Похожие презентации:
Reach - lecture
1. REACH - Lecture
Registration, Evaluation, Authorization andRestriction of Chemicals
2. Lecture content - Block 1
Overview REACHScope and Objectives
Definitions
Registration, Evaluation
Authorization and restriction of Chemicals
Safety Data Sheet
Essential related regulations
2
3. Lecture content - Block 2
Chemical Safety Assessment and Chemical Safety ReportGuidance and Support
incl. downstream user information transfer in the supply chain
Recycling, articles, intermediates, substance identity, etc.
Use of IT tools for REACH incl. guidance documents
IUCLID5 and REACH-IT
Data sharing in SIEFs and consortia
Overview and Summary of the contents of the lecture
3
4. C17 H21 N04 = „Cocaine“
is subject to REACH!!CAS number:
EINECS number:
IUPAC name:
50-36-2
200-032-7
1R-(exo,exo)]-3-(Benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]octan-2carboxylsäuremethylester
Registration deadline: 30.11.2010 (>1000 t/a)
CAS
EINECS
Use Germany: Eye surgeries (20%), Ointment (2%)
In Austria, cocaine is subject to the Narcotic Substances Act.
IUPAC
Chemical Abstract Service
European Inventory of
Existing Commercial
Chemical Substances
International Union of Pure
and Applied Chemistry
There are suppliers on the Internet.
Safety data sheet required, since classified as "toxic”.
4
5.
56. On the methodical implementation of the new European chemicals management according to REACH
7. Content
Background and content of REACHIndustry’s reaction to REACH
Impact of REACH
7
8. New processes for EU-wide uniform regulation of chemicals through REACH
Deficits of "old" chemicals legislation inthe EU
Intended gap-filling by the REACH regulation
Equalization of the different data stocks of old substances compared
to new substances
Too little information on the safety of
existing substances in use
Mandatory communication along the product supply chains
Creation of mechanisms for long-term substitution of particularly
hazardous substances
Different EU legislation regulating chemical
substances
Low incentives for new substance
development due to high bureaucratic
requirements
Existing regulations are now united in only one law, the REACH
regulation
Establishment of a central European Chemicals Agency (ECHA) as a
superordinate institution
Improvement in the registration of new substances
8
9. REACH stands for Registration, Evaluation, Authorization and Restriction of Chemicals
Registration: The industry must comprehensively evaluate its substances that aremanufactured or imported into the EU in quantities > 1t/a, especially with regard to
uses, risks and risk reduction measures
The documentation takes place in a substance dossier, which is submitted to the
European Chemicals Agency (ECHA) in the course of registration.
Depending on the quantity threshold and hazard characteristics, different registration deadlines
apply (2010, 2013, 2018)
Two-stage procedure consisting of pre-registration and registration
Without a valid registration no substances may be manufactured or imported into the EU
in the future “NO Data NO Market“
ECHA and national authority evaluate registration dossiers for completeness and
plausibility
For substances of very high concern, an authorisation procedure is carried out and
restrictions on the use of substances are taken over from existing regulations.
9
10. Broad scope of application
1011. Content
Background and content of REACHIndustry’s reaction to REACH
Impact of REACH
11
12. Uniform implementation methodology
1213. Creating and network organizational structures
REACH only works with structures13
14. Changed framework conditions for raw material procurement in the EU due to REACH
AGeneral importer
B
C
Central import (to the EU) by the general importer
Decentralized import (into the EU) with existing
purchasing logistics
Saving resources through central implementation of the
REACH agendas
Decentralised implementation of the REACH agendas
Saving registration costs
Multiple registrations in the company group
Flexible purchasing oranization ergo supplier selection
Restrictions on purchasing structure and choice of
supplier (at European level)
14
15. Content
Background and content of REACHIndustry’s reaction to REACH
Impact of REACH
15
16. Novel processes trigger unforeseen industry response
"Discrepancy between pre-registrationand registration".
Trigger
Initial position / Consequence
Fundamentally new content in European chemicals law
due to REACH
Industry and authorities do not yet have practical
experience in implementing
Pre-registration is free and easy to carry out
Hedging idea of the industry
Registration means high costs and requires high effort
Optimisation idea of the industry
16
17. Key Findings
Positive aspectsNegative aspects
Structuring of the company's own substance portfolio
Expenditure and costs high compared to the previous
benefit
Common goal of the industry promotes formation of
networks
First registration deadline short compared to the required
data volume
Adequate support from European and national authorities
Underestimating the impact of the new registration
process by authorities
17
18. REACH Registration, Evaluation, Authorization and Restriction of Chemicals
Part: Scope and Objectives19. New processes for the EU-wide uniform regulation of chemicals through REACH
Shortcomings of "old" legislation onchemicals in the EU
Intended gaps to be closed by the REACH Regulation
Compensation of the different data stocks of existing substances
compared to new substances
Too little information on the safety of
existing substances in use
Mandatory communication along the product supply chains
Creation of mechanisms for long-term substitution of particularly
hazardous substances
Different EU legislation regulating chemical
substances
Low incentives for new substance
development due to high bureaucratic
requirements
Existing regulations are now united in only one law, the REACH
regulation
Establishment of a central European Chemicals Agency (ECHA) as a
superordinate institution
Improvement in the registration of new substances
19
20. Broad scope of application
2021. REACH – Recital Nr. 1
This regulation should ensure a high level of protection of humanhealth and the environment and the free movement of substances
on their own, in mixtures or in the finished article, while enhancing
competitiveness and innovation.
This regulation should also encourage the development of
alternative assessment methods for the hazards of substances.
Note: Considerations and intentions underlying the Regulation also recitals 1 to 4
21
22. REACH – Recital Nr. 2
The Community internal market for substances can only functioneffectively if the requirements for substances do not differ
significantly from one Member State to another.
22
23. REACH – Recital Nr. 3
The approximation of legislation on substances should ensure a highlevel of protection of human health and the environment with the
objective of sustainable development. Legislation should be applied
without discrimination according to whether substances are traded
within the Community or internationally in accordance with the
Community's international obligations.
23
24. REACH – Recital Nr. 4
In accordance with the Plan of Implementation adopted by theWorld Summit on Sustainable Development in Johannesburg on 4
September 2012, the European Union aims to achieve by 2020 that
chemicals are produced and used in ways that minimise significant
adverse effects on human health and the environment.
24
25. Expected risk reduction with the use of REACH
Source: Fuchs O., 200925
26. Article 1 – Aim and Scope
(1) The purpose of this Regulation is to ensure a high level ofprotection of human health and the environment, including the
promotion of alternative methods for assessment of hazards of
substances, as well as the free circulation of substances on the
internal market while enhancing competitiveness and innovation.
26
27. Article 1 – Aim and Scope
(2) This Regulation lays down provisions on substances andpreparations within the meaning of Article 3. These provisions shall
apply to the manufacture, placing on the market or use of such
substances on their own, in preparations or in articles and to the
placing on the market of preparations.
27
28. Article 1 – Aim and Scope
(3) This Regulation is based on the principle that it is formanufacturers, importers and downstream users to ensure that they
manufacture, place on the market or use such substances that do
not adversely affect human health or the environment. Its provisions
are underpinned by the precautionary principle.
28
29. Precautionary Principle
• 'Better safe than sorry'• Essential component of current environmental and health policy in
Europe
• Avoidance or at
least reduction of burdens or damage to the
environment and human health in advance
• Risk or hazard prevention
29
30. Hazard vs Risk
Dangerous processIntensity and probability of
occurrence
Damage
Values, vulnerability,
probability of presence
Risk = probability of occurrence X extent of damage
30
31. Article 2 – Application
(1) REACH does not apply toRadioactive materials
Products under customs seal
Non-insulated intermediates
Transport of dangerous goods
(2) Waste is not a substance, mixture or article according to REACH
(3) Possibility of exemption for military purposes
National Defense
(4) REACH applies regardless of
The EU worker and environmental legislation
The existing restrictions and prohibitions on testing on vertebrate animals
31
32. Article 2 – Application
(5) Exemptions from registration, downstream user, evaluation andauthorisation for
Medicinal products for human or veterinary use
• Certain substances in food or feed
Food additives
Flavoring substances
Additives for animal feed
Animal feed
Note: Exceptions do not apply to raw materials or intermediate products for the
production of the above mentioned products!
32
33. Article 2 – Application
(6) The following are excluded from the disclosure of informationMedicinal products for human or veterinary use
Cosmetic products
Medical Products
Food or feed
33
34. Article 2 – Application
(7) Excluded from registration, downstream user, evaluation areDerogations contained in Annex IV
Derogations contained in Annex V
Reimported substances (in the same supply chain)
Recovery (Recycling!) of a substance already registered (independent of the
supply chain)
34
35. Exemptions Annex IV - Extract
Ascorbic acid (CAS-Nr. 50-81-7)
Sucrose, pure (CAS-Nr. 57-50-1)
Galactose (CAS-Nr. 59-23-4)
Lactose (CAS-Nr. 63-42-3)
Noble gases (Ar, Xe, Ne, He, Kr)
Nitrogen (N2)
Water distilled, conductivity water or water of similar purity (CAS-Nr. 7732-18-5)
(Starch, cellulose, tallow, matodextrin, fatty acids, etc.)
35
36. Exemptions Annex V - Extract
No. 2: substances which have been produced by a chemical reaction which has
occurred during the storage of other substances, mixtures or products;
No. 7: the following natural substances, provided they have not been chemically
altered: Minerals, ores, ore concentrates, natural gas, crude and processed,
crude oil and coal;
No. 10.: the following substances, provided they have not been chemically
altered: LPG, natural gas condensate, process gases and their components, coke,
cement clinker and magnesia;
36
37. Article 2 – Application
(8) On-site isolated and transported isolated intermediates shall beexempted from
General registration obligation for substances (simplified
requirements for intermediates according to Articles 17 and 18)
the registration requirement
registration
Polymers do not have to be registered and are therefore not subject to evaluation
37
38. Article 2 – Application
(8) On-site isolated and transported isolated intermediates shall beexempted from
Title II, Chapter 1 (Exception to Articles 8 & 9)
Title VII
(9) Title II and VI do not apply to polymers
38
39. Summary – Aim and Scope
• High level of protection for human health and theenvironment
• Promotion of alternative assessment methods
• Free movement of substances in the internal market
• Improving competitiveness and innovation
39
40. REACH Review – 5-Year Review Report of the EU Commission of 05.02.2013
• Human health and environmentProgress is being made - but it is too early to quantify. Shortcomings in registration
dossiers, the evaluation of PBT substances, the content and format of the safety data
sheet, the determination of substance identity. With regard to substance identity, it
reserves the right to propose further measures, including implementing rules.
• Promotion of alternative assessment methods
ECHA concludes that the main needs of industry at this stage are stability and reliability
and does not support any changes to REACH in the short term. Member States should
enforce compliance with the requirements for testing proposals.
40
41. REACH Review – 5-Year Review Report of the EU Commission of 05.02.2013
• Free movement of substances within the internal marketThe Commission notes market concentration and higher prices for certain substances, but
sees positive effects in the fact that greater specialisation and new business models could
increase safety. It identifies "regulatory incompatibilities" between the EU and key markets
as a risk to the EU's external competitiveness. It aims to mitigate the financial impact of
REACH on SMEs. Member States are invited to interpret the 0.1% criterion for SVHC
substances in articles in a consistent way.
• Improving competitiveness and innovation
The Commission considers that REACH fulfils the objective in terms of innovation. The
Commission will continue to monitor the impact of REACH on innovation, particularly in
new technology areas, and will present a report by 1 January 2019.
41
42. REACH Review – 5-Year Review Report of the EU Commission of 05.02.2013
The EU Commission sees substantive problems in the implementation ofREACH in three areas that need to be addressed:
Quality of registration dossiers, including the description of substance identities
• Insufficient PBT/vPvB assessments by registrants
• difficulties with the content and format of safety data sheets
The report (also in German) and a more detailed background document, as well as the
thematic studies, can be downloaded from the EU Commission at:
• http://ec.europa.eu/environment/chemicals/reach/review_2012_en.htm
42
43. REACH Review – 10-Year Review Report of the EU Commission of 05.03.2018
“Some 10 years after its entry into force, REACH is fully operational and delivering results towardsachieving its objectives. Although progress towards the objectives is lagging behind initial expectations, it
has steadily improved as experience was gained.”
Costs until 2018: 1,7 billion Euro.
Expected potential benefits for human health and the environment: 100 billion euros over the next
25-30 years.
“…further opportunities to improve and simplify have been identified, in particular for extended Safety
Data Sheets, evaluation, authorization and restriction. The issues requiring most urgent action are:
Non-compliance of registration dossiers;
Simplification of the authorization process;
Ensuring a level playing field with non-EU companies through effective restrictions and enforcement;
Clarifying the interface between REACH and other EU legislation, in particular that on occupational safety and
health (OSH) and on waste.
https://ec.europa.eu/growth/sectors/chemicals/reach/review_de
43
44. Summary – Application
Not regulated by REACH, but specifically regulated are, among others:• Waste, radioactive materials, materials for military purposes, transport of
dangerous goods, customs
Partially exempted from REACH:
• Human or veterinary medicinal products, cosmetic products, medical
products, food or feed
Special rules / simplifications for:
• Recycling and reimportation
• Isolated intermediates
• Polymers
• Substances with low hazard potential (Annex IV and V)
44
45. REACH Registration, Evaluation, Authorization and Restriction of Chemicals
Part: Definitions46. Essential terms according to Article 3
Term definition in REACHDefined in Article (Art.)
Substance
Preparation
Article
Art. 3 Nr. 1
Art. 3 Nr. 2
Art. 3 Nr. 3
Manufacturer (in the EU)
Importer (to the EU)
Downstream User
Art. 3 Nr. 9
Art. 3 Nr. 11
Art. 3 Nr. 13
Distributer (a distributer is not an actor in the supply chain)
Art. 3 Nr. 14
Agency
Art. 3 Nr. 18
Competent Authority
Art. 3 Nr. 19
Phase-in Substance
Art. 3 Nr. 20
Intermediate
Art. 3 Nr. 15
Polymer and Monomer
Art. 3 Nr. 5 and 6
46
47. Assign definitions correctly ?! Substance – Preparation – Article
methanollacquer
pan
car
cutlery
cleaning agent
hydrochloric acid
concrete
PC
47
48. A few introductory examples
Term definition inREACH
General example
Example from the steel industry
Substance
methanol, hydrochloric acid
Iron, sinter, slag, scale
Preparation
Lacquer, cleaning agent, concrete
Corrosion protection oil, ferroalloy
Article
PC, car, pan, cutlery
Slab, shaped steel parts
48
49. Article 3 No. 1 - Substance
„a chemical element and its compounds in the natural state or obtained byany manufacturing process, including any additive necessary to preserve its
stability and any impurity deriving from the process used, but excluding any
solvent which may be separated without affecting the stability of the
substance or changing its composition."
More details on the description of the substance identification in the
„Guidance for Identification and Naming of Substances under REACH and
CLP“
• http://echa.europa.eu/en/guidance-documents/guidance-on-reach
49
50. Article 3 No. 20 – Phase-in Substance
A substance that meets at least one of the following criteria:• it is listed in the European Inventory of Existing Commercial Chemical
Substances (EINECS)
• the substance was manufactured but not placed on the market 15 years
before REACH entered into force (01.06.2007); proof required
• The substance is included in the so-called No-Langer-Polymer (NLP) list
and was also placed on the market; proof of this is required
Important definition for registration obligations under REACH
50
51. A substance may contain
• oneor more main constituent(s): constituent(s) which constitute(s) a
significant part of the substance. The main constituent(s) shall be clearly
distinguishable from:
• Impurities:
any unintentional component resulting from the
manufacturing process or deriving from the starting material(s). These
impurities may be the result of secondary or incomplete reactions during
production and may be present in the final substance even if the
manufacturer did not intend their presence.
• Additives: all ingredients added to maintain the stability of the substance
and only added intentionally.
51
52. Groups of substances
• Precisely defined substances: Substances with a defined qualitative andquantitative composition that can be adequately identified using the
identifiers in Annex VI, Section 2 of REACH.
• UVCB-Substances (Substances of Unknown or Variable composition,
Complex reaction products or Biological materials): Substances with an
unknown or variable composition, complex reaction products or biological
materials. These substances cannot be sufficiently identified using the
above mentioned parameters.
52
53. Precisely defined substances
• mono-constituent substance• multi-constituent substance
Decision based on the „80% : 20%“- and „80% : 10%“- rule
• Typical
concentrations or concentration ranges must be reported or
determined by appropriate analytical methods (not further specified here)
• At least the following must be indicated
Chemical name (IUPAC and/or CAS-name)
• The CAS number
• EG-number and/or the molecular formula
• Possible exceptions are not further discussed - for details see the guide
53
54. Mono-constituent substance
• Main constituent at least 80% present and fully specified• Impurities or additives not more than 20%
• To be specified if > 1% is present
• Intentionally added substances that have been added for a purpose
other than to maintain the stability of the substance shall be
considered as separate substances that need not be taken into
account when establishing the mass balance of the main constituents
54
55. Multi-constituent substance
• Several main components present in a concentration between 10% and 80%Example
Main
component
Aniline
Upper
limit (%)
Typical
content
(%)
Lower
limit (%)
90
75
65
Contaminat
ion
Phenanthren
Naphtalin
35
20
10
Upper
limit (%)
5
Typical
content
(%)
4
Lower
limit (%)
Substance
identity
1
„Reaction
mass“ of
Aniline and
Naphtalin
• According to the rules in this guidance, this substance is a „Multi-constituent-
substance“. Although the range of a component is > 80%, this happens only
occasionally and the typical concentration is < 80%.
55
56. UVCB Substances
• Chemicalcomposition: variable, unknown, fluctuating,
predictable unambiguous identification not possible
not
• Therefore identification on the basis of:
• Origin (z.B. plant or animal species)
• Manufacturing process (e.g. extraction, iron production, gas purification)
• Others (e.g. Enzyme index, mineral constituents)
• Variable concentrations and concentration ranges
56
57. Four main subgroups of UVCB substances
UVCB-subgroup 1: This subgroup includes substances synthesized from material of
biological origin. The biological material is modified by a (bio)chemical process, whereby
new components are produced.
UVCB-subgroup 2: This subgroup includes substances whose material is of chemical or
mineral origin and in which new molecules have been synthesized by (bio)chemical
reactions.
UVCB-subgroup 3: This subgroup includes substances whose material is of biological
origin and which have been produced by grafting with deliberate creation of new
molecules.
UVCB-subgroup 4: This subheading covers substances whose material is of chemical or
mineral origin and which have been obtained by a process of finishing without any
intentional chemical reaction.
57
58. Substance identification
Requirements for the full identification of a substance under REACH (Annex VI Section 2):the chemical composition of the substance, taking into account impurities and additives
in addition to the main constituent(s), where appropriate, and respective typical
concentrations and concentration ranges
• chemical identity of the constituent(s) in the form of the IUPAC name and other
identifiers where available, e.g. EC number or CAS number; for UVCB substances
information on the origin and manufacturing process is also required
• molecular and structural information, where available and appropriate, to be indicated
by molecular and structural formula, information on optical activity, proportion of
isomers, molecular weight or molecular weight range
• Spectral and analytical data sufficient to confirm the structure and composition of the
substance
58
59. Substance name
Precisely defined mono-constituent substances: Name after the main constituent. Its IUPAC name
shall be used. Other internationally accepted names may be given as additional information.
Precisely defined multi-component substances: Name according to the reaction mass of the main
constituents of the substance. In general, the format "Reaction mass of [names of main
constituents]" shall be used, supplemented by a list of the constituents which shall be arranged
alphabetically and separated by the conjunction "and".
UVCB-Substances: Designation by combining origin and method (in that order). Depending on
whether the material is of biological or non-organic origin, the names of the species (genus, species,
family) or the source material (IUPAC names) shall be used. The process shall be indicated by stating
the chemical reaction where new molecules are synthesized or the nature of the processing step.
Sometimes, for example in the case of combined processing, several steps must be indicated in
addition to the indication of origin. There are also borderline cases where UVCB substances can be
labelled according to their constituents.
59
60. Determination / verification of identical substances
• Verification by different manufacturers/importers whether their substancescan be considered identical
OSOR Principle: One Substance One Registration
• Starting point for identification and designation of a substance are rules
for
mono-constituent substances
multi-constituent substances
UVCB-Substances
Any substantial change in origin or procedure another substance
60
61. Importance of "correct" and uniform substance identification
Importance of "correct" and uniformsubstance identification
• Ensure that the chemical identity of the substance is traceable for the
authorities in enforcement (e.g. ECHA, national authorities)
ECHA's conclusion from Evaluation Report 2015: 2/3 of the dossiers examined were
not sufficiently identified
• To be able to determine whether one (or more) substances are actually the
same
• Basis for common data sharing and registration
• To correctly identify or communicate hazards and risks arising from a
substance
61
62. Summary substance identification
6263. Article 3 No. 2 - Preparation
Preparations, mixtures or solutions consisting of two or moresubstances
63
64. Article 3 No. 3 - Article
An object which during production is given a special shape, surfaceor design which determines its function to a greater degree than
does its chemical composition.
Details of articles and obligations,
requirements for substances in articles“:
etc.:
"Guidance
on
• https://echa.europa.eu/guidance-documents/guidance-on-
reach
64
65. What is an article?
• Shape means the three-dimensional shape of an object, such as depth, widthand height.
• Surface comprises the outermost layer of an object.
• Form means the arrangement of the design elements in a way that is most
suitable to fulfil a specific purpose.
• The term 'function' in the definition of 'article' should be interpreted as a
principle determining the use of the subject matter and not as a degree of
technical complexity. In this sense, the function of a printer cartridge is to put
ink on the paper and the function of a battery is to provide electrical power.
65
66. Example Delimitation substance - preparation - article
6667. Example aluminium industry: Delimitation substance - preparation - article
Bauxite(Natural substance)
Extraction
Aluminium
oxide
Electrolyis
Aluminium
(Substance)
(Substance)
Aluminium alloy
(Preparation)
Casting
Rolling Ingots
(Preparation)
mill
String bars
(Preparation)
Extrusion
Sheet metal
(article)
Press profile
(article)
Aluminium casting
(article)
67
68. Summary substance - preparation - article
*) currently still discussions at EU level**) if no predefined form
Material
Metal pipe
Article
Blasting material
Substance or Preparation *)
Magnetic foils
Article
Aluminium foil
Article
Wire
Article
Welding rod
Source: „REACH Info 6“ of BAuA
Classification
Substance or Preparation
Metal balls (ball bearing)
Article
CD cases (plastic)
Article
Paper
Article
Textiles
Article
Polyester fibres
Article
Packaging
Article
Pen
Preparation (in a container)
Printer/Toner Cartridge
Preparation (in a container)
Candles
Substance or Preparation *)
Battery/Accu
Material
adhesive tape
Classification
Article
Wet cleaning wipes
Preparation (on a carrier
material)
Metal ingots **)
Substance or Preparation
Machine (oiled)
Article
Car Tyres
Article
Buckling light
Preparation
Article
68
69. Monomers and Polymers
Article 3 No. 5 - PolymerPolypropylene
A substance consisting of molecules characterized by the sequence of one or more types of
monomer units. Such molecules must be distributed over a range of molecular weights
wherein differences in the molecular weight are primarily attributable to differences in the
number of monomer units. A polymer comprises the following:
a simple weight majority of molecules containing at least three monomer units which are
covalently bound to at least one other monomer unit or other reactant;
less than a simple weight majority of molecules of the same molecular weight.
In the context of this definition a "monomer unit“ means the reacted form of a monomer
substance in a polymer;
69
70. Monomers and Polymers
Article 3 No. 6 - MonomerA substance which is capable of forming covalent bonds with a sequence of additional like or
unlike molecules under the conditions of the relevant polymer-forming reaction used for the
particular process.
Details on monomers and obligations, etc.: „Guidance on Monomers and Polymers":
https://echa.europa.eu/documents/10162/23036412/polymers_en.pdf/9a74545f-05be4e10-8555-4d7cf051bbed
70
71. Delimination
7172. Article 3 No. 15 - Intermediate
A substance that is manufactured for and consumed in or used for chemical processing inorder to be transformed into another substance (hereinafter referred to as synthesis).
non-isolated intermediate: an intermediate that during synthesis is not intentionally removed
(except for sampling) from the equipment in which the synthesis takes place. Such equipment includes
the reaction vessel, its ancillary equipment, and any equipment through which the substance(s)
pass(es) during a continuous flow or batch process as well as the pipework for transfer from one vessel
to another for the purpose of the next reaction step, but it excludes tanks or other vessels in which the
substance(s) are stored after the manufacture
on-site isolated intermediate: an intermediate not meeting the criteria of a non-isolated
intermediate and where the manufacture of the intermediate and the synthesis of (an)other
substance(s) from that intermediate take place on the same site, operated by one or more legal
entities;
transported isolated intermediate: an intermediate not meeting the criteria of a non-isolated
intermediate and transported between or supplied to other sites;
72
73. Intermediates - Interpretation of terms according to REACH Guide
A substance is an intermediate, if all the following conditions aremet:
• The substance is manufactured to be transformed into another
substance at an industrial site.
• The result of the chemical processing is not another substance
in an article, but another manufactured substance as such.
73
74. Examples of delimitation
IntermediatesNo intermediates are e.g.
catalysts
Processing aids
Hardener
Surface treatment agents
(z.B. silver cyanide)
Source: Guidance on Intermediates:
https://www.echa.europa.eu/documents/10162/23036412/i
ntermediates_en.pdf
74
75. Intermediates
Different tasks and obligations depending on the status of the intermediatenon-isolated intermediates
isolated intermediates
on-site (non-transported) isolated intermediates
transported isolated intermediates
"Simplified" requirements and reduced registration fees compared to substances
BUT: Proof of manufacture and use under certain conditions according to Articles 17 and 18
in REACH
Keyword: strict containment
75
76. Intermediates
More information on intermediates and commitments, etc.: “Guidance onintermediates“
https://www.echa.europa.eu/documents/10162/23036412/intermediates_en.pdf
Concrete examples from the steel industry
• Raw tar
• Light oil, coal
• Zinc slag
76
77. Intermediates
Additional requirements for the registration dossiers:https://echa.europa.eu/de/-/echa-receives-updates-for-intermediate-dossiers-andannounces-further-follow-up-actions
77
78. "Roles" in REACH
"Roles" in REACH• Manufacturer (in the EU)
• Importer (to the EU)
• Downstream users
• Distributor
78
79. Article 3 No. 17 – Actors in the supply chain
All manufacturers and/or importers and/or downstream users in asupply chain;
User 3
upwards
User 3 is the downstream
user of user 2
User 2
User 1
downwards
Manufacturer is the
upstream actor of user 1
Manufacturer
79
80. Article 3 No. 9 – Manufacturer
Any natural or legal person established within the Community whomanufactures a substance within the Community;
Article 3 No. 8 - Manufacturing
production or extraction of substances in the natural state;
80
81. Article 3 No. 11 – Importer
Any natural or legal person established within the Community whois responsible for import;
Article 3 No. 10 – Import
the physical introduction into the customs territory of the
Community
Article 3 No. 12 – Placing on the market
supplying or making available, whether in return for payment or
free of charge, to a third party. Import shall be deemed to be
placing on the market;
81
82. Article 3 No. 13 – Downstream User
Any natural or legal person established within the Community,other than the manufacturer or the importer, who uses a
substance, either on its own or in a preparation, in the course of his
industrial or professional activities. A distributor or a consumer is
not a downstream user. A re-importer exempted pursuant to Article
2(7)(c) shall be regarded as a downstream user;
82
83. Article 3 No. 14 – Distributer
Any natural or legal person established within the Community,including a retailer, who only stores and places on the market a
substance, on its own or in a preparation, for third parties;
83
84. Summary – Roles in REACH
Role in REACHExample from the steel industry
Manufacturer (of a substance) in the EU
pig iron production
Importer (of a substance) into the EU
Import of iron ore pellets e.g. from South
Africa
Downstream User
Formulator of a paint mixture; user of
pickling acid, lubricants, alloys, ..: Use of
hydrochloric acid in the laboratory
Distributor
Chemical dealers (no import from outside
the EU)
84
85. Authorities
Article 3 No. 18 - Agencythe European Chemicals Agency as established by this Regulation
• Short: ECHA
• Headquarters in Helsinki (Finland)
• Founded in 2007
Article 3 No. 19 – Competent authority
the authority or authorities or bodies established by the Member States
to carry out the obligations arising from this Regulation
• Competent national authority in AUT: BM for sustainability and tourism
85
86. Other terms defined in Article 3
Producer of an article
Registrant
Site
Notified substance
Product and process orientated research
and development
scientific research and development
Use
Registrants own use
Identified use
Full study report
Robust study summary
Study summary
Per Year
Restriction
supplier of a substance or a preparation
supplier of an article
recipient of a substance or a preparation
Recipient of an article:
SME
Exposure scenario
Use and exposure category
Substances which occur in nature
Not chemically modified substance
alloy
86
87. REACH Registration, Evaluation, Authorization and Restriction of Chemicals
Part: Registration and Evaluation88. Registration
Article 5 – “No Data, No Market”Subject to Articles 6, 7, 21 and 23, substances on their own, in
preparations or in articles shall not be manufactured in the Community
or placed on the market unless they have been registered in accordance
with the relevant provisions of this Title where this is required.
88
89. Obligation to register
• Manufactured and / or in the EU imported substances > 1 ton peryear per registrant (Art. 6)
• Information to ECHA in the form of a registration dossier
• Content: Information on properties and uses of the substance
• adverse effects and, where appropriate, an assessment of the risks that may
arise from the use of the substance and how to control those risks
• ONE Substance, ONE Registration = OSOR-Principle
89
90. General registration requirements
• Registration obligation (Art. 6) ab 1 Ua for• Substance as such manufacturer or importer into the EU
• Substances in preparations Importer in the EU (or downstream user)
• Special cases (substances in articles, PPORD, intermediates, polymers)
• in consideration of the uses
• independent of the properties (hazardous or non-hazardous)
• in consideration of the scope
90
91. Special case polymers (Art. 6 (3))
• Registration of polymers by monomers or other substances• Which have not yet been registered
• And at least 2 % by mass in the form of monomer units or chemically bound
• ≥ 1 t/a
• Monomer must be fully registered; no facilitation of registration as an
isolated intermediate (Article 6 (2))
• See also Art. 2 (9)
91
92. Article 23 - Transitional periods for registration
9293. Article 23 - Transitional periods for registration
• End of the first transitional period on 01.12.2010Phase-in Substances ≥ 1.000 tones per year
Phase-in Substances, classified with R50/53 (potential PBT or vPvB substances) ≥ 100 t/a
CMR phase-in substances (Cat. 1 and 2; where known) ≥ 1 ton per year (per
manufacturer/importer)
• End of the second transitional period on 01.06.2013
Other phase-in substances (not R50/53) ≥ 100 t/a
• End of the last transitional period on 01.06.2018
Other phase-in substances (not CMR) ≥ 1 t/a
93
94. Article 28 – Pre-Registration
• 01.06.2008 until 01.12.2008:Possibility for pre-registration of phase-in substances (Art. 28)
Those subject to the general registration requirement (Art. 5 and 28)
Those intentionally released from articles (Art. 7)
On-site isolated intermediates (Art. 17)
Transported isolated intermediates (Art. 18)
Conditions for the use of the transitional periods under Art. 23
• Pre-registration also possible after 01.12.2008, if
substance was manufactured or imported into the EU for the first time
there is still one year to go before the end of the relevant transitional period
94
95. Later manufacturing or import into the EU Now what?
Event after entry into force of REACHRegulated in
REACH
Requirements
Article 22
Substance has
already been
registered
Article 26
Substance still has
to be registered
Company is closed or name of a company
changes
New use of a substance becomes known
Increasing the manufactured quantity of a
substance, or increasing the quantity imported
into the EU and thus exceeding a tonnage
threshold
Company which manufactures or imports substances
into the EU will be founded later
Company starts manufacturing or importing a
substance into the EU later
95
96. Article 10 – Information requirements for registration
Technical dossier• i. Identity of the manufacturer/importer
• ii. Identity of the substance
• iii. Information on production and use
• iv. Classification and labelling
• v. Guidelines for safe use
• vi. Study summaries
• vii. Qualified study summaries
• viii. Information on quality assurance
• ix. Testing proposals
• x. Exposure data for substances between 1 and 10 t/a
• xi. Confidentiality justification
Chemical Safety Report (CSR) according to Annex 1
Link to the registration dossier of the
iron industry:
Link to the public registration dossier
for iron:
http://apps.echa.europa.eu/registered/data/do
ssiers/DISS-9ea2736e-faca-51db-eo4400144f67d031/DISS-9es2736e-faca-51dbe044-00144f67d031_DISS-9ea2736e-faca51db-e044-00144f67d031.htm
96
97. Article 13 – Obtaining information on substances for the registration dossier
• General provisions on the procurement of informationExisting Data
„In-vitro“-Methods
(Q)SAR, group formation, analogy concepts
In accordance with the requirements of Annex XI
• Test methods Regulation (EC) No 440/2008
• If animal testing is intended - only testing proposals
For review by ECHA as part of the evaluation
• Information to be collected in accordance with Annexes VII to X
• Toxicological and ecotoxicological tests according to GLP
97
98. Registration of isolated intermediates
• On-site isolated intermediates - Article 17• Simplified registration requirements only if manufacturer confirms that the
substance is only manufactured and used under strictly controlled conditions in that
it is rigorously contained by technical means during its whole lifecycle. Control and
procedural technologies shall be used to minimize emission and any resulting
exposure.
98
99. Registration of isolated intermediates
• Transported isolated intermediate - Article 18• Simplified registration requirements only if the manufacturer or importer confirms
himself or states that he has received confirmation from the user that the synthesis
of (an)other substance(s) from that intermediate takes place on other sites under
strictly controlled conditions.
99
100. Information requirements for the registration of intermediates (Art. 17 and 18)
On-site isolated intermediates• Identity of the manufacturer
• Identity of the intermediate product
• Classification of the intermediate
product
• All available information
• Brief general information on use
• Applied risk management measures
Transported isolated intermediate
• Identity of the manufacturer
• Identity of the intermediate product
• Classification of the intermediate product
• All available information
• Brief specification for use
• Applied and recommended risk
management measures
• For intermediates above 1 000 t/a, the
data referred to in Annex VII
100
101. What are strictly controlled conditions? (Art. 18 (4))
The substance is rigorously contained by technical means during its whole lifecycle including
manufacture, purification, cleaning and maintenance of equipment, sampling, analysis, loading and
unloading of equipment or vessels, waste disposal or purification and storage.
Procedural and control technologies shall be used that minimize emission and any resulting
exposure.
Only properly trained and authorized personnel handle the substance.
In the case of cleaning and maintenance works, special procedures such as purging and washing are
applied before the system is opened and entered.
in cases of accident and where waste is generated, procedural and/or control technologies are used
to minimize emissions and the resulting exposure during purification or cleaning and maintenance
procedures.
substance-handling procedures are well documented and strictly supervised by the site operator.
101
102. Proof of compliance with the strictly controlled conditions
• Under strictly controlled conditions, a combination of technical measuressupported by established procedures and management systems.
• Procedure according to the guidance on intermediates!
https://echa.europa.eu/view-article/-/journal_content/title/guidance-onintermediates
• Example: Registration of an intermediate product in industry
Expert opinion on the substance flow from independent civil engineer
• Confirmation from customers
102
103. Common registration requirements
Duties of the European Chemicals Agency (Article 20)• Assignment of a submission number
• Completeness check generally within 3 weeks
In case of incompleteness: indication of what information is missing or setting of a
reasonable period of time for completion.
• Refusal of registration: registrant does not comply with the late submission deadline
• Assignment of a registration number
• Communication to the competent authority or authorities of the Member
States
• Information regarding new information after registration
103
104. Common registration requirements
Update of the registration by the registrant (Article 22)• Change in manufacturer/importer status or identity (legal succession - see Art. 36)
• Change in substance composition
• Change of quantity range (including stop of production or import)
• New identified uses or uses advised against
• New knowledge about substance risks leading to changes e.g. SDS, CSR
• Changed classification and labelling
• Testing proposals for Annex IX or X
• Change in the accessibility of information
104
105. Registration statistics
Source: https://echa.europa.eu/de/registration-statistics105
106. Registration statistics
RegistrationsUnique
Substances
93685
22119
-
15666
Registrations
Substances
Registered as full registration (Art. 10)
69077
15626
Registered as intermediate (Art. 17 &18)
16581
8551
Total (2019)
Phase-in
Source: https://echa.europa.eu/de/registration-statistics
106
107. Public registration dossiers
• Registration dossier for iron:http://apps.echa.europa.eu/registered/data/dossiers/DISS-9ea2736e-faca51dbe044-00144f67d031/DISS-9ea2736e-faca-51db-e044-00144f67d031_DISS9ea2736e-faca-51db-e044-00144f67d031.htm
107
108. Evaluation (Assessment)
Recital 20• The evaluation provisions should provide for follow-up to registration, by allowing
for checks on whether registrations are in compliance with the requirements of
this Regulation and if necessary by allowing for generation of more information
on the properties of substances. If the Agency in cooperation with the Member
States considers that there are grounds for considering that a substance constitutes
a risk to human health or the environment, the Agency should, after having
included the substance in the Community rolling action plan for substance
evaluation, relying on the competent authorities of Member States, ensure that this
substance is evaluated.
108
109. Evaluation - Overview
TaskWho?
Evaluation according to REACH
ECHA
Member States
Dossier evaluation
Procedure
Assessment of
compliance
with the requirements
Assessment of
testing proposals
Substance evaluation
Source: ECHA
http://www.echa.europa.eu/de/regulations/reach/evaluation/substance-evaluation
109
110. Dossier evaluation
• Examination of testing proposals (Art. 40)• Compliance check of dossier registrations (Art. 41)
• Check of information submitted and follow-up to dossier evaluation (Art. 42)
• Procedure and time periods for examination of testing proposals
(Art. 43)
• Registrants' and downstream users' rights (Art. 50)
• Adoption of decisions under dossier evaluation (Art. 51)
110
111. Substance evaluation
• Criteria for substance evaluation (Art. 44)• Competent authority (Art. 45)
• Requests for further information and check of information submitted (Art. 46)
• Coherence with other activities (Art. 47)
• Follow-up to substance evaluation (Art. 48)
• Registrants' and downstream users' rights (Art. 50)
• Adoption of decisions under substance evaluation (Art. 52)
111
112. Overview of the substance evaluation process
Source: ECHAhttps://echa.europa.eu/documents/10
162/13628/sub_eval_under_reach_le
aflet_en.pdf/98a7b249-e407-403d82c9-2704a10cd774
112
113. Ongoing Community Action Plan
• = Community Rolling Action Plan (CoRAP)Continuously updated action plan for a period of three years, listing the substances to
be subject to substance evaluation.
• Is updated annually
• Need for action for registrants whose substance is listed in CoRAP!
• Current CoRAP
https://echa.europa.eu/de/information-on-chemicals/evaluation/community-rollingaction-plan/corap-table
113
114. Current CoRAP – Extract
• In 2022 and 2023 it is planned to evaluate 50 substancesSource
Year
2024
2024
Member
State
NL
FR
EC
Number
205-739-4
200-467-2
CAS
Number
149-44-0
60-29-7
Substance
Name
Initial Ground for
concren
Sodium
hydroxymeth
anesulphinat
e
Suspected Carcinogenic
Suspected Mutagenic
Suspected Reprotoxic
Exposure of workers
High (aggregated)
tonnage
Wide dispersive use
Not started
Diethyl ether
Suspected Carcinogenic
Suspected Mutagenic
Suspected Reprotoxic
Other hazard based
concern
Consumer use
Exposure of
environment
High (aggregated)
tonnage
Wide dispersive use
Not started
Status
114
115. Current status on dossier and substance evaluation
• Publication of a report on progress in dossier and substance evaluation (Art.54)
• Progress report from 2011 and 2017:
https://echa.europa.eu/documents/10162/13628/evaluation_report_en.pdf/0598c959-8fbd-40719523-5ca7151f5df5
https://echa.europa.eu/documents/10162/13628/evaluation_report_recommendations_2017_en.pd
f/c2cb9cd3-e2b5-662a-c359-c5d998444853
• Publication of decisions on dossier evaluation see link
https://echa.europa.eu/information-on-chemicals/dossier-evaluation-status
115
116. Interim Status Evaluation of Progress Report 2011
Tests carried out in 2011 for compliance with the requirementsTotal number
Number of compliance checks initiated in 2011
158
Number of compliance checks carried over from 2010
81
Total number of dossiers to be checked for compliance 2011
239
Number of complete
registration dossiers
received by end 2011:
25,378
Examination of testing proposals carried out in 2011
Phase-in
NotPhase-in
Number of examinations of testing proposals initiated in 2011
448
24
Number of examinations of testing proposals taken over from 2010
94
21
Total number of dossiers under examination for testing proposals in
2011
587
116
117. Interim Status Evaluation of Progress Report 2011
Number of completeregistration dossiers
received by the end of
2012: 28.009
117
118. First evaluation result of 06.03.2013
• The Member States have evaluated 36 substances listed in the communityrolling action plan (CoRAP) for 2012
• In four cases, the result was that the evaluating Member State concluded that
there is no need to ask for further information as the suspected concern
could already be clarified with the available information. These substances
are:
Ethylene oxide, EC 200-849-9, CAS 75-21-8 (MS Austria)
• Tributyl phosphate, EC 204-800-2, CAS 126-73-8 (MS Hungary)
• M-Tolylidene diisocyanate, EC 247-722-4, CAS 26471-62-5 (MS Poland)
• Toluene, EC 203-625-9, CAS 108-88-3 (MS Finland)
118
119. Interim status Evaluation from Progress Report 2016
Tests carried out in 2016 for compliance with the requirementsTotal number
Number of compliance checks initiated in 2016
184
Number of compliance checks adopted since 2013
164
Total number of dossiers to be checked for compliance 2016
348
Number of complete registration dossiers received by the end of 2016: 40,878
Link: https://echa.europa.eu/documents/10162/13628/evaluation_report_2016_en.pdf/f43e244f7c90-75bd-e1b2-3771bcb1f8e8
119
120. First evaluation results of February 2017
• The Member States have evaluated 182 substances listed in the communityrolling action plan (CoRAP) until 2016
• In 50 cases, the result was that the evaluating Member State concluded that
there is no need to ask for further information as the suspected concern
could already be clarified with the available information.
2 substances with drafted conclusions
• 48 substances with published conclusions
120
121. Summary
• Registration „No data no market“Dependence on quantity and risk
• Substance, preparation, article
• Isolated intermediates
• OSOR
• Registration dossier and chemical safety report
• Evaluation
Dossier evaluation by ECHA
• SSubstance evaluation by the national authorities of the Member States (CoRAP)
Need for action for manufacturers, importers, users,…
121
122. REACH Registration, Evaluation, Authorization and Restriction of Chemicals
Part: Authorization and Restriction123. Approval (authorisation)
• Completely new system in European chemicals legislation with theaim of designing the use of substances of very high concern, so
that
• the risks are adequately controlled, or
• be replaced by suitable material or technical (and economically viable)
alternatives (substitution principle)
123
124. Authorization - Scope
• The following, among others, are exempt from the authorizationrequirement:
Use in human or veterinary medicinal products [Article 2 (5) lit. a]
• Use in food or feed [Article 2 (5) lit. b]
• On-site isolated intermediates and transported isolated intermediates [Article 2 (8) lit. b]
• Other exempted uses:
Article 56 (3)
• Article 56 (4) a - d
• Article 56 (5) a - b
• Article 56 (6) a - b
R&D
Plant protection, biocides, fuels, mineral oils
cosmetic products, materials in contact with food
Substances in preparations < 0,1%-(w/w)
124
125. Substances of Very High Concern (SVHC)
Hazard characteristic according to Article 57 REACHCancerogenic (carcinogenic), mutagenic or toxic for
reproduction categories 1 and 2
Abbreviation
CMR Kat. 1
and 2
Resistant or bioaccumulative with toxic properties
PBT
Very Persistent (highly persistent) or very Bioaccumulative
(highly bioaccumulative)
vPvB
Individual cases: Substances of very high concern (e.g.
endocrine disrupters)
---
125
126. Indication for SVHC substances fulfilling the criteria according to Article 57
• SIN List from International Chemical Secretariat (ChemSec)• ChemSec = Swedish NGO (non-governmental organization)
• Contains a total of 626 potential SVHCs
• The SIN list can be found at: http://w3.chemsec.org/
• Trade Union Priority List for REACH Authorization der ETUC
• ETUC = European Trade Union Confederation
• Contains a total of 320 potential SVHCs
• TU list to be found at:
https://www.etuc.org/en/trade-union-priority-list
126
127. The authorization procedure overview
REACH Article 591
Indication for identification
as SVHC
Tasks
What: Preparation of Annex XV dossier Submission to ECHA
Who: National authorities of the Member States ECHA on behalf of
the EU Commission
2
List of admission candidates
(ECHA-Website)
Obligation
What: Information requirements up the supply chain
Who: concerned companies (Article 62(2))
REACH Article 58
Prioritisation of substances on
3
the candidate list for authorisation
4
List of Authorisations
(REACH Annex XIV)
Tasks
What: Prioritisation of substances according to specific criteria
Who: ECHA to EU Commission and national authorities of the
Member States
Obligations
What: Application for authorisation or substitution from a given
date
Who: concerned companies (Article 62(2))
127
128. 1. Indication for identification as SVHC
• Criteria referred to in Article 57 shall be metClassification and labeling in accordance with the relevant legislation
• Preparation of an Annex XV dossier by a Member State authority or by ECHA
(if mandated by the EU Commission)
• Publication of the Annex XV dossier by ECHA on its website
Opportunity for public consultation
• Published Annex XV dossiers:
https://echa.europa.eu/de/registry-of-svhc-intentions
• http://echa.europa.eu/de/proposals-to-identify-substances-of-very-high-concernprevious-consultations
128
129. 2. Inclusion in the list of candidates for authorization
• After completion of the public consultation of the Annex XV dossier,publication on the ECHA website and thus legally valid
• Need for action: Information requirements for companies that manufacture
substances on the candidate list for authorization and place them on the
market, etc.
Article 7, 31 (1) and 33
e.g. Notification for substances in articles
Summary of obligations:
https://echa.europa.eu/de/candidate-list-obligations
129
130. 3. Prioritization of substances on the candidate list for authorization
• At least every 2 years - recommendation which substances from thecandidate list for authorisation should be included in the list of substances
subject to authorisation (Annex XIV)
ECHA to Member State authorities or EU Commission
• Thereby prioritising of substances that (Art. 58 (3))
are highly dangerous to the environment (PBT and vPvB properties)
be widely used
are produced in large quantities or imported into the EU
• Current recommendations see under:
https://echa.europa.eu/de/recommendation-for-inclusion-in-the-authorisation-list
130
131. 4. Inclusion in the list of substances subject to authorization (Annex XIV)
• At the end of the public consultation on ECHA's recommendation (3 months)• Inclusion in the list of substances subject to authorization (Annex XIV)
• Need for action: Submission of an application for authorization in due time
before the expiry of the period set out in Annex XIV
Payment of a fee to ECHA according to REACH Fee Regulation
131
132. Extract from the list of admission candidates
Source: https://echa.europa.eu/de/candidate-list-table132
133. Extract from the list of admission candidates
2-(2H-benzotriazol-2-yl)-4,6-ditertpentylphenol (UV-328) - PBT;
2-(2H-benzotriazol-2-yl)-4-(tert-butyl)-6-(sec-butyl)phenol (UV-350), PBT;
2 4-di-tert-butyl-6-(5-chlorobenzotriazol-2-yl)phenol (UV-327), PBT;
2-benzotriazol-2-yl-4,6-di-tert-butylphenol (UV-320), PBT;
ammonium pentadecalfluorootanoate (APFO), - CMR) and PBT;
pentadecafluorooctanoic acid (PFOA) - CMR) and PBT;
4-nonlyphenol, branched and linear, ethoxylated - endocrine disrupting substance (of equivalent concern)
cadmium - CMR, equally concerning
cadmium oxide - CMR, equally concerning
di-n-pentyl phthalat - CMR
Possibility to submit comments:
https://echa.europa.eu/de/substances-of-very-high-concern-identification
133
134. Excerpt from the list of substances subject to authorization (Annex XIV)
Source: https://echa.europa.eu/de/authorisation-list134
135. Previous admissions - examples
List of authorization candidates• https://echa.europa.eu/de/candidate-list-table
List of substances subject to authorization - Annex XIV
• https://echa.europa.eu/de/authorisation-list
135
136. Roadmap on SVHC of the EU Commission
• 138 substances on the candidate list for authorization by end 2012Objective: By 2020 all SVCH's on the list of authorization candidates
All substances meeting the criteria of Article 57 (approximately 440 substances estimated)
CMR , PBT, vPvB, endocrine disruptive substances, etc.
All registered substances according to Article 5 shall be recorded.
Intermediate products possibly after 2020, as exempted from authorization according to
Art 2(8)
Definition of responsibilities and obligations
improving planning, predictability and communication
TOOL for systematic recording: Risk Management Option Analysis (RMO)
136
137. Application for authorization
Guidance on the preparation of an application for authorization• https://echa.europa.eu/documents/10162/13643/authorisation_ap
plication_en.pdf/8f8fdb30-707b-4b2f-946f-f4405c64cdc7
137
138. Restriction Background
• Enables the introduction of additional EU-wide risk reductionmeasures where necessary
• Not to be confused with authorization, but an additional safety
net
• Valid for any substance on its own, in preparation, or in an article
• Valid for manufacturers, distributors, and users
138
139. Restriction Delimitation and scope
• Restriction not valid for dedicated exemptions from REACH according toArticle 2 (1) to (4)
• Explicit exemption for substances in cosmetic products according to Art. 67
(2) or in Annex XVII
• Starting point for restrictions in REACH is the existing EU Restriction Directive
(76/769/EEC)
• legal and valid from 01.06.2009 in REACH
• If, in addition, national restrictions exist, these may remain in place until
01.06.2013
• Admission of new restrictions Restriction procedure
139
140. Restriction procedure (Articles 68-73) Overview
140141. Actors in the restriction procedure
• Submitter (Member State or ECHA)• Interested parties (citizens, organizations, companies etc.)
• ECHA
Secretariat
• Committees and Forum
RAC (Committee for Risk Assessment)
• SEAC (Committee for Socio-Economic Analysis)
• „Forum“ (The Forum for Exchange of Information on Enforcement )
• EU Commission
• Industry
• National authorities of the Member States
141
142. Restriction procedure - steps
• Announcement of intention to make a proposal to restrict a chemical• Transmission of the proposal
• Checking the conformity of the proposal
• Public consultation (6 months)
• Consultation on enforceability through the Forum
• Statement of the Committee for Socio-economic Analysis
• Public consultation on the opinion of the Committee for Socio-economic Analysis (2
months)
• Statement of the Committee for Socio-economic Analysis
• Draft amendment of Annex XVII by the Commission
• EU-Decision
142
143. Example Restriction (Annex XVII)
The following phthalates (or otherCAS and EC numbers identifying
the substance)
a) Diisononyl Phthalate (DINP)
CAS-Nr. 28553-12-0 and
68515-48-0
EG-Nr. 249-079-5 and 271090-9
b) Diisodecyl Phthalate (DIDP)
CAS-Nr. 26761-10-0 and
68515-49-1
EG-Nr. 247-977-1 and 271091-4
c) Dinoctyl phthalate (DNOP)
CAS-Nr. 117-84-0
EG-Nr. 204-214-7
1. Shall not be used as substances or in preparations in
concentrations greater than 0,1 % by weight of the
plasticised material in toys and childcare articles
which can be placed in the mouth by children
2. Toys and childcare articles containing these
phthalates in concentrations exceeding 0,1 % by
weight of the plasticised material shall not be placed
on the market
3. The Commission shall, by 16 January 2010, reevaluate the measures provided for in relation to that
entry in the light of new scientific information on
those substances and their substitutes and, if
necessary, amend the measures accordingly
4. For the purposes of this entry, 'childcare article'
means any product intended to facilitate sleep,
relaxation, hygiene, feeding and sucking of children
Green handle,
orange key (if 5cm
in one dimension)
The main body and
wheels (if 5 cm in all
dimesnions)
External parts if 5
cm in one
dimension
External parts if 5
cm in one
dimension
Edges can be taken
into the mouth
143
144. Ongoing restriction procedures
Source: https://echa.europa.eu/de/restrictions-under-consideration144
145. Latest news on restrictions
ECHA• https://echa.europa.eu/de/substances-restricted-under-reach
Website of the EU Commission on restrictions
• https://ec.europa.eu/growth/sectors/chemicals/reach/restrictions_en
145
146. REACH Registration, Evaluation, Authorization and Restriction of Chemicals
Part: Safety Data Sheet147. Sharing information in the supply chain
User 3User 3 is the downstream
actor of user 2
Upwards
User 2
User 1
Manufacturer is the
upstream actor of user 1
Downwards
Manufacturer
Supply chain direction
Supply chain actors
147
148. Obligation to prepare a safety data sheet Article 31 (1)
A safety data sheet in accordance with Annex II shall be prepared when• a substance (and since 1. June 2015 a preparation) meets the criteria for
classification as hazardous under the CLP Regulation;
a preparation meets the criteria for classification as dangerous according to the
Dangerous Preparations Directive 1999/45/EC ("DPD") (until 1 June 2015);
• a substance is persistent, bioaccumulative and toxic (PBT) or very persistent
and very bioaccumulative (vPvB) according to the criteria in Annex XIII of
REACH; or
• a substance has been included for other reasons in the candidate list of
substances for authorisation under Article 59(1) of REACH.
148
149. Requirements for safety data sheets
• Must correspond to a predefined layout (Art. 31 (6))• If required to prepare a Chemical Safety Report, the exposure
scenarios for the identified uses shall be annexed to the Safety Data
Sheet (Art. 31 (7))
• Must be provided on paper or electronically free of charge (Art. 31
(8))
• To be updated on relevant occasions (see Art. 31 (9)) and made
available to all former customers within the last 12 months
149
150. Information requirements for substances and preparations for which a safety data sheet is not required [Article 32 (1)].
• the registration number(s) referred to in Article 20(3), if available, forsubstances for which information on:
• a possible authorization requirement and details of authorizations
granted or refused in this supply chain
• details of restrictions
• any other available and relevant information on the substance that is
necessary to identify and apply appropriate risk management
measures, including the specific conditions resulting from the
application of the substance-specific exposure-based assessment
(Section 3 of Annex XI)
150
151. Duty to communicate information on substances in articles (Art. 33)
• If a substance is included on the candidate list for authorization and iscontained in the article in a concentration of more than 0.1 mass
percent (w/w) at least the name of the substance shall be passed on
to the recipients of the article by the supplier; in addition, the
information available to him and sufficient for safe use of the article
shall be made available
• For end users, this information shall be provided free of charge on
request
• Transmission of information within 45 days
151
152. Access to the information in the safety data sheet
• The employer shall grant employees and their representativesaccess to the information referred to in Articles 31 and 32 (Art. 35)
• The information shall be kept for at least 10 years ((calculated
from the last manufacture, import, supply or use of a substance or
mixture (Art. 36 (1))
• legal retention period usually 7 years!
152
153. Obligation to transmit information down the supply chain (Art. 34)
Each actor in the supply chain of a substance or preparation shall provide thefollowing information to the actor or distributor immediately upstream in the
supply chain:
• new information on hazardous properties
irrespective of the uses concerned
• Information that may call into question the suitability of the risk
management measures specified in the safety data sheet
for identified uses
Distributors shall forward this information to the actor or distributor
immediately upstream in the supply chain
153
154. Content of the safety data sheet according to Art. 31(6)
1.2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
identification of the substance/preparation and of the company/undertaking;
hazards identification;
composition/information on ingredients;
first-aid measures;
fire-fighting measures;
accidental release measures;
handling and storage;
exposure controls/personal protection;
physical and chemical properties;
stability and reactivity;
toxicological information;
ecological information;
disposal considerations;
transport information;
regulatory information;
other information.
154
155. Example: Safety Data Sheet
Source: https://www.moellerchemie.com/fileadmin/media/pdf/security_certificates/5513.pdf155
156.
156157. Example: Safety Data Sheet
157158. Example: Safety Data Sheet
158159. Example: Safety Data Sheet
159160. Example: Safety Data Sheet
160161. Example: Safety Data Sheet
161162. Example: Safety Data Sheet
162163. Example: Safety Data Sheet
163164. Example: Safety Data Sheet
Quelle: https://www.carlroth.com/downloads/sdb/de/X/SDB_X863_AT_DE.pdf164
165. Example: Safety Data Sheet
165166. Example: Safety Data Sheet
166167. Example: Safety Data Sheet
167168. Example: Safety Data Sheet
168169. Example: Safety Data Sheet
169170. Example: Safety Data Sheet
170171. Example: Safety Data Sheet
171172. Example: Safety Data Sheet
172173. Example: Safety Data Sheet
173174. Example: Safety Data Sheet
174175. Example: Safety Data Sheet
175176. Example: Safety Data Sheet
176177. Further information
REACH Regulation• Article 31 to 36
• Annex II
Guidance on the preparation of safety data sheets:
• https://echa.europa.eu/de/guidance-documents/guidance-on-reach
177
178. REACH Registration, Evaluation, Authorization and Restriction of Chemicals
Part: Related Regulations179. Overview of related regulations
• Fees Regulation (EC) No 340/2008• Test Methods Regulation (EC) No 440/2008
• Regulation on the Board of Appeal (EC) No 771/2008
• Directive on the protection of animals used for scientific purposes
• Regulation on classification, labelling and packaging of substances
and preparations (CLP Regulation)
• Biocidal Products Directive (BPR)
• Regulation on Prior Informed Consent (PIC Regulation)
179
180. Regulation related to REACH
• Fees Regulation (EC) No 340/2008• Setting of fees for registration, authorisation, oppositions, etc.
• Test Methods Regulation (EC) No 440/2008
• Specification of test methods for registration, evaluation, authorisation and
restriction of chemicals under REACH.
• Regulation on the Board of Appeal (EC) No 771/2008
• Laying down rules on the organisation and procedures of the Board of
Appeal of the European Chemicals Agency.
180
181. Directive 2010/63/EU on the protection of animals used for scientific purposes
• Principally: Execution of the necessary tests in accordance with theabove-mentioned guideline for REACH required
• Specified are: Requirements concerning the care of laboratory
animals
• Scientifically satisfactory methods or experimental strategies using
live animals should not be used if the results can be obtained by any
other scientifically satisfactory method
• Requirements see test methods according to Regulation (EC) No 440/2008
181
182. Methods for obtaining information on substances
• In-vivo studies (using live animals)• Ex-vivo studies (e.g. using tissues from animals)
• In-vitro studies (e.g. using bacteria or cultured cells)
• Information on human exposure
• Predictions based on information available from structurally similar
substances (i.e. by "analogues" and "chemical categories").
• Predictions from valid predictive calculation methods, e.g.
(quantitative) structure-activity relationships ((Q)SAR).
182
183. Regulation on classification, labelling and packaging of substances and preparations
Entry into force of the CLP Regulation in January 2009• Based on the globally applicable GHS system
Objective:
• provide information on the risks of chemical substances and preparations for
workers and consumers
Regulation of classification and labelling
• Identification of risks to human health and the environment before placing
on the market (classification corresponding to the identified risks)
• Further information e.g. https://echa.europa.eu/de/regulations/clp/legislation
183
184. Regulation on classification, labelling and packaging of substances and preparations (CLP-VO)
• Communication of the hazardous properties of chemicals bymeans of standard information and pictograms on identification
labels and in safety data sheets
• Over time, the CLP Regulation replaces two previous regulations
• The Dangerous Substances Directive (67/548/EEC)
• The Dangerous Preparations Directive (1999/45/EC)
• There was a transitional period until 2017
184
185. Regulation on classification, labelling and packaging of substances and preparations (CLP-VO)
185186. Regulation on classification, labelling and packaging of substances and preparations (CLP-VO)
CLP Quiz: https://echa.europa.eu/de/regulations/clp/clp-quiz186
187. Biocidal Products Directive (BPD) Regulation (EU) No. 528/2012
• Regulation of the placing on the market and use of biocidalproducts which, due to the activity of the active substances they
contain, are used to protect humans, animals, materials or
products against harmful organisms
• Objective of the Regulation
• Improving the functioning of the biocidal products market in the EU
• To ensure a high level of protection of human and animal health and the
environment
187
188. Biocidal Products Directive (BPD) Regulation (EU) No. 528/2012
• Annex V of the BPD classifies biocidal products into 22 biocidalproduct types, which in turn are grouped into four main groups
• Main group 1: Disinfectants
• Main group 2: Protective agents
• Main group 3: Pesticides
• Main group 4: other biocidal products
• Biocidal products used as protective agents for food and feed are
excluded from the scope
188
189. Example
Source: https://echa.europa.eu/regulations/biocidal-products-regulation/product-types189
190. Regulation (EU) No 649/2012 on prior informed consent (PIC Regulation)
• Regulates the export of certain dangerous chemicals• Obligations for companies wishing to export these chemicals to countries
outside the EU
• The objectives are
• Promoting shared responsibility and cooperation in international trade of
hazardous chemicals
• To protect human health and the environment by providing developing
countries with information on how to store, transport, use and dispose of
dangerous chemicals safely
190
191. Regulation (EU) No 649/2012 on prior informed consent (PIC Regulation)
• Applies to chemicals banned or severely restricted within theCommunity, such as industrial chemicals and pesticides, e.g.
• Benzene
• Chloroform
• Nicotine (Pesticide)
• The export of chemicals listed in Annex 1, Parts 2 and 3 of the PIC
Regulation is not only subject to notification but also requires a
valid explicit consent from the national authority of the importing
country outside the EU
191
192. Example Nanotechnology
Flocculants in wastewater treatment192
193. Nanomaterials
• Rapid growth of nanotechnology in areas such as• healthcare, consumer goods such as cosmetics, electronics, energy technologies,
food, environmental technology and agriculture
• Specific physicochemical properties that may differ from those of the
corresponding substance in larger particles
• Dimensions from about 1 to 100 nm
higher risks?
• Although there are no explicit requirements for nanomaterials in
REACH or CLP, they meet the definitions of substances in these
Regulations and therefore the relevant provisions apply!
• Further information: https://echa.europa.eu/regulations/nanomaterials
193
194. Example of a background paper on nanomaterials and REACH
• Paper of the German federal authorities BAuA, BfR and UBA• Content is how they believe nanomaterials should be considered under
REACH
• The paper also identifies areas where regulation beyond REACH is seen as
necessary. As an example, the BAuA cites processes on its website in which
respirable dust or fibers are released
• The paper can be found at: https://mobil.bfr.bund.de/cm/343/nanomaterialienund-reach.pdf
194
195. REACH Registration, Evaluation, Authorization and Restriction of Chemicals
Part: Summary Block 1196. Scope and objectives
• Deficits of old legislation - new gaps closed by REACH• Precautionary principle
• Exemptions from REACH
Total exception
Partly exempted
Waste, radioactive materials, materials for military purposes, transport of dangerous goods, customs
Human or veterinary medicinal products, cosmetic products, medical products, food or feed
Special rules / simplifications
Recycling and reimportation
Isolated intermediates
Polymers
Substances with low hazard potential (Annex IV and V)
196
197. Definitions
Definitions Substance / Preparation / Article:
sulphuric acid, liquid household cleaner, metal pipe, car tyres steel slab, colour pigment, graphite
Central term: substance
Definition
Phase-in substance
Group of substances and distinguishing characteristics
Substance identification
Preparation, Article
• Intermediate
• supply chain actors
• Manufacturer, importer, downstream user
• Agency for REACH
197
198. Registration and Evaluation
• „No data no market“• Transitional periods for registration
• First manufacture or import into the EU after REACH enters into force
• Registration of intermediates
• Strictly controlled conditions
• Update of registrations
• Substance evaluation and dossier evaluation
• Ongoing Action Plan of the European Communities (CoRAP)
198
199. Authorisation (Approval) and restriction
• Objective and delimitation (exceptions)• Difference between authorisation and restriction
• SVHC – What is it?
• Individual steps in the authorisation procedure
• Roadmap on SVHC
• Obligations resulting from "SVHC status“
• Individual steps in the restriction procedure
199
200. Safety Data Sheet (SDS)
• Prerequisite for creation• Requirements for safety data sheets
• 16 chapters
• Exposure Scenarios (eSDB)
• Information requirements if no SDS is required
• Substances, Preparation, Article
• Information requirements
• Employers, actors in the supply chain
200
201. Safety Data Sheet (SDS)
• Fees Regulation (EC) No 340/2008• Test methods Regulation (EC) No 440/2008
• Regulation on the Board of Appeal (EC) No 771/2008
• Directive on the protection of animals used for scientific purposes
• Regulation on classification, labelling and packaging of
substances and mixtures (CLP Regulation)
• Biocidal Products Directive (BPD)
• Regulation on Prior Informed Consent (PIC Regulation)
• Nanomaterials
201

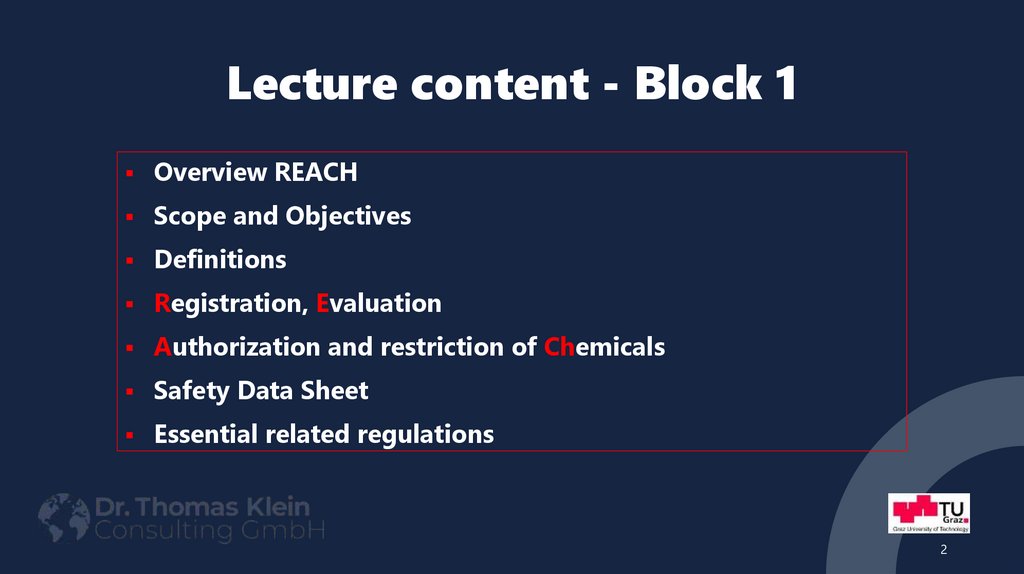




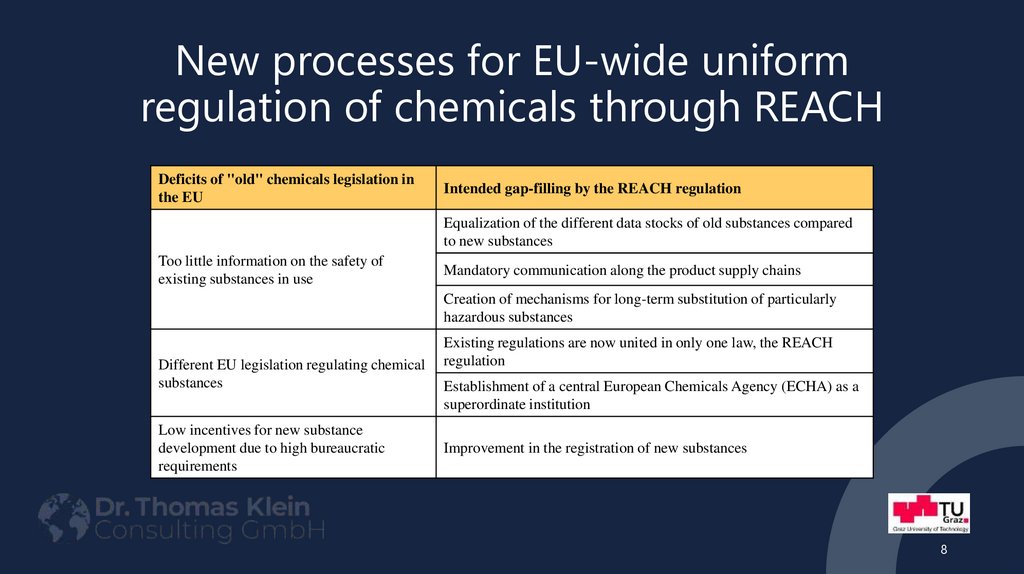




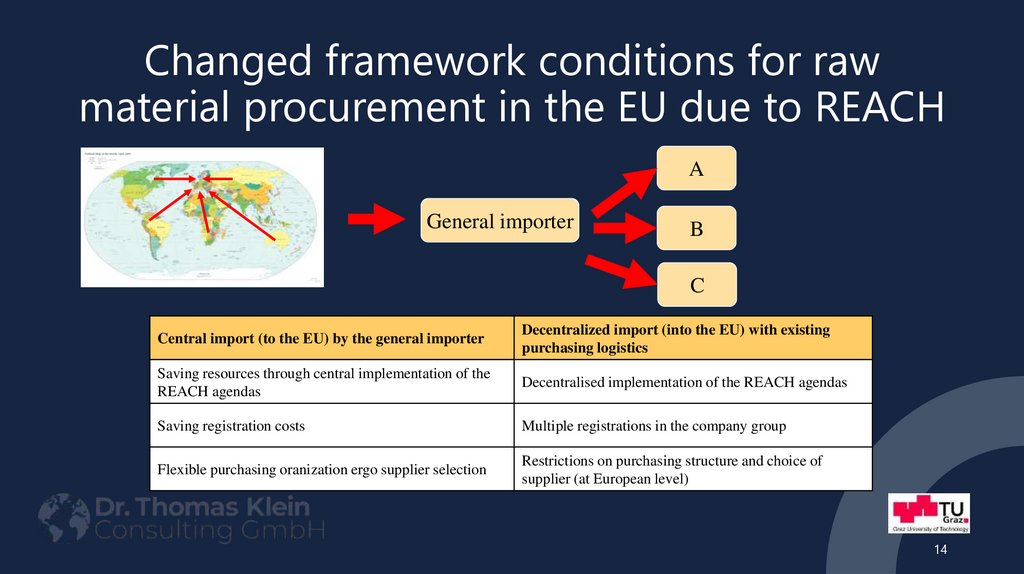

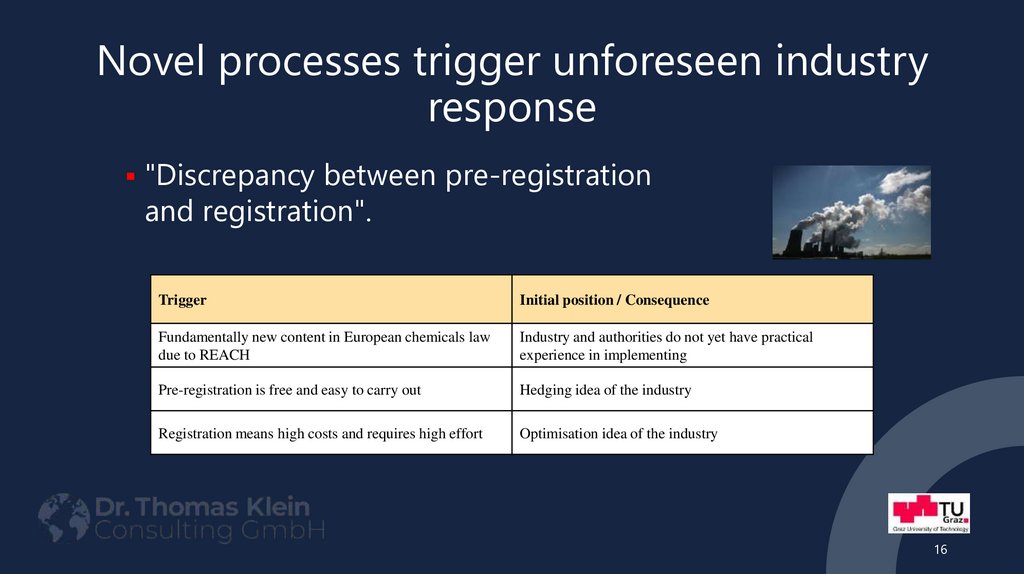
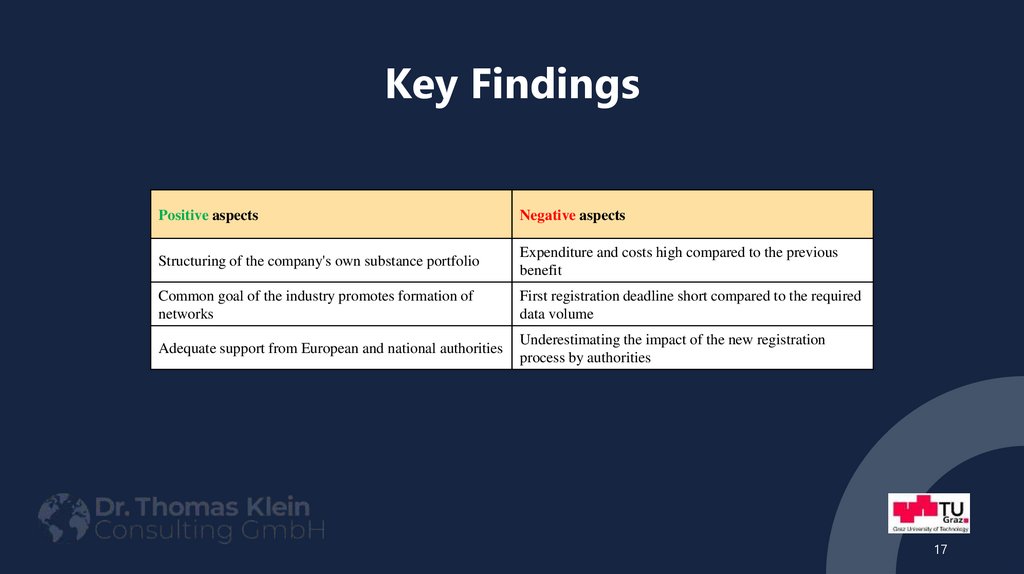

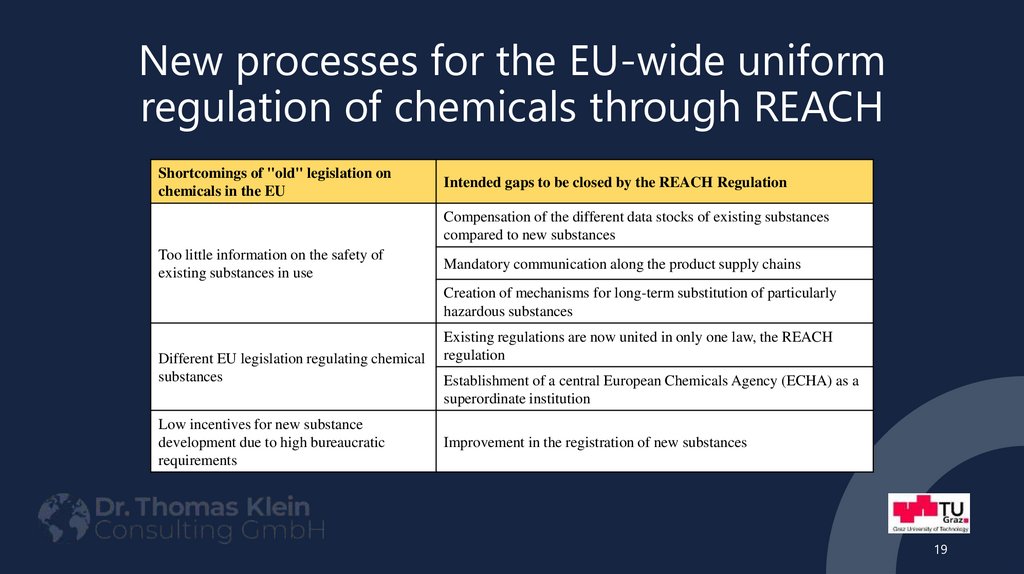










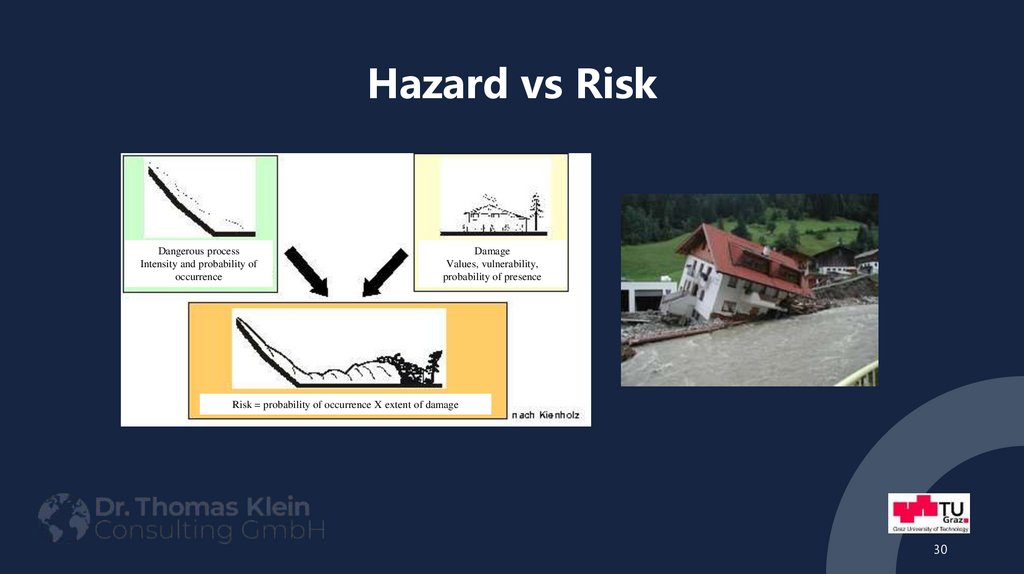















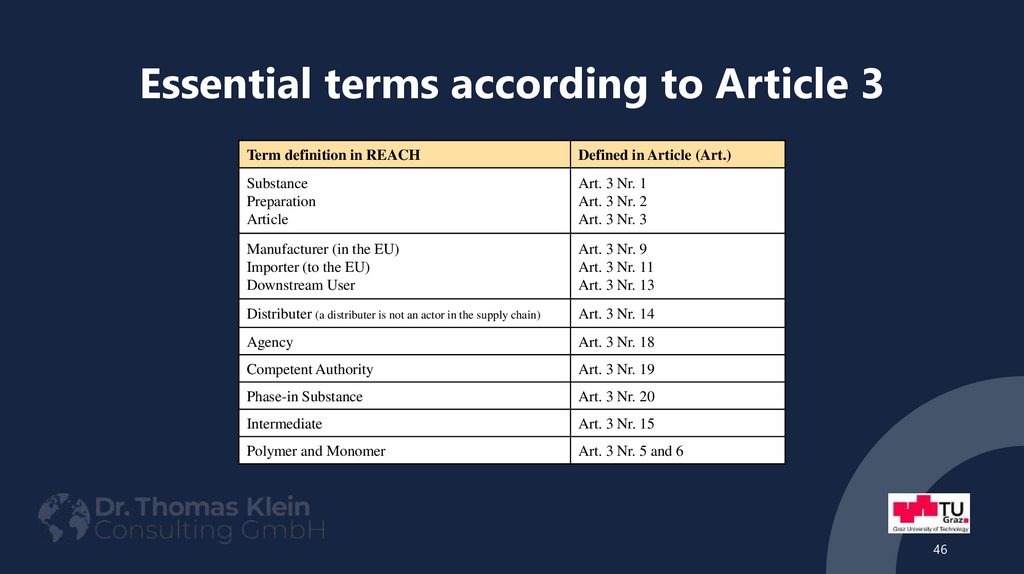

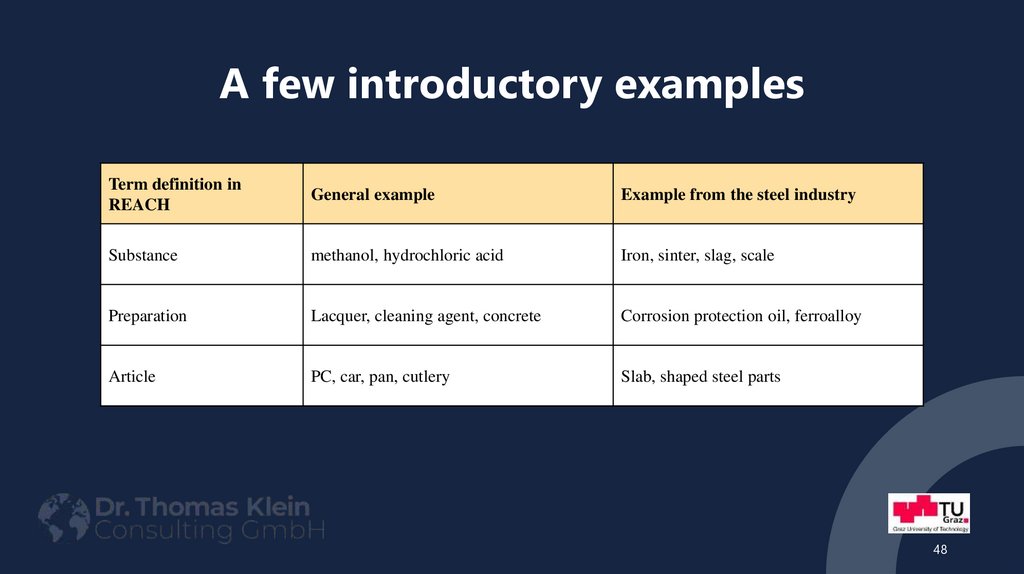






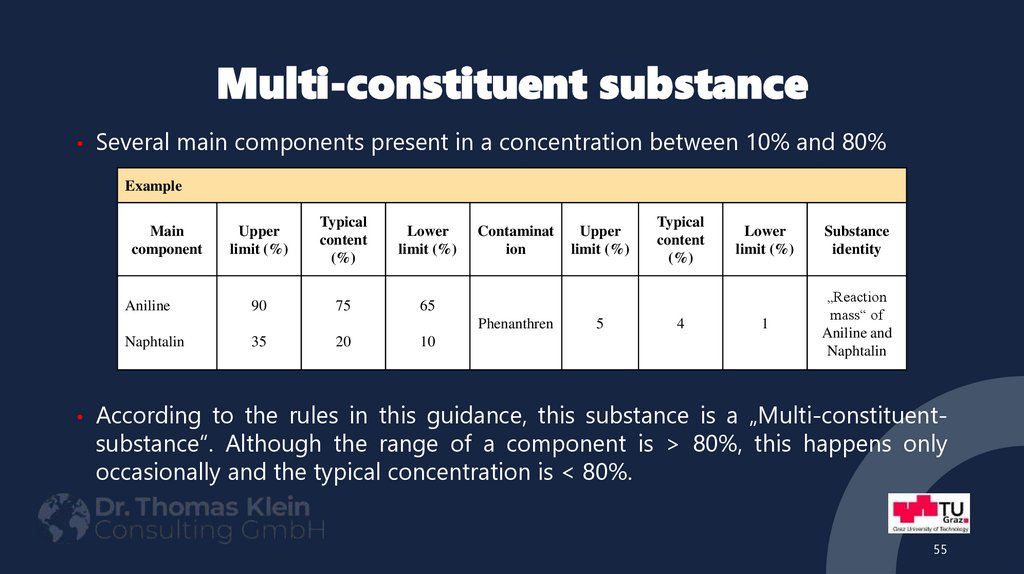


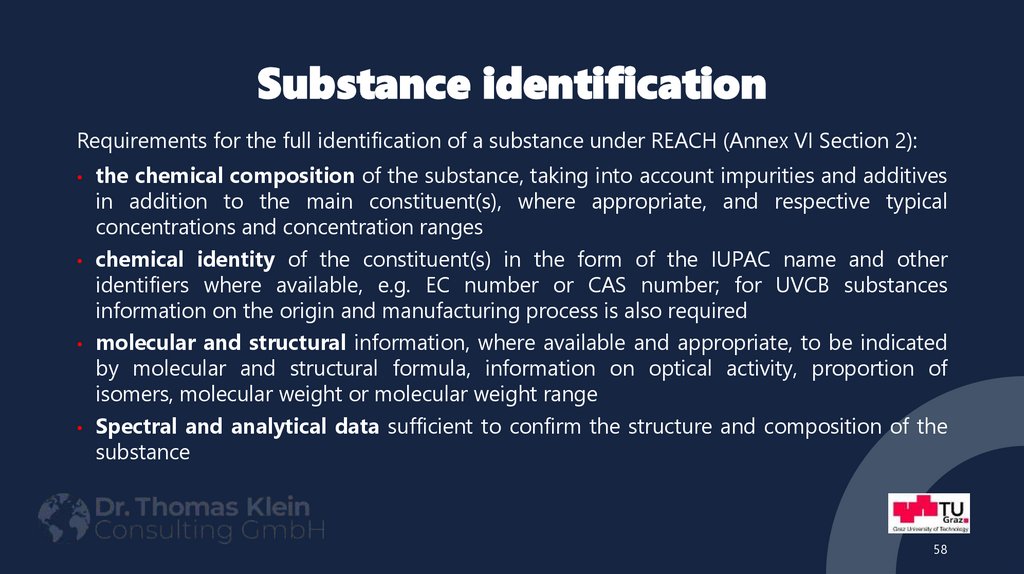

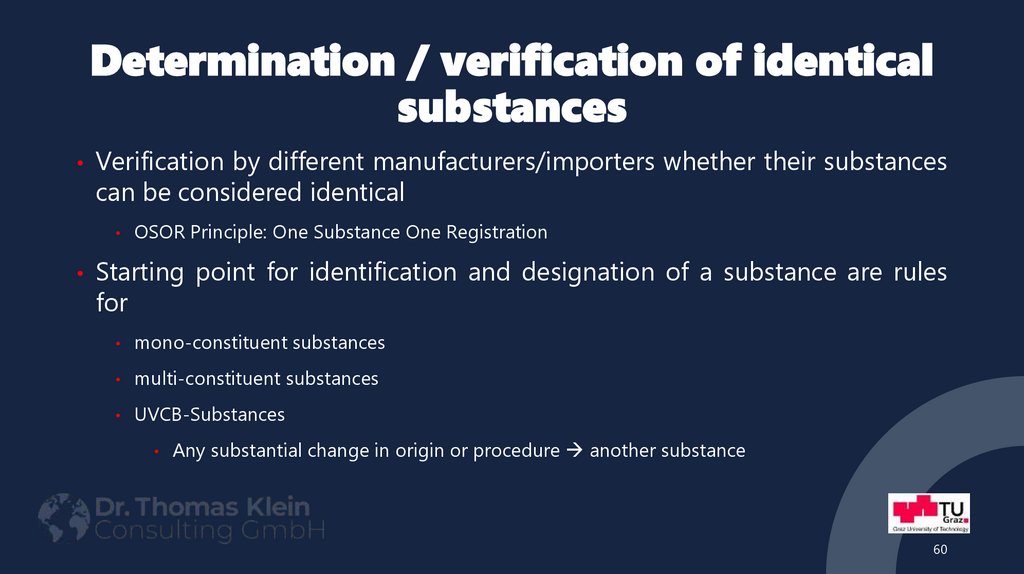




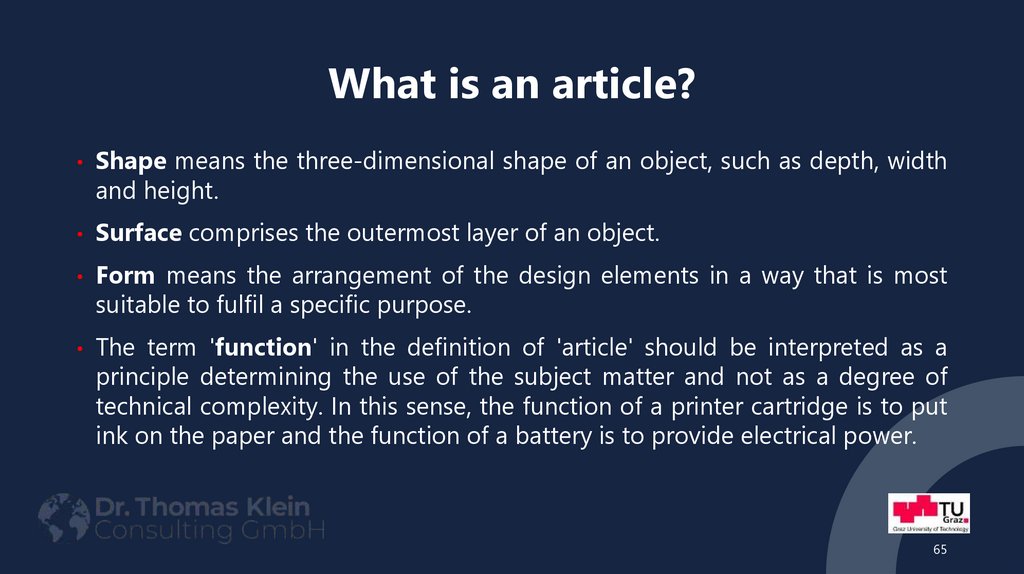

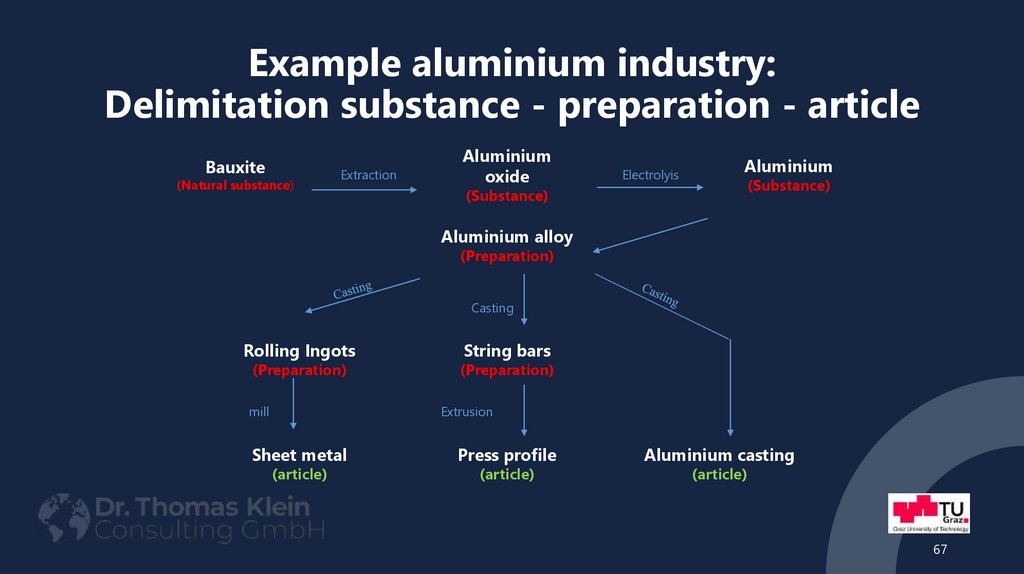
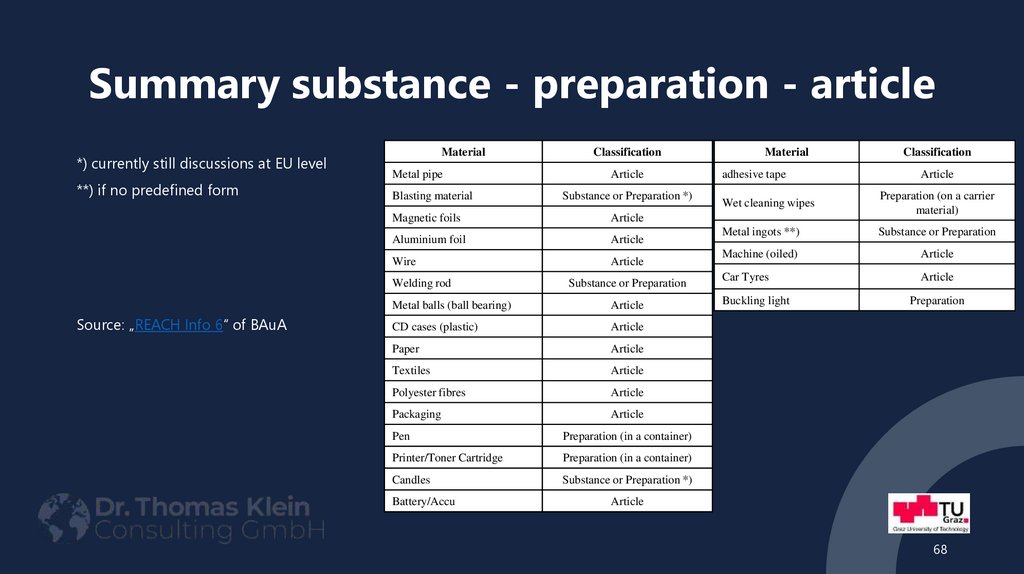
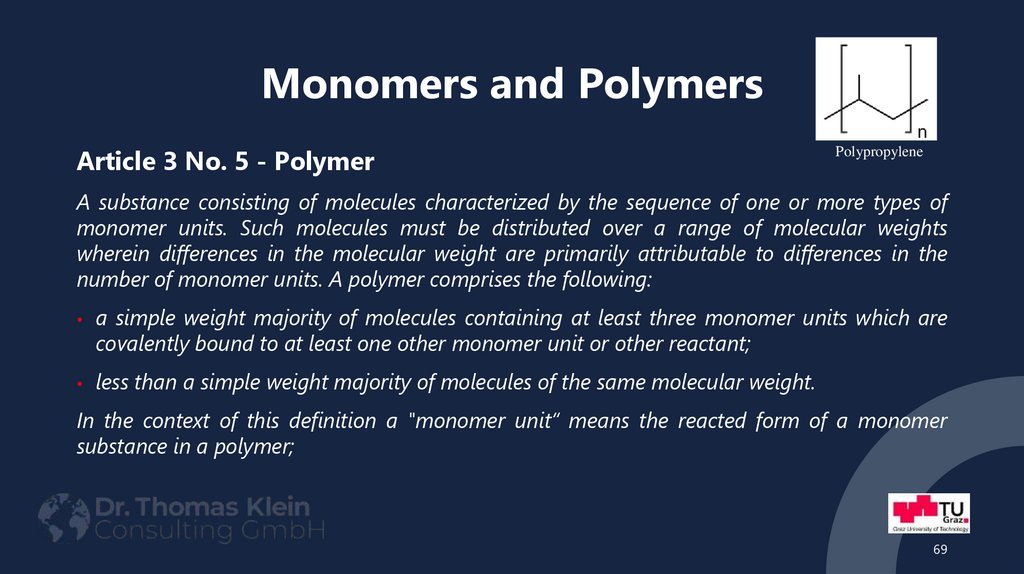

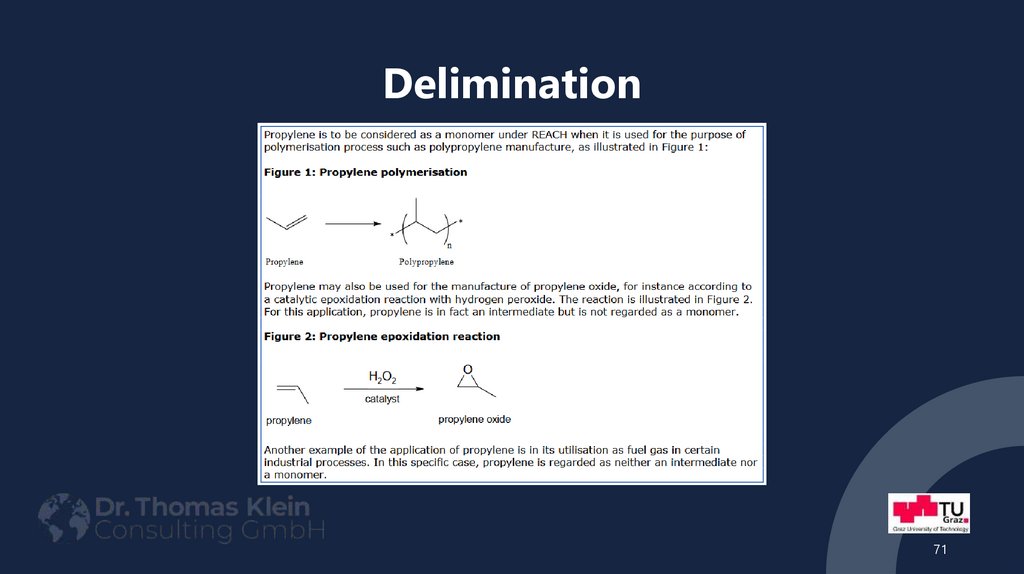


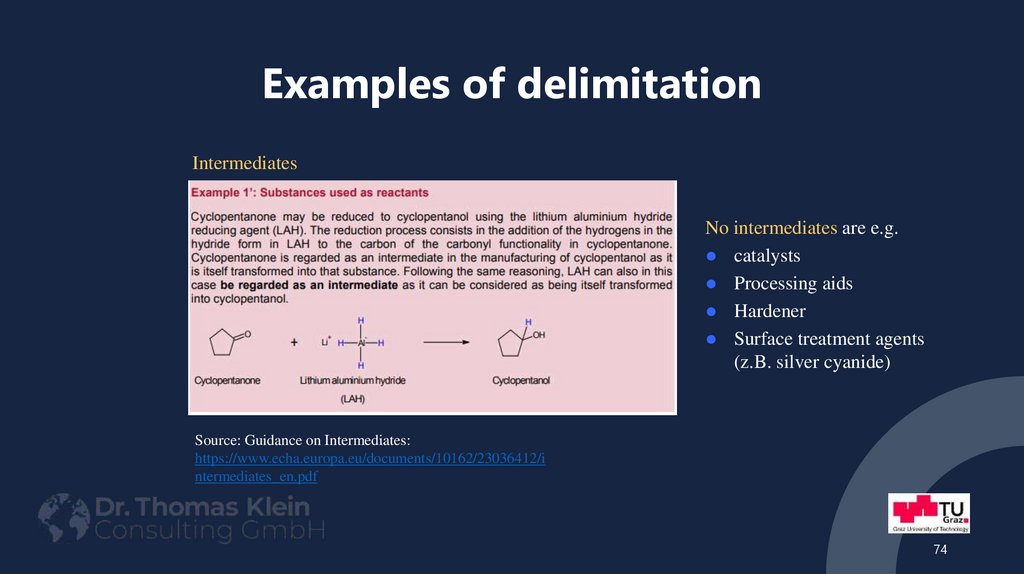




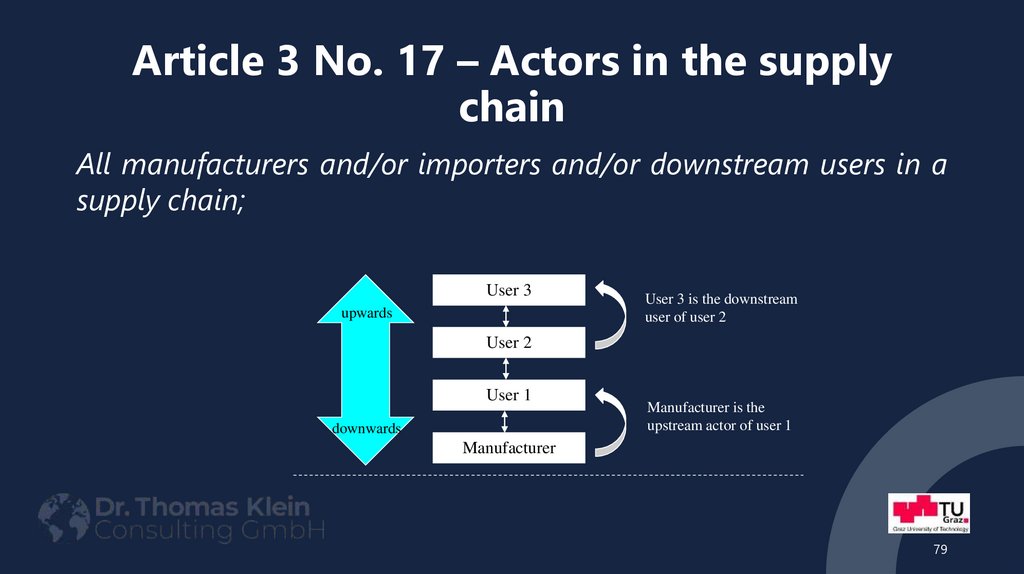












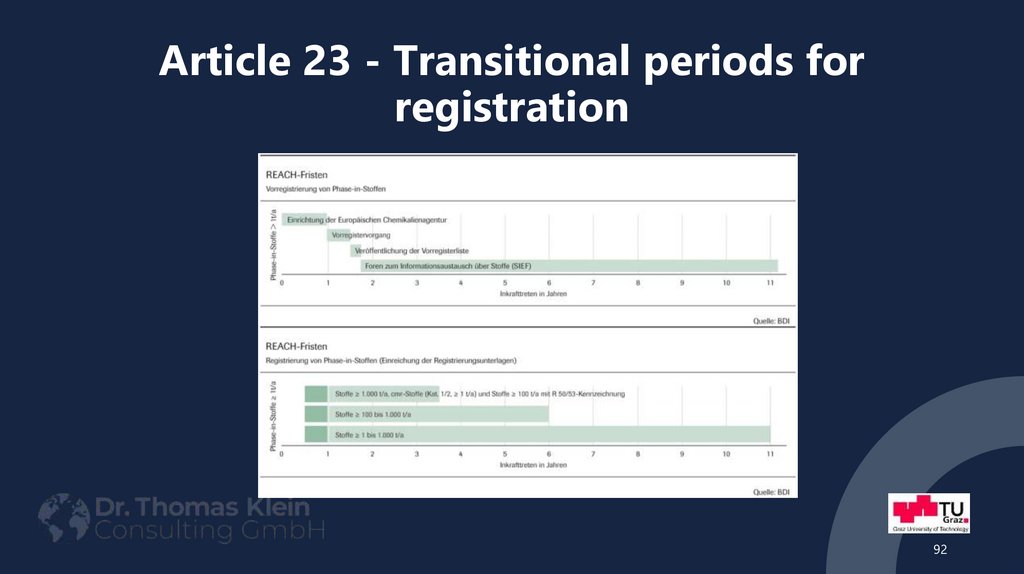


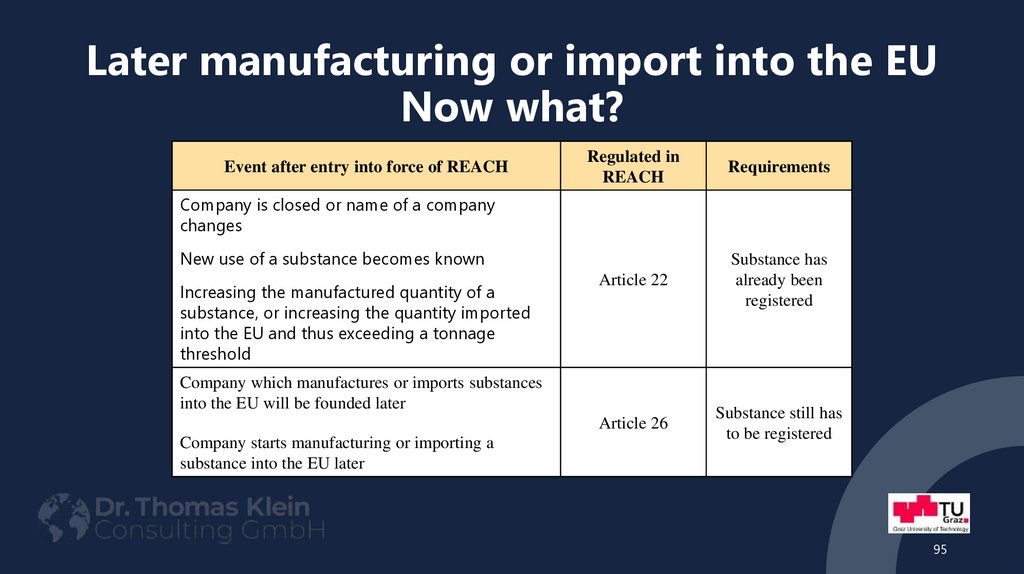










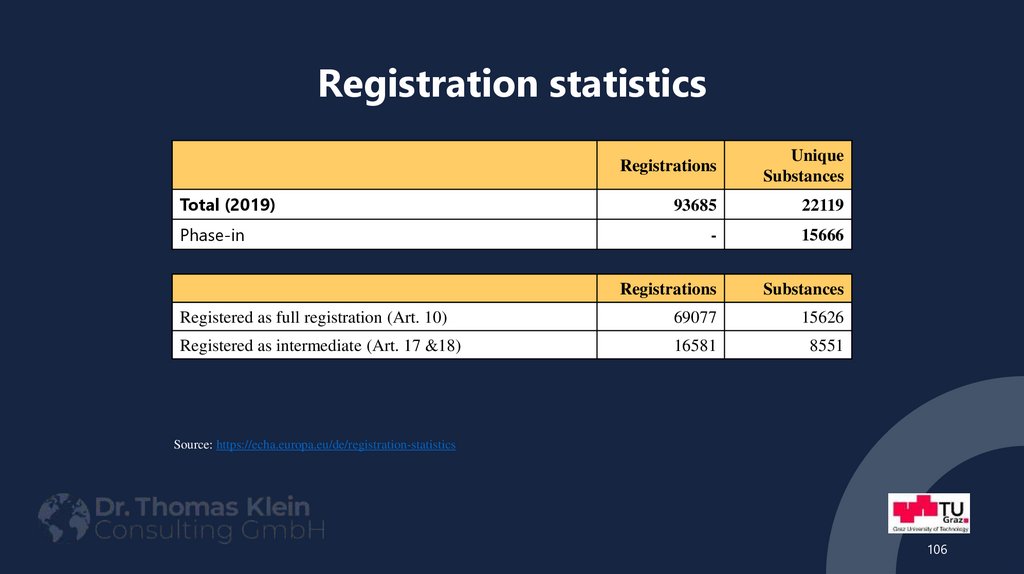


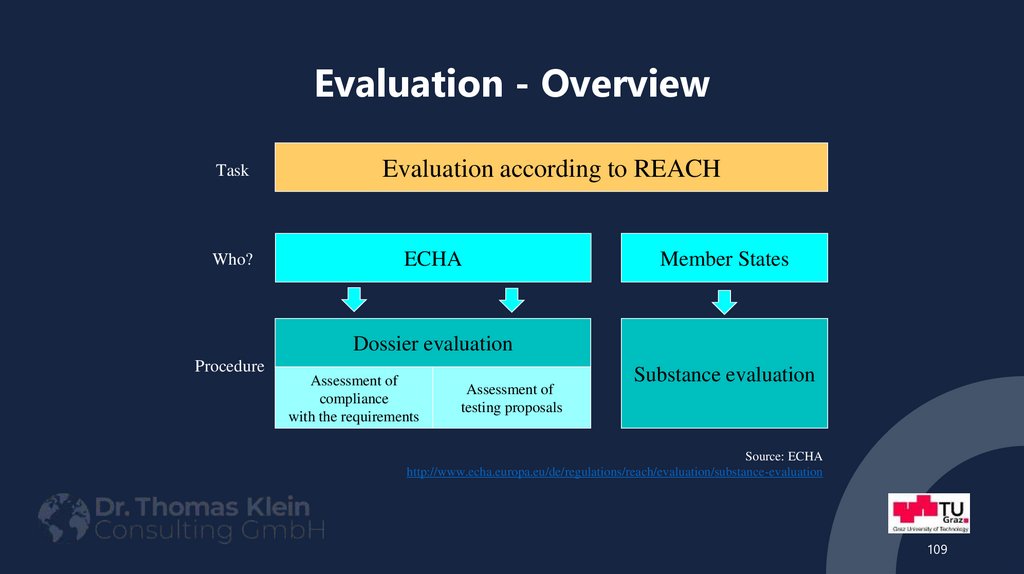


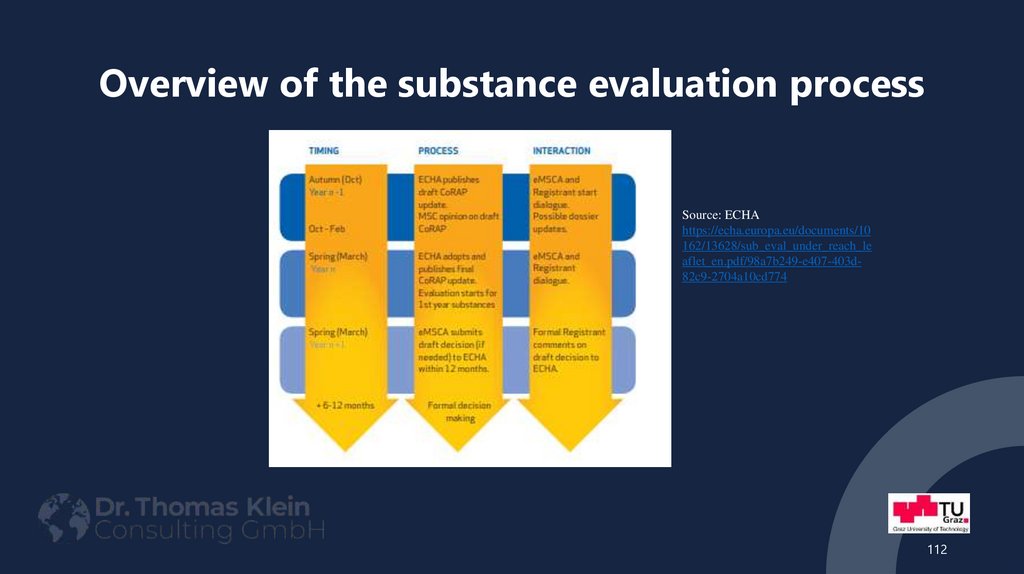



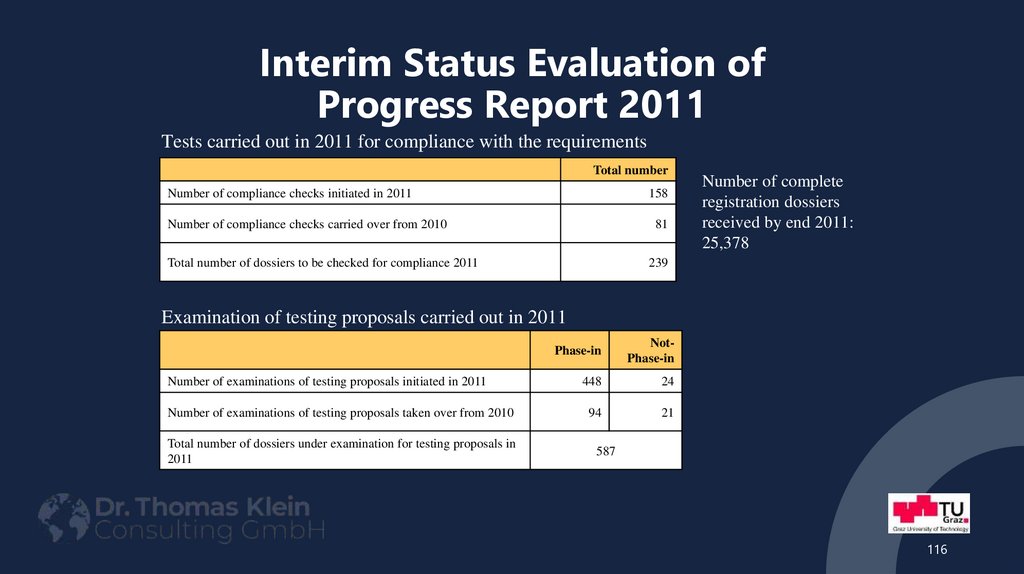


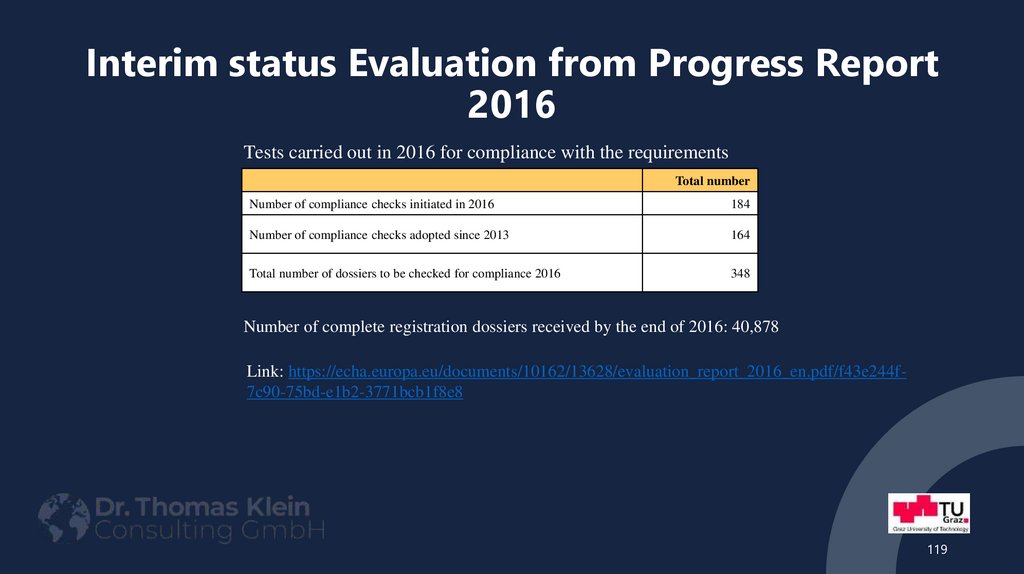




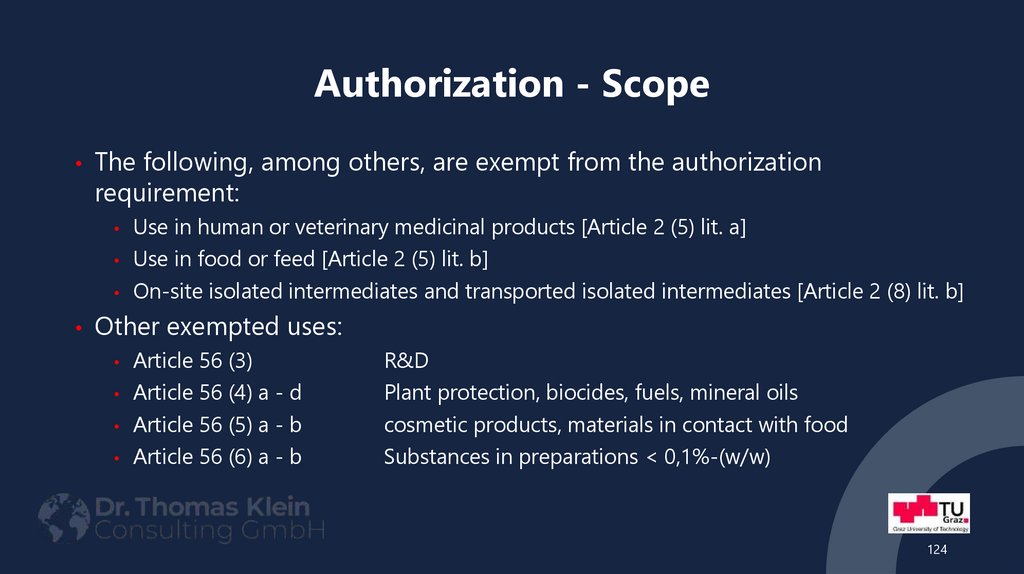
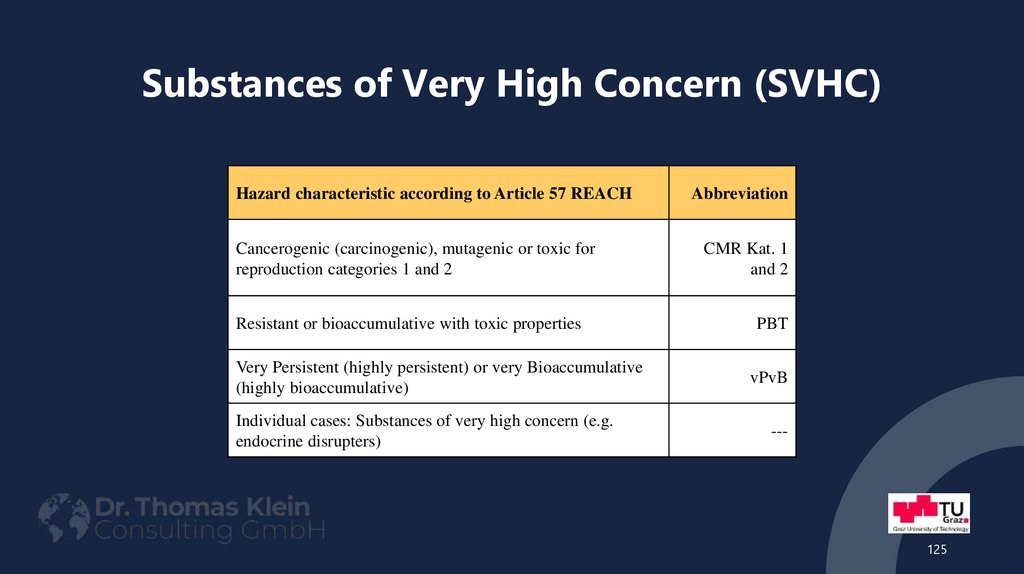

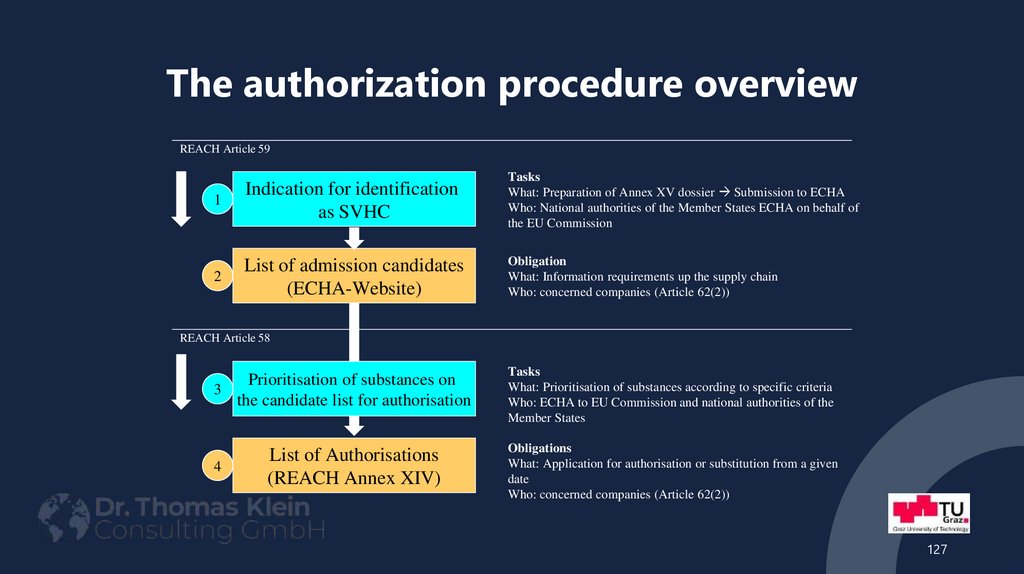




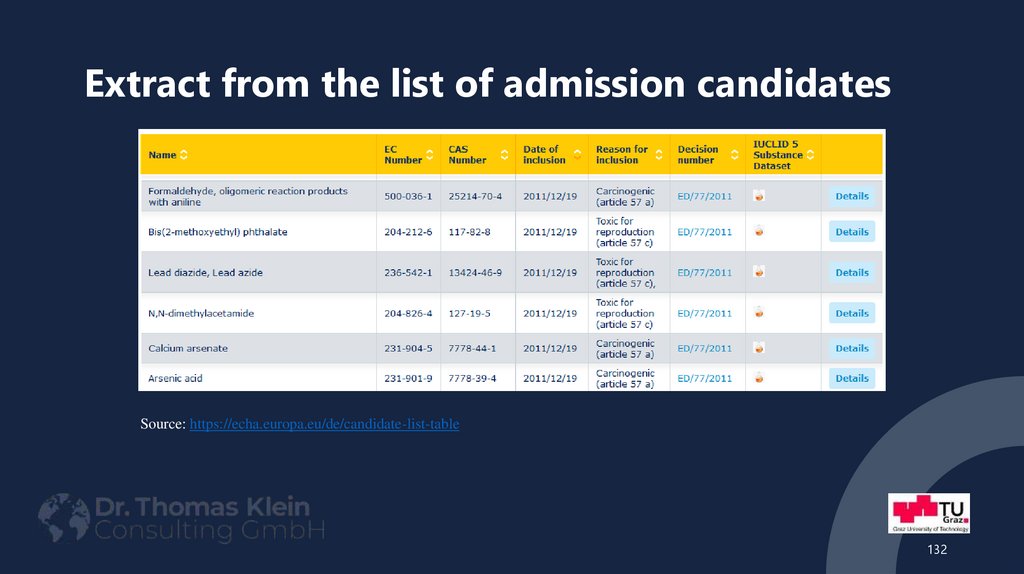







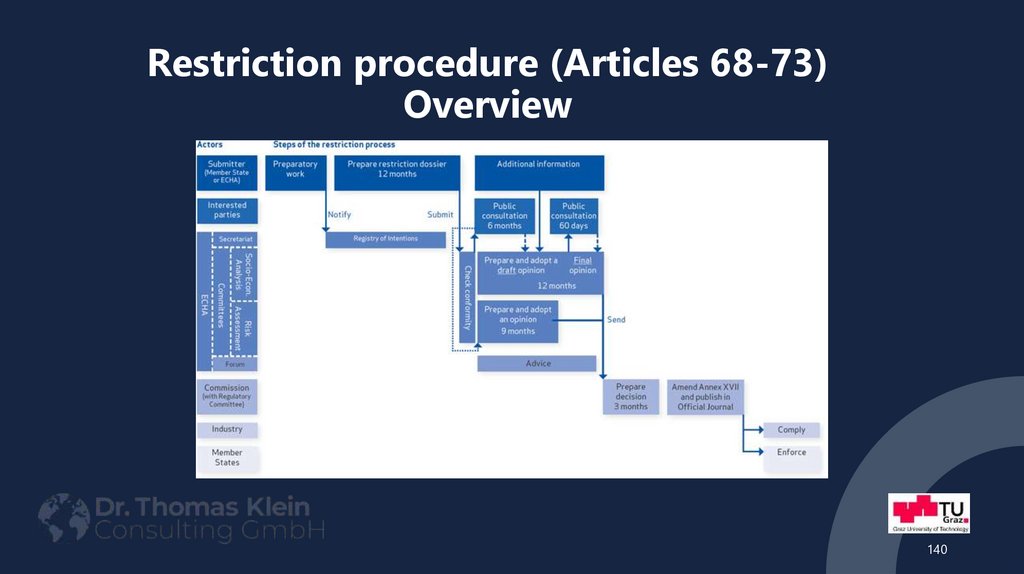


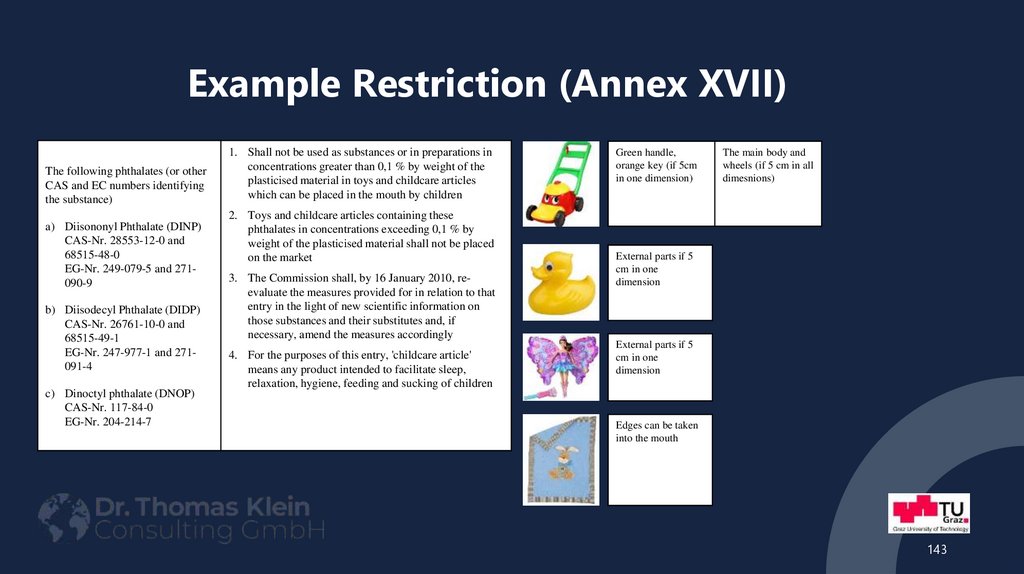
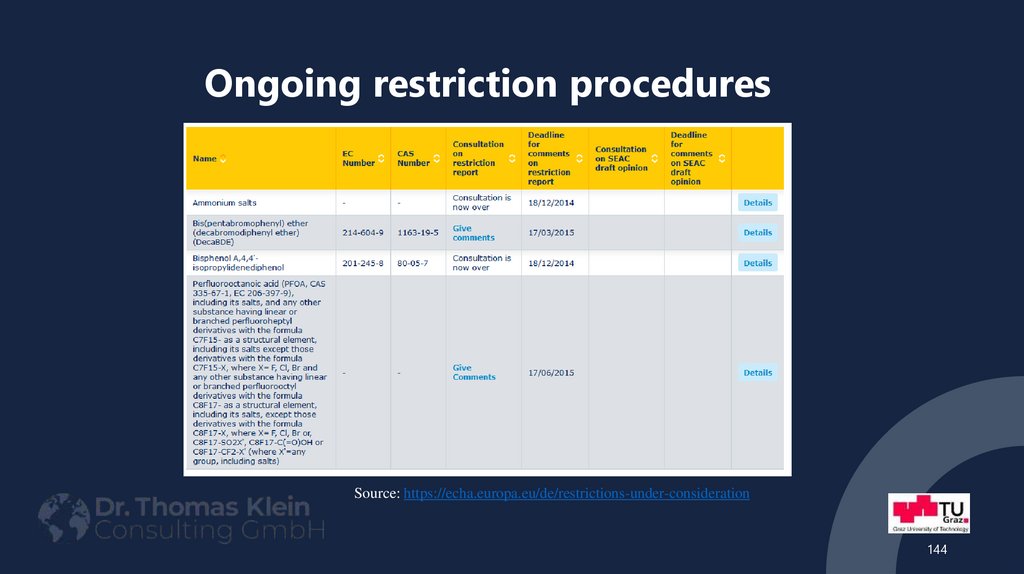


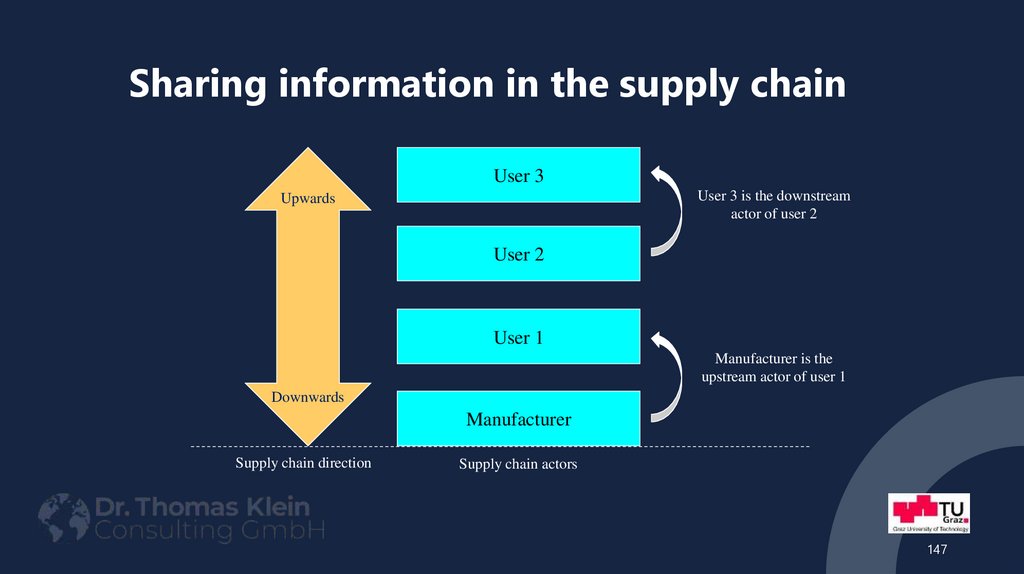


![Information requirements for substances and preparations for which a safety data sheet is not required [Article 32 (1)]. Information requirements for substances and preparations for which a safety data sheet is not required [Article 32 (1)].](https://cf4.ppt-online.org/files4/slide/u/umPTeEidX75UrWJOoRD8hSkL10Z4lFBKjztVqM/slide-149.jpg)





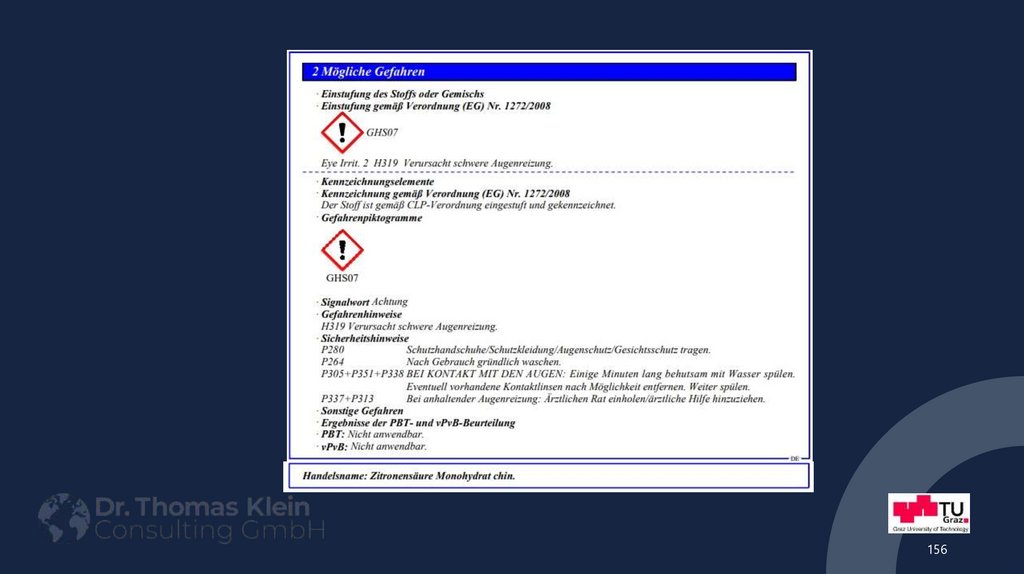
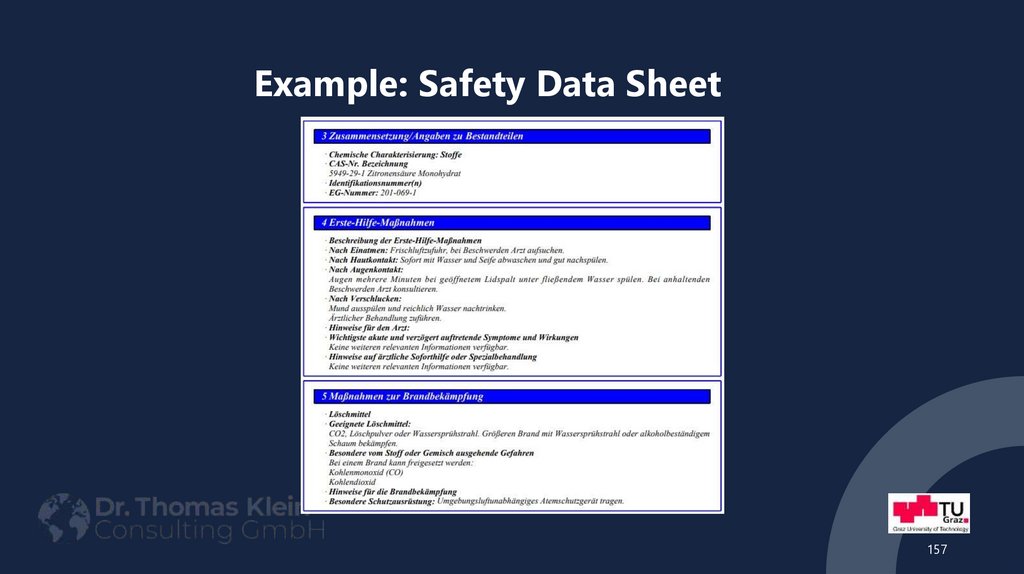


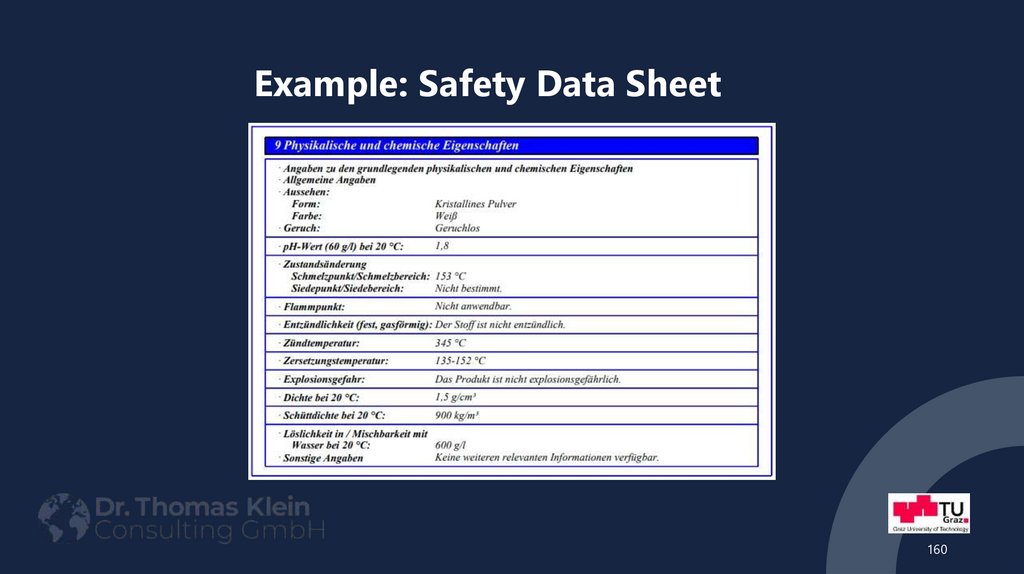
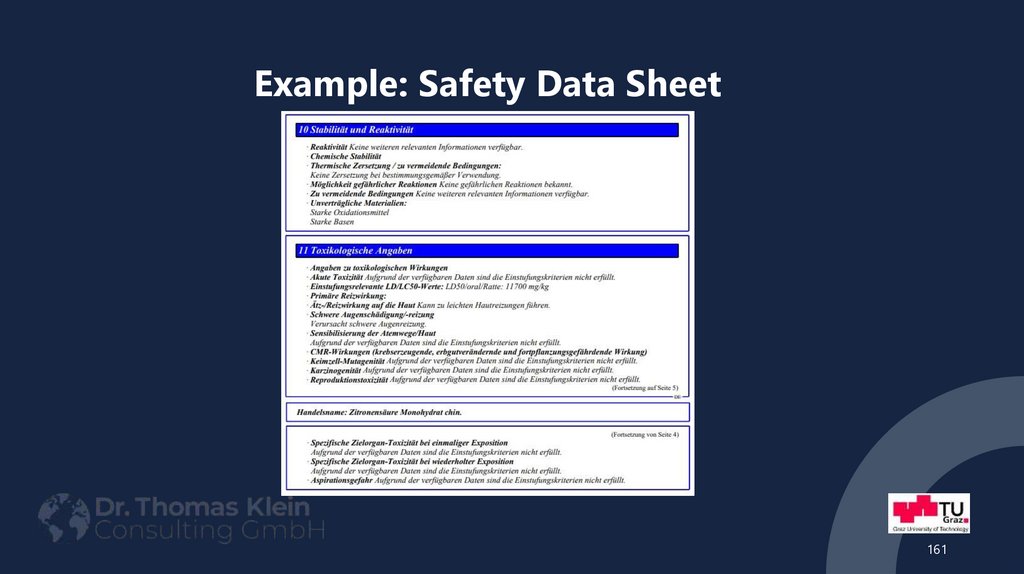
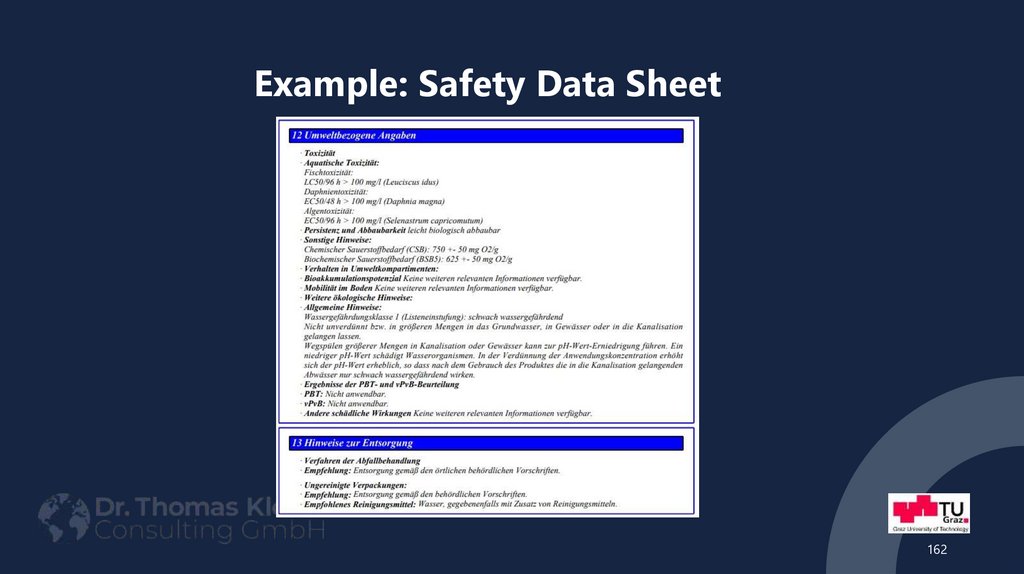

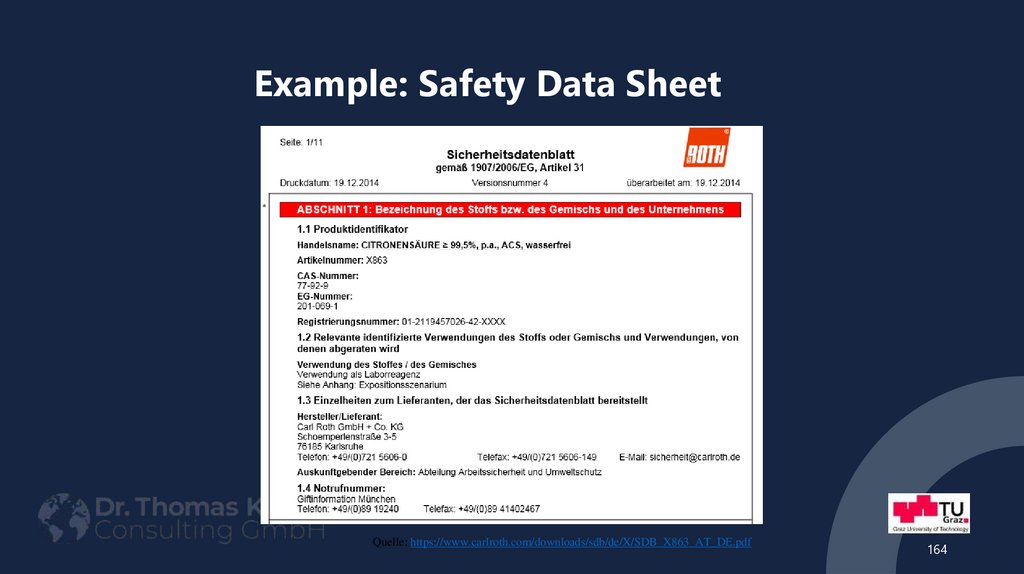





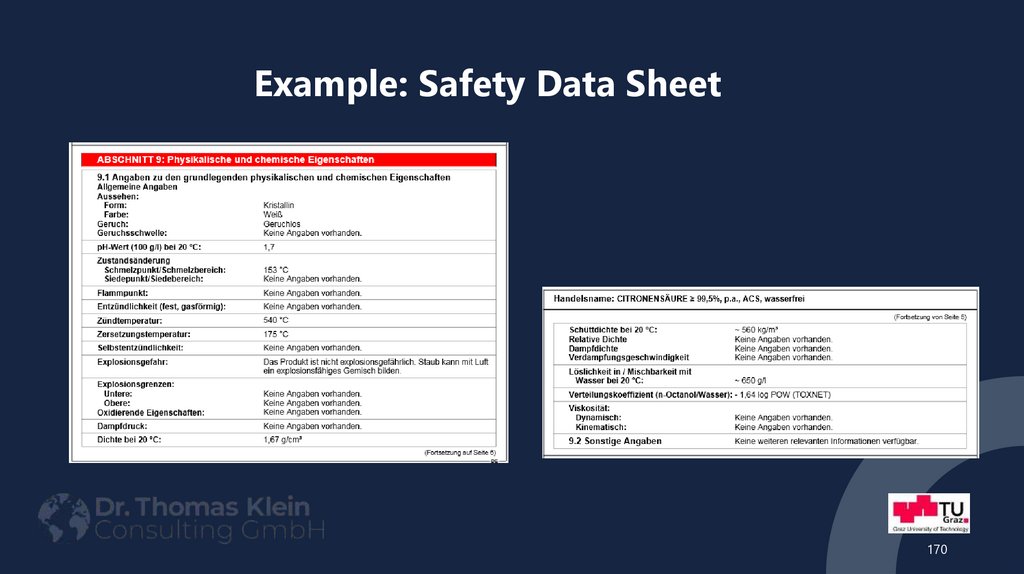































 Химия
Химия


