Похожие презентации:
CHE1226 Physical Chemistry School of Chemical Engineering Lecture 4 – Enthalpy. Hess’s Law
1. CHE1226 Physical Chemistry
School of Chemical EngineeringLecture 4 – Enthalpy. Hess’s Law
2. Table of contents
• Enthalpy• Heat capacity
• Thermochemistry
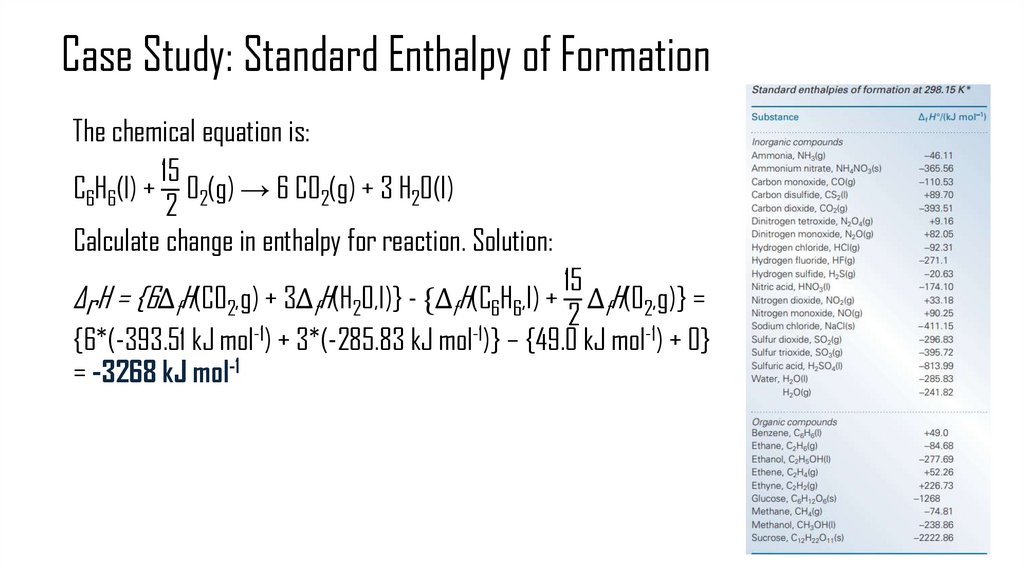
• Hess’s Law
Learning Objective: Quantify the heat transferred at constant pressure as Enthalpy and
apply it to chemical systems.
References:
P. Atkins and J. de Paula Ch.2 and 3
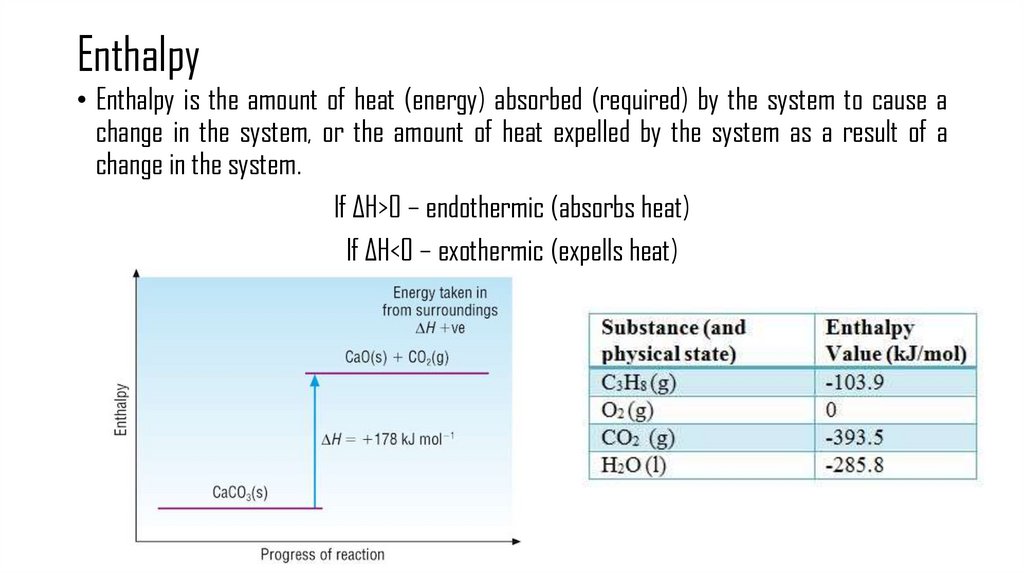
3. Enthalpy
• Enthalpy is the amount of heat (energy) absorbed (required) by the system to cause achange in the system, or the amount of heat expelled by the system as a result of a
change in the system.
If ΔH>0 – endothermic (absorbs heat)
If ΔH<0 – exothermic (expells heat)
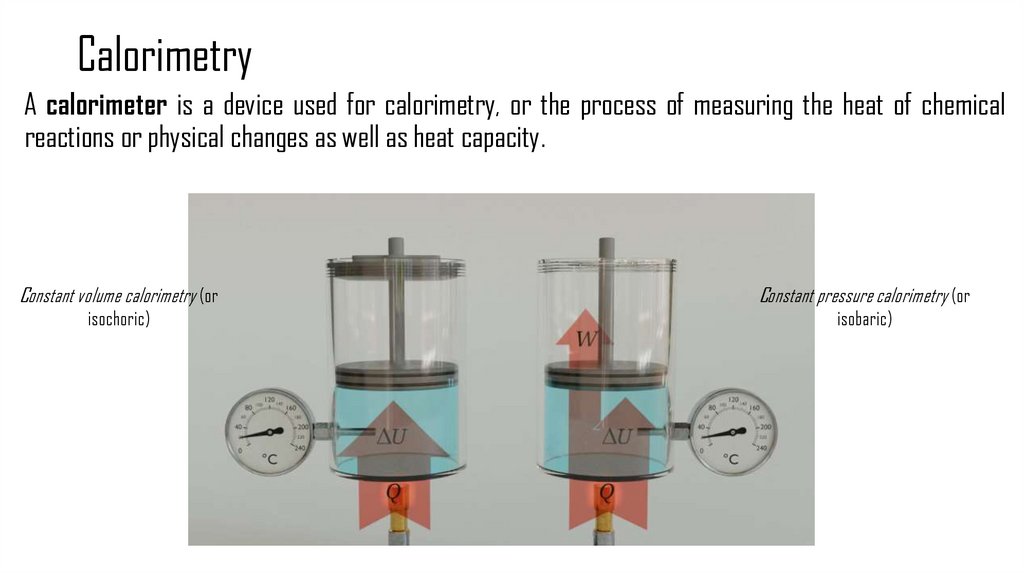
4. Calorimetry
A calorimeter is a device used for calorimetry, or the process of measuring the heat of chemicalreactions or physical changes as well as heat capacity.
Constant volume calorimetry (or
Constant pressure calorimetry (or
isochoric)
isobaric)
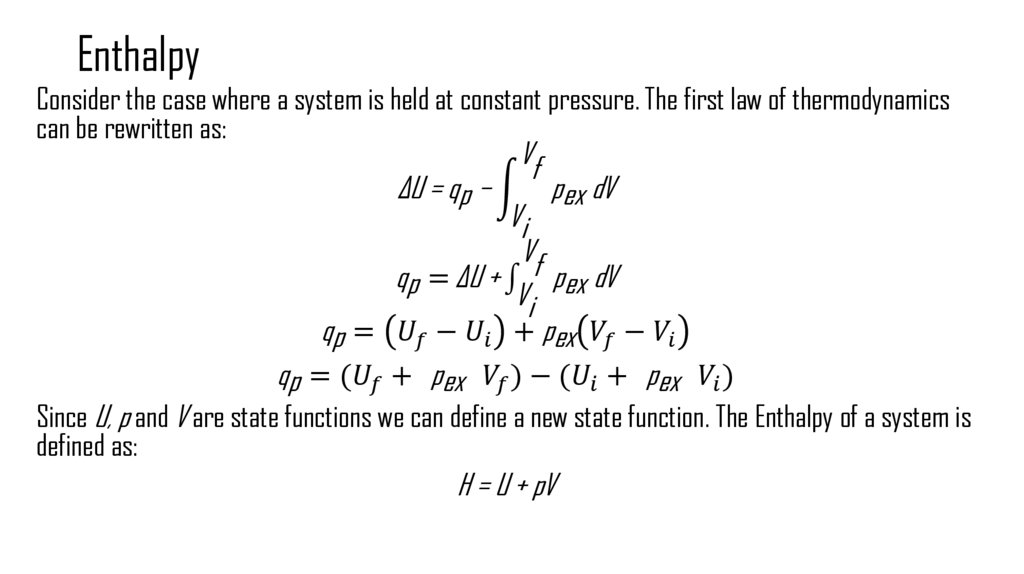
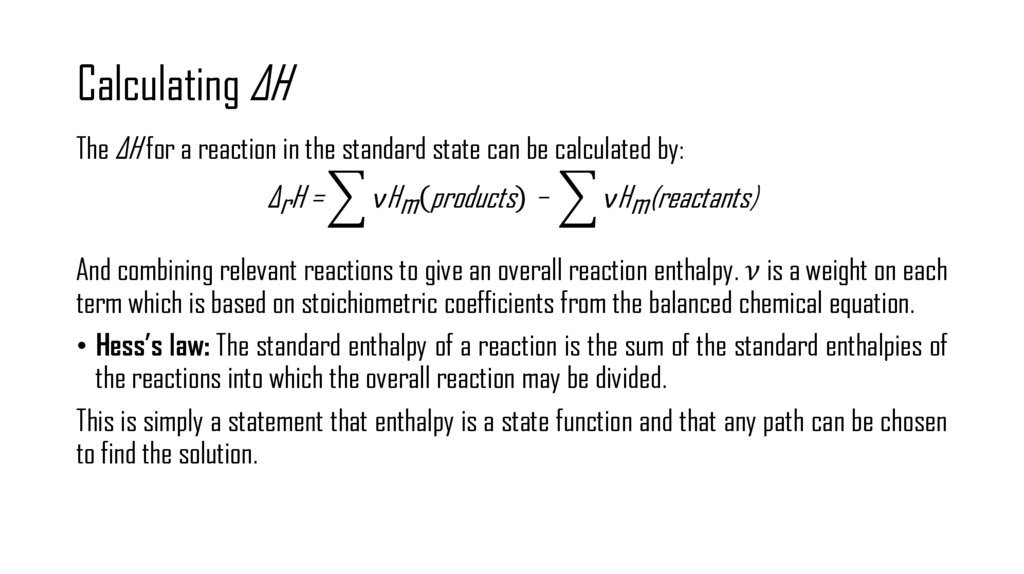
5. Enthalpy
Consider the case where a system is held at constant pressure. The first law of thermodynamicscan be rewritten as:
Vf
ΔU = qp − න pex dV
Vi
Vf
qp = ΔU + pex dV
Vi
qp =



 Химия
Химия








