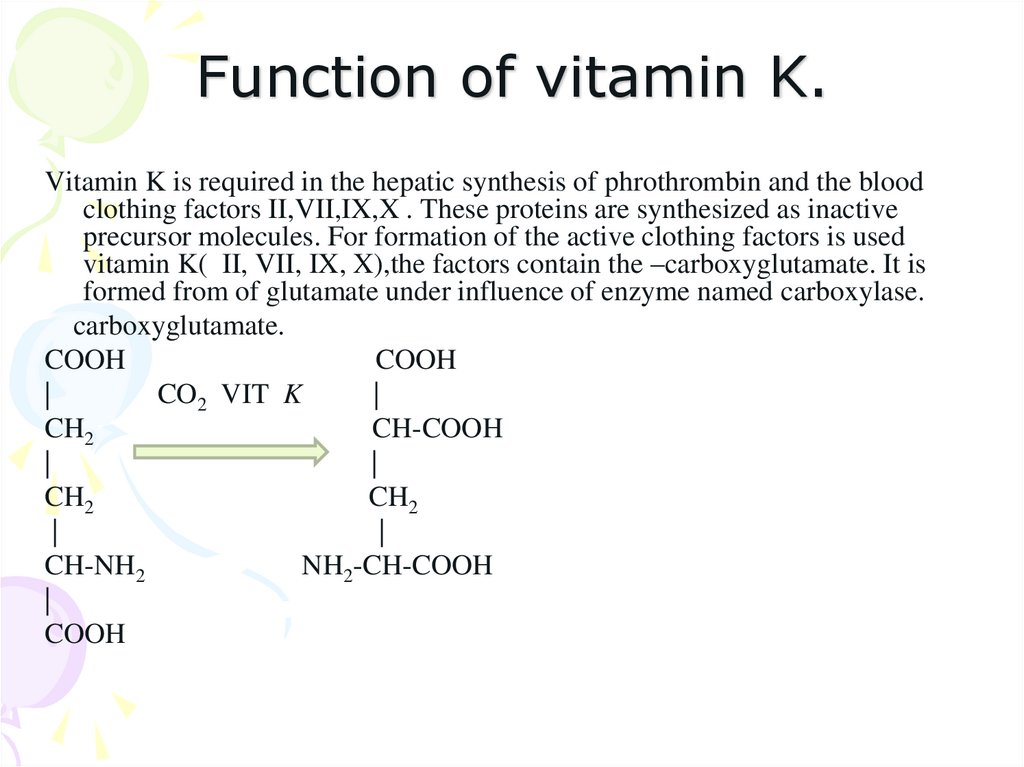
36. Function of vitamin K.
Vitamin K is required in the hepatic synthesis of phrothrombin and the bloodclothing factors II,VII,IX,X . These proteins are synthesized as inactive
precursor molecules. For formation of the active clothing factors is used
vitamin K( II, VII, IX, X),the factors contain the –carboxyglutamate. It is
formed from of glutamate under influence of enzyme named carboxylase.
carboxyglutamate.
COOH
COOH
|
CO2 VIT K
|
CH2
CH-COOH
|
|
CH2
CH2
|
|
CH-NH2
NH2-CH-COOH
|
COOH
37. Anticoagulants
• Several substances, known as anticoagulants,are in use to inhibit the blood clotting.
• Calcium is essentially required for certain
reactions of blood coagulation. The
substances which bind the Ca²⁺are very
effective as anticoagulants.
38.
Heparin in an anticoagulant used to maintain normalhemostasis.
It is a heteropolysaccharide found in many tissues including
mast cells in the endothelium of blood vessels.
Heparin combines with antithrombin III which in turn,
inhibits the clotting factors II, Ix, X, XI, XII and
kallikrein. Heparin can be administered to patients during
and after surgery to retard blood clotting.
The blood contains another anticoagulant – namely
protein C – which is activated by thrombin. Active protein
C hydrolysis and inactivates clotting factors V and VIII.
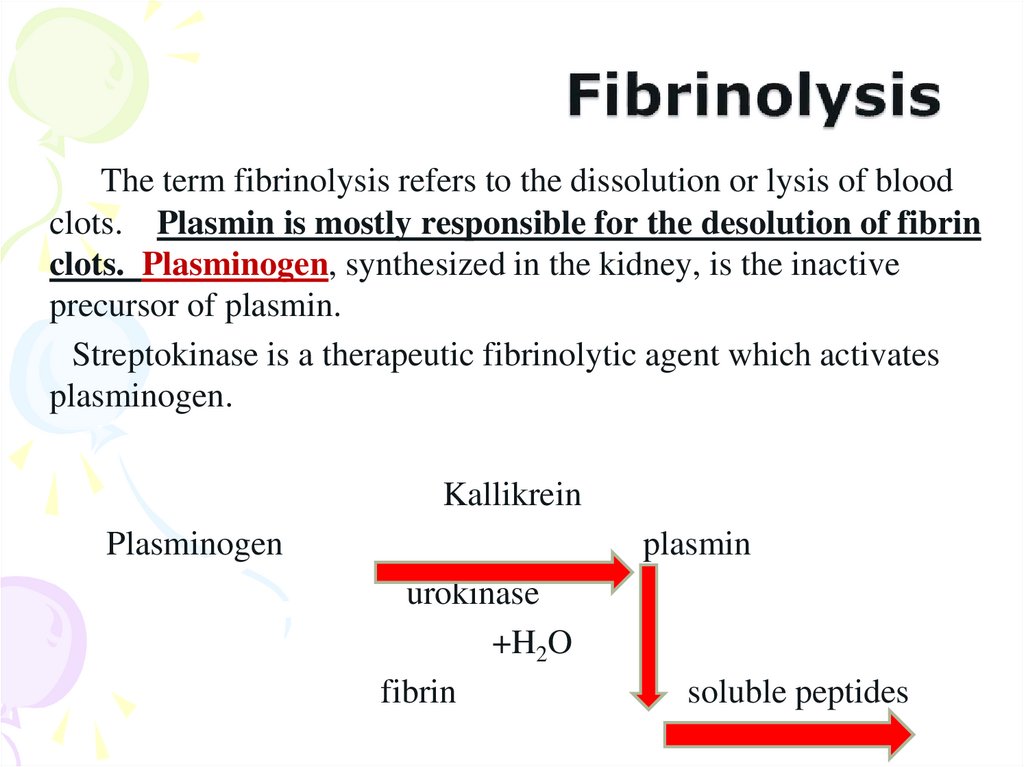
39. Fibrinolysis
The term fibrinolysis refers to the dissolution or lysis of bloodclots. Plasmin is mostly responsible for the desolution of fibrin
clots. Plasminogen, synthesized in the kidney, is the inactive
precursor of plasmin.
Streptokinase is a therapeutic fibrinolytic agent which activates
plasminogen.
Kallikrein
Plasminogen
plasmin
urokinase
+H2O
fibrin
soluble peptides
40. Abnormalites in blood clotting
Several abnormalities associated with blood clotting areknown. These are due to defects in clotting factors which
may be inherited or acquired. Hemophilia, Von
Willebrand`s disease etc., are examples of inherited
disorder while afibrinogiemia is an acquired diseases.
Hemophilia A (classical hemophilia) : This is a sexlinked disorder transmitted by females affecting males.
Hemophelia A is the most common clotting abnormality
and is due to the deficiency of antihemophilic factor
(VIII). The affected individuals have prolonged clotting
time and suffer from internal bleeding. Hemophilia A has
gained importance due to the fact that the Royal families of
Britain are among the affected individuals.
41. General question ?????
1. Components of blood?2. Factors of coagulation of blood ?
3. Haw many pathways of blood
coagulation?
4. What is fibrinolysis ?
5. Significance of antioxidant blood
system?

























 Биология
Биология