Похожие презентации:
Period 3
1.
Period 3 ElementsWednesday, 17 December 2025
2. Atomic Radii
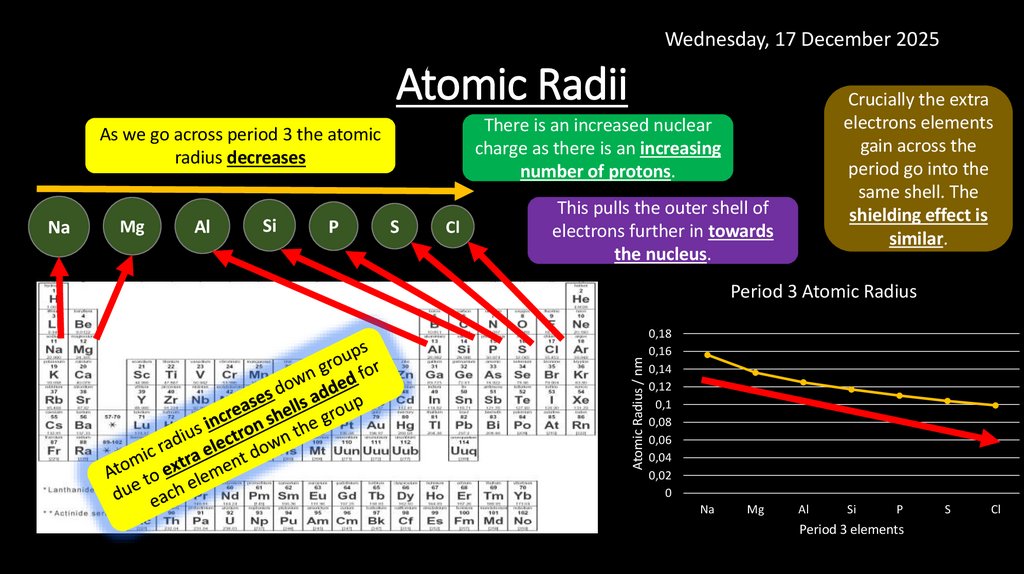
Wednesday, 17 December 2025Atomic Radii
There is an increased nuclear
charge as there is an increasing
number of protons.
Mg
Al
Si
P
S
Cl
This pulls the outer shell of
electrons further in towards
the nucleus.
Period 3 Atomic Radius
Atomic Radius / nm
As we go across period 3 the atomic
radius decreases
Na
Crucially the extra
electrons elements
gain across the
period go into the
same shell. The
shielding effect is
similar.
0,18
0,16
0,14
0,12
0,1
0,08
0,06
0,04
0,02
0
Na
Mg
Al
Si
P
Period 3 elements
S
Cl
3. Melting Points
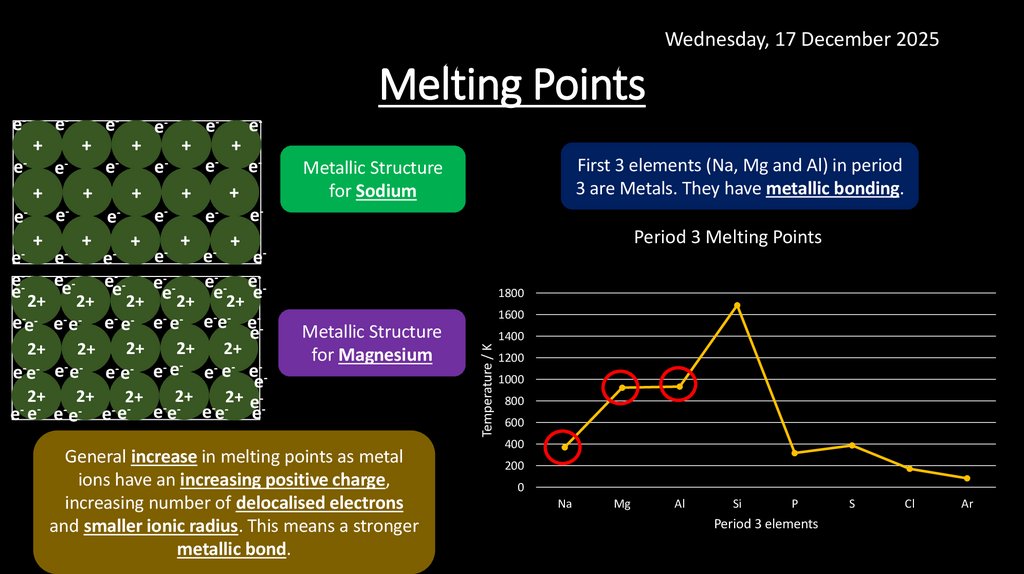
Wednesday, 17 December 2025Melting Points
e-
e+
+
e-
ee-
e-
e-
e-
+
e-
+
e-
+
+
+
e-
e-
e+
+
e-
e-
e-
e-
e+
+
+
e-
+
e-
e-
e-
+
e-
First 3 elements (Na, Mg and Al) in period
3 are Metals. They have metallic bonding.
Metallic Structure
for Sodium
+
Period 3 Melting Points
e-
e-ee- - ee- - e- e- - e- e 2+
2+
2+ e 2+ e 2+ e
2+
e-e-
2+
e- e-
e- e- e- e2+
e- e-
2+
e
- e-
e-e- e-e
2+
e- e-
e-
1600
Metallic Structure
for Magnesium
e2+
2+
2+
2+
2+ ee e e e e e e e e e e-
General increase in melting points as metal
ions have an increasing positive charge,
increasing number of delocalised electrons
and smaller ionic radius. This means a stronger
metallic bond.
1400
Temperature / K
e-e- e- e-
1800
1200
1000
800
600
400
200
0
Na
Mg
Al
Si
P
Period 3 elements
S
Cl
Ar
4. Melting Points
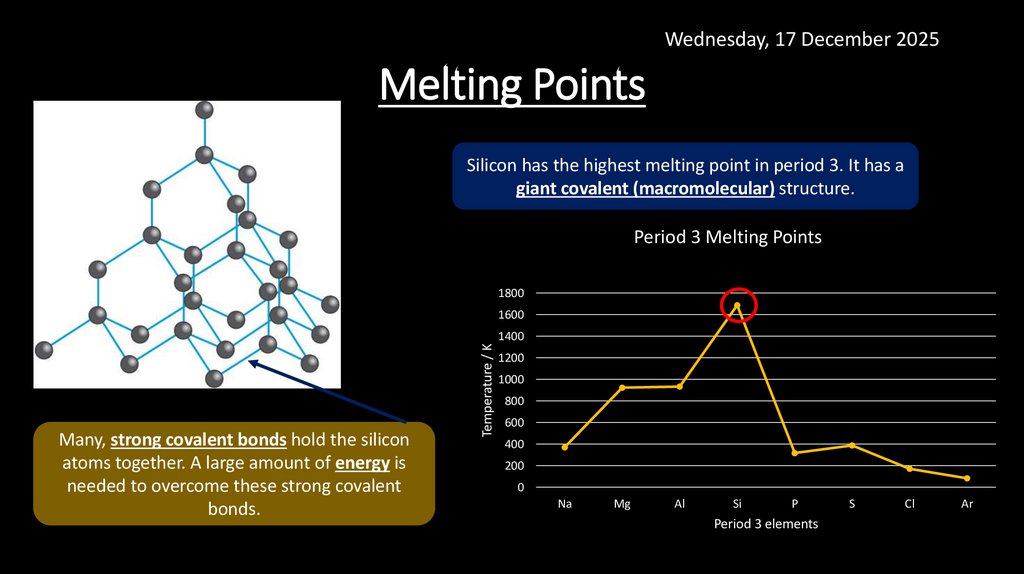
Wednesday, 17 December 2025Melting Points
Silicon has the highest melting point in period 3. It has a
giant covalent (macromolecular) structure.
Period 3 Melting Points
1800
1600
Many, strong covalent bonds hold the silicon
atoms together. A large amount of energy is
needed to overcome these strong covalent
bonds.
Temperature / K
1400
1200
1000
800
600
400
200
0
Na
Mg
Al
Si
P
Period 3 elements
S
Cl
Ar
5. Melting Points
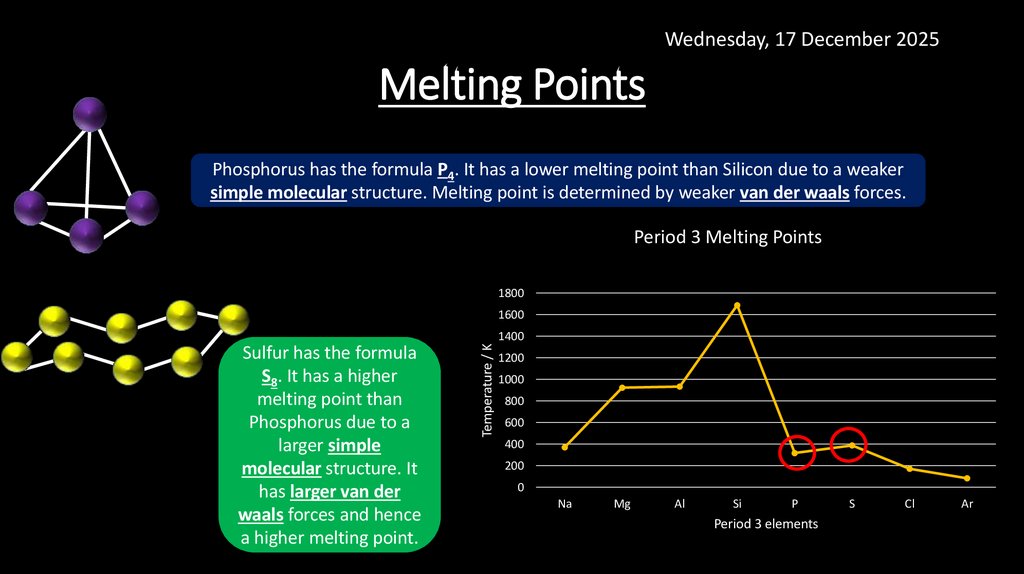
Wednesday, 17 December 2025Melting Points
Phosphorus has the formula P4. It has a lower melting point than Silicon due to a weaker
simple molecular structure. Melting point is determined by weaker van der waals forces.
Period 3 Melting Points
1800
1600
Sulfur has the formula
S8. It has a higher
melting point than
Phosphorus due to a
larger simple
molecular structure. It
has larger van der
waals forces and hence
a higher melting point.
Temperature / K
1400
1200
1000
800
600
400
200
0
Na
Mg
Al
Si
P
Period 3 elements
S
Cl
Ar
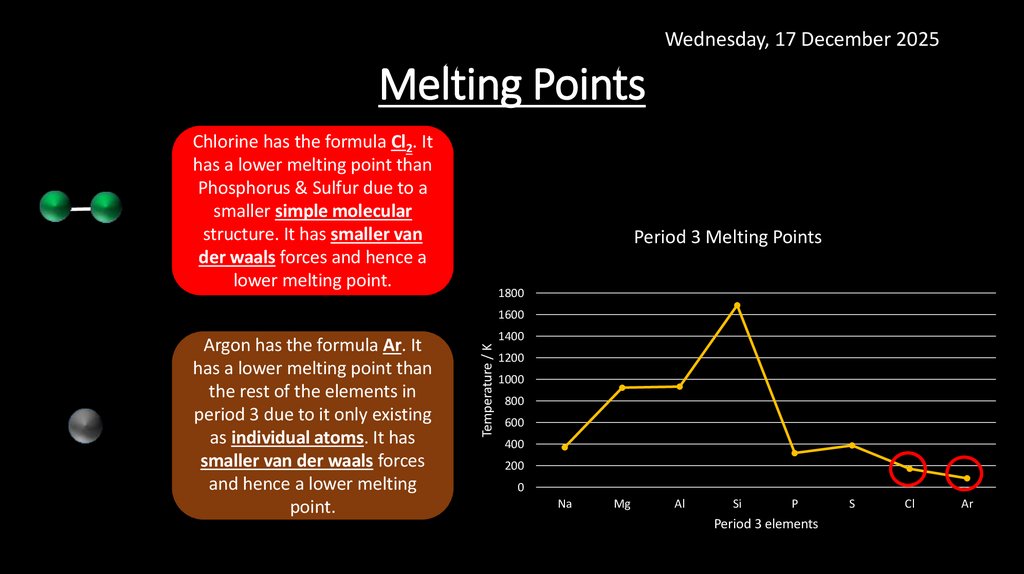
6. Melting Points
Wednesday, 17 December 2025Melting Points
Chlorine has the formula Cl2. It
has a lower melting point than
Phosphorus & Sulfur due to a
smaller simple molecular
structure. It has smaller van
der waals forces and hence a
lower melting point.
Period 3 Melting Points
1800
1600
1400
Temperature / K
Argon has the formula Ar. It
has a lower melting point than
the rest of the elements in
period 3 due to it only existing
as individual atoms. It has
smaller van der waals forces
and hence a lower melting
point.
1200
1000
800
600
400
200
0
Na
Mg
Al
Si
P
Period 3 elements
S
Cl
Ar
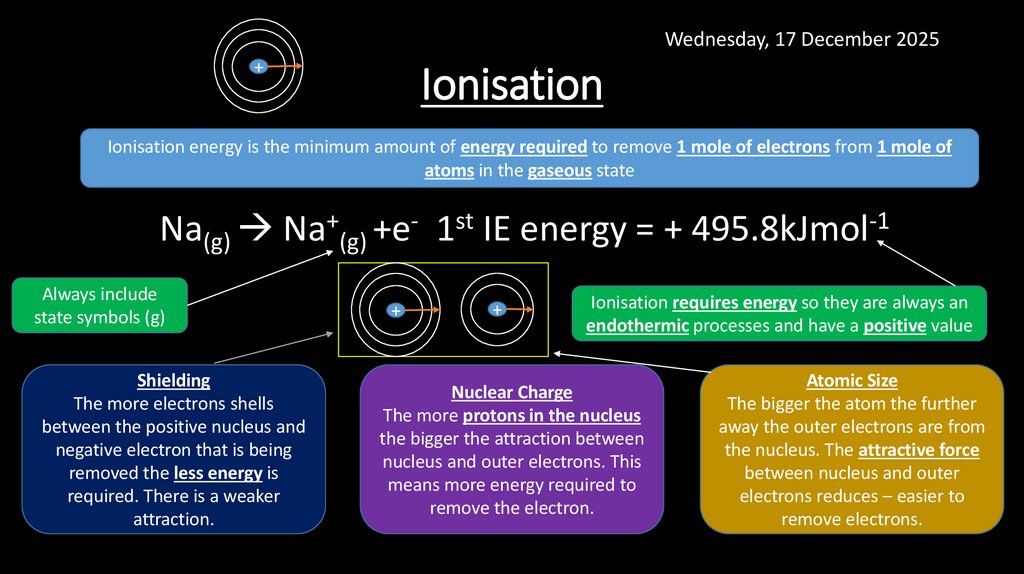
7. Ionisation
Wednesday, 17 December 2025+
Ionisation
Ionisation energy is the minimum amount of energy required to remove 1 mole of electrons from 1 mole of
atoms in the gaseous state
Na(g) Na+(g) +e- 1st IE energy = + 495.8kJmol-1
Always include
state symbols (g)
+
Shielding
The more electrons shells
between the positive nucleus and
negative electron that is being
removed the less energy is
required. There is a weaker
attraction.
Nuclear Charge
The more protons in the nucleus
the bigger the attraction between
nucleus and outer electrons. This
means more energy required to
remove the electron.
+
Ionisation requires energy so they are always an
endothermic processes and have a positive value
Atomic Size
The bigger the atom the further
away the outer electrons are from
the nucleus. The attractive force
between nucleus and outer
electrons reduces – easier to
remove electrons.
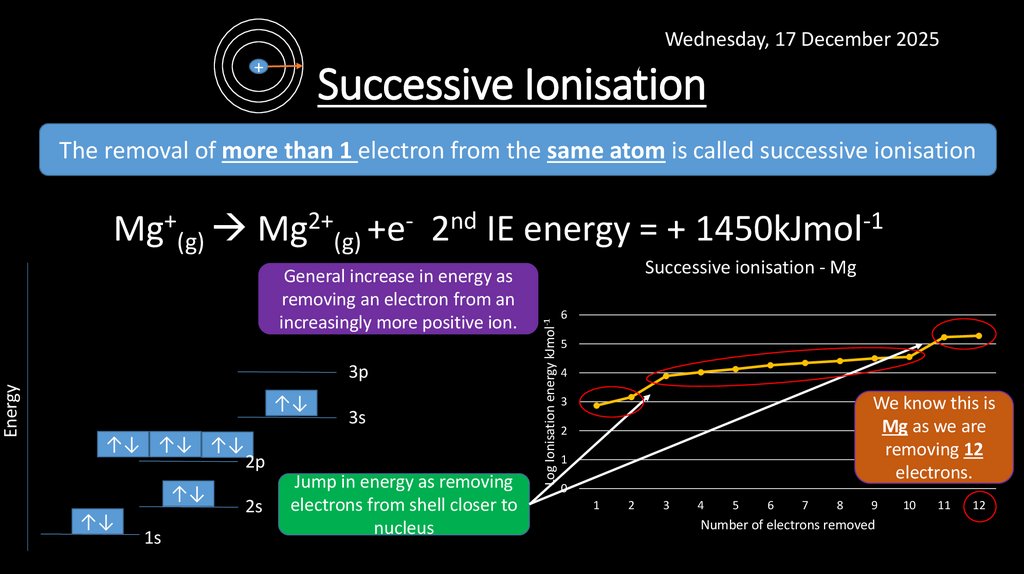
8. Successive Ionisation
Wednesday, 17 December 2025+
Successive Ionisation
The removal of more than 1 electron from the same atom is called successive ionisation
Mg+(g) Mg2+(g) +e- 2nd IE energy = + 1450kJmol-1
Energy
3p
↑↓
↑↓ ↑↓ ↑↓
↑↓
↑↓
1s
3s
2p
2s
Jump in energy as removing
electrons from shell closer to
nucleus
Successive ionisation - Mg
Log Ionisation energy kJmol-1
General increase in energy as
removing an electron from an
increasingly more positive ion.
6
5
4
We know this is
Mg as we are
removing 12
electrons.
3
2
1
0
1
2
3
4
5
6
7
8
9
Number of electrons removed
10
11
12
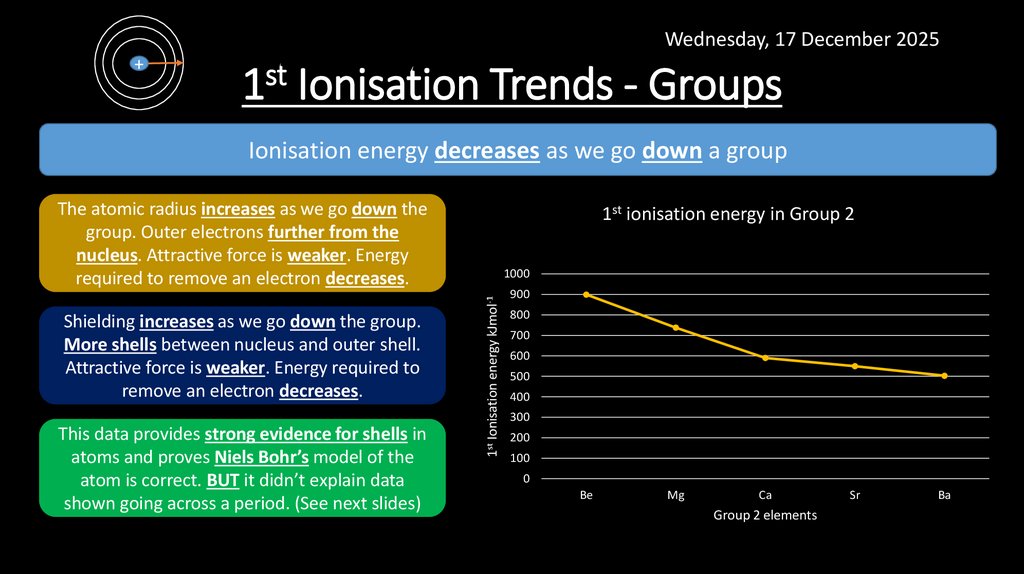
9. 1st Ionisation Trends - Groups
Wednesday, 17 December 2025+
1st Ionisation Trends - Groups
Ionisation energy decreases as we go down a group
The atomic radius increases as we go down the
group. Outer electrons further from the
nucleus. Attractive force is weaker. Energy
required to remove an electron decreases.
This data provides strong evidence for shells in
atoms and proves Niels Bohr’s model of the
atom is correct. BUT it didn’t explain data
shown going across a period. (See next slides)
1000
1st Ionisation energy kJmol-1
Shielding increases as we go down the group.
More shells between nucleus and outer shell.
Attractive force is weaker. Energy required to
remove an electron decreases.
1st ionisation energy in Group 2
900
800
700
600
500
400
300
200
100
0
Be
Mg
Ca
Group 2 elements
Sr
Ba
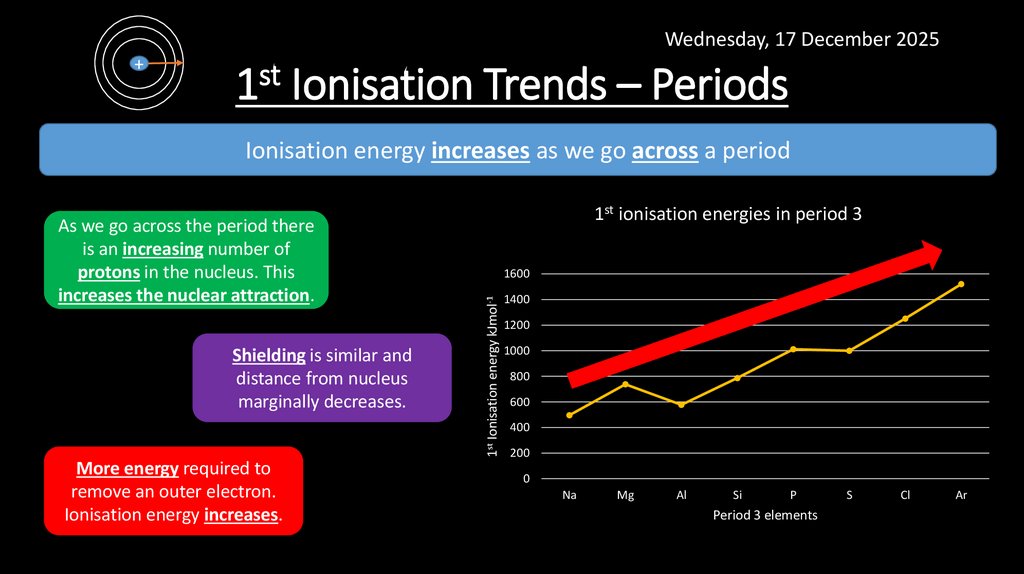
10. 1st Ionisation Trends – Periods
Wednesday, 17 December 2025+
1st Ionisation Trends – Periods
Ionisation energy increases as we go across a period
Shielding is similar and
distance from nucleus
marginally decreases.
More energy required to
remove an outer electron.
Ionisation energy increases.
1600
1st Ionisation energy kJmol-1
As we go across the period there
is an increasing number of
protons in the nucleus. This
increases the nuclear attraction.
1st ionisation energies in period 3
1400
1200
1000
800
600
400
200
0
Na
Mg
Al
Si
P
Period 3 elements
S
Cl
Ar
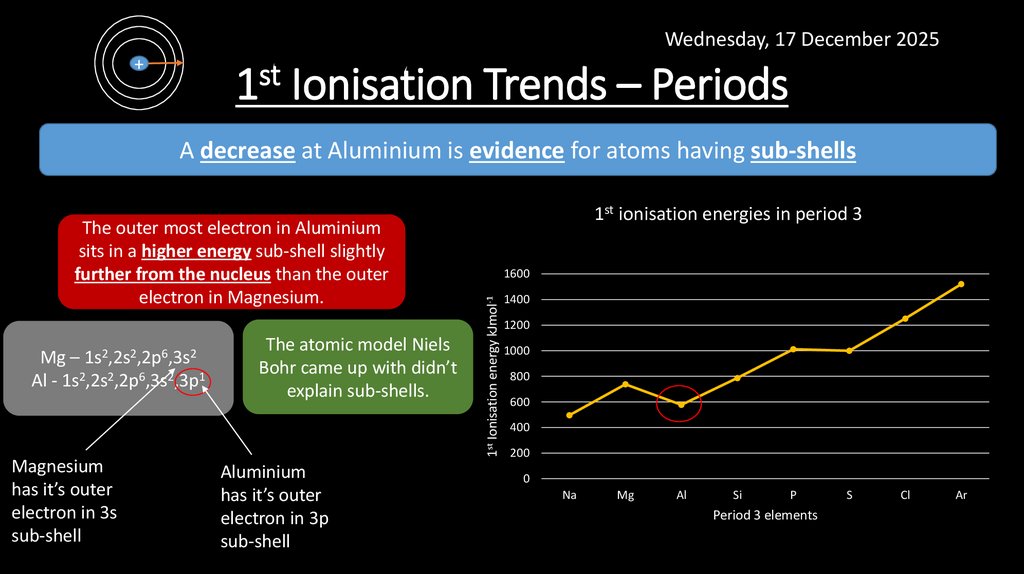
11. 1st Ionisation Trends – Periods
Wednesday, 17 December 2025+
1st Ionisation Trends – Periods
A decrease at Aluminium is evidence for atoms having sub-shells
Mg – 1s2,2s2,2p6,3s2
Al - 1s2,2s2,2p6,3s2,3p1
Magnesium
has it’s outer
electron in 3s
sub-shell
The atomic model Niels
Bohr came up with didn’t
explain sub-shells.
Aluminium
has it’s outer
electron in 3p
sub-shell
1600
1st Ionisation energy kJmol-1
The outer most electron in Aluminium
sits in a higher energy sub-shell slightly
further from the nucleus than the outer
electron in Magnesium.
1st ionisation energies in period 3
1400
1200
1000
800
600
400
200
0
Na
Mg
Al
Si
P
Period 3 elements
S
Cl
Ar
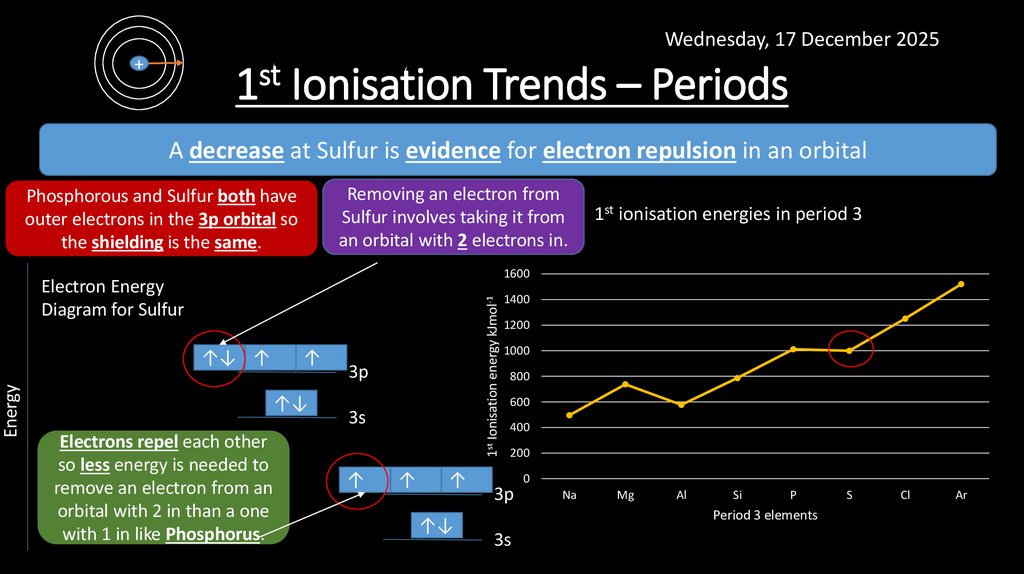
12. 1st Ionisation Trends – Periods
Wednesday, 17 December 2025+
1st Ionisation Trends – Periods
A decrease at Sulfur is evidence for electron repulsion in an orbital
Removing an electron from
Sulfur involves taking it from
an orbital with 2 electrons in.
Phosphorous and Sulfur both have
outer electrons in the 3p orbital so
the shielding is the same.
1600
↑↓ ↑
↑
↑↓
Electrons
each
other
↑↓repel
↑↓
↑↓
so less energy is needed2p
to
remove an electron
↑↓ from an
2s
orbital with 2 in than a one
↑↓
with 1 in like
1s Phosphorus.
1st Ionisation energy kJmol-1
Electron Energy
Diagram for Sulfur
Energy
1st ionisation energies in period 3
3p
3s
↑
↑
↑
↑↓
1400
1200
1000
800
600
400
200
3p
0
Na
Mg
Al
Si
P
Period 3 elements
3s
S
Cl
Ar

 Химия
Химия








