Похожие презентации:
Atomic Models & Atomic Structure
1.
Atomic ModelsAtomic and Nuclear Structure
2.
Topics/ Learning ObjectivesAtomic models throughout history
Scientists and their contributions to the study of the
atom
Geiger-Marsden (Rutherford) alpha particle scattering
experiments
Structure of the atom
Some properties of atoms and its constituents
Atomic notation and its meaning
3.
Atomic ModelsDemocritus (460–370 BC)
Greek philosopher
Everything is made of atoms
“atom” comes from the Greek ”indivisible”
• Atoms are indestructible;
• Between atoms there is empty space;
• Atoms are continuously in motion;
• There are an infinite number of atoms, and kinds of atoms, which
differ in shape, and size.
4.
Atomic ModelsAristotle (384–322 BC)
Greek philosopher
Atoms do not exist
Matter can be divided infinitely
Aristotle’s ideas (on this and other matters) influenced the scientific thought
for 2000 years..!
5.
Atomic ModelsJohn Dalton (1766–1844)
English chemist, physicist, and meteorologist
Elements are made of extremely small particles called atoms
Atoms of different elements combine in simple whole-number ratios to
form chemical compounds
6.
Atomic Models: Solid SphereSolid Sphere Model (proposed by John Dalton in 1803)
Dalton saw the atom as a compact hard solid sphere
• Atoms of a given element are identical in size, mass, and other
properties;
• Atoms cannot be subdivided, created, or destroyed;
• In chemical reactions, atoms are combined, separated, or rearranged.
7.
Atomic ModelsJ. J. Thomson (1856–1940)
English physicist; Nobel Prize in 1906
Thomson discovered the electron
(1897)
• Investigation of cathode rays led to the discovery of the electron
Thomson concluded that the rays were composed of negatively charged “corpuscles”
whose mass was over 1000 times smaller than the mass of hydrogen atom;
Thomson measured the charge-to-mass (q/m) ratio of the electron;
Thomson proved that atoms were divisible (by discovering a subatomic particle: electron)
8.
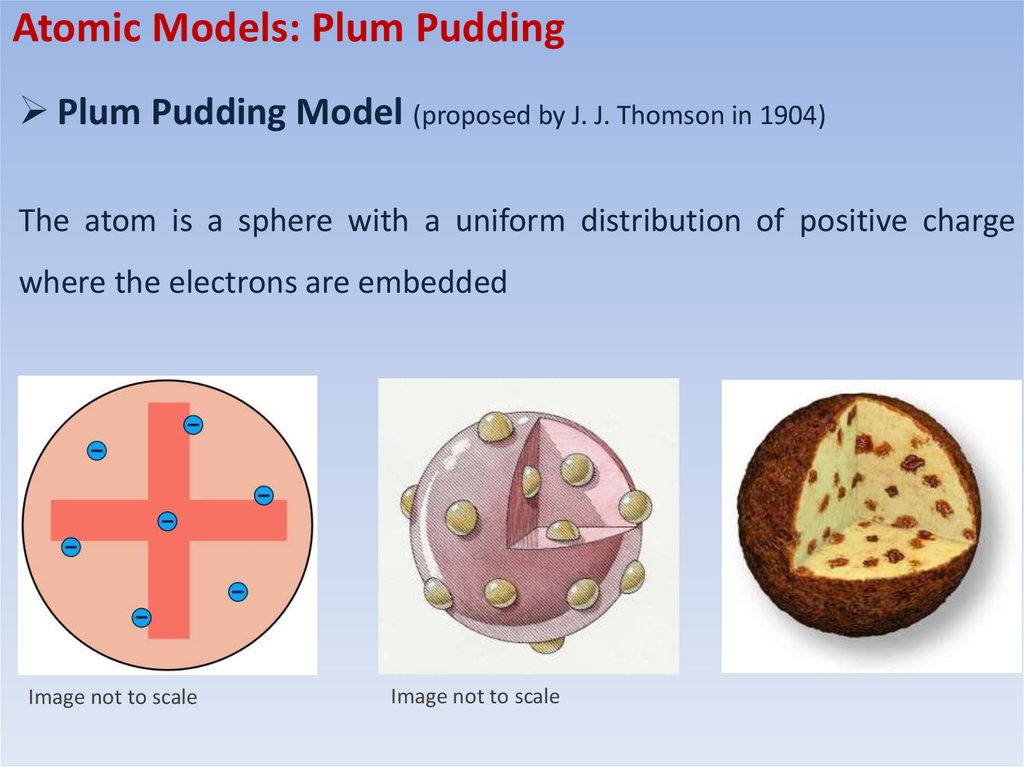
Atomic Models: Plum PuddingPlum Pudding Model (proposed by J. J. Thomson in 1904)
The atom is a sphere with a uniform distribution of positive charge
where the electrons are embedded
Image not to scale
Image not to scale
9.
Atomic ModelsE. Rutherford (1871–1937)
New Zealand-born British physicist; Nobel Prize in 1908
• Proved that the Plum Pudding Model was not correct (1911)
• Discovered that atoms have a tiny, positive and heavy nucleus (1911)
• Split the nucleus and discovered the proton (1920)
10.
Geiger-Marsden Experiments (Rutherford)Geiger-Marsden experiments (Rutherford)
These experiments were suggested by Rutherford and carried out by Hans Geiger and Ernest
Marsden from 1908 to 1913 under the direction of Rutherford
• Alpha particles were fired against gold and other metallic foils
In 1907, Rutherford and Thomas Royds proved that alpha particles were helium nucleus
• Foils of different thickness were used and the scattering pattern was
measured using a fluorescent screen
• One of the main objectives was to measure how much these alpha
particles were dispersed by the atoms in the foils
11.
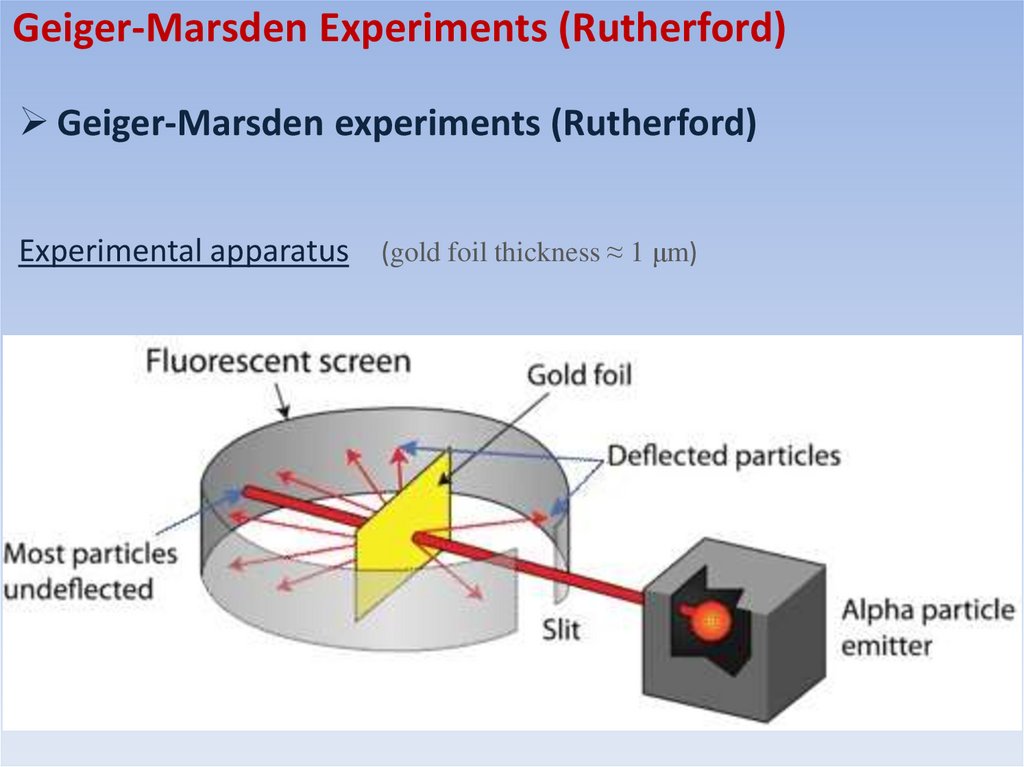
Geiger-Marsden Experiments (Rutherford)Geiger-Marsden experiments (Rutherford)
Experimental apparatus (gold foil thickness ≈ 1 μm)
12.
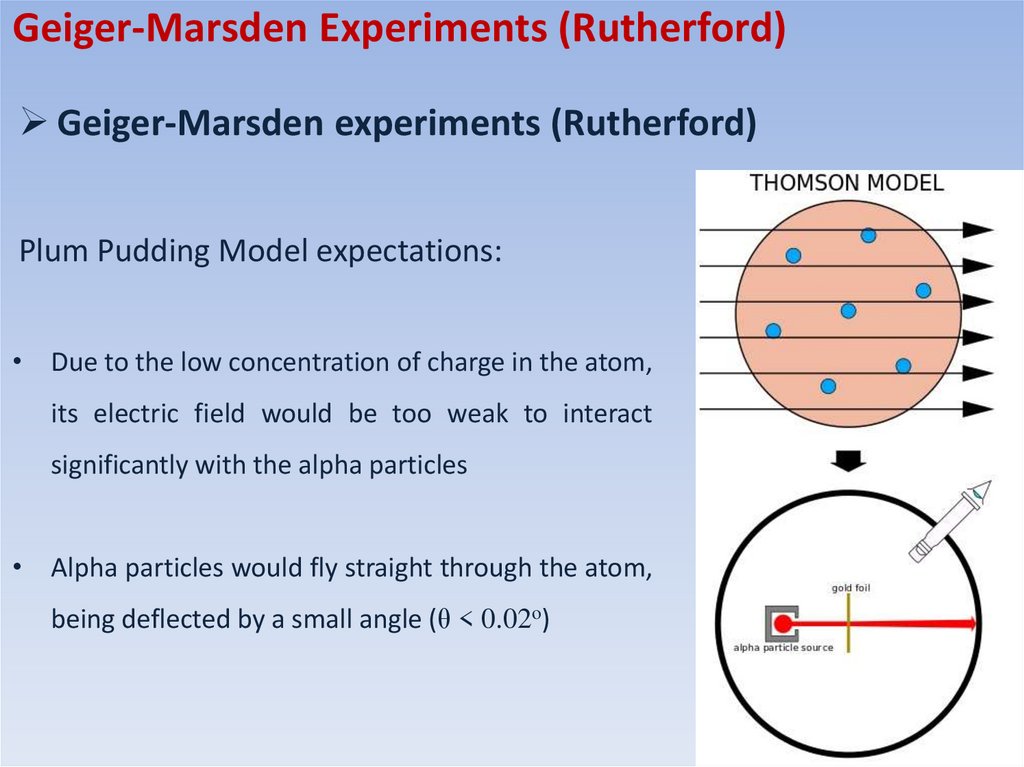
Geiger-Marsden Experiments (Rutherford)Geiger-Marsden experiments (Rutherford)
Plum Pudding Model expectations:
• Due to the low concentration of charge in the atom,
its electric field would be too weak to interact
significantly with the alpha particles
• Alpha particles would fly straight through the atom,
being deflected by a small angle (θ < 0.02o)
13.
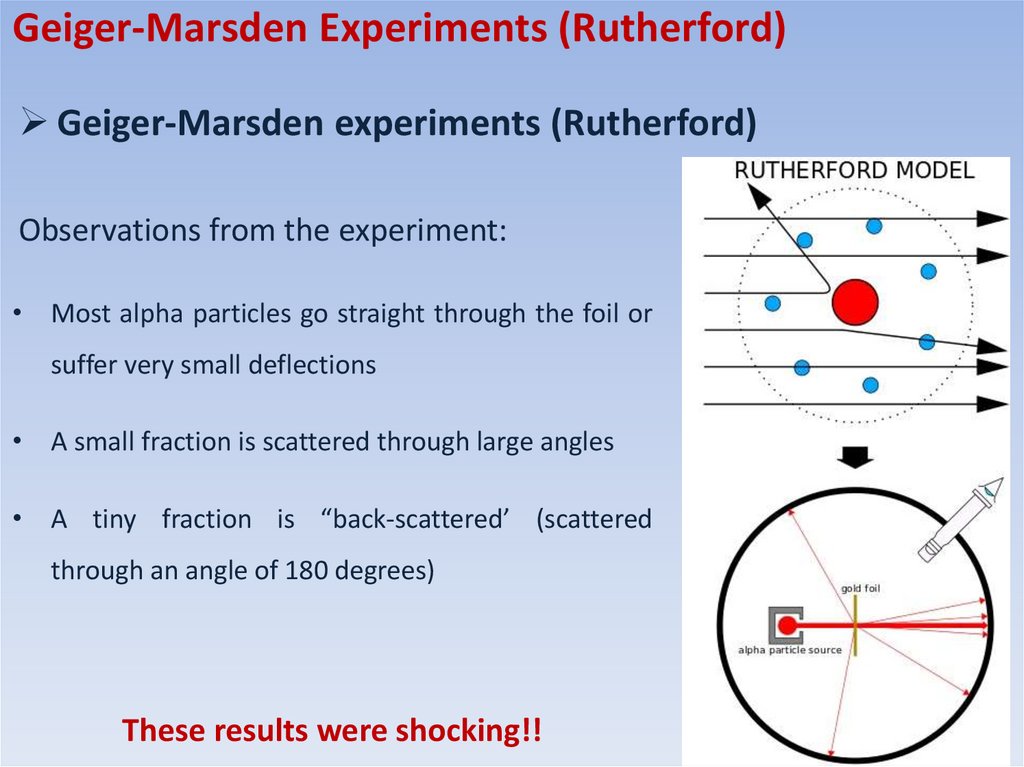
Geiger-Marsden Experiments (Rutherford)Geiger-Marsden experiments (Rutherford)
Observations from the experiment:
• Most alpha particles go straight through the foil or
suffer very small deflections
• A small fraction is scattered through large angles
• A tiny fraction is “back-scattered’ (scattered
through an angle of 180 degrees)
These results were shocking!!
14.
Geiger-Marsden Experiments (Rutherford)Geiger-Marsden experiments (Rutherford)
Rutherford and his collaborators were stunned with their results:
“I remember... later Geiger coming to me in great excitement and
saying, 'We have been able to get some of the α-particles coming
backwards...‘
It was quite the most incredible event that has ever happened to me in
my life. It was almost incredible as if you fired a 15-inch shell at a piece
of tissue paper and it came back and hit you.”
(Rutherford, 1938)
15.
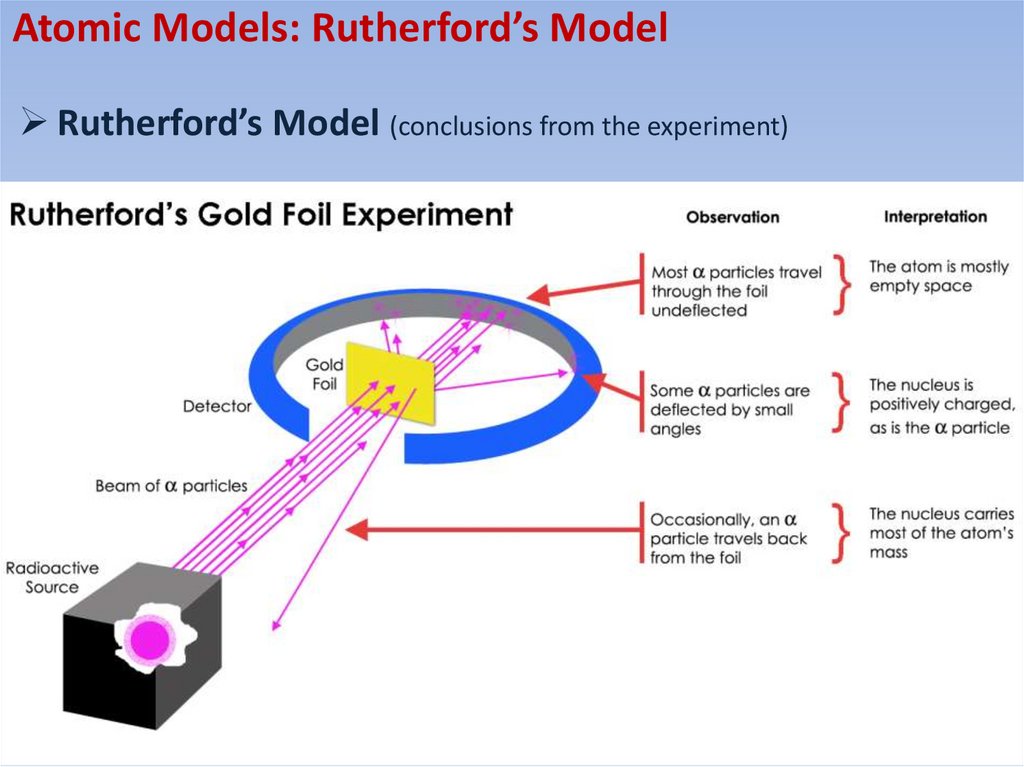
Atomic Models: Rutherford’s ModelRutherford’s Model (conclusions from the experiment)
16.
Atomic Models: Rutherford’s ModelRutherford’s Model (proposed by Rutherford in 1911)
• Most of the mass of the atom and all its positive charge is
concentrated in its nucleus
• The nucleus is about 100 000 (105) times smaller than the atom
If the nucleus was a sphere of radius 1 cm, the closest electron would be 1 km away..!!
• The atom is mostly empty space
The atom is more than 100 times emptier than the solar System..!!
17.
Atomic Models: Rutherford’s ModelRutherford’s Model (proposed by Rutherford in 1911)
Although Rutherford could not draw any conclusions about the electrons in
the atom, he mentioned a model suggested by Japanese physicist Hantaro
Nagaoka, where electrons orbited the nucleus (similar to a planetary system)
18.
Atomic ModelsNiels Bohr (1885–1962)
Danish physicist; Nobel Prize in 1922
• Bohr developed the Bohr model of the atom (1913)
• Energy of the electrons in the atom is quantized
• Electrons go around the nucleus in stable orbits but can jump from
one energy level (or orbit) to another
19.
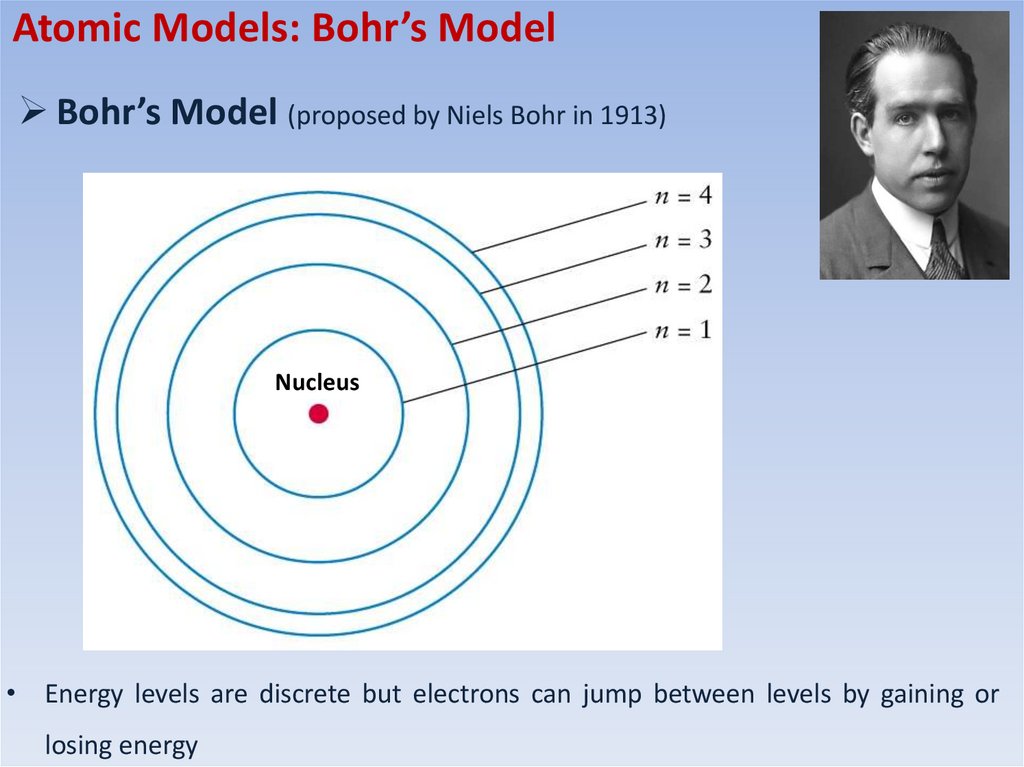
Atomic Models: Bohr’s ModelBohr’s Model (proposed by Niels Bohr in 1913)
Nucleus
• Energy levels are discrete but electrons can jump between levels by gaining or
losing energy
20.
Atomic ModelsErwin Schrödinger (1887–1961)
Austrian physicist; Nobel Prize in 1933
Schrödinger developed the Quantum
Mechanical Model
• Schrödinger gave priceless contributions to Quantum Mechanics
• The concept of “orbit” was replaced by “orbital”
An orbital is a space around the nucleus were is most probable to find the electrons
21.
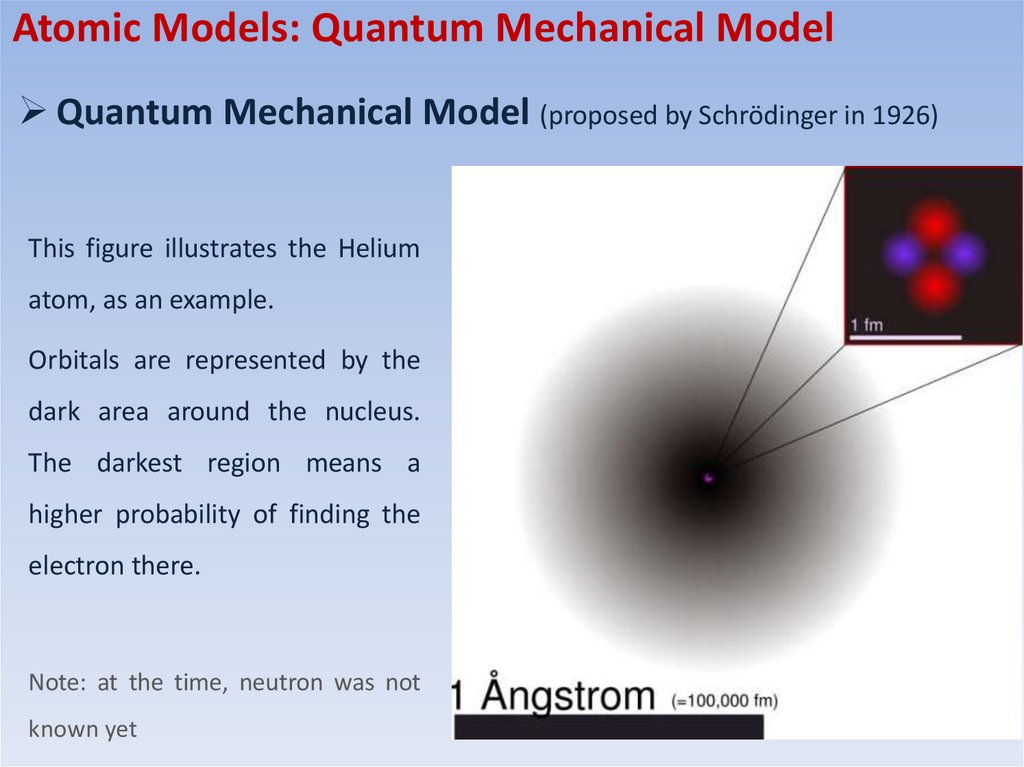
Atomic Models: Quantum Mechanical ModelQuantum Mechanical Model (proposed by Schrödinger in 1926)
This figure illustrates the Helium
atom, as an example.
Orbitals are represented by the
dark area around the nucleus.
The darkest region means a
higher probability of finding the
electron there.
Note: at the time, neutron was not
known yet
22.
Atomic ModelsJames Chadwick (1891–1974)
English physicist; Nobel Prize in 1935
Chadwick discovered the neutron
• Rutherford split the atom and discovered the proton (1920)
• Chadwick discovered the neutron (1932)
• Chadwick and Goldhaber measured the mass of the neutron (1935)
23.
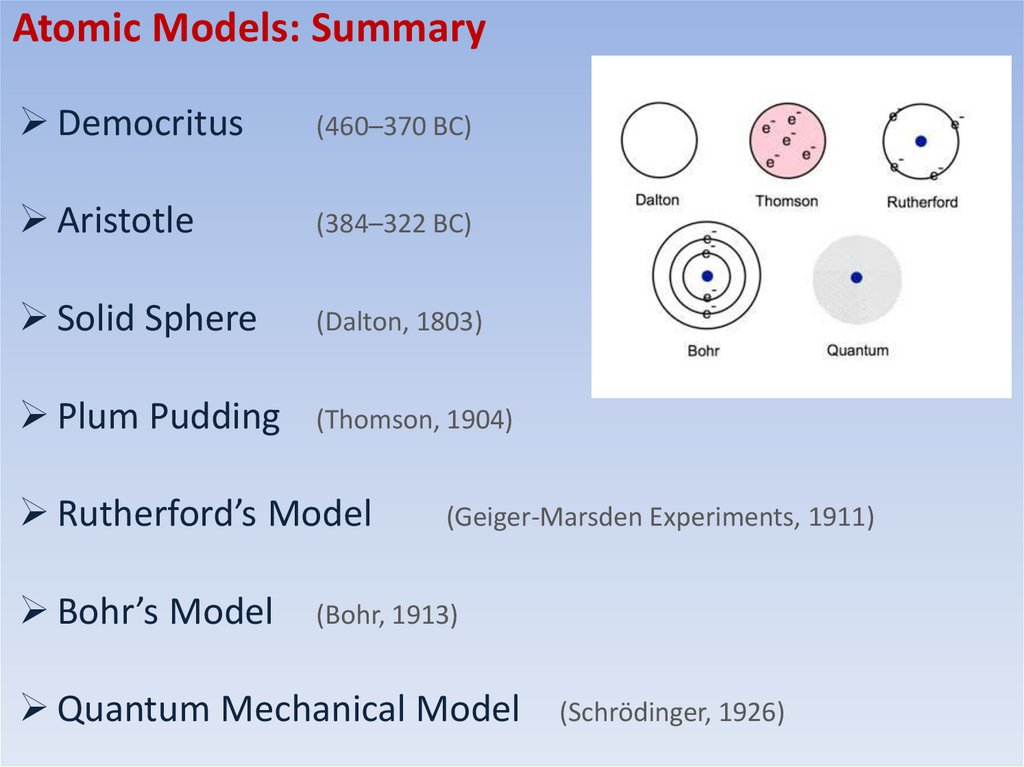
Atomic Models: SummaryDemocritus
(460–370 BC)
Aristotle
(384–322 BC)
Solid Sphere
(Dalton, 1803)
Plum Pudding
(Thomson, 1904)
Rutherford’s Model
Bohr’s Model
(Geiger-Marsden Experiments, 1911)
(Bohr, 1913)
Quantum Mechanical Model
(Schrödinger, 1926)
24.
Exercises and Problems1. Which of the following statements are true?
A. Aristotle proposed an atomic model in which electrons
orbited the nucleus.
B. Dalton was the first to suggest the existence of atoms.
C. Rutherford proved that the positive charge of the atom is
concentrated in its nucleus.
D. Thomson discovered that the negative charge was
distributed uniformly throughout the atom
C
25.
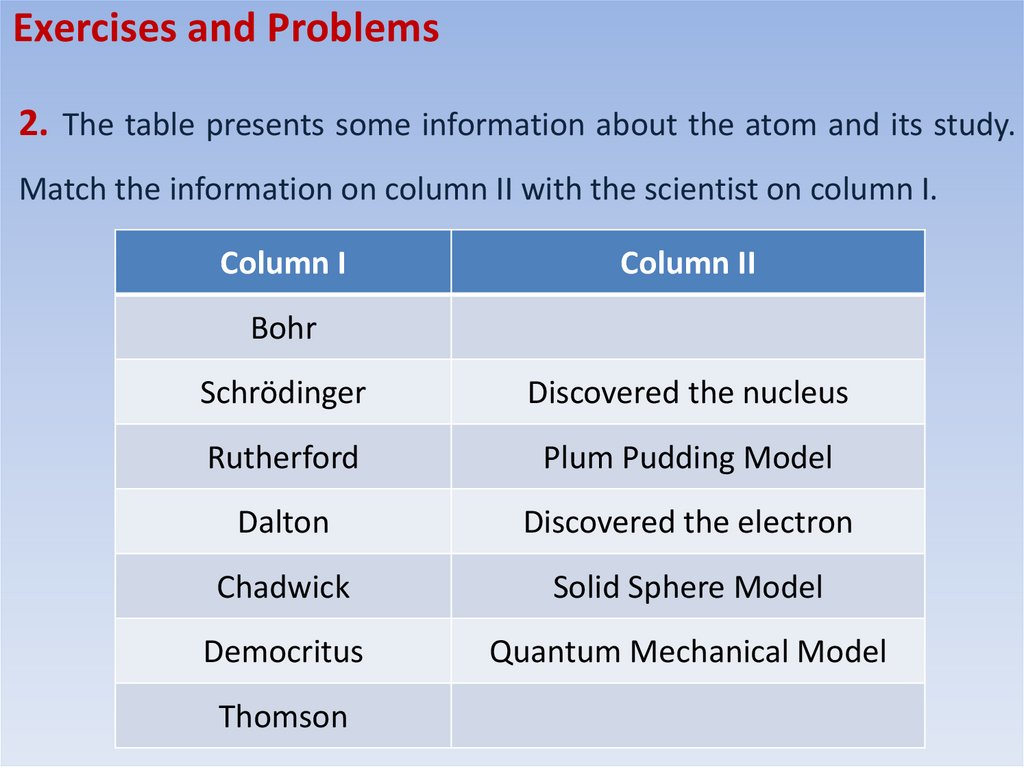
Exercises and Problems2. The table presents some information about the atom and its study.
Match the information on column II with the scientist on column I.
Column I
Column II
Bohr
Schrödinger
Discovered the nucleus
Rutherford
Plum Pudding Model
Dalton
Discovered the electron
Chadwick
Solid Sphere Model
Democritus
Quantum Mechanical Model
Thomson
26.
Exercises and Problems3. Which statements relate to conclusions drawn from the GeigerMarsden experiment?
A. Electrons go around the nucleus in stable orbits.
B. Almost all mass of the atom is in its nucleus.
C. The positive charge is spread uniformly throughout the atom.
D. The atom is mostly empty space.
E. The positive charge is concentrated in the nucleus of the atom.
F. The size of the nucleus is just a tiny fraction of the size of the atom.
B, D, E, F
27.
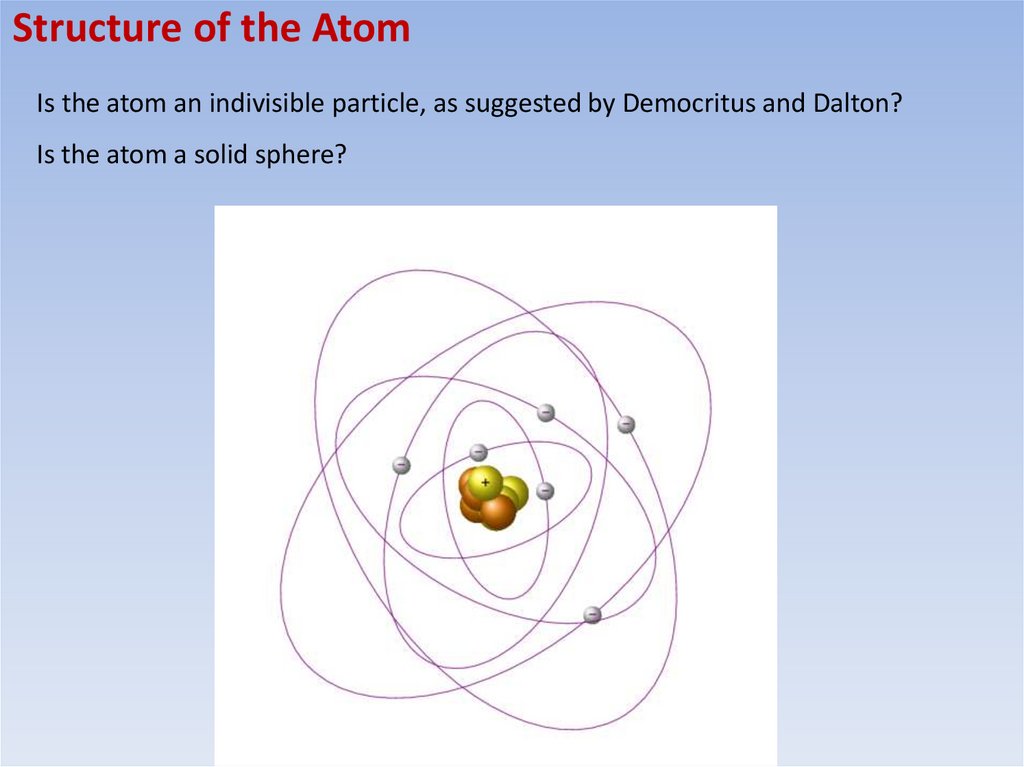
Structure of the AtomIs the atom an indivisible particle, as suggested by Democritus and Dalton?
Is the atom a solid sphere?
28.
Structure of the AtomAtoms consist of electrons surrounding a nucleus that contains
protons and neutrons (protons and neutrons are also known as nucleons)
Hydrogen-1 is the only atom that does not contain neutrons
29.
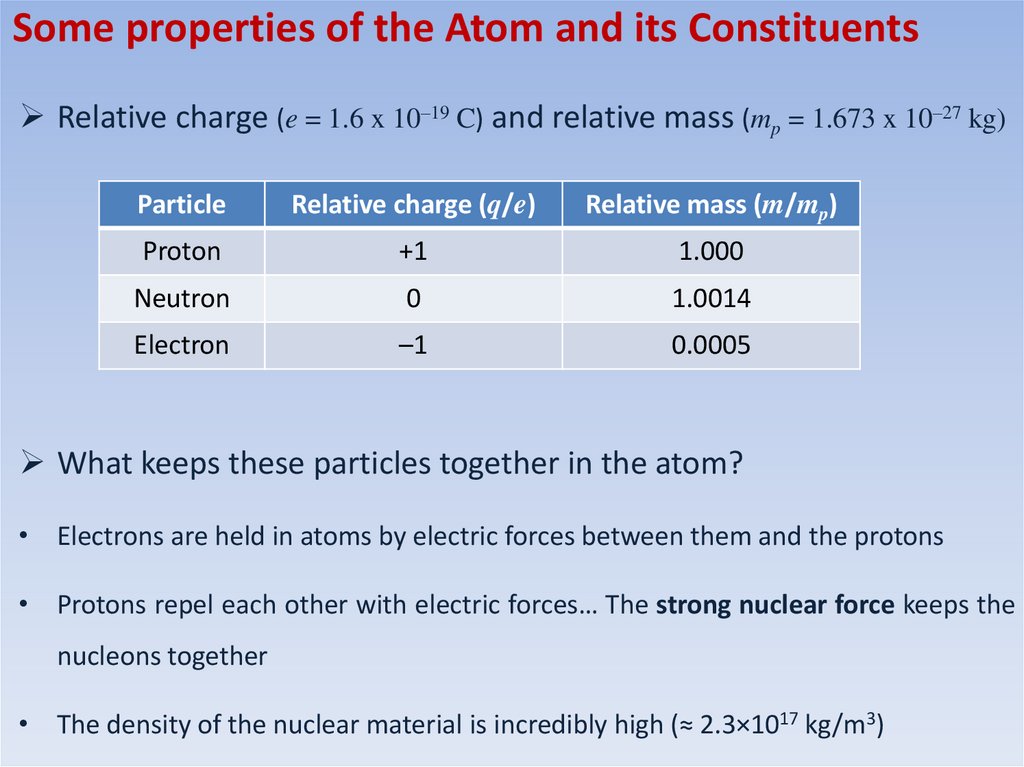
Some properties of the Atom and its ConstituentsRelative charge (e = 1.6 x 10–19 C) and relative mass (mp = 1.673 x 10–27 kg)
Particle
Relative charge (q/e)
Relative mass (m/mp)
Proton
+1
1.000
Neutron
0
1.0014
Electron
–1
0.0005
What keeps these particles together in the atom?
• Electrons are held in atoms by electric forces between them and the protons
• Protons repel each other with electric forces… The strong nuclear force keeps the
nucleons together
• The density of the nuclear material is incredibly high (≈ 2.3×1017 kg/m3)
30.
Some properties of the Atom and its ConstituentsAtom: some facts
• Atoms are neutral, so the number of electrons must be equal to
the number of protons;
• The nucleus has 99.9% of the mass of the atom;
• Size of the nucleus ≈ 10–15 m;
• Size of the atom ≈ 10–10 m
31.
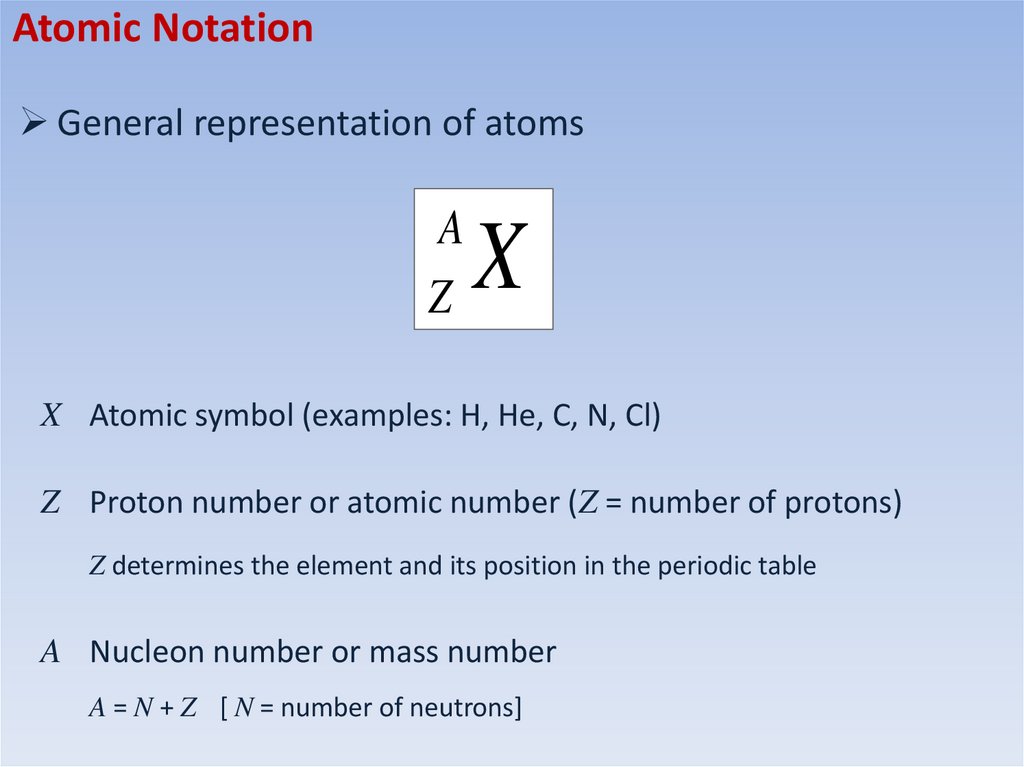
Atomic NotationGeneral representation of atoms
A
Z
X
X Atomic symbol (examples: H, He, C, N, Cl)
Z Proton number or atomic number (Z = number of protons)
Z determines the element and its position in the periodic table
A Nucleon number or mass number
A = N + Z [ N = number of neutrons]
32.
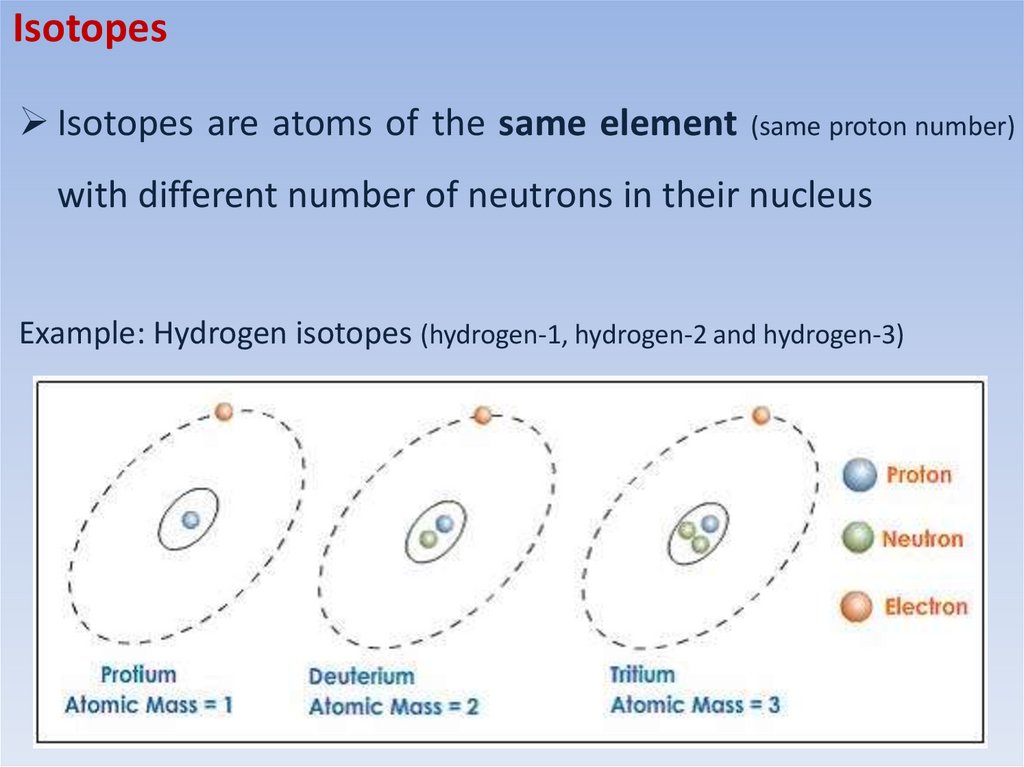
IsotopesIsotopes are atoms of the same element (same proton number)
with different number of neutrons in their nucleus
Example: Hydrogen isotopes (hydrogen-1, hydrogen-2 and hydrogen-3)
33.
Exercises and Problems1. An atom "Y" has 13 protons and 12 neutrons
a) What is its mass number?
b) What is its atomic number?
25
13
c) How many nucleons does this atom have?
d) How many electrons does this atom have?
25
13
e) Represent the atom using the atomic notation.
25
13
Y
34.
Exercises and Problems2.
Element
Z
A
6
12
17Cl
Uranium-235
N
18
92
18
14C
Uranium-238
a) Complete the table.
b) Which ones, if any, are isotopes?
12C and 14C
235U and 238U
10
35.
Exercises and Problems3. Three isotopes of Lithium are Lithium-6, Lithium-7 and
Lithium-8.
a) Represent these isotopes using the notation
A
Z
X
b) How many neutrons does each isotope have? 3, 4, 5
c) How many electrons does each isotope have?
3
6
3
Li 37 Li
8
3 Li
36.
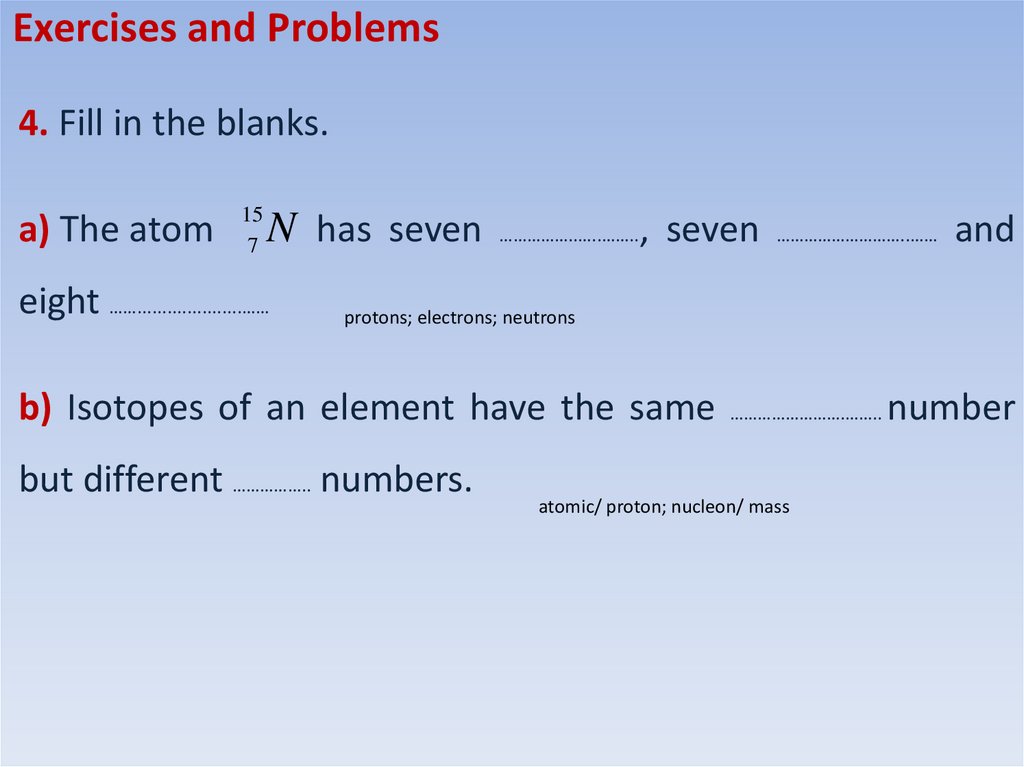
Exercises and Problems4. Fill in the blanks.
a) The atom 157 N has seven ……………..…..…….., seven ………………………..…… and
eight …….....................……
protons; electrons; neutrons
b) Isotopes of an element have the same …………………….…….. number
but different …………….. numbers.
atomic/ proton; nucleon/ mass














 Химия
Химия








