Похожие презентации:
Soil Chemistry 13- lect (1)_b3c02146ef2619bf4a99009ba2649eaf
1. Soil Chemistry
2. Brief Contents
Soil pH importanceGeneral soil pH conditions
Causes of soil basicity. Hydrolysis of basic
cations
Causes of soil acidity. Accumulation of
soluble acids
Problems associated with acidity
Alkaline, Saline, and Sodic of Soils
3. Ion Exchange
Ions adsorbed to soil surfaces can beexchanged with ions in soil solution.
Cations and anions
4. Ion exchange
Organic colloids and inorganic micelles(clays) are sites of ion exchange
Where do ions in soil come from?
Release from organic matter
Rain
Weathering of parent material
5. Ion exchange
Exchangeable cations (on soil surfaces)cannot be removed by leaching.
Soluble cations (in solution)
can be removed by leaching.
6.
When soil is dried……exchangeable cations hold to adsorption sites
on soil surfaces.
…soluble cations (and anions) precipitate or
crystallize as salts.
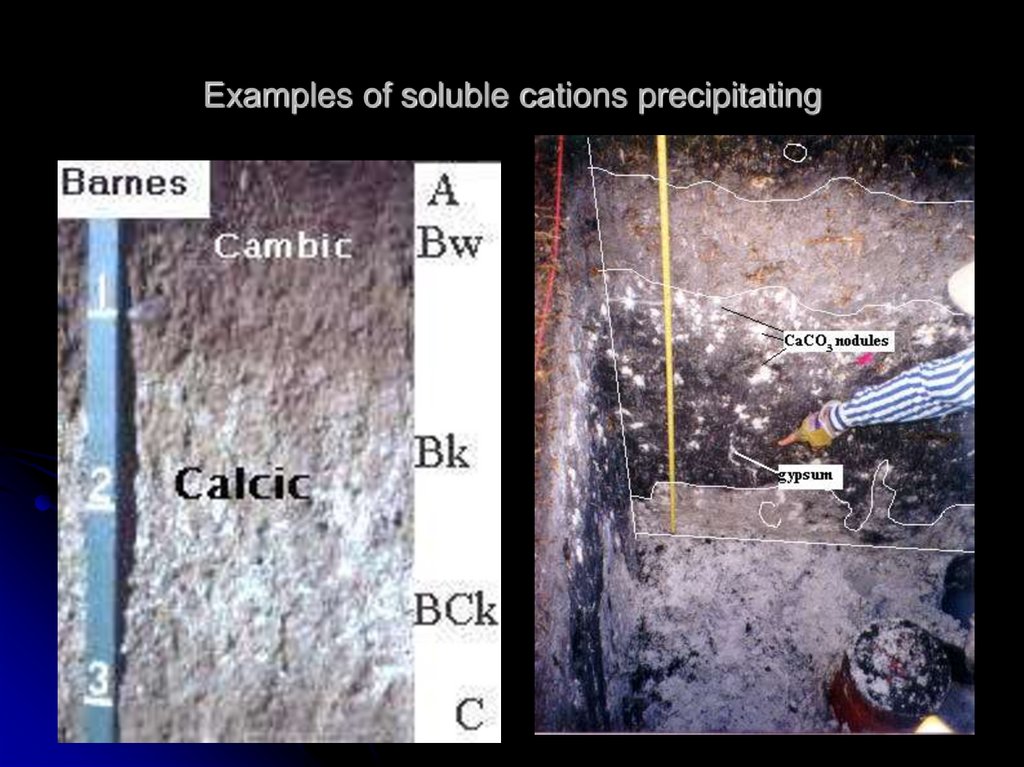
7. Examples of soluble cations precipitating
8. Ion exchange
Exchangeable ions on soil surface tradingplaces with ions in solution.
9. On soil surfaces, there are: Exchangeable and Nonexchangeable Ions :
Exchangeable: weakly held, in contact with soilsolution, ready for quick replacement.
“outer sphere complex”
Nonexchangeable:
“inner sphere complex”
adsorbed by strong bonds or held in
inaccessible places
(e.g., the K+ between layers of illite)
not part of ion exchange !
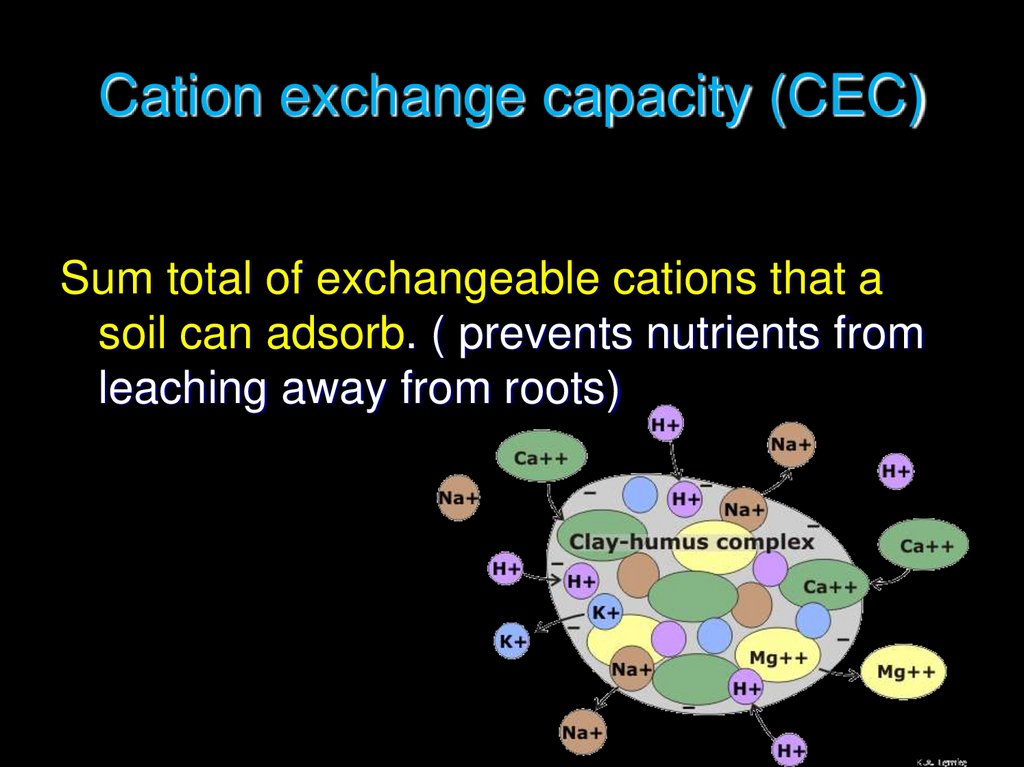
10. Cation exchange capacity (CEC)
Sum total of exchangeable cations that asoil can adsorb. ( prevents nutrients from
leaching away from roots)
11. CEC
Expressed in:milliequivalents per 100 g (meq/100g)
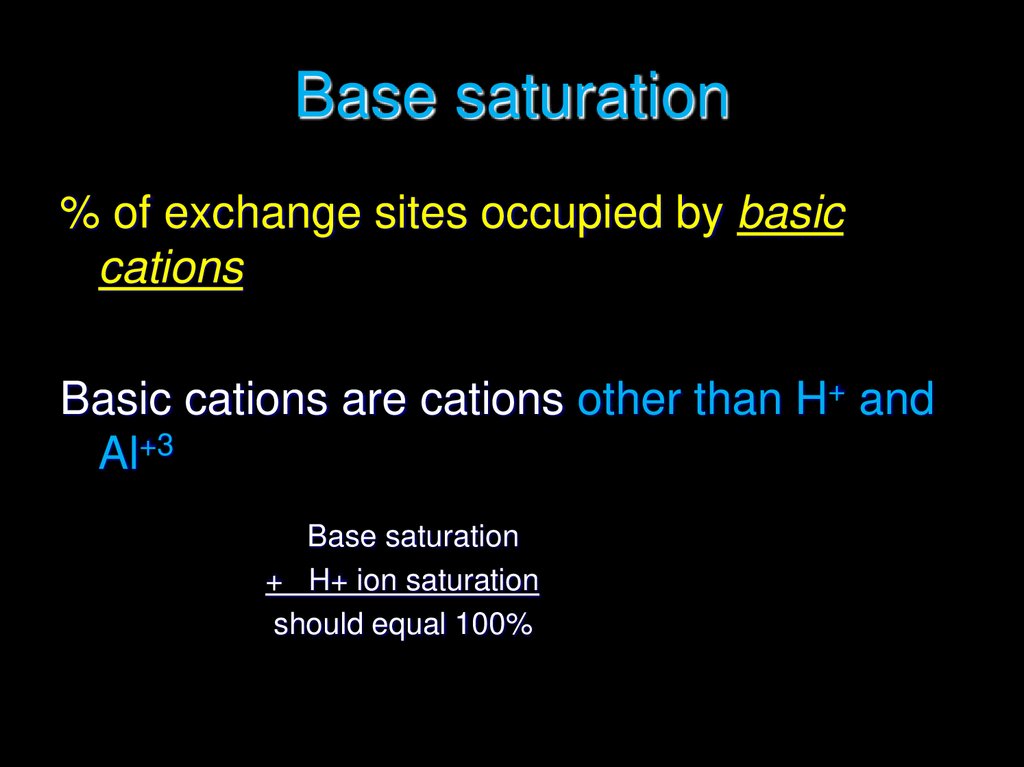
12. Base saturation
% of exchange sites occupied by basiccations
Basic cations are cations other than H+ and
Al+3
Base saturation
+ H+ ion saturation
should equal 100%
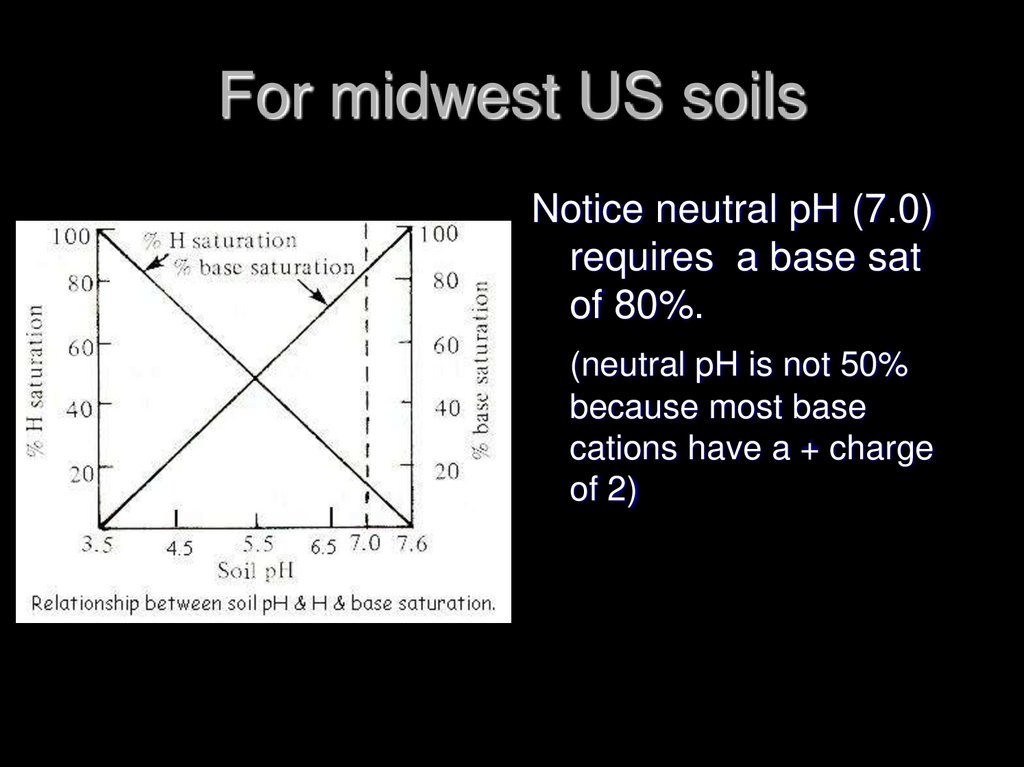
13. For midwest US soils
Notice neutral pH (7.0)requires a base sat
of 80%.
(neutral pH is not 50%
because most base
cations have a + charge
of 2)
14. equilibrium
Strive for equivalent proportions of solutionand exchangeable ions.
Upset equilibrium by:
removal by plants
leaching
fertilization
weathering
Initiate ion exchange
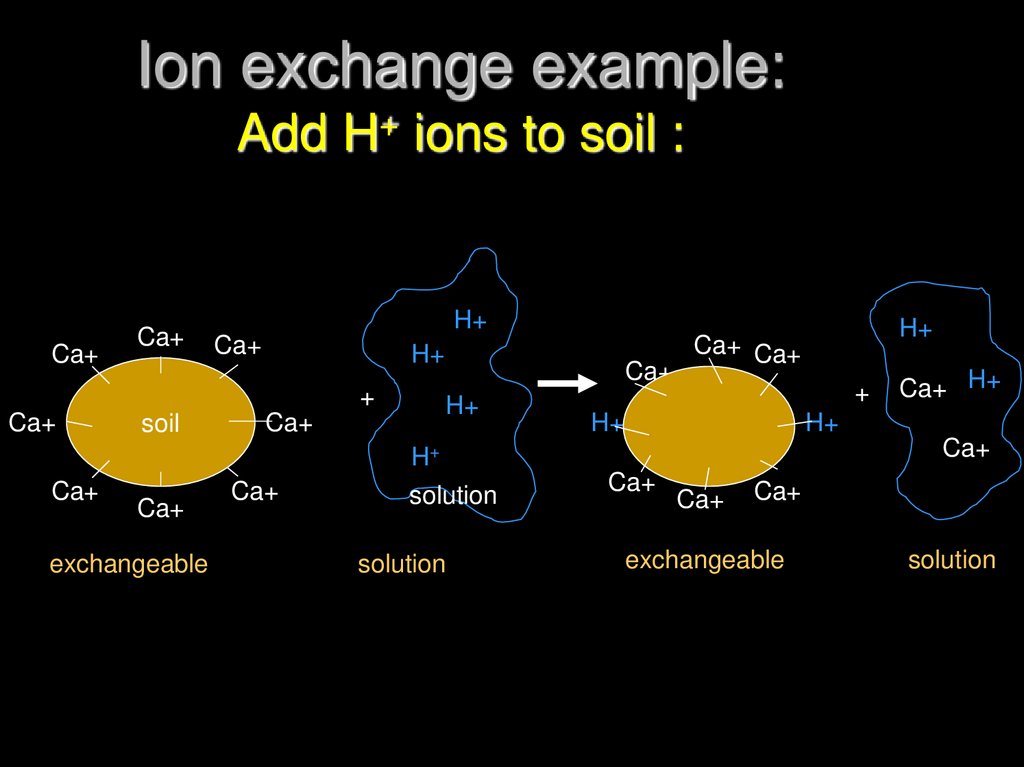
15. Ion exchange example: Add H+ ions to soil :
Ca+Ca+
H+
Ca+
H+
+
Ca+
Ca+
soil
Ca+
exchangeable
Ca+
Ca+
Ca+
H+
H+
solution
solution
H+
Ca+ Ca+
+
H+
Ca+ H+
H+
Ca+
Ca+
Ca+
Ca+
exchangeable
solution
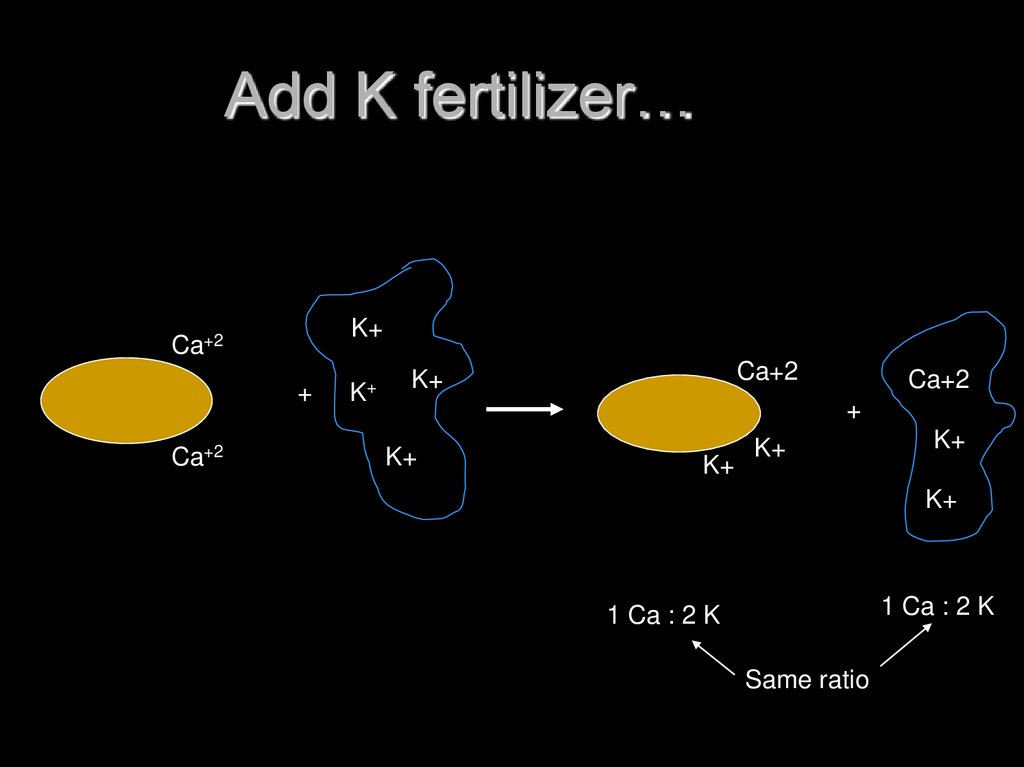
16. Rules of ion exchange
Process is ReversibleRatio Law:
ratio of exchangeable cations will be
same as ratio of solution cations
17. Add K fertilizer…
K+Ca+2
+
Ca+2
K+
Ca+2
K+
Ca+2
+
K+
K+
K+
K+
K+
1 Ca : 2 K
1 Ca : 2 K
Same ratio
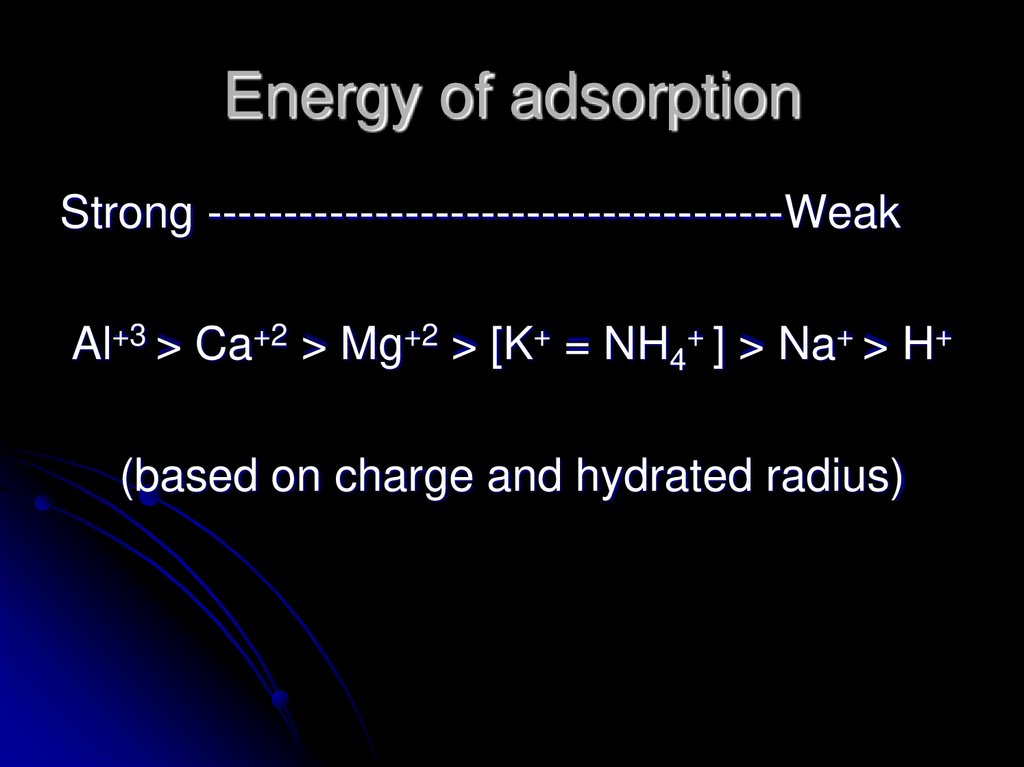
18. Energy of adsorption
Strong --------------------------------------WeakAl+3 > Ca+2 > Mg+2 > [K+ = NH4+ ] > Na+ > H+
(based on charge and hydrated radius)
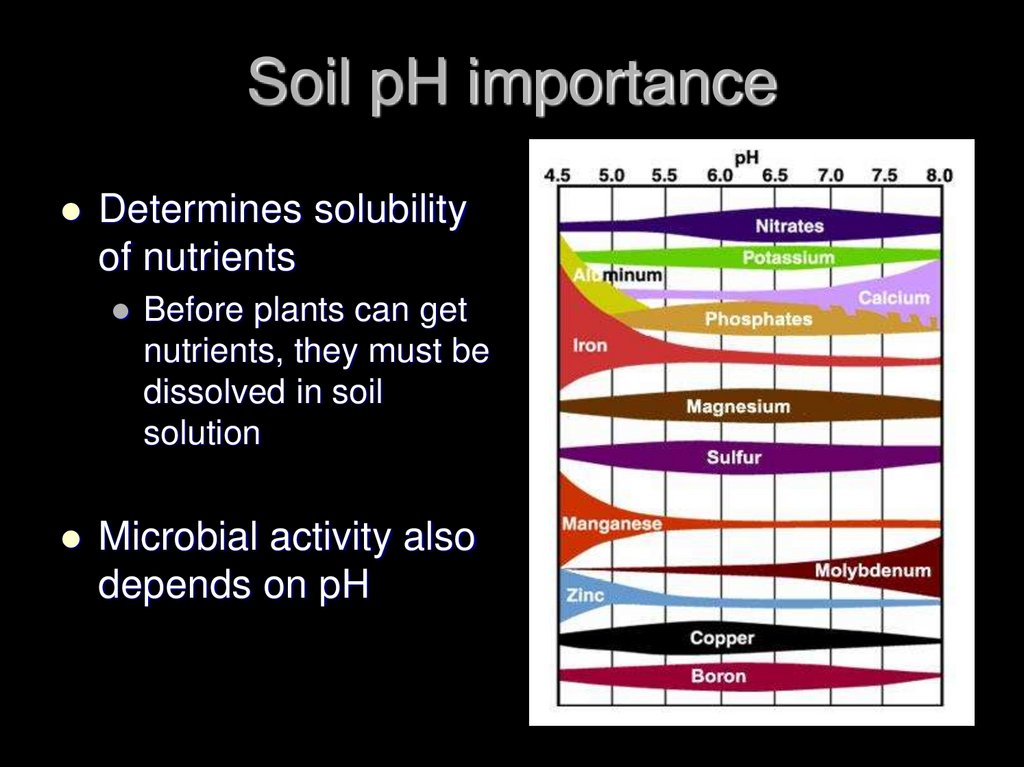
19. Soil pH importance
Determines solubilityof nutrients
Before plants can get
nutrients, they must be
dissolved in soil
solution
Microbial activity also
depends on pH
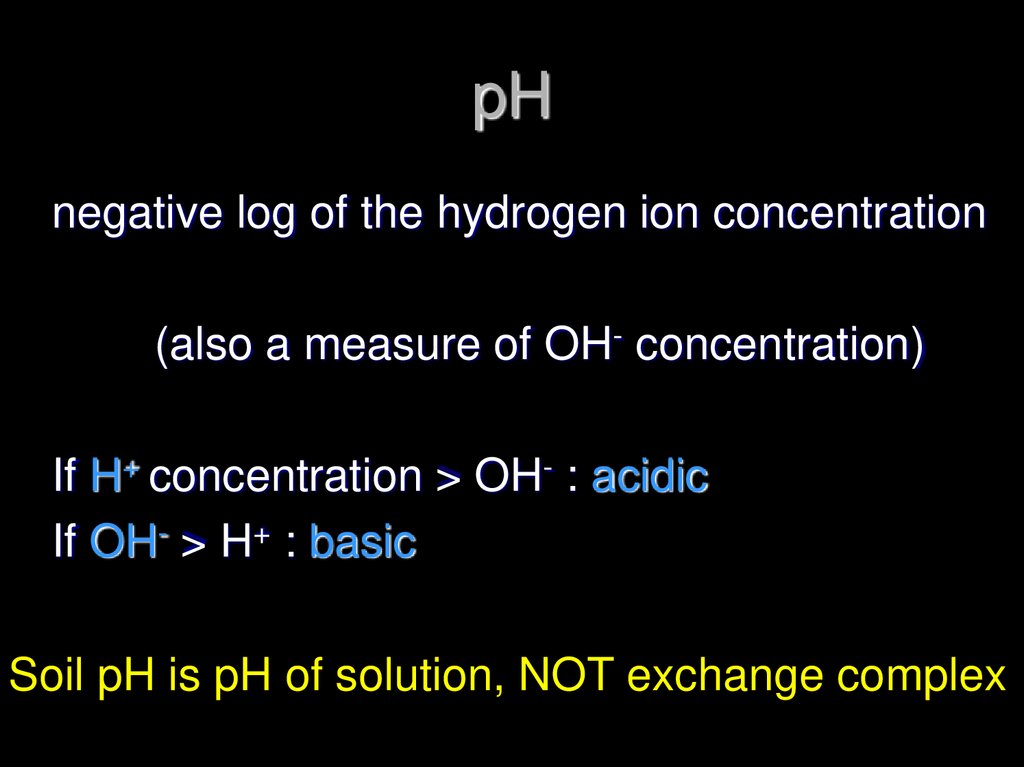
20. pH
negative log of the hydrogen ion concentration(also a measure of OH- concentration)
If H+ concentration > OH- : acidic
If OH- > H+ : basic
Soil pH is pH of solution, NOT exchange complex
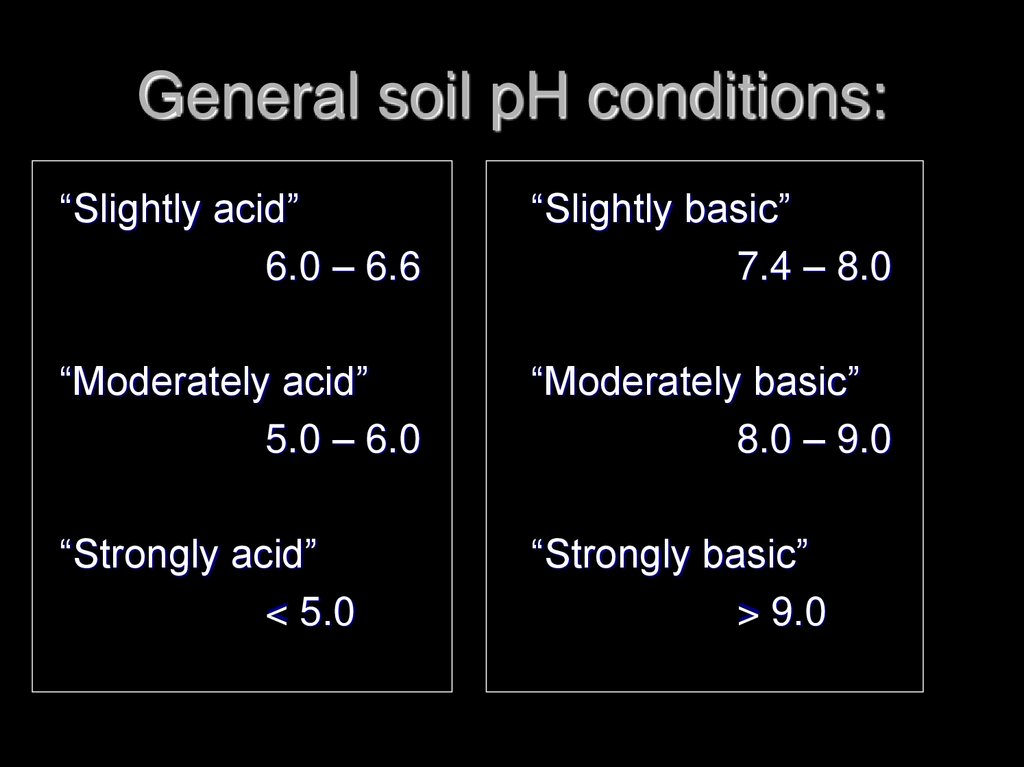
21. General soil pH conditions:
“Slightly acid”6.0 – 6.6
“Slightly basic”
7.4 – 8.0
“Moderately acid”
5.0 – 6.0
“Moderately basic”
8.0 – 9.0
“Strongly acid”
< 5.0
“Strongly basic”
> 9.0
22.
In soil, both H+ and Al+3 ions produce acidityAl+3 produces H+ ions when it reacts with
water.
(when pH below 6: Al+3 is the cause of acidity)
23. Causes of soil basicity
1.2.
Hydrolysis of basic cations
Hydrolysis of carbonates
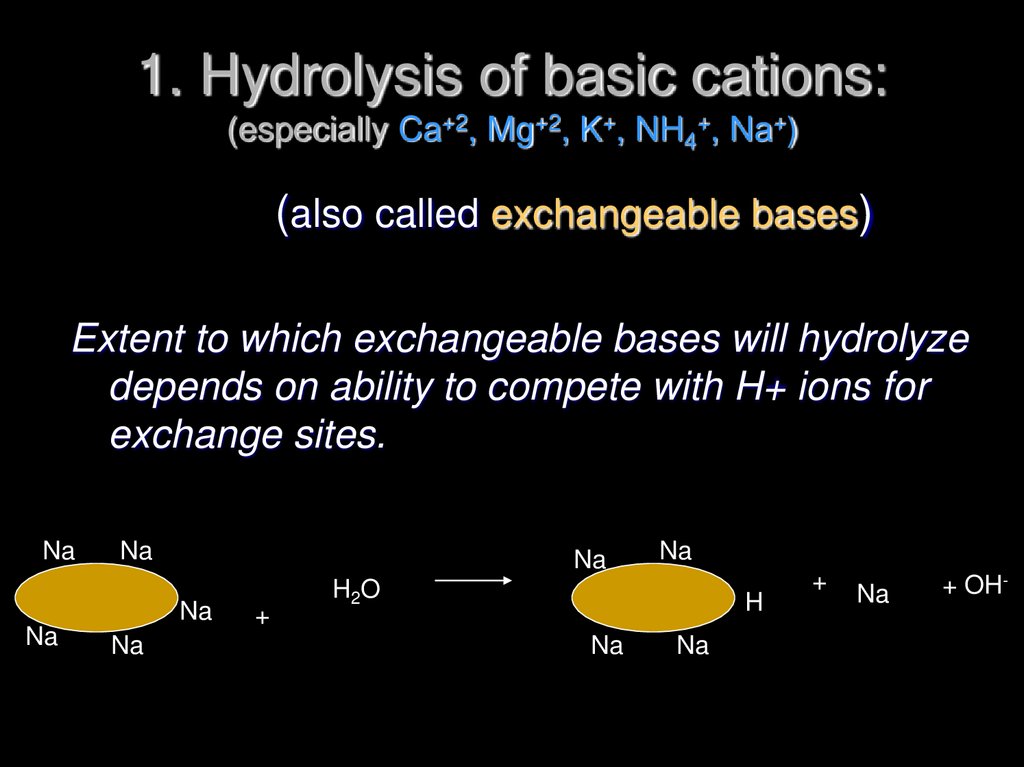
24. 1. Hydrolysis of basic cations: (especially Ca+2, Mg+2, K+, NH4+, Na+)
(also called exchangeable bases)Extent to which exchangeable bases will hydrolyze
depends on ability to compete with H+ ions for
exchange sites.
Na
Na
Na
Na
Na
Na
+
Na
H2O
H
Na
Na
+
Na
+ OH-
25.
K+ and Na+ are weakly held compared toCa+2 and Mg+2.
Recall energy of adsorption
So, K+ and Na+ are hydrolyzed easily and
yield higher pHs .
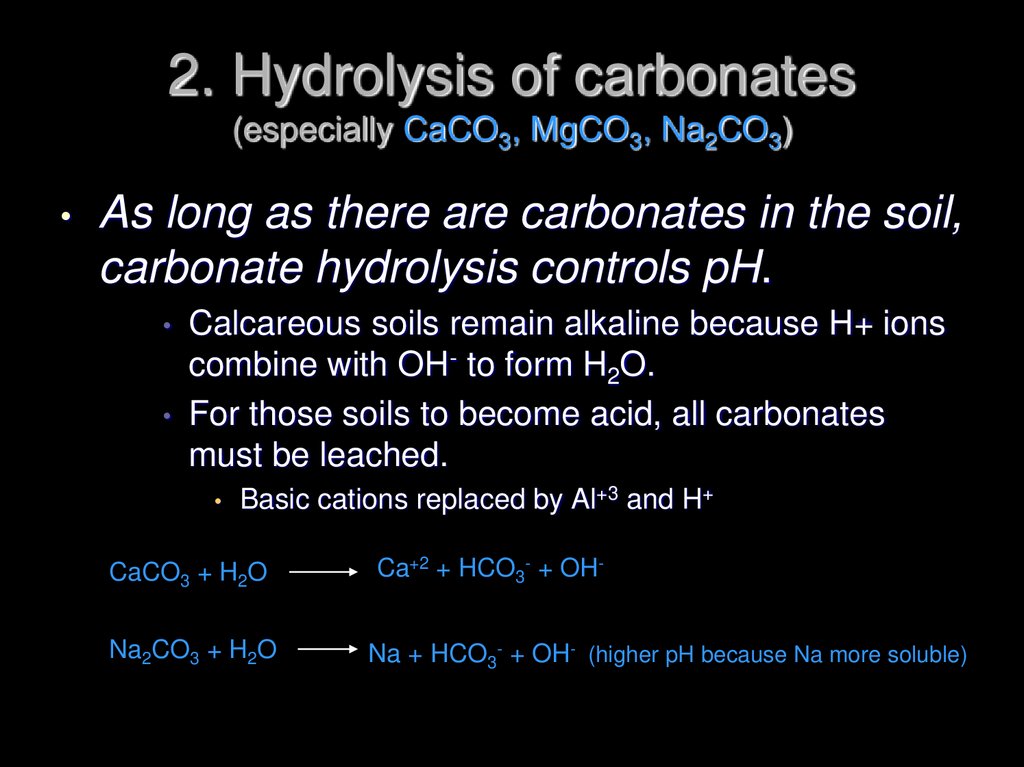
26. 2. Hydrolysis of carbonates (especially CaCO3, MgCO3, Na2CO3)
As long as there are carbonates in the soil,
carbonate hydrolysis controls pH.
• Calcareous soils remain alkaline because H+ ions
combine with OH- to form H2O.
• For those soils to become acid, all carbonates
must be leached.
Basic cations replaced by Al+3 and H+
CaCO3 + H2O
Ca+2 + HCO3- + OH-
Na2CO3 + H2O
Na + HCO3- + OH- (higher pH because Na more soluble)
27. Causes of soil acidity
1.2.
Accumulation of soluble acids
Exchangeable acids (Al+3, H+)
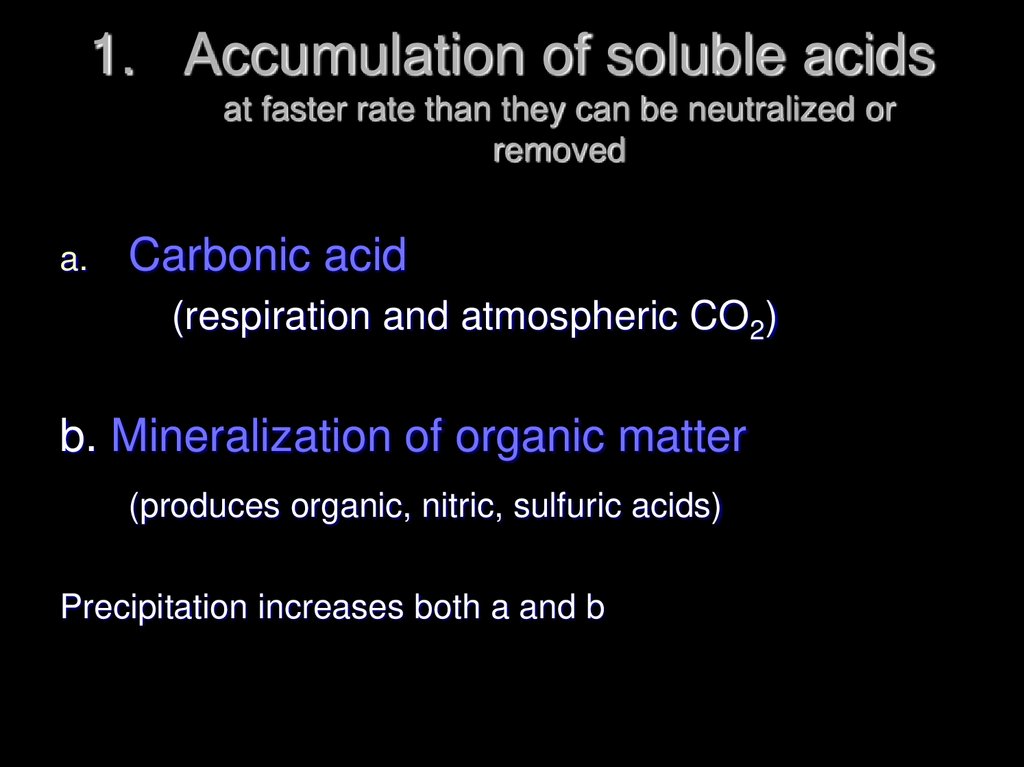
28. Accumulation of soluble acids at faster rate than they can be neutralized or removed
1. Accumulation of soluble acidsat faster rate than they can be neutralized or
removed
a.
Carbonic acid
(respiration and atmospheric CO2)
b. Mineralization of organic matter
(produces organic, nitric, sulfuric acids)
Precipitation increases both a and b
29. Distribution of acid soils
Acidic soils usually occur where rainfallleaches the cations out of the soil over
time. In the U.S. there is a fairly strong
correlation between precipitation and pH,
with soils receiving more than about 30
inches of annual precipitation having a pH
<6
30. Problems associated with acidity
Aluminum toxicity: Aluminum becomesmore available when pH is pH < 5
Manganese toxicity: This may occur in soil
that are high in Mn and that have a pH < 5.
31. 2. Exchangeable acids
Exch. H+ or Al+3 dissociateAl+3 ties up OH- from water, releases an
equivalent amount of H+ ions.
Al+3 + H2O
AlOH+2 + H+
32.
Acid soils and liming Lime (calciumcarbonate) is added to acid soils to raise
the pH. Calcium (Ca2+) replaces hydrogen
and aluminum on the exchange sites. For
a good reference on liming, see the Soil.
33. CEC and pH
Only 2:1 silicate clays do not have pH-dependent CECs.Others are pH-dependent:
1:1 kaolinite:
low pH: low CEC
high pH: high CEC
Oxidic clays
34. Alkaline, Saline, and Sodic Soils
Alkalinity and acidity: Soils that vary from aneutral pH have varying degrees of
alkalinity (pH > 7) or acidity (pH < 7). The
mean soil pH in the U.S. is around 6.4.
35. Salinity:
Soils that have excess soluble salts in thesoil solution have varying degrees of
salinity
36. Sodicity
Soils that specifically have excess sodiumin the soil solution are called sodic
37. Alkalinity
Alkalinity Soils in arid and semi-arid areascan lack enough rainfall to leach cations,
especially calcium (Ca2+), magnesium
(Mg2+), potassium (K+) and sodium (Na+),
from the soil. These cations bind many of the
CEC sites, blocking hydrogen (H+) ions from
binding and making the soil alkaline. This can
also happen if irrigating with water high in
calcium bicarbonate or magnesium
bicarbonate.
38. Salinity
Salinity A soil containing sufficient solublesalts (these salts include Mg2+, Na+, Ca2+,
chloride (Cl- ), sulfate (SO4 2-), bicarbonate
(HCO3 -) and carbonate
(CO3 2-). Saline
Sodicity
soils mainly occur in dry areas, again, where
there is not enough precipitation to leach the
salts from the soil, so the salts build up over
time. In order for there to be salts in the soil,
there must be a source for them.
39. Sodicity
A soil containing sufficient exchangeablesodium to adversely affect crop production
and soil structure under most conditions of
soil and plant types. Many saline soils are
also sodic, although not necessarily. Sodium
is toxic to plants. It also causes soil particles
to disperse (separate), which causes
cracking and sealing of the soil surface,
leading to poor soil structure and decreased
water intake.
40.
Sodic soils can be reclaimed with a two-step process. First the sodium is flushed
from CEC sites by adding amendments
high in calcium (such as lime, gypsum, or
dolomite) or by adding sulfur followed by
lime. (The sulfur is converted to sulfuric
acid by microbial activity.
41.
The sulfuric acid then reacts with lime tofree calcium.) In either case, the Ca2+
ions replace the Na+ cations, freeing the
Na+ in the soil solution. The second step
is to leach out the sodium ions by irrigating
in excess of what the plant needs.
42. Quantitative definitions
Specifically, alkaline, saline, and sodicsoils are defined as such:
a) Alkaline soil: Has a pH of > 8.5 or with
an exchangeable sodium percentage
(ESP, that is, the percent of the CEC
occupied just by sodium) greater than
15%. Soils at this ESP contain sufficient
sodium to interfere with the growth of most
crop plants.
43.
b) Saline soil: Soil salinity is determined bymeasuring the electrical conductivity (EC)
of a saturated paste of soil: if the EC is
greater than 4 dS/m (decisiemens per
meter), the soil is classified as saline.
However this is a rough range: saltsensitive plants can be affected at half this
EC and highly tolerant plants can handle
up to about twice this EC.
44. Conclusion
Soil chemistry plays a fundamental role in determiningsoil fertility, plant nutrition, and overall ecosystem health.
Understanding ion exchange processes, cation
exchange capacity, and base saturation helps explain
how nutrients are retained and made available to plants.
Soil pH strongly influences nutrient solubility and
microbial activity, while the balance between acidity and
basicity is shaped by factors such as rainfall, mineral
weathering, organic matter decomposition, and the
presence of carbonates.
45.
Acidic soils often present challenges such as aluminumand manganese toxicity, whereas alkaline, saline, and
sodic soils can restrict plant growth through excess salts
or sodium-induced structural problems. Effective
management strategies—including liming acid soils and
reclaiming sodic soils with calcium
amendments—are essential for maintaining soil
productivity. Overall, knowledge of soil chemical
properties is crucial for sustainable agriculture,
environmental protection, and long-term soil health.




























 Химия
Химия








