Похожие презентации:
2024-08-21 INH-023WO Replacement Drawings5704890.1
1.
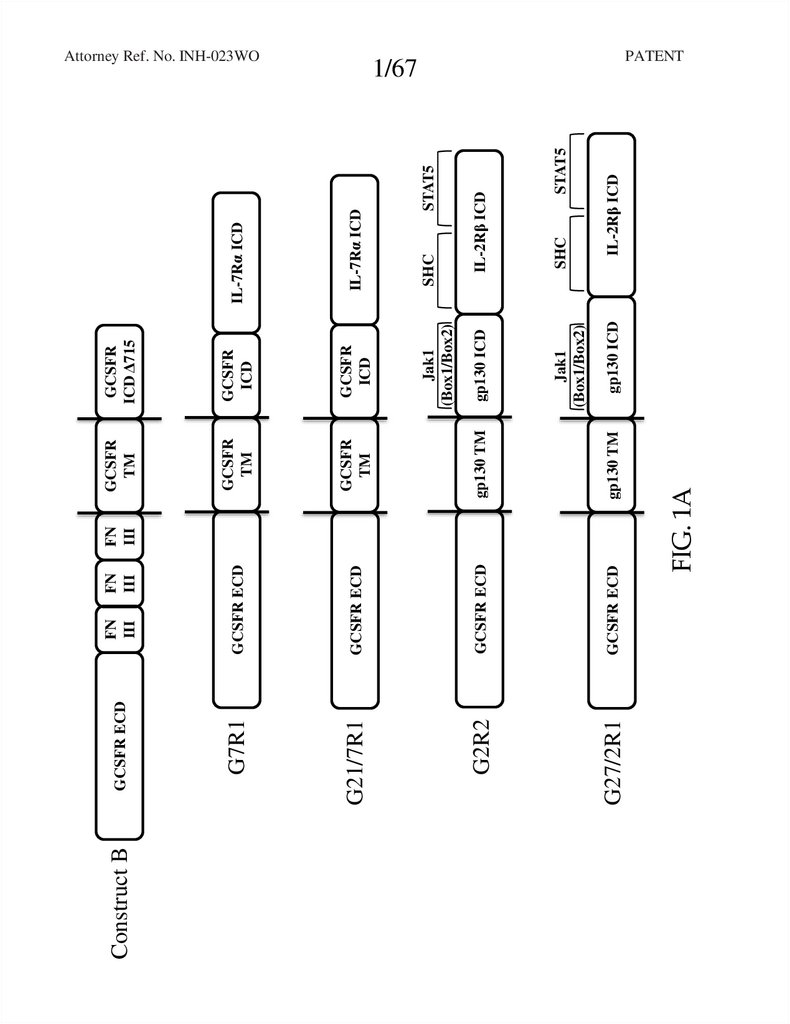
Construct BGCSFR ECD
GCSFR ECD
G2R2
G27/2R1
FN
III
GCSFR
ICD
GCSFR
TM
gp130 TM
gp130 TM
GCSFR
ICD
GCSFR
TM
gp130 ICD
IL-2Rβ ICD
STAT5
SHC
Jak1
(Box1/Box2)
STAT5
IL-2Rβ ICD
SHC
IL-7Rα ICD
IL-7Rα ICD
gp130 ICD
Jak1
(Box1/Box2)
GCSFR
ICD Δ715
GCSFR
TM
1/67
FIG. 1A
GCSFR ECD
G21/7R1
FN
III
GCSFR ECD
FN
III
G7R1
GCSFR ECD
Attorney Ref. No. INH-023WO
PATENT
2.
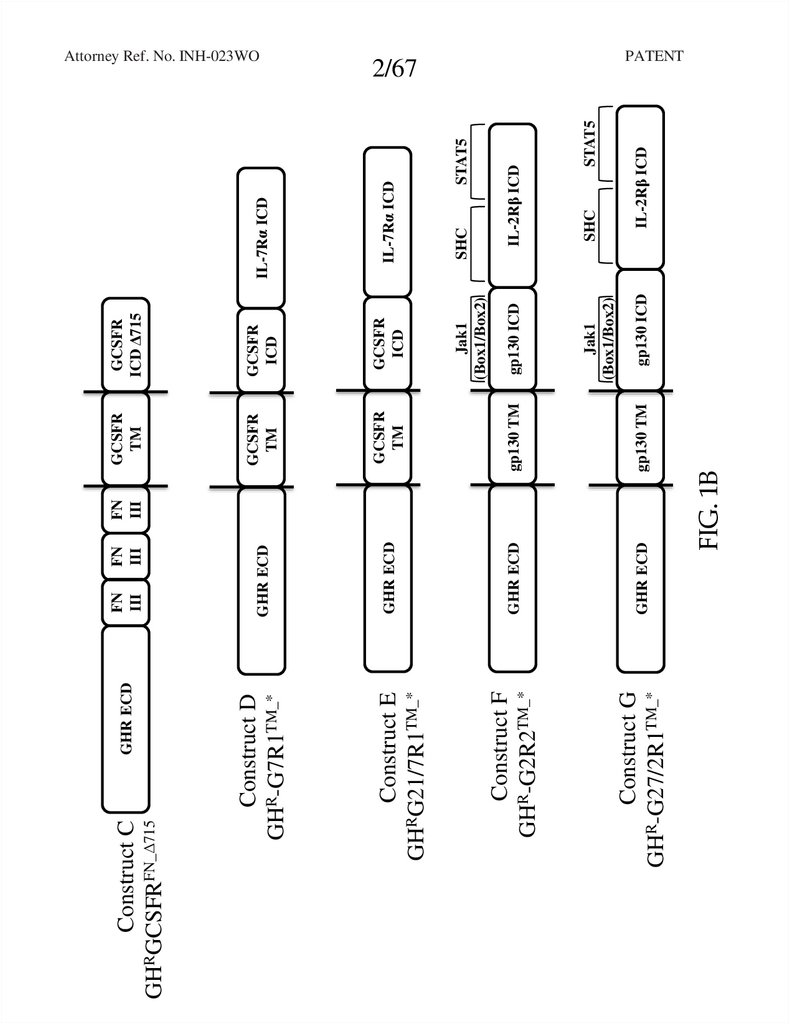
GHR ECDFN
III
GHR ECD
Construct G
GHR-G27/2R1TM_*
FN
III
gp130 TM
gp130 TM
GCSFR
ICD
GCSFR
TM
gp130 ICD
IL-2Rβ ICD
STAT5
SHC
Jak1
(Box1/Box2)
STAT5
IL-2Rβ ICD
SHC
IL-7Rα ICD
IL-7Rα ICD
gp130 ICD
Jak1
(Box1/Box2)
GCSFR
ICD
GCSFR
ICD Δ715
GCSFR
TM
GCSFR
TM
2/67
FIG. 1B
GHR ECD
GHR ECD
GHR ECD
FN
III
Construct F
GHR-G2R2TM_*
Construct E
GHRG21/7R1TM_*
Construct D
GHR-G7R1TM_*
Construct C
GHRGCSFRFN_Δ715
Attorney Ref. No. INH-023WO
PATENT
3.
3/67PATENT
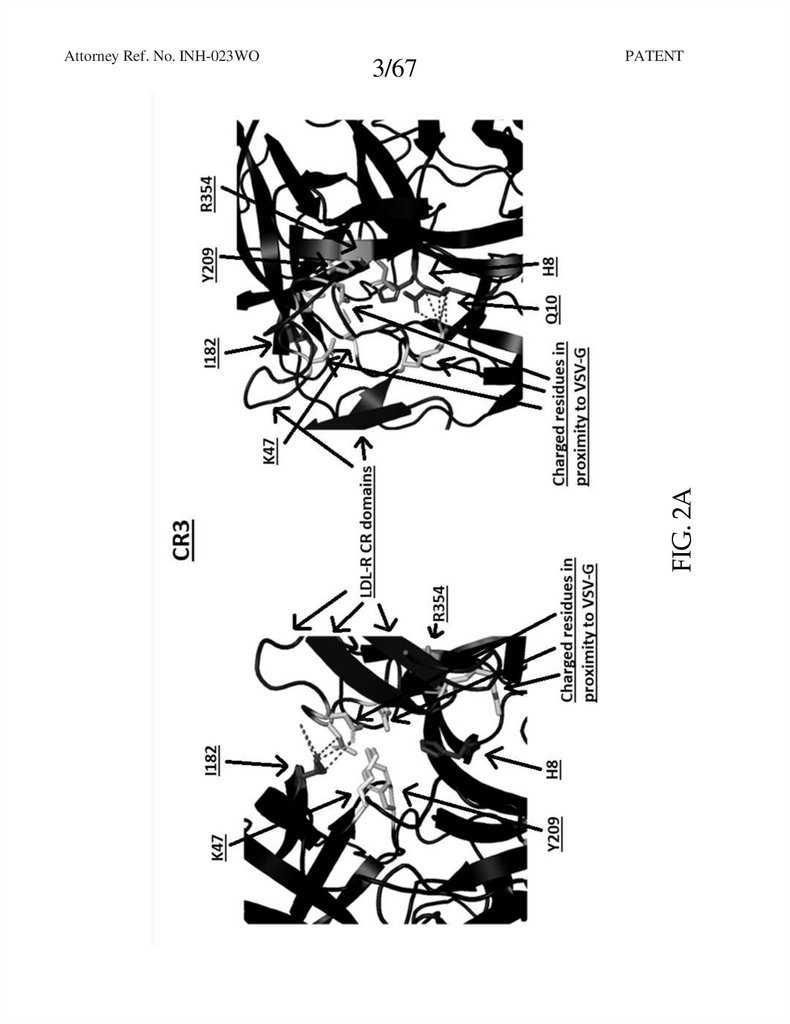
FIG. 2A
Attorney Ref. No. INH-023WO
4.
4/67PATENT
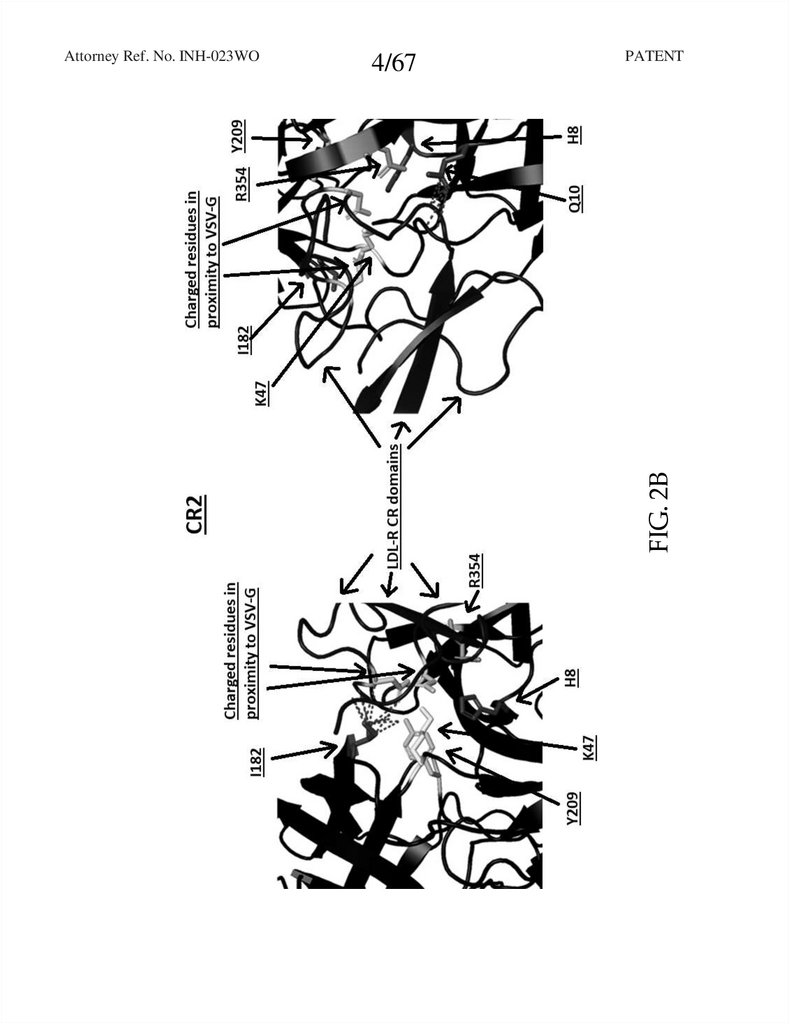
FIG. 2B
Attorney Ref. No. INH-023WO
5.
Attorney Ref. No. INH-023WO5/67
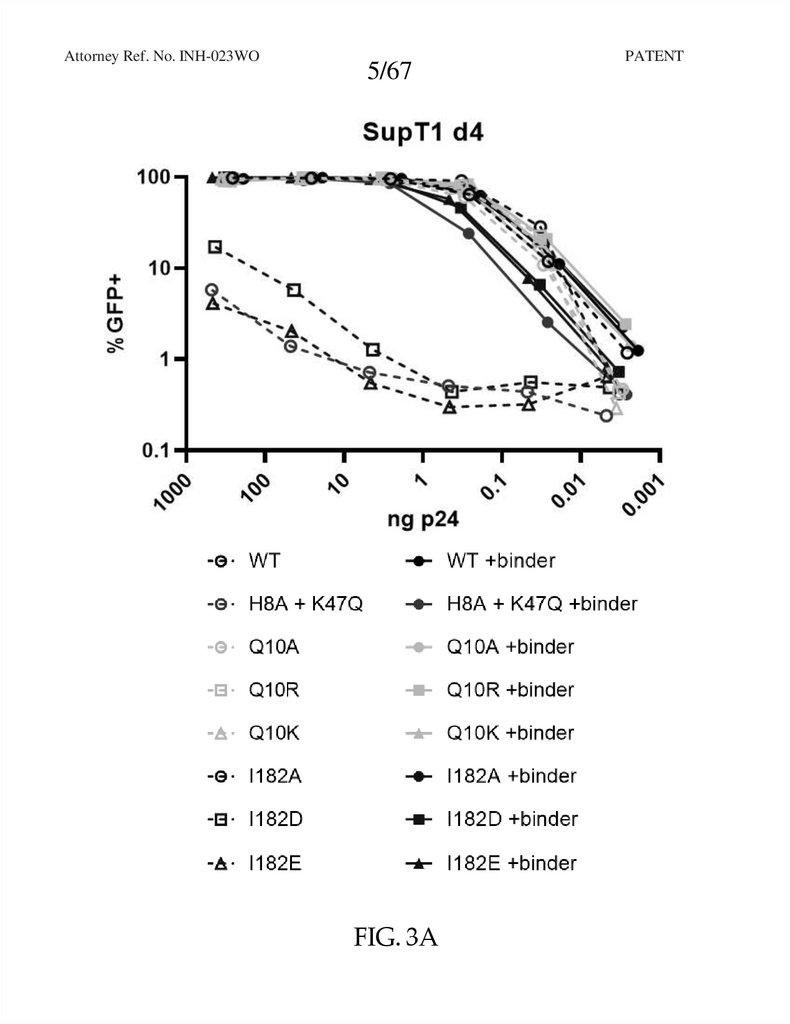
FIG. 3A
PATENT
6.
6/67PATENT
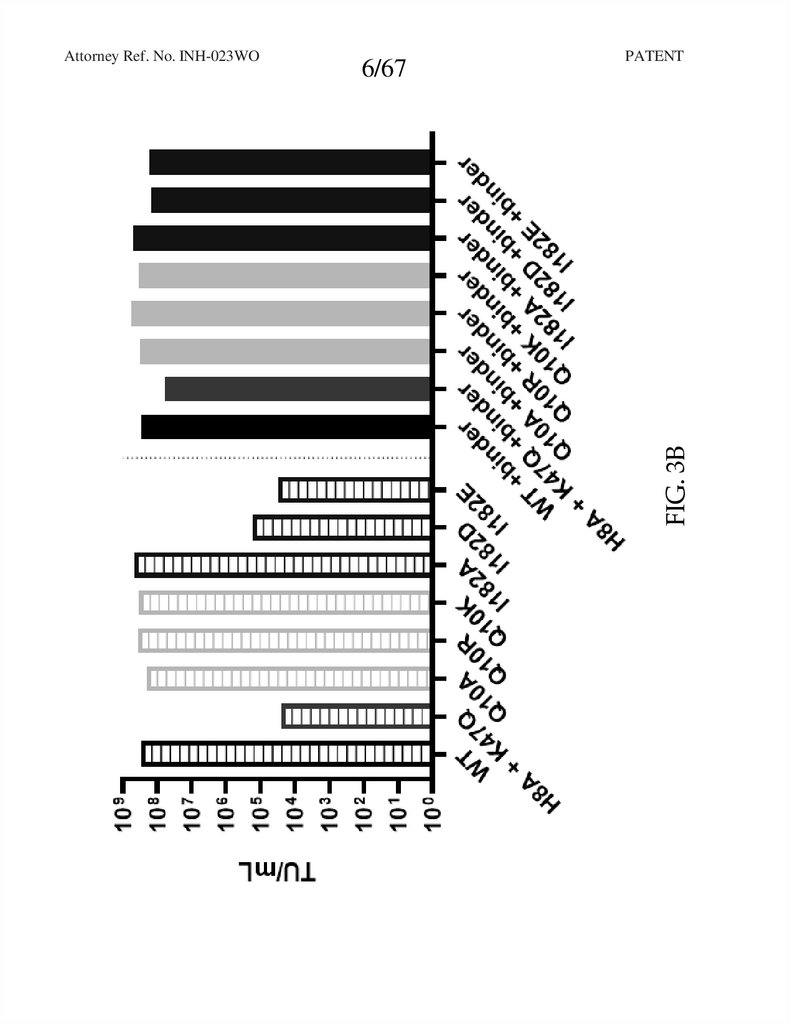
FIG. 3B
Attorney Ref. No. INH-023WO
7.
PATENT7/67
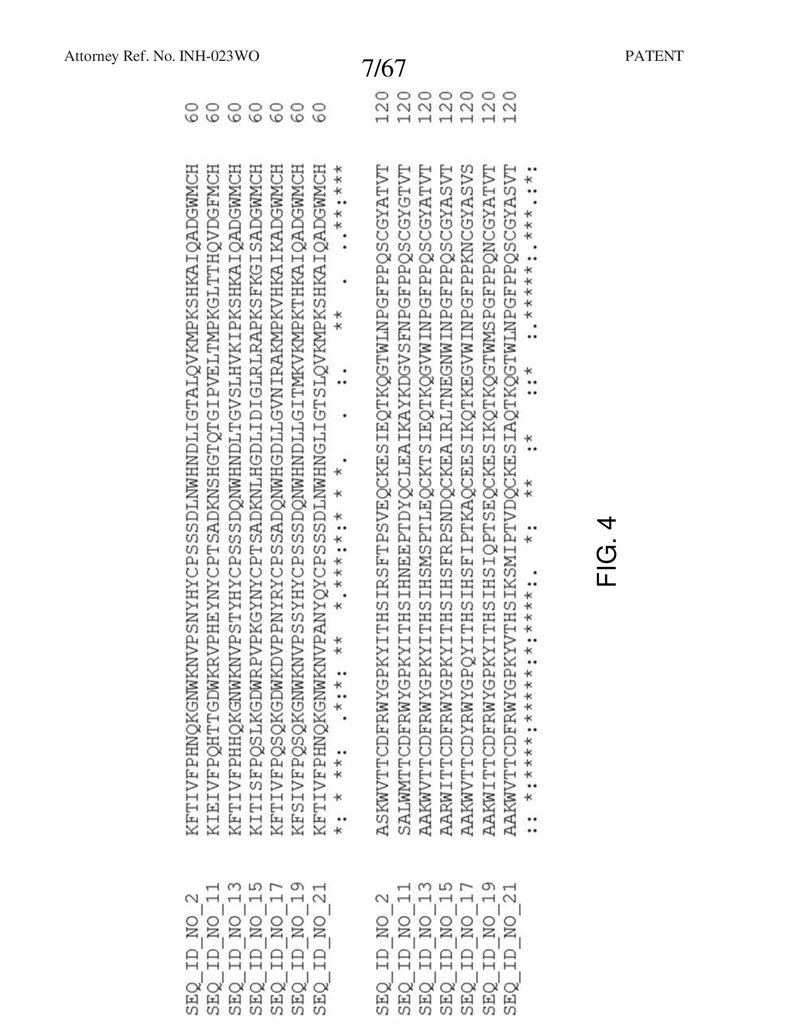
FIG. 4
Attorney Ref. No. INH-023WO
8.
PATENT8/67
FIG. 4 (Continued)
Attorney Ref. No. INH-023WO
9.
PATENT9/67
FIG. 4 (Continued)
Attorney Ref. No. INH-023WO
10.
10/67PATENT
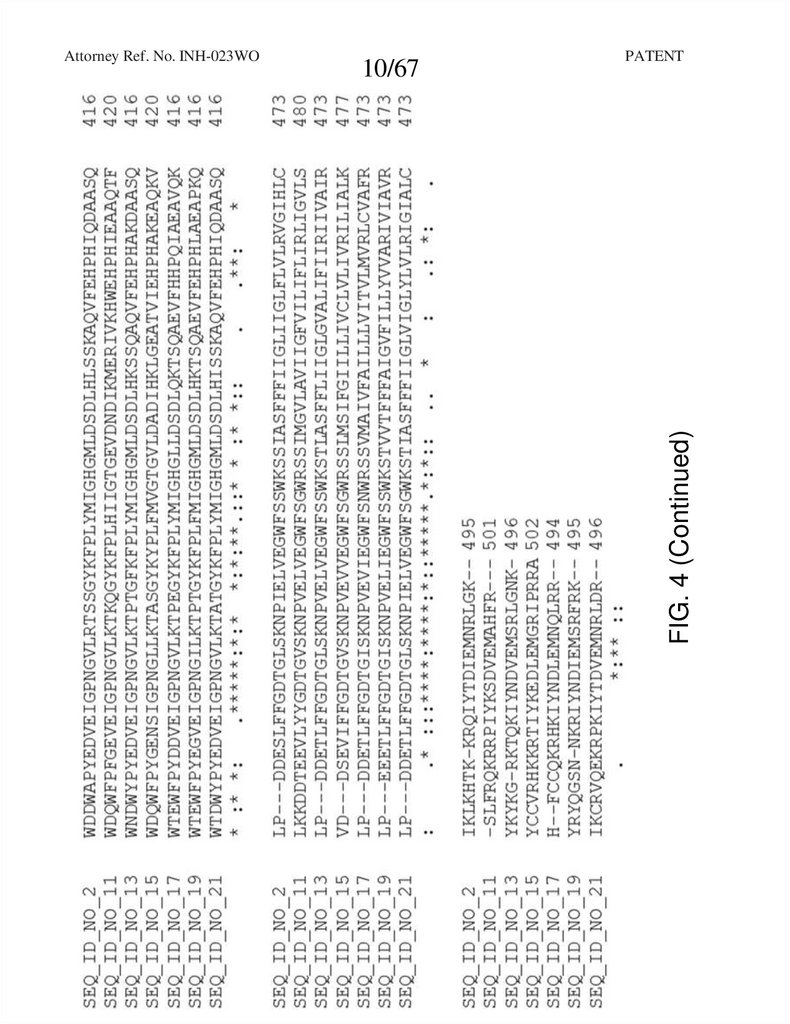
FIG. 4 (Continued)
Attorney Ref. No. INH-023WO
11.
11/67PATENT
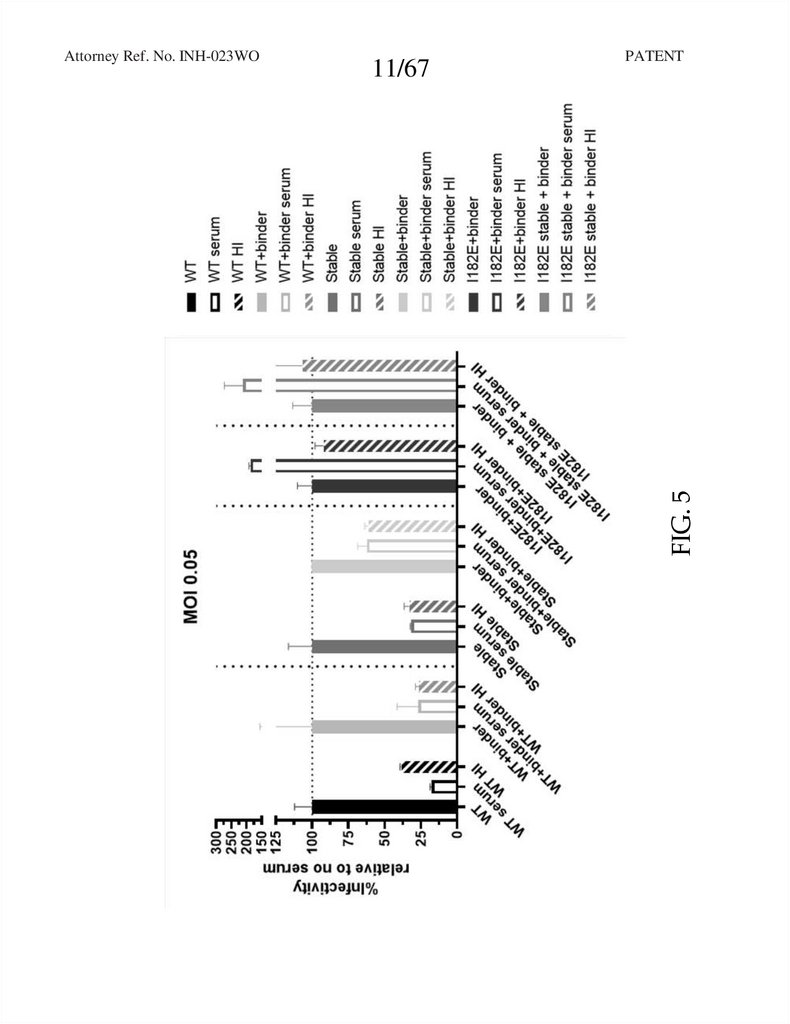
FIG. 5
Attorney Ref. No. INH-023WO
12.
12/67PATENT
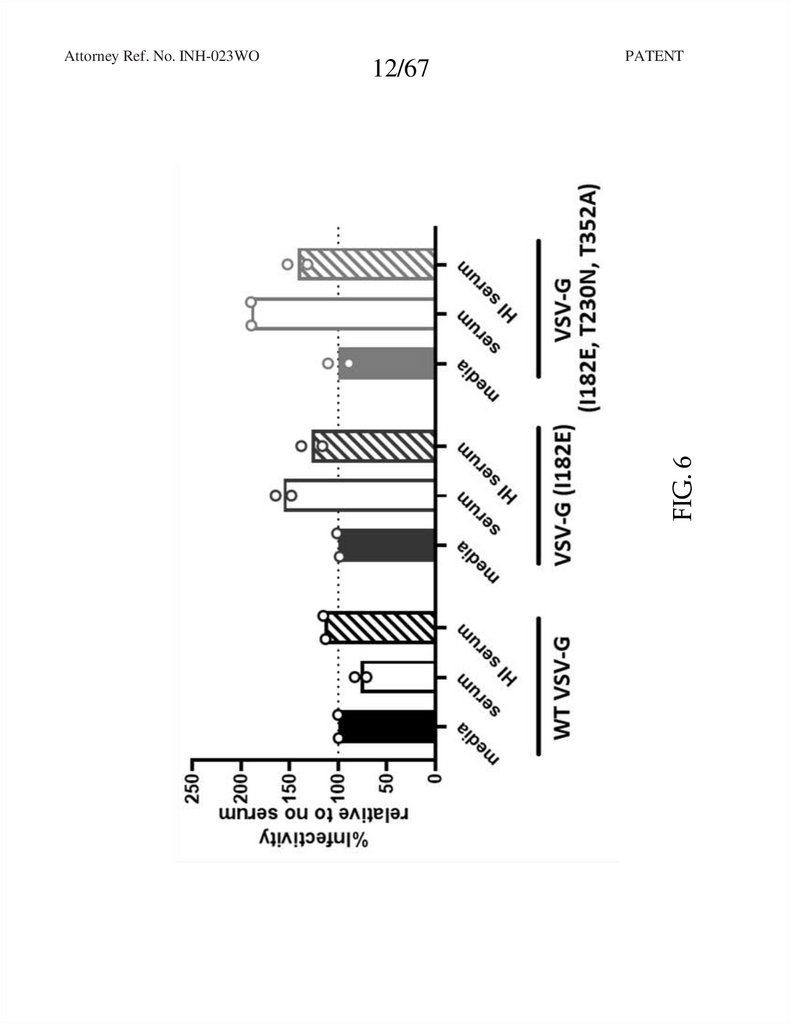
FIG. 6
Attorney Ref. No. INH-023WO
13.
13/67PATENT
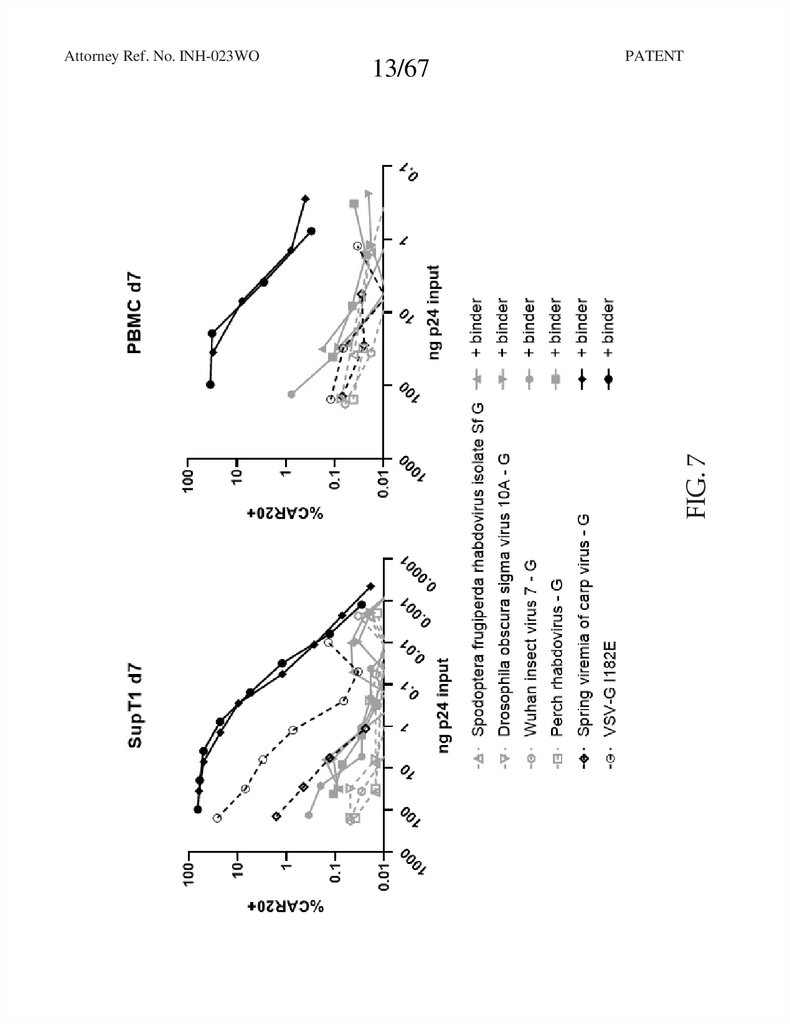
FIG. 7
Attorney Ref. No. INH-023WO
14.
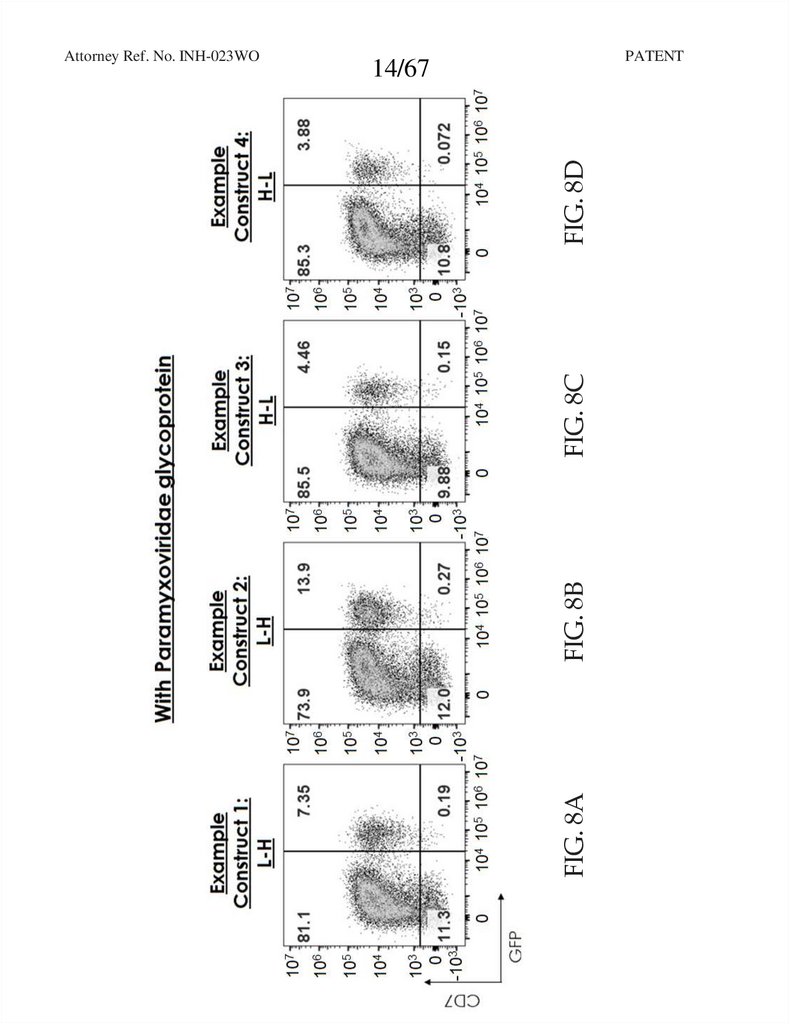
PATENTFIG. 8B
FIG. 8C
FIG. 8D
14/67
FIG. 8A
Attorney Ref. No. INH-023WO
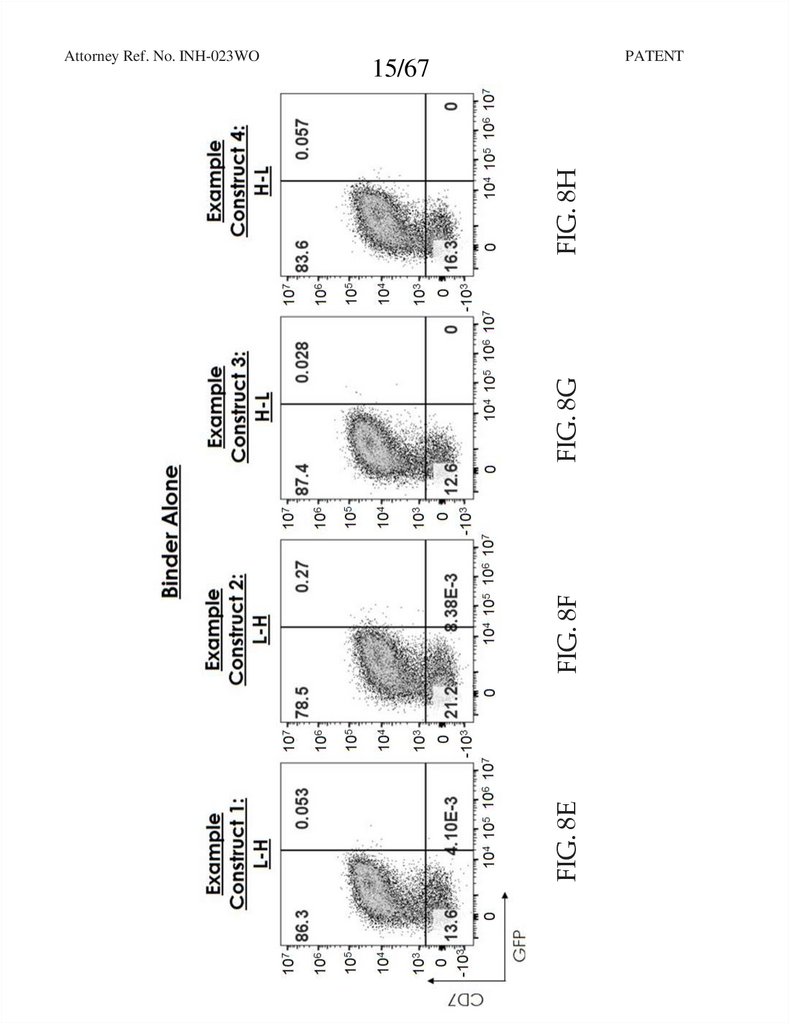
15.
PATENTFIG. 8F
FIG. 8G
FIG. 8H
15/67
FIG. 8E
Attorney Ref. No. INH-023WO
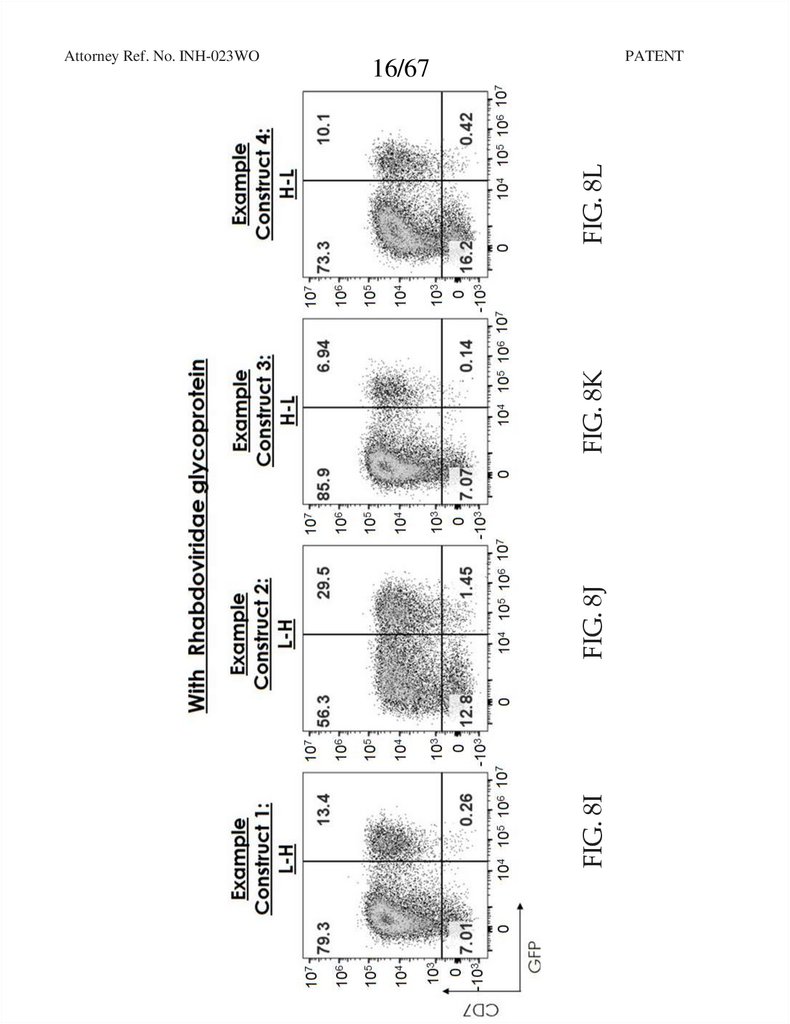
16.
PATENTFIG. 8J
FIG. 8K
FIG. 8L
16/67
FIG. 8I
Attorney Ref. No. INH-023WO
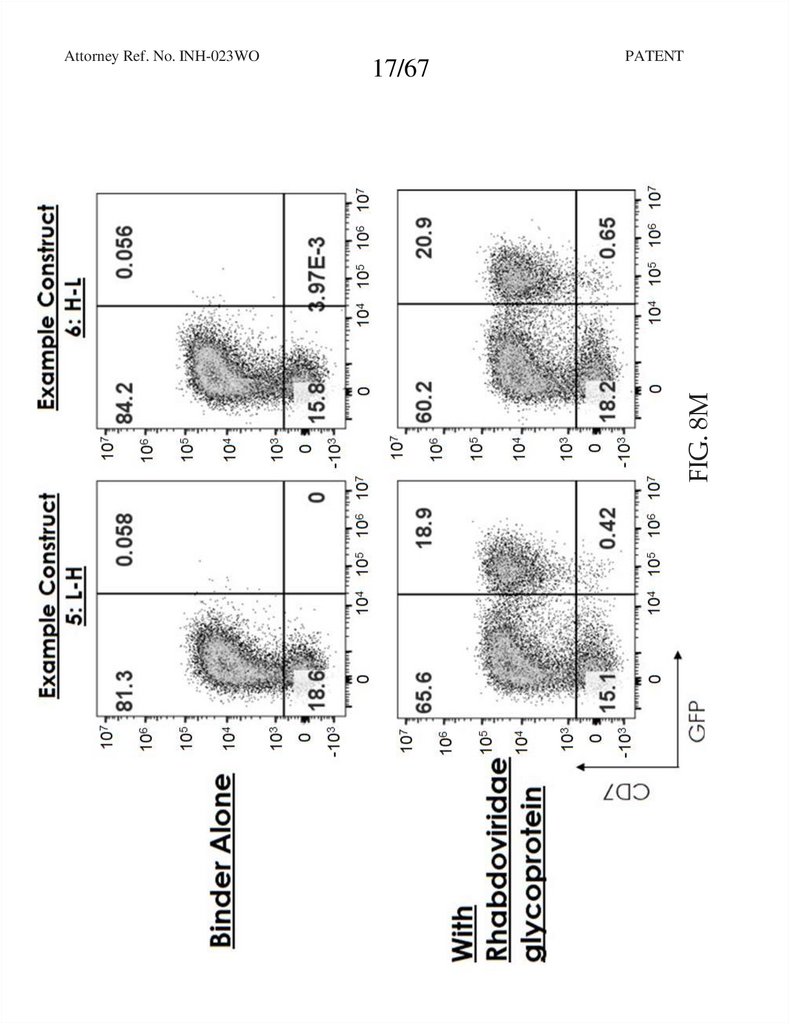
17.
17/67PATENT
FIG. 8M
Attorney Ref. No. INH-023WO
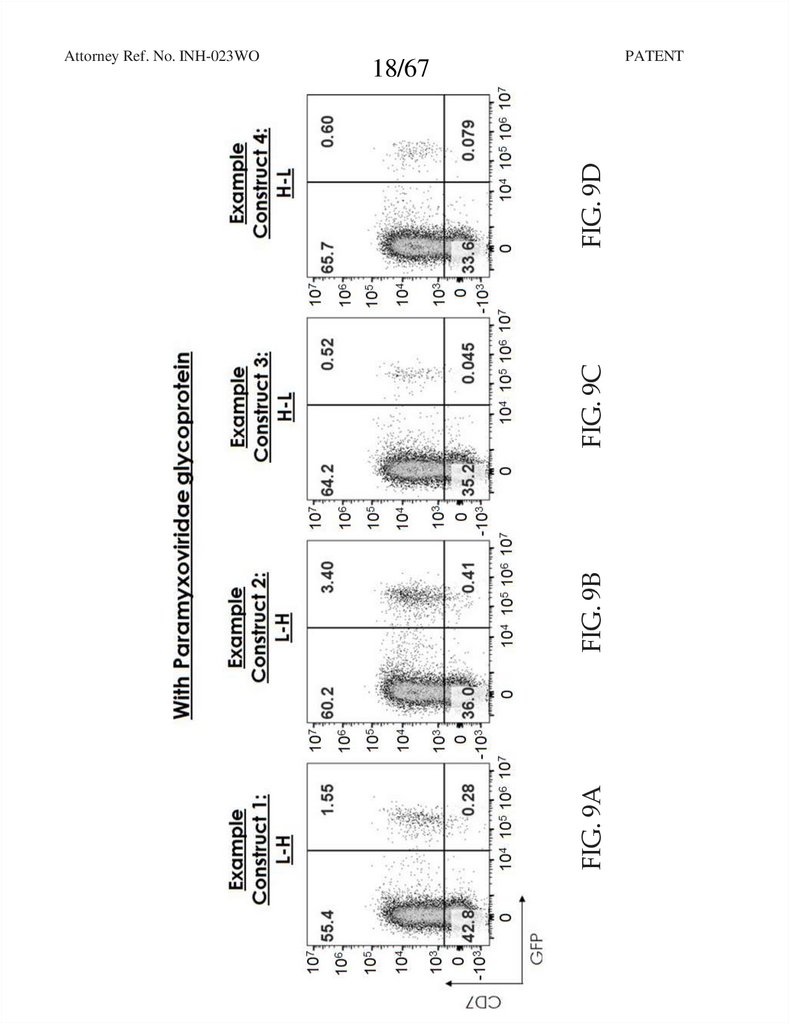
18.
PATENTFIG. 9B
FIG. 9C
FIG. 9D
18/67
FIG. 9A
Attorney Ref. No. INH-023WO
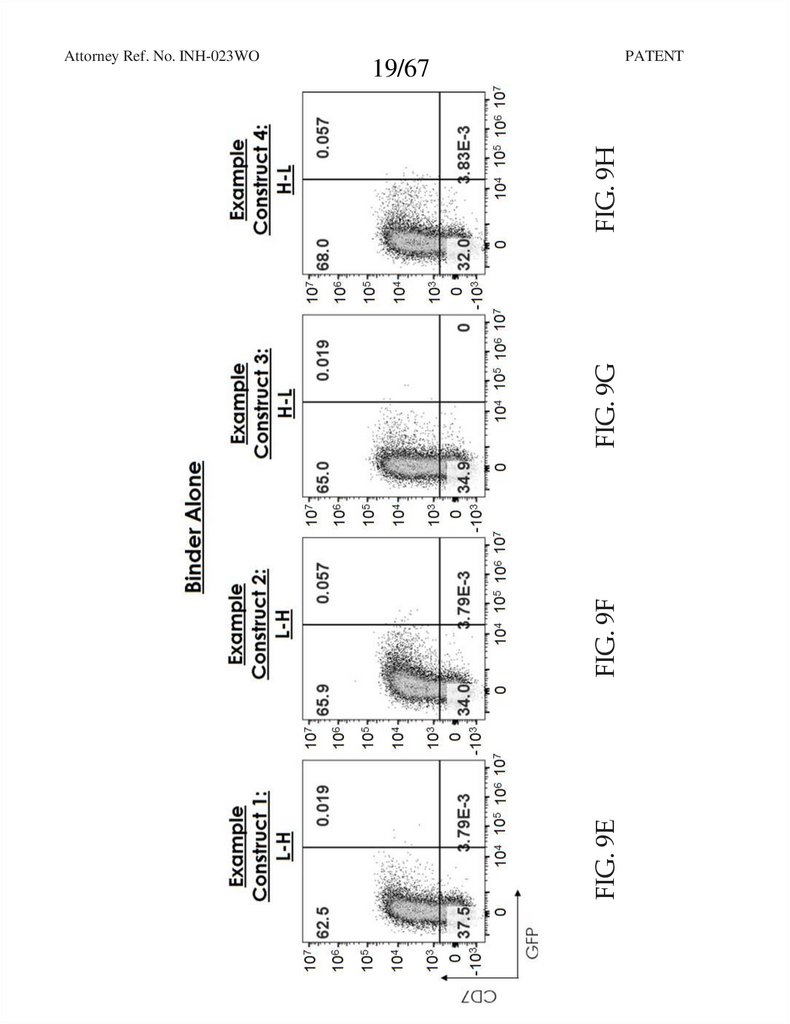
19.
PATENTFIG. 9F
FIG. 9G
FIG. 9H
19/67
FIG. 9E
Attorney Ref. No. INH-023WO
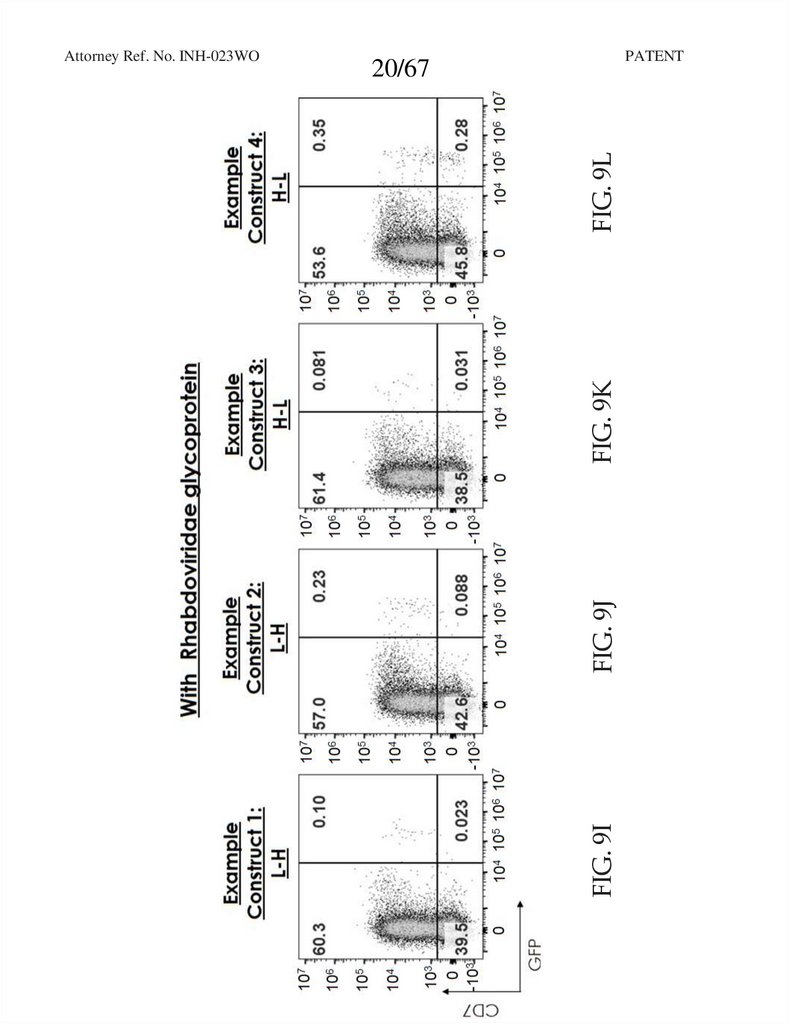
20.
PATENTFIG. 9J
FIG. 9K
FIG. 9L
20/67
FIG. 9I
Attorney Ref. No. INH-023WO
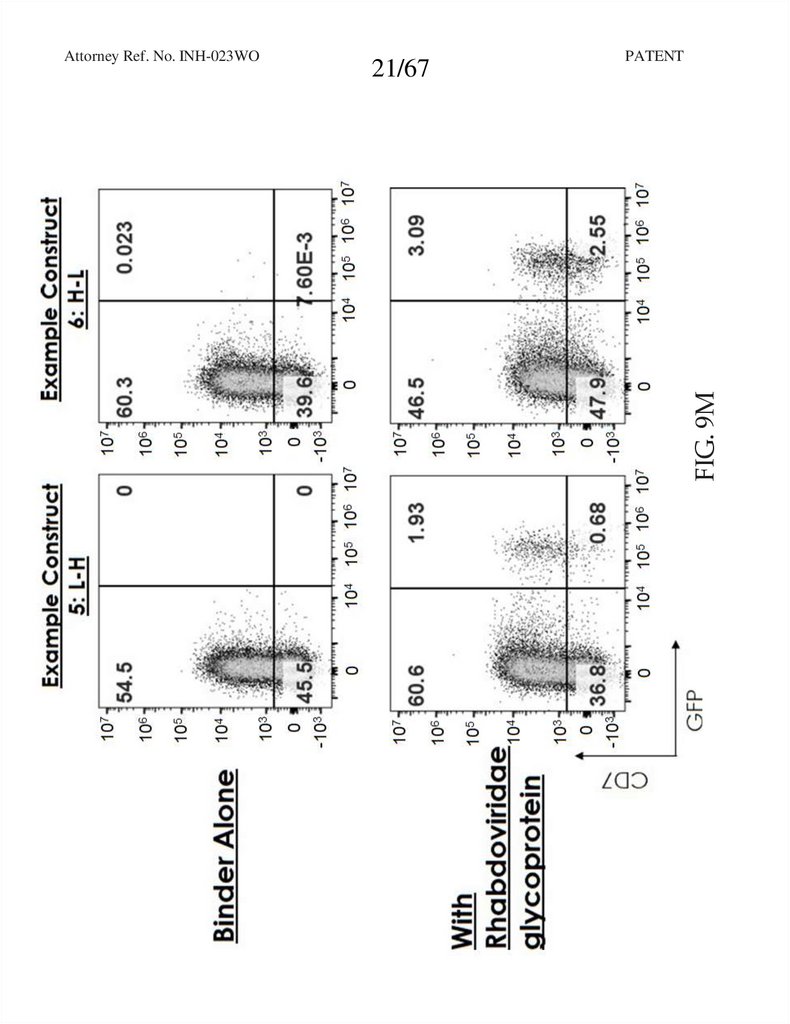
21.
21/67PATENT
FIG. 9M
Attorney Ref. No. INH-023WO
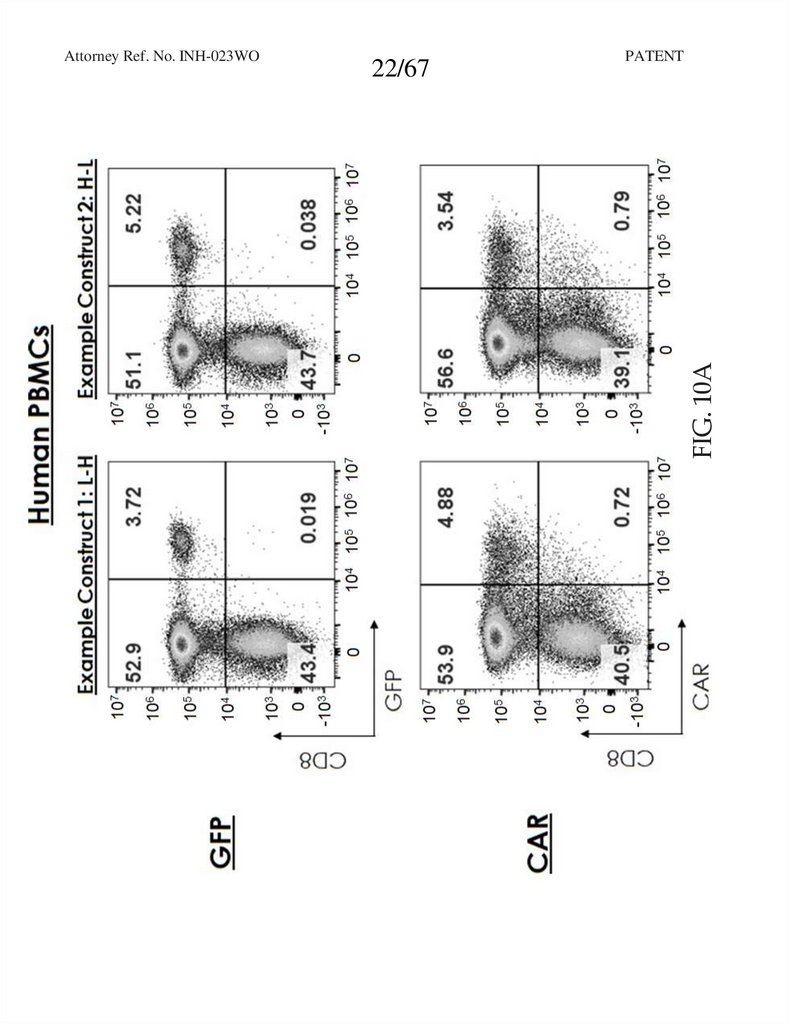
22.
22/67PATENT
FIG. 10A
Attorney Ref. No. INH-023WO
23.
23/67PATENT
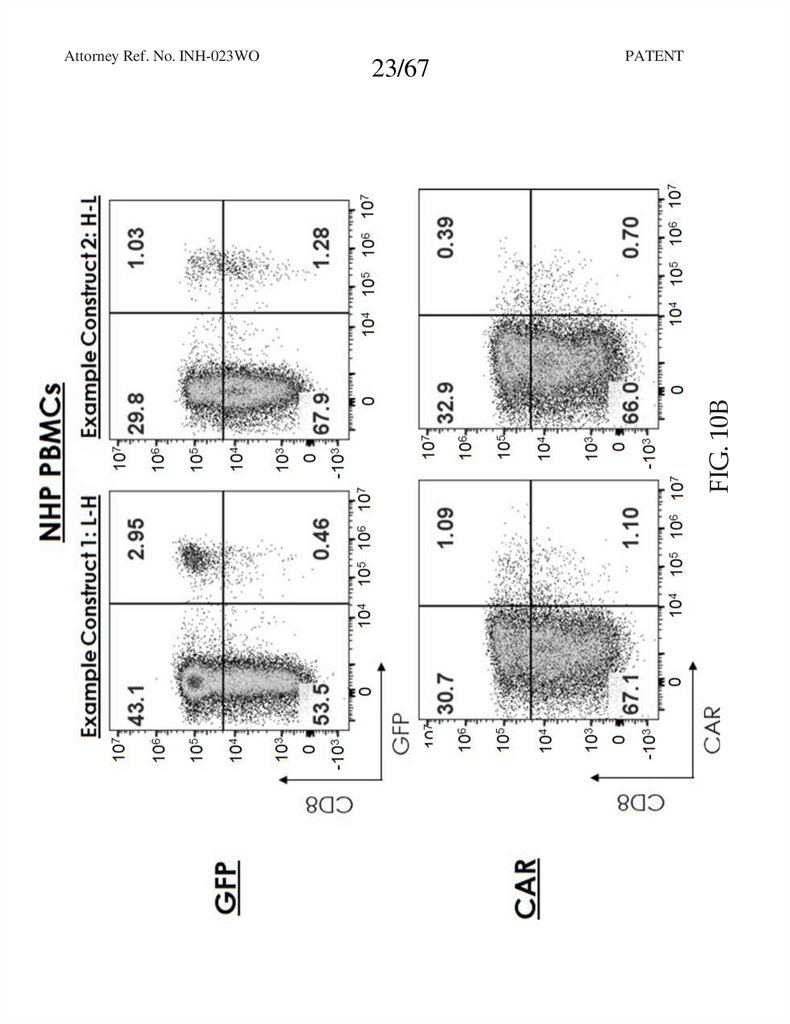
FIG. 10B
Attorney Ref. No. INH-023WO
24.
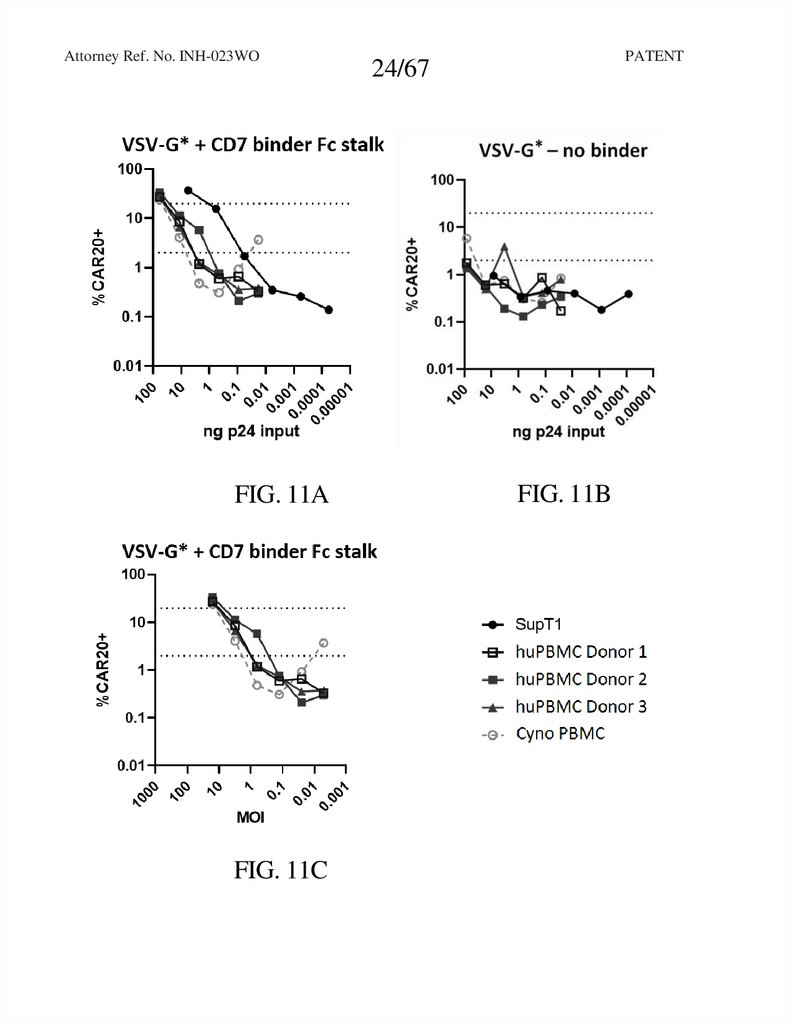
Attorney Ref. No. INH-023WOFIG. 11A
FIG. 11C
PATENT
24/67
FIG. 11B
25.
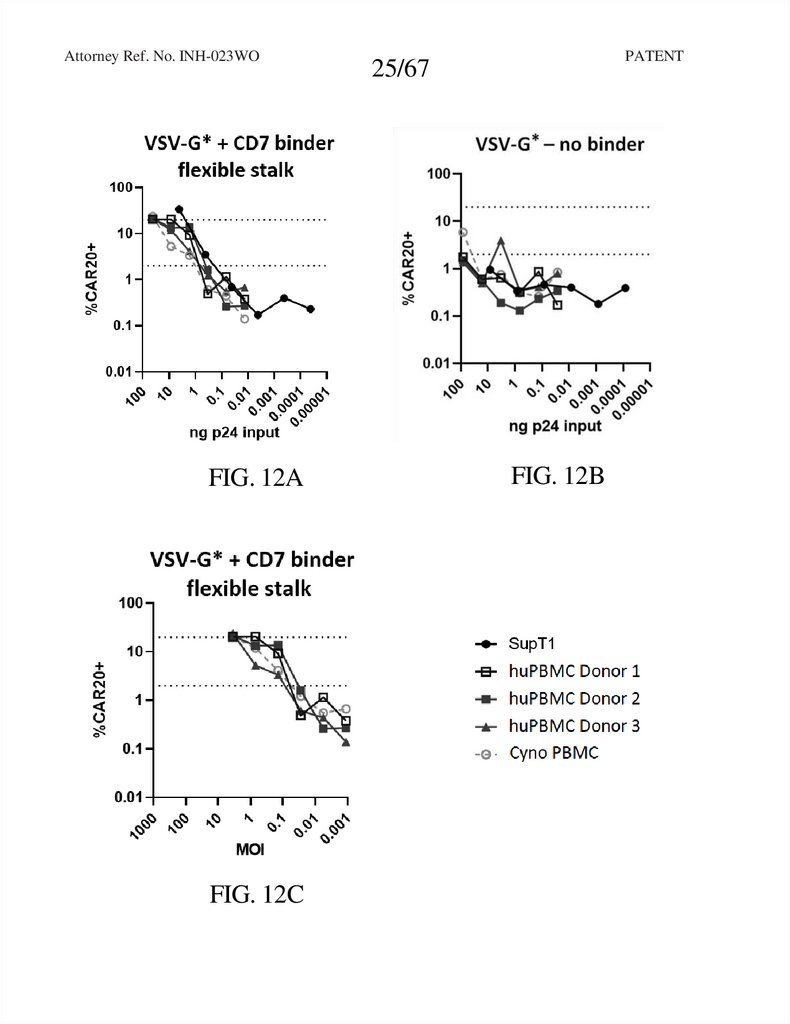
Attorney Ref. No. INH-023WOFIG. 12A
FIG. 12C
PATENT
25/67
FIG. 12B
26.
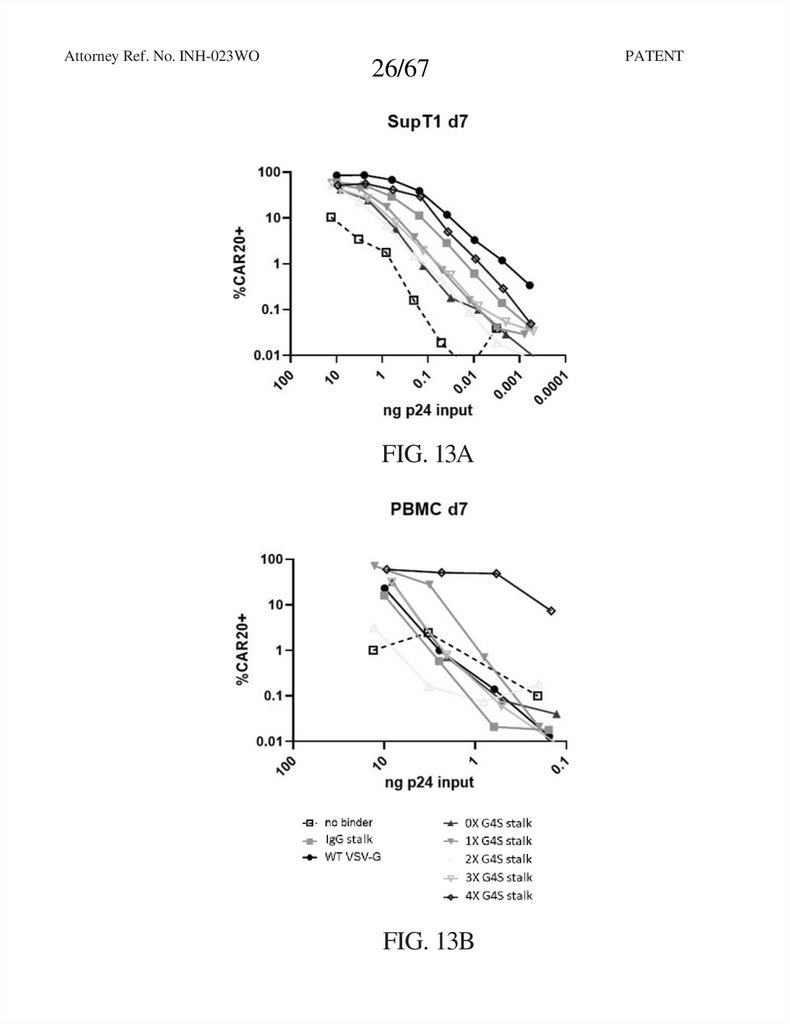
Attorney Ref. No. INH-023WO26/67
FIG. 13A
FIG. 13B
PATENT
27.
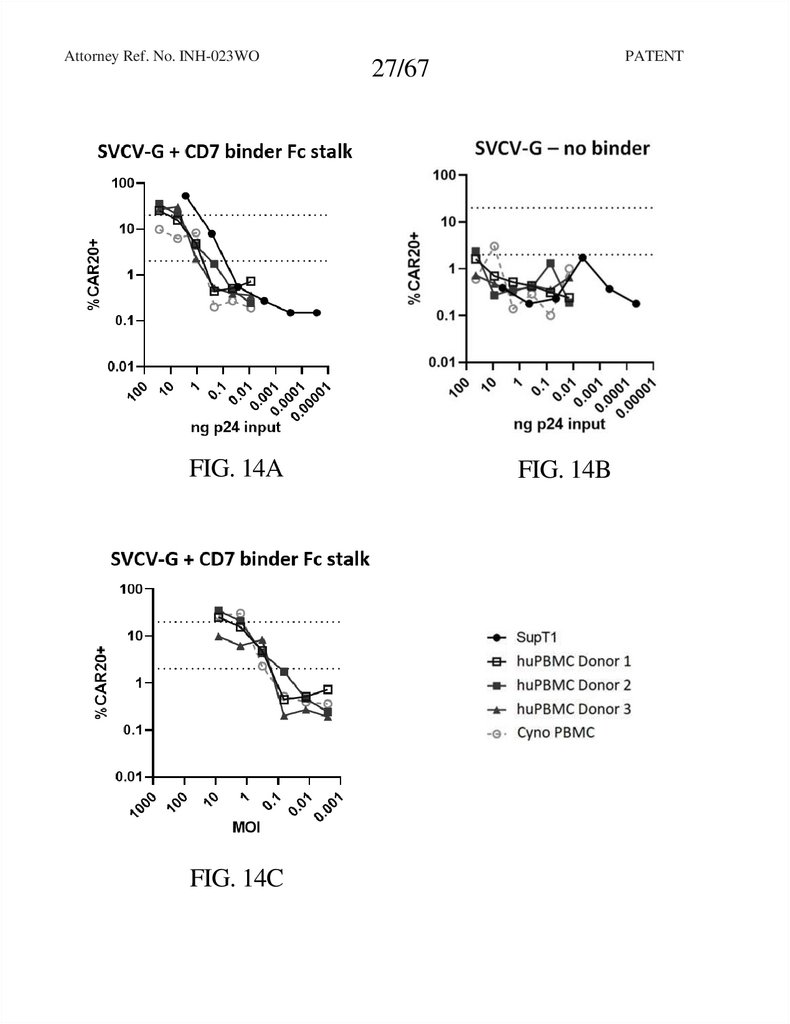
Attorney Ref. No. INH-023WOFIG. 14A
FIG. 14C
PATENT
27/67
FIG. 14B
28.
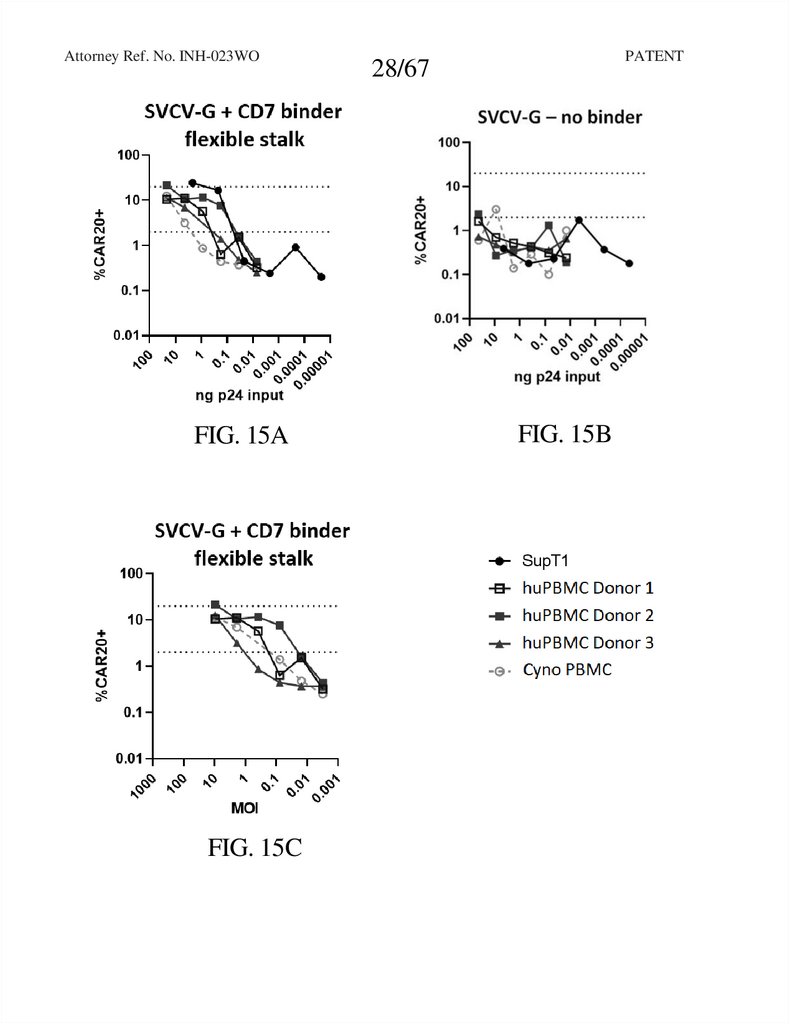
Attorney Ref. No. INH-023WOFIG. 15A
FIG. 15C
PATENT
28/67
FIG. 15B
29.
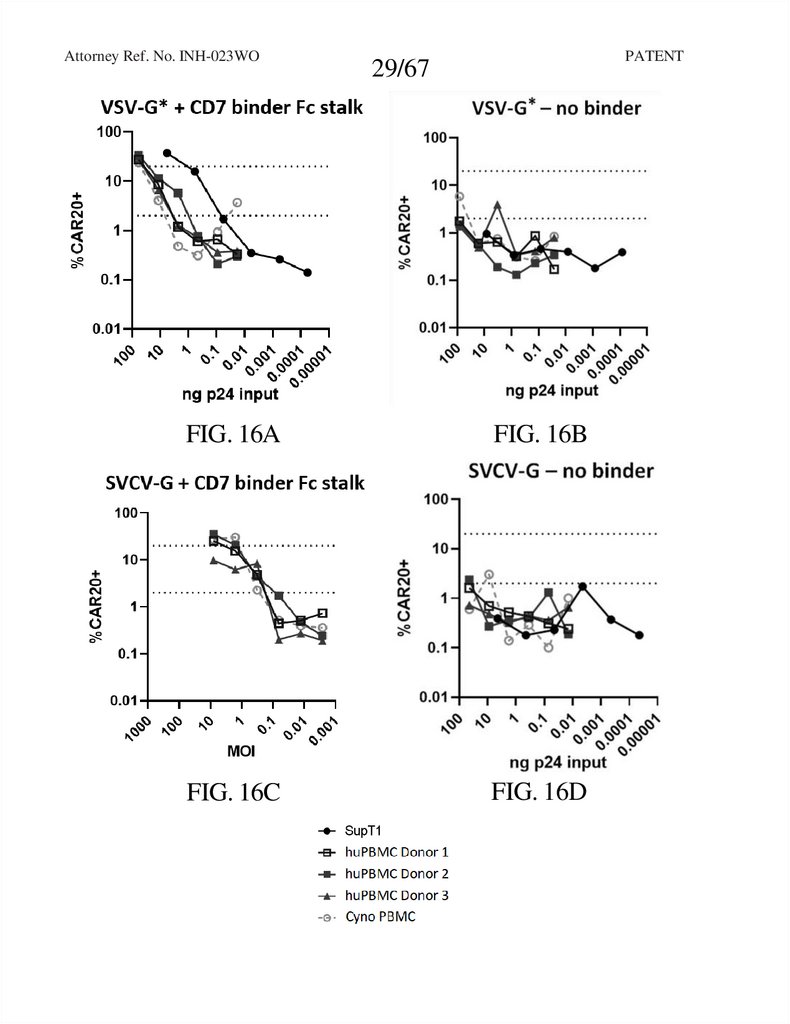
Attorney Ref. No. INH-023WOPATENT
29/67
FIG. 16A
FIG. 16B
FIG. 16C
FIG. 16D
30.
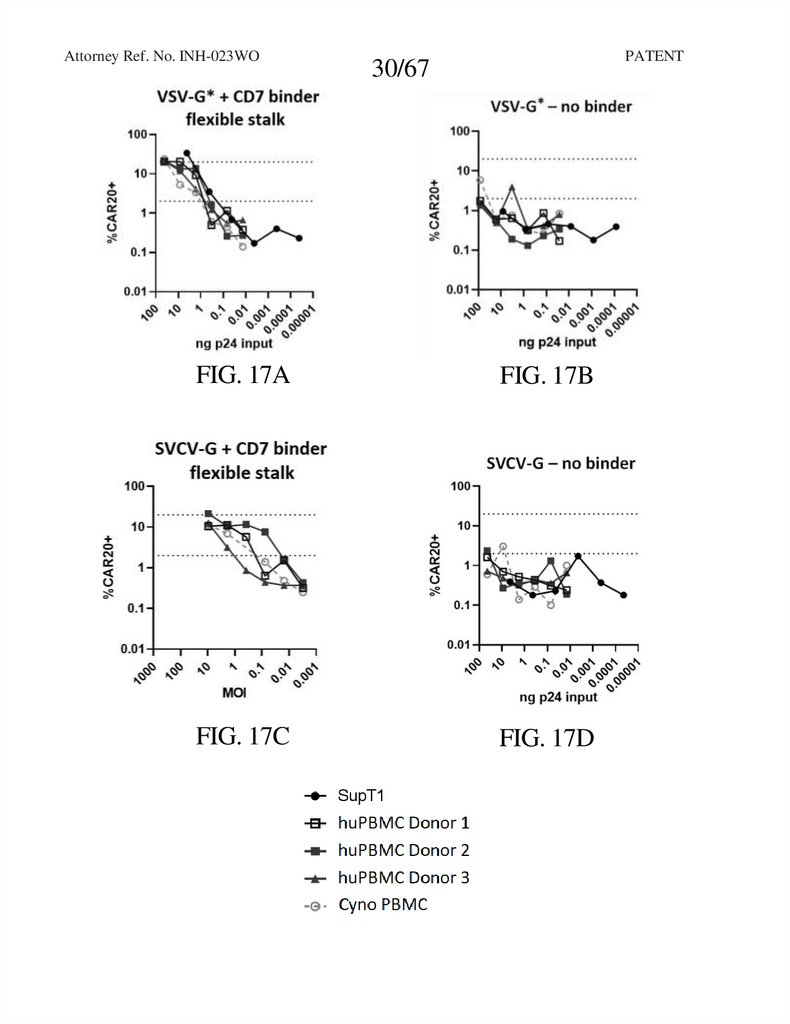
Attorney Ref. No. INH-023WOPATENT
30/67
FIG. 17A
FIG. 17B
FIG. 17C
FIG. 17D
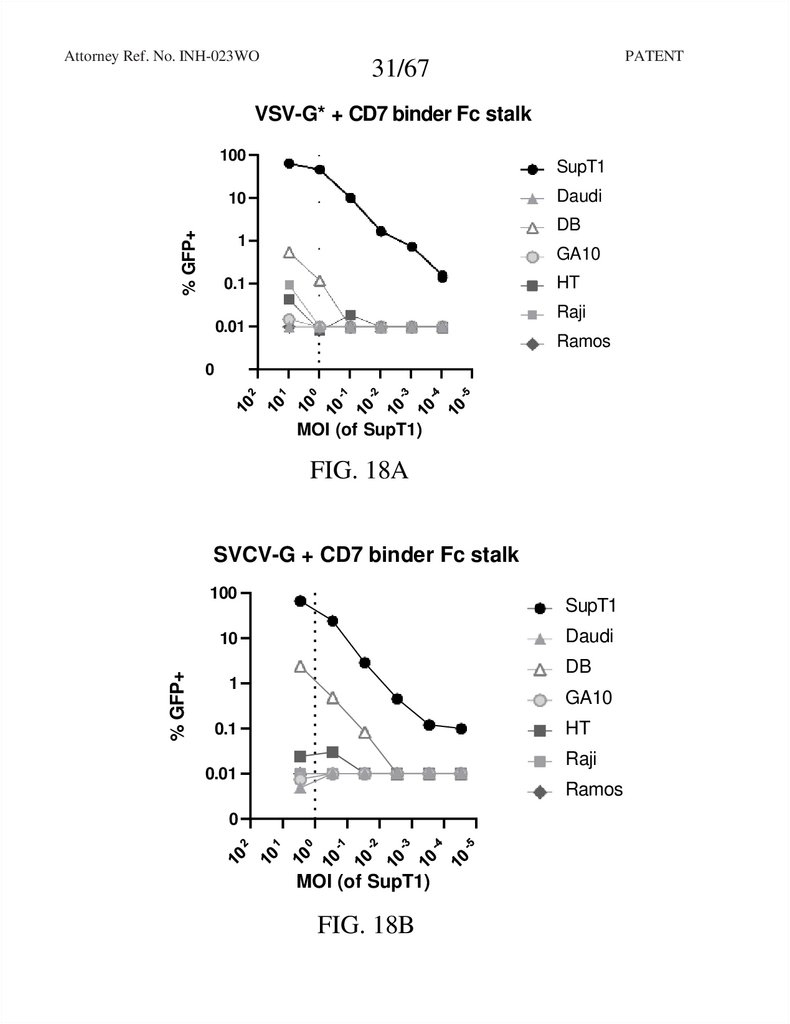
31.
Attorney Ref. No. INH-023WOPATENT
31/67
VSV-G* + CD7 binder Fc stalk
100
SupT1
Daudi
10
% GFP+
DB
1
GA10
HT
0.1
Raji
0.01
Ramos
0
MOI (of SupT1)
FIG. 18A
SVCV-G + CD7 binder Fc stalk
100
SupT1
Daudi
% GFP+
10
DB
1
GA10
HT
0.1
Raji
0.01
Ramos
0
MOI (of SupT1)
FIG. 18B
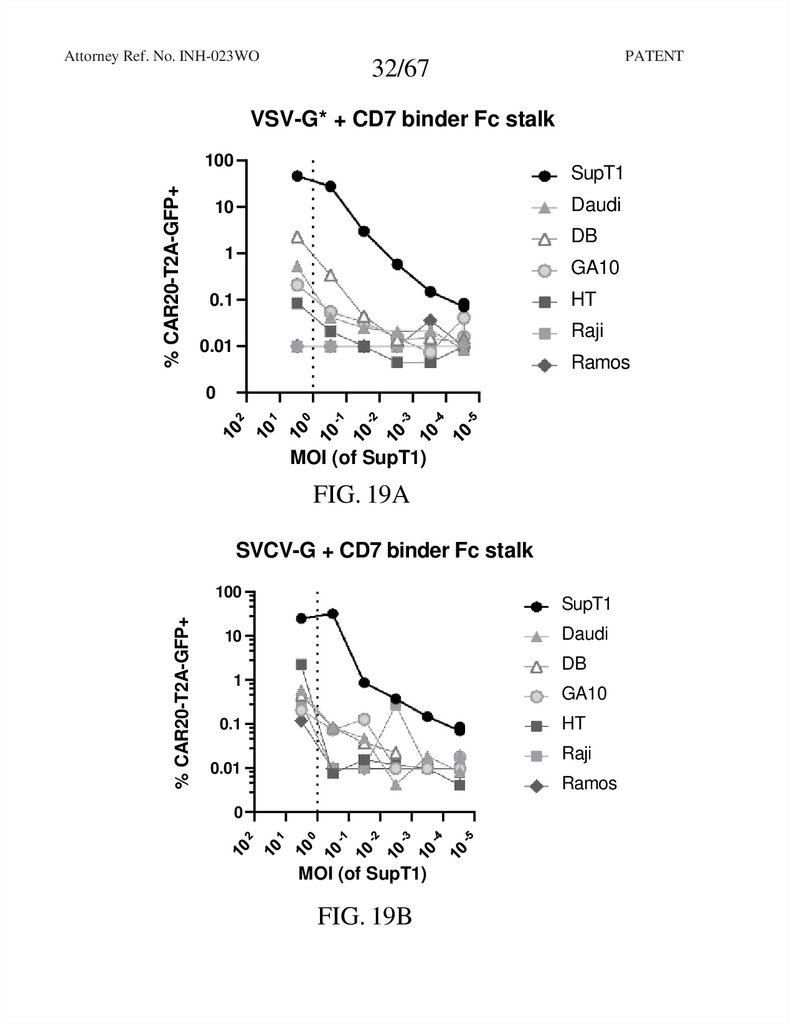
32.
Attorney Ref. No. INH-023WOPATENT
32/67
VSV-G* + CD7 binder Fc stalk
% CAR20-T2A-GFP+
100
SupT1
Daudi
10
DB
1
GA10
HT
0.1
Raji
0.01
Ramos
0
MOI (of SupT1)
FIG. 19A
SVCV-G + CD7 binder Fc stalk
% CAR20-T2A-GFP+
100
SupT1
Daudi
10
DB
1
GA10
HT
0.1
Raji
0.01
Ramos
0
MOI (of SupT1)
FIG. 19B
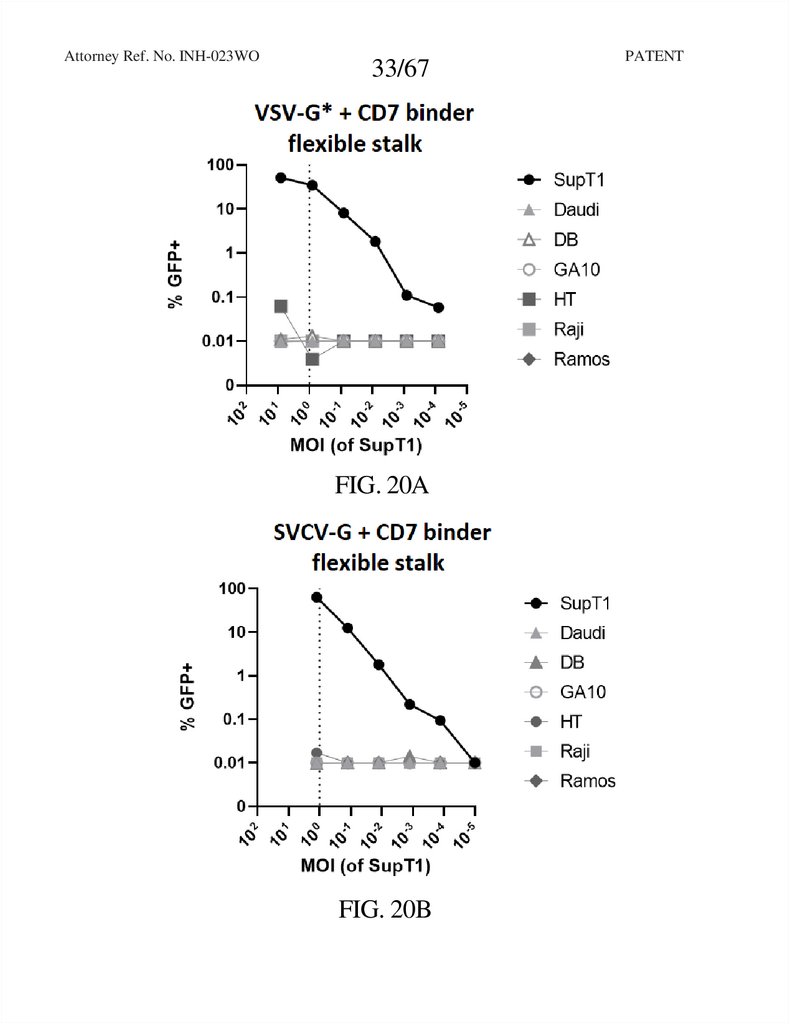
33.
Attorney Ref. No. INH-023WO33/67
FIG. 20A
FIG. 20B
PATENT
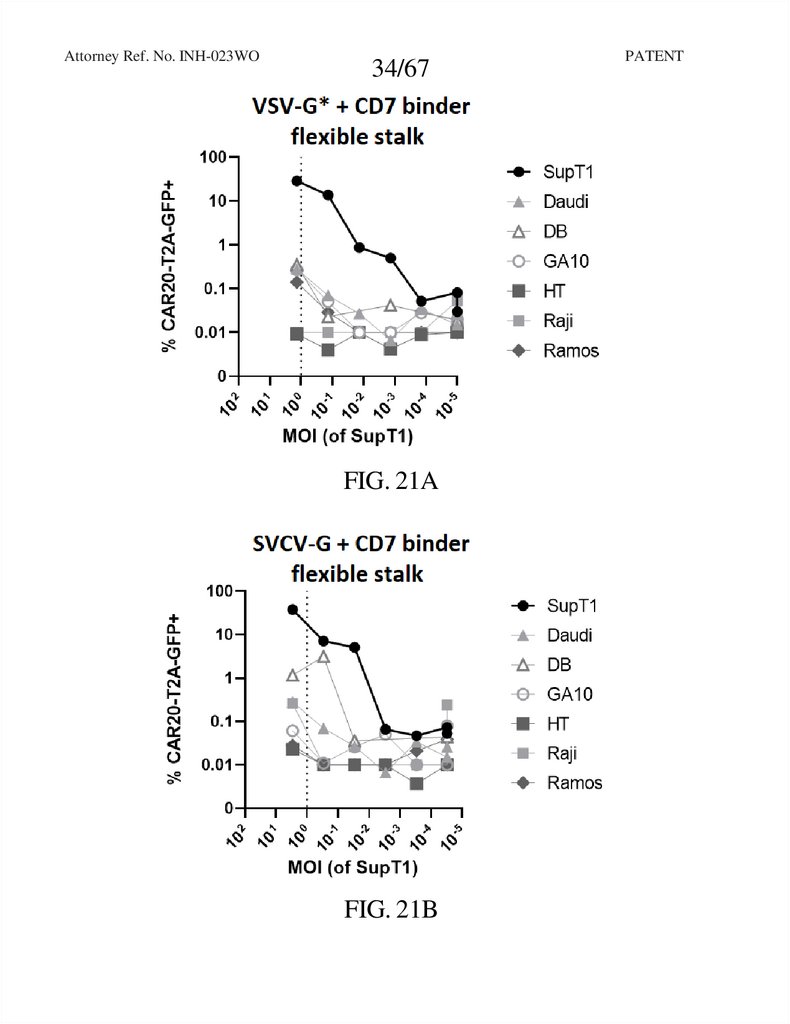
34.
Attorney Ref. No. INH-023WO34/67
FIG. 21A
FIG. 21B
PATENT
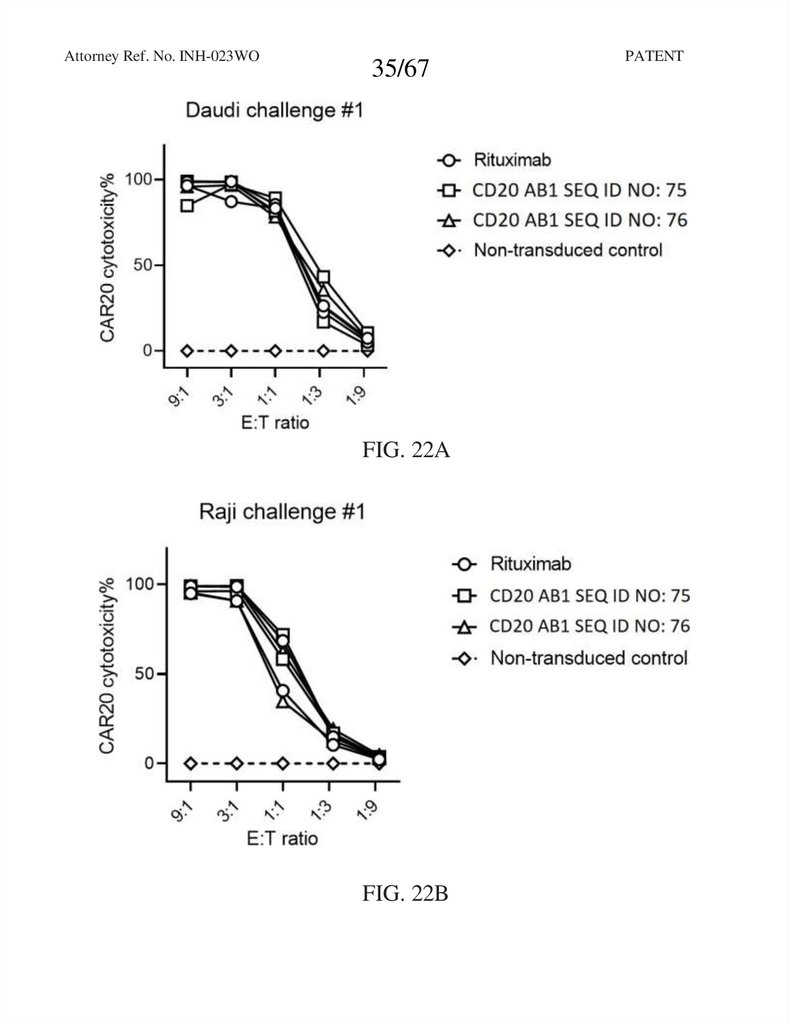
35.
Attorney Ref. No. INH-023WO35/67
FIG. 22A
FIG. 22B
PATENT
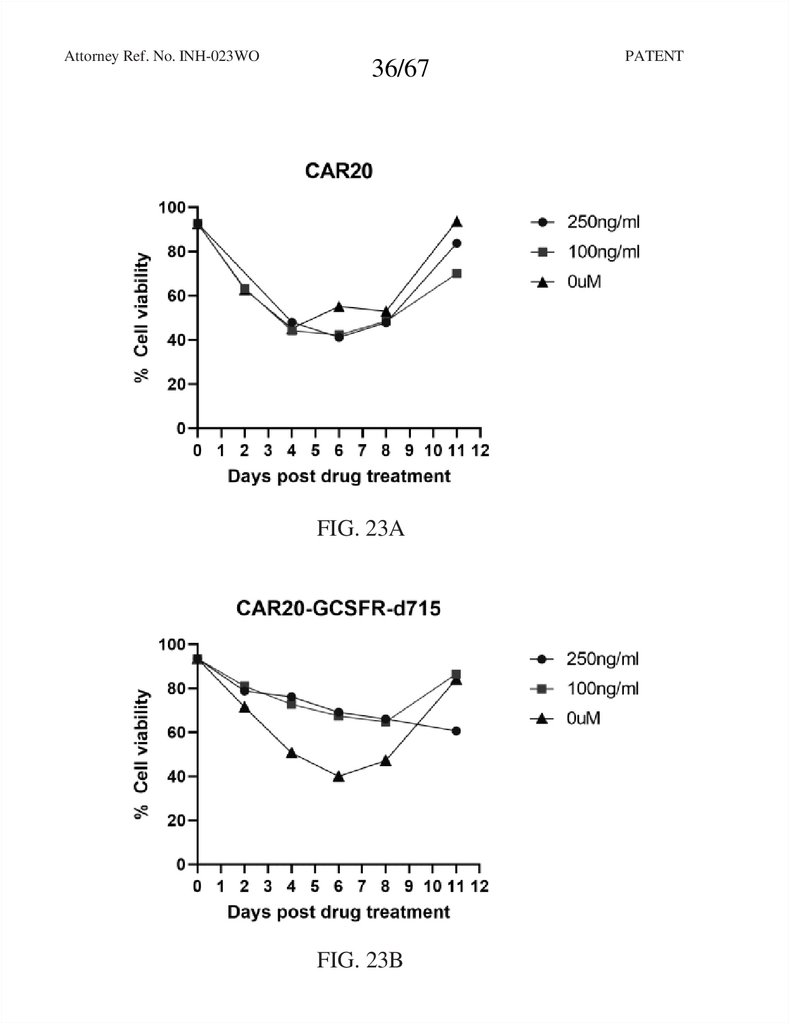
36.
Attorney Ref. No. INH-023WO36/67
FIG. 23A
FIG. 23B
PATENT
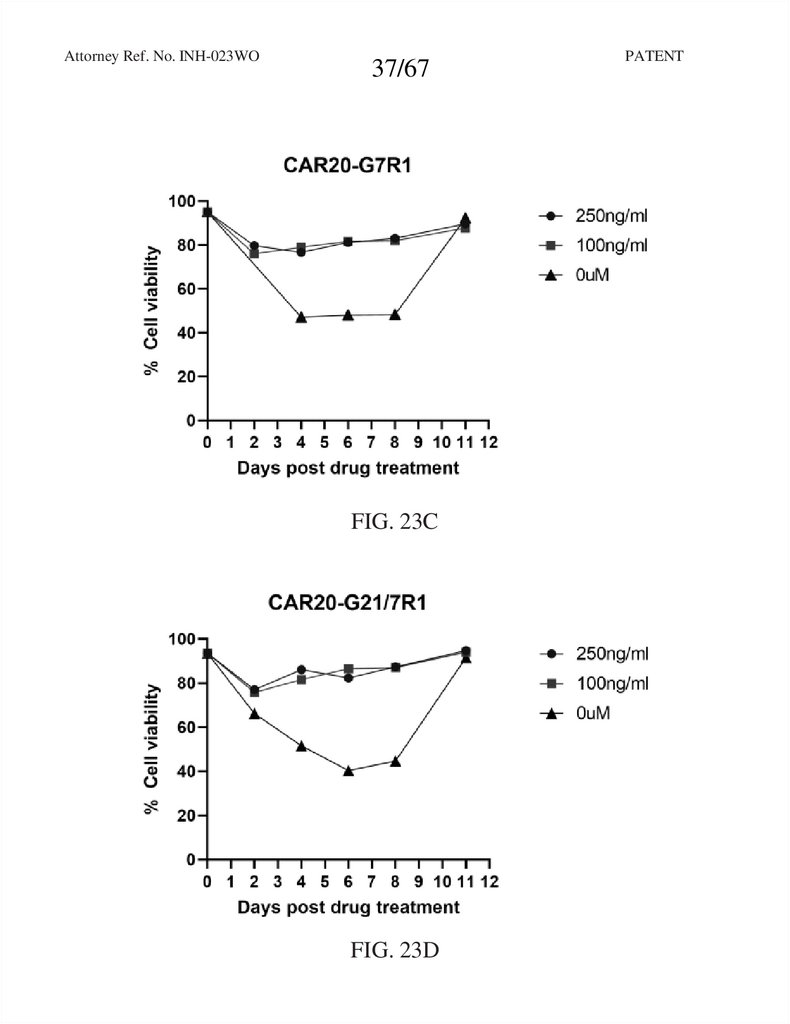
37.
Attorney Ref. No. INH-023WO37/67
FIG. 23C
FIG. 23D
PATENT
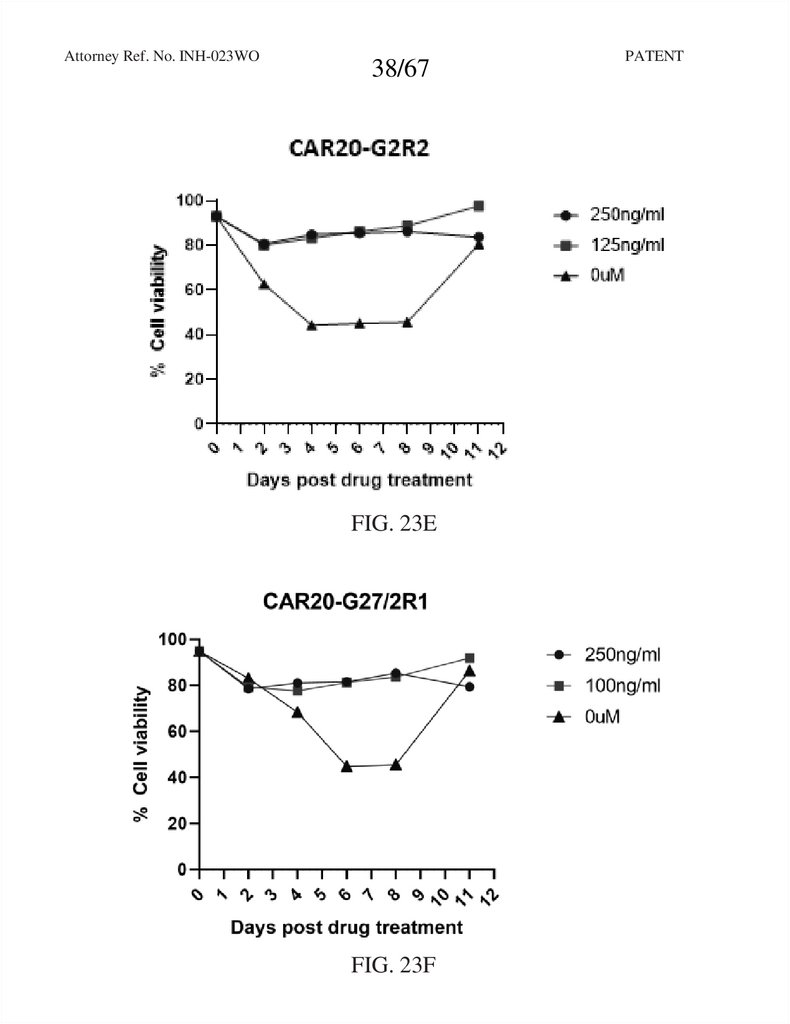
38.
Attorney Ref. No. INH-023WO38/67
FIG. 23E
FIG. 23F
PATENT
39.
Attorney Ref. No. INH-023WO39/67
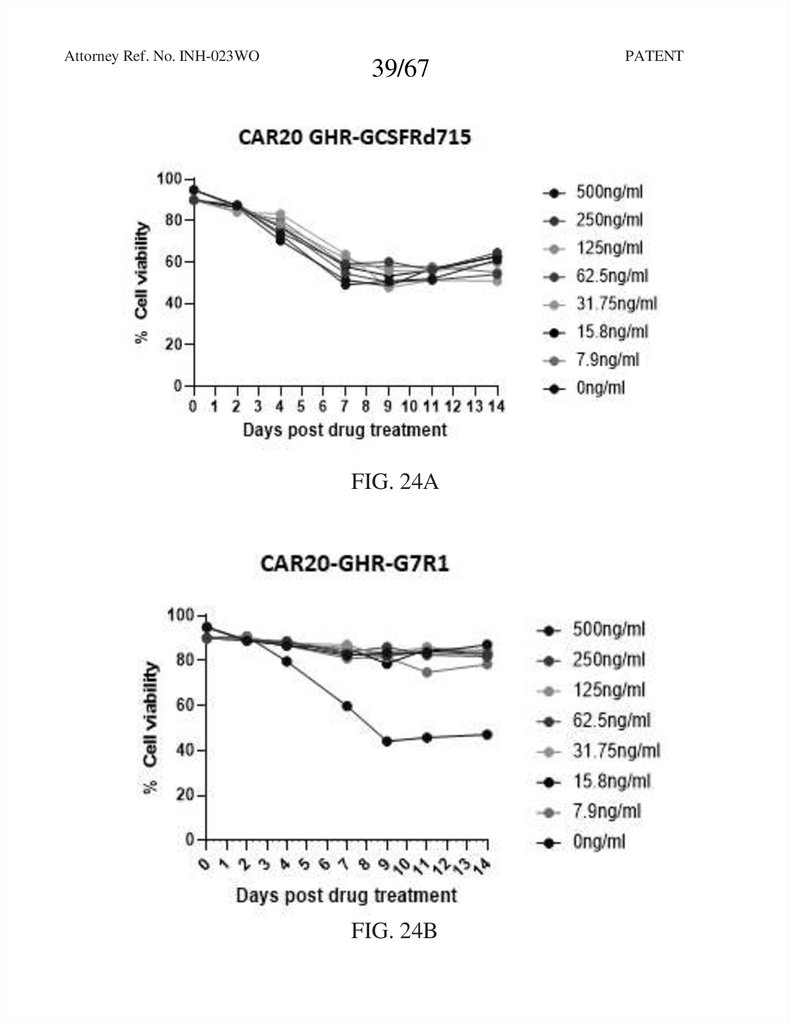
FIG. 24A
FIG. 24B
PATENT
40.
Attorney Ref. No. INH-023WO40/67
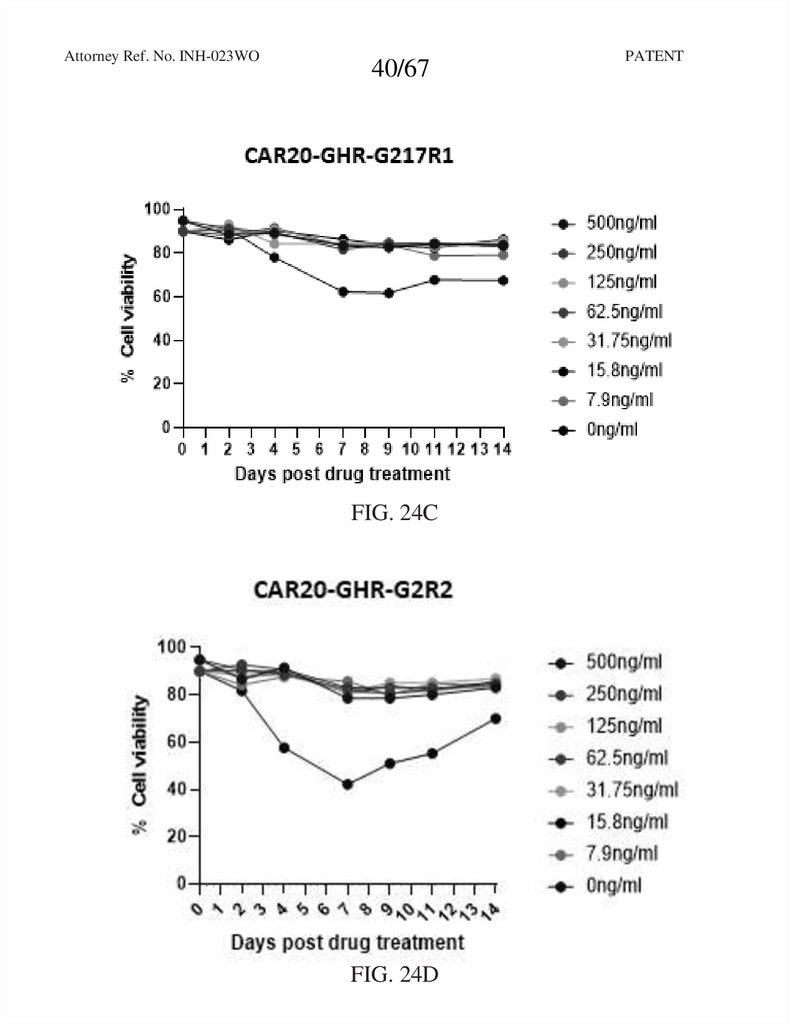
FIG. 24C
FIG. 24D
PATENT
41.
Attorney Ref. No. INH-023WO41/67
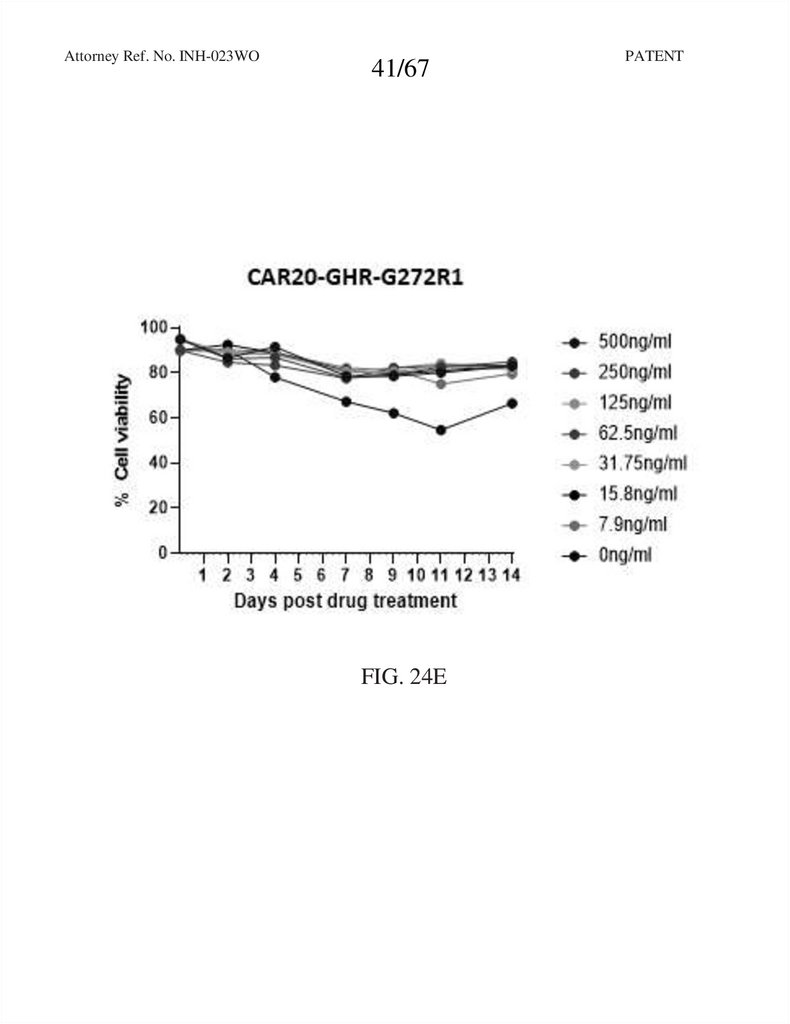
FIG. 24E
PATENT
42.
Attorney Ref. No. INH-023WOPATENT
42/67
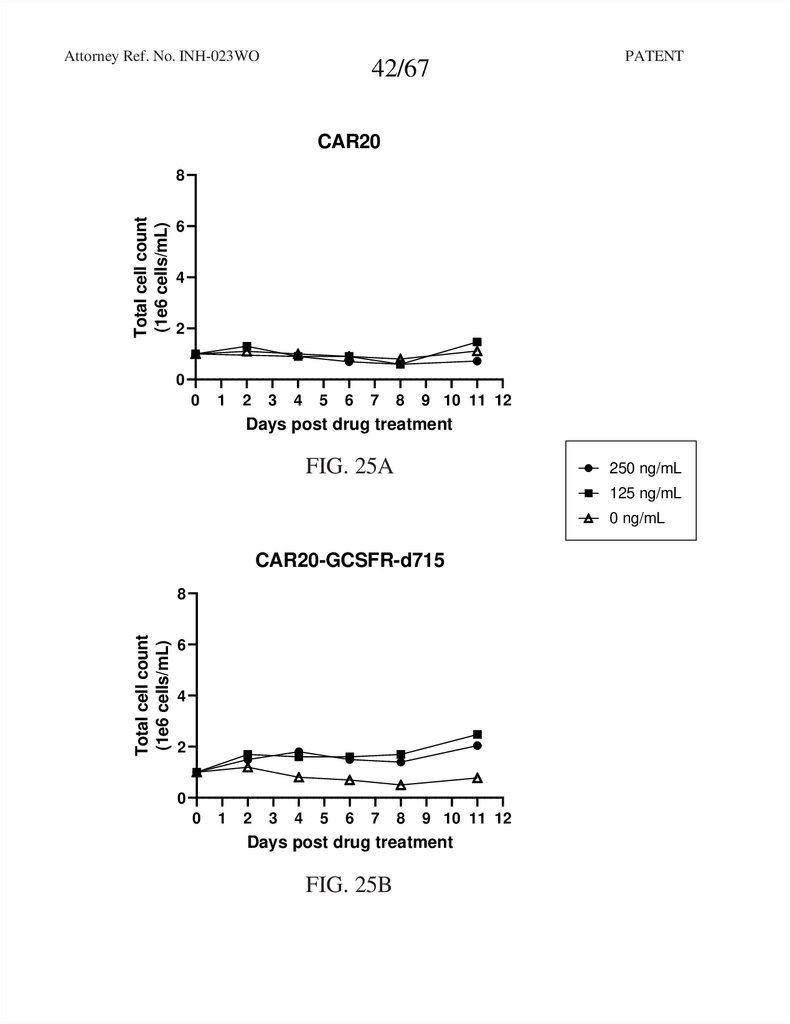
CAR20
Total cell count
(1e6 cells/mL)
8
6
4
2
0
0
1
2
3
4
5
6
7
8
9 10 11 12
Days post drugCAR20
treatment
Total cell count
(1e6 cells/mL)
8
Total cell count
(1e6 cells/mL)
8
FIG. 25A
250 ng/mL
125 ng/mL
6
0 ng/mL
4
CAR20-GCSFR-d715
2
0
6
0
1
2
3
4
5
6
7
8
9 10 11 12
Days post drug treatment
4
2
0
0
1
2
3
4
5
6
7
8
9 10 11 12
Days post drug treatment
FIG. 25B
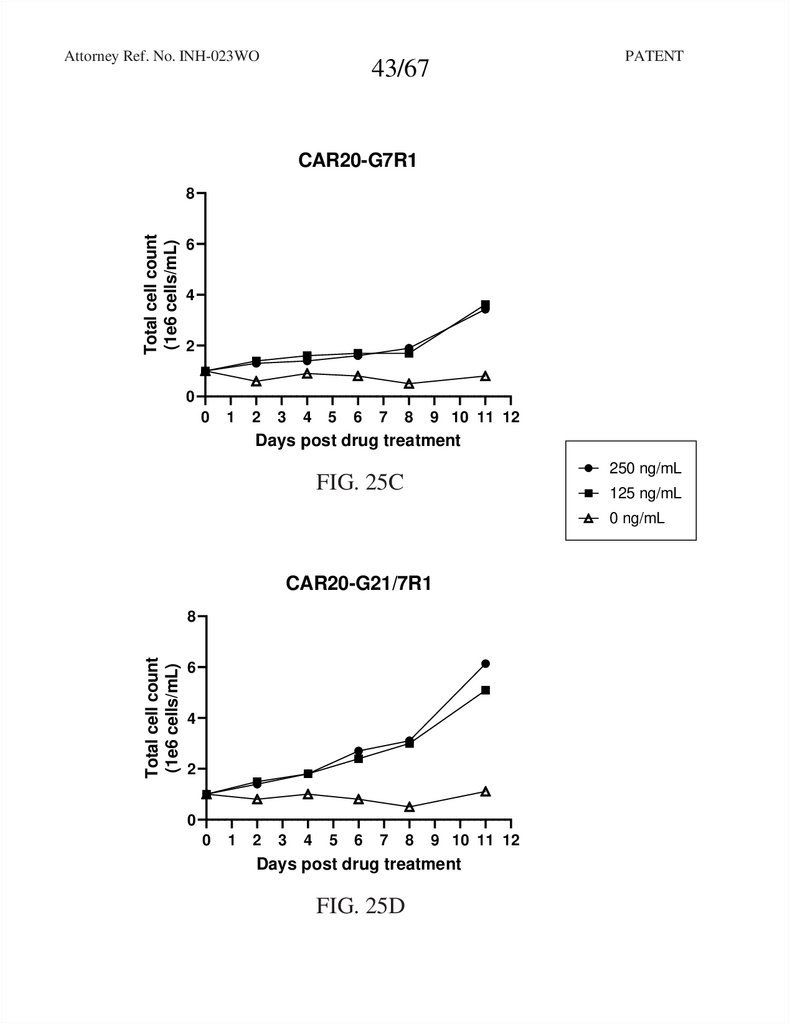
43.
Attorney Ref. No. INH-023WOPATENT
43/67
CAR20-G7R1
Total cell count
(1e6 cells/mL)
8
6
4
2
0
0
1
2
3
4
5
6
7
8
CAR20
9 10 11 12
Days post drug treatment
Total cell count
(1e6 cells/mL)
8
250 ng/mL
FIG. 25C
125 ng/mL
6
0 ng/mL
4
CAR20-G21/7R1
2
8
Total cell count
(1e6 cells/mL)
0
0
6
1
2
3
4
5
6
7
8
9 10 11 12
Days post drug treatment
4
2
0
0
1
2
3
4
5
6
7
8
9 10 11 12
Days post drug treatment
FIG. 25D
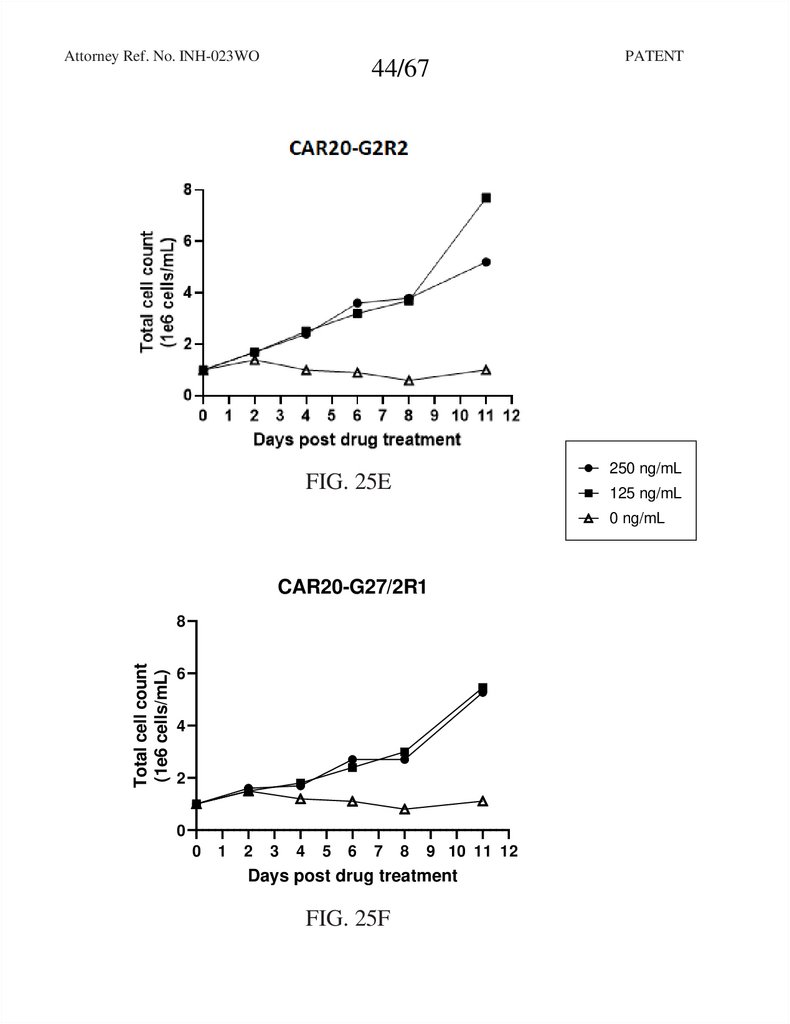
44.
Attorney Ref. No. INH-023WOPATENT
44/67
CAR20
Total cell count
(1e6 cells/mL)
8
250 ng/mL
FIG. 25E
125 ng/mL
6
0 ng/mL
4
CAR20-G27/2R1
2
8
Total cell count
(1e6 cells/mL)
0
0
6
1
2
3
4
5
6
7
8
9 10 11 12
Days post drug treatment
4
2
0
0
1
2
3
4
5
6
7
8
9 10 11 12
Days post drug treatment
FIG. 25F
45.
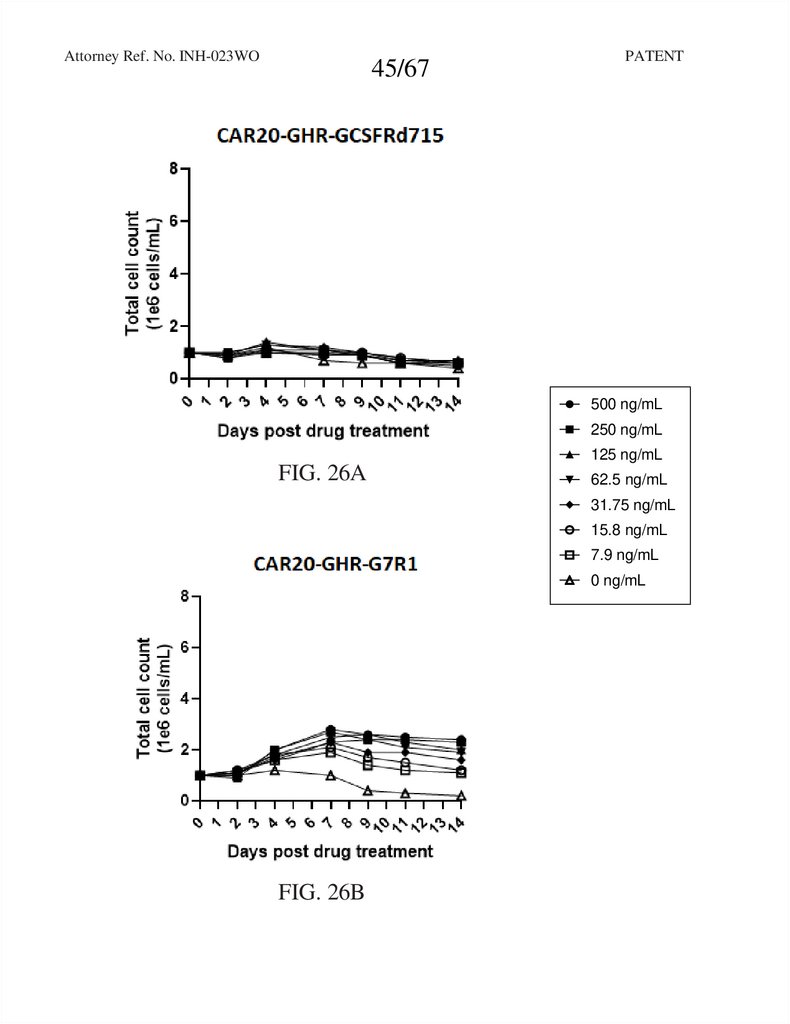
Attorney Ref. No. INH-023WO45/67
PATENT
RTXCAR20-T2A-GHR-GCSFRd715
500 ng/mL
250 ng/mL
6
125 ng/mL
4
FIG. 26A
62.5 ng/mL
31.75 ng/mL
15.8 ng/mL
2
7.9 ng/mL
0
0 ng/mL
0
1
2
3
4
5
6
7
8
9
10
11
12
13
14
Total cell count
(1e6 cells/mL)
8
Days post drug treatment
FIG. 26B
46.
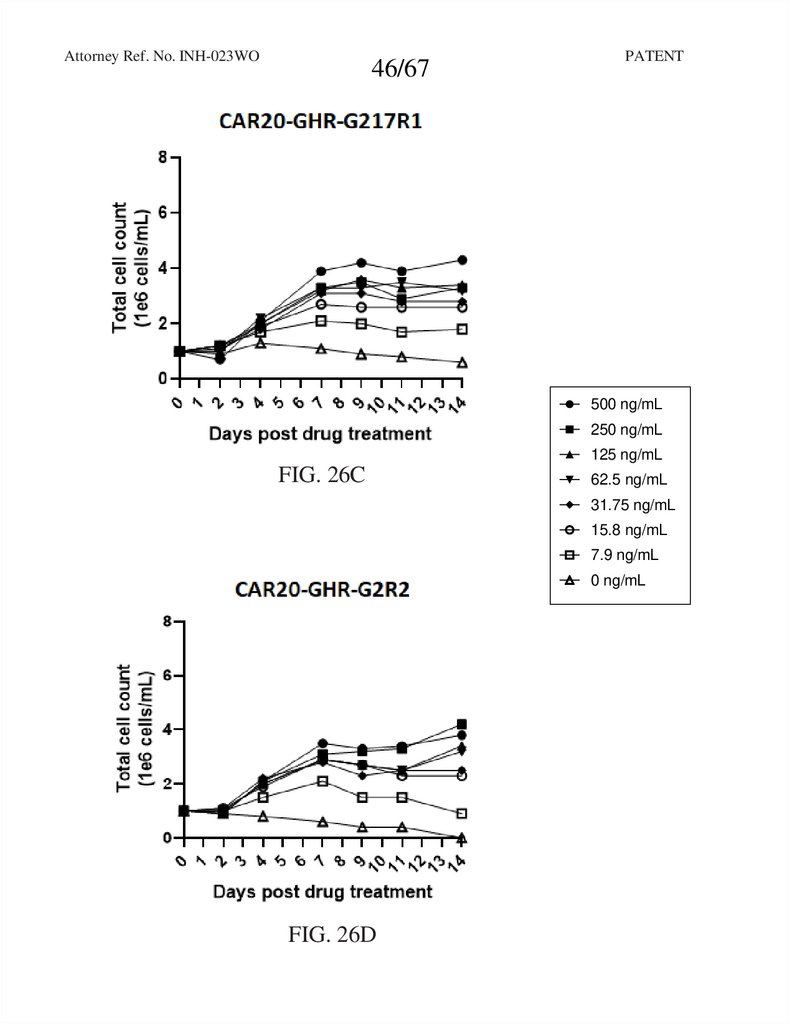
Attorney Ref. No. INH-023WO46/67
PATENT
RTXCAR20-T2A-GHR-GCSFRd715
500 ng/mL
250 ng/mL
6
125 ng/mL
4
FIG. 26C
62.5 ng/mL
31.75 ng/mL
15.8 ng/mL
2
7.9 ng/mL
0
0 ng/mL
0
1
2
3
4
5
6
7
8
9
10
11
12
13
14
Total cell count
(1e6 cells/mL)
8
Days post drug treatment
FIG. 26D
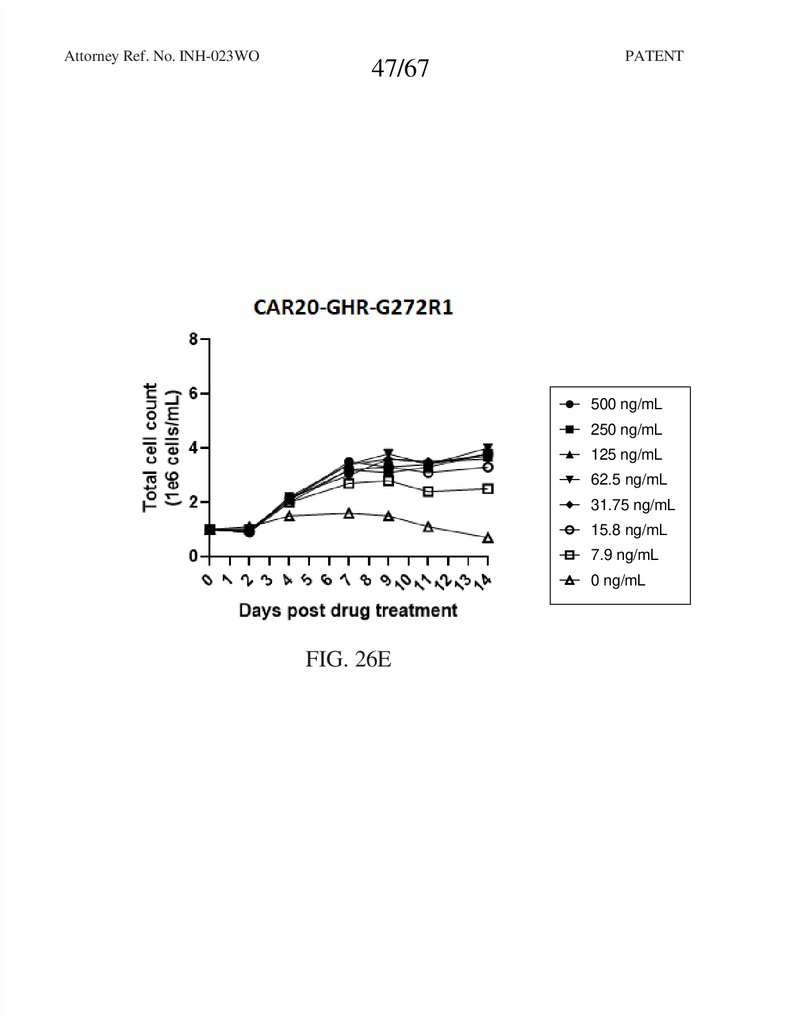
47.
Attorney Ref. No. INH-023WO47/67
PATENT
RTXCAR20-T2A-GHR-GCSFRd715
500 ng/mL
250 ng/mL
6
125 ng/mL
62.5 ng/mL
4
31.75 ng/mL
15.8 ng/mL
2
7.9 ng/mL
0
0 ng/mL
0
1
2
3
4
5
6
7
8
9
10
11
12
13
14
Total cell count
(1e6 cells/mL)
8
Days post drug treatment
FIG. 26E
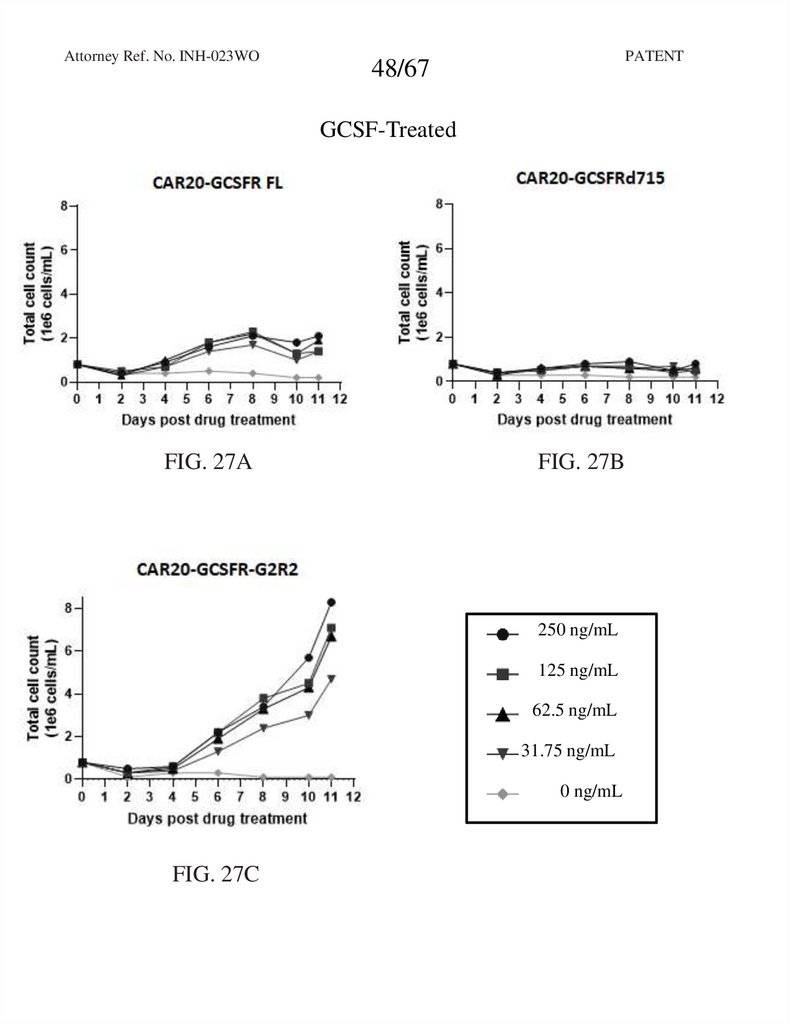
48.
Attorney Ref. No. INH-023WOPATENT
48/67
GCSF-Treated
FIG. 27A
FIG. 27B
250 ng/mL
125 ng/mL
62.5 ng/mL
31.75 ng/mL
0 ng/mL
FIG. 27C
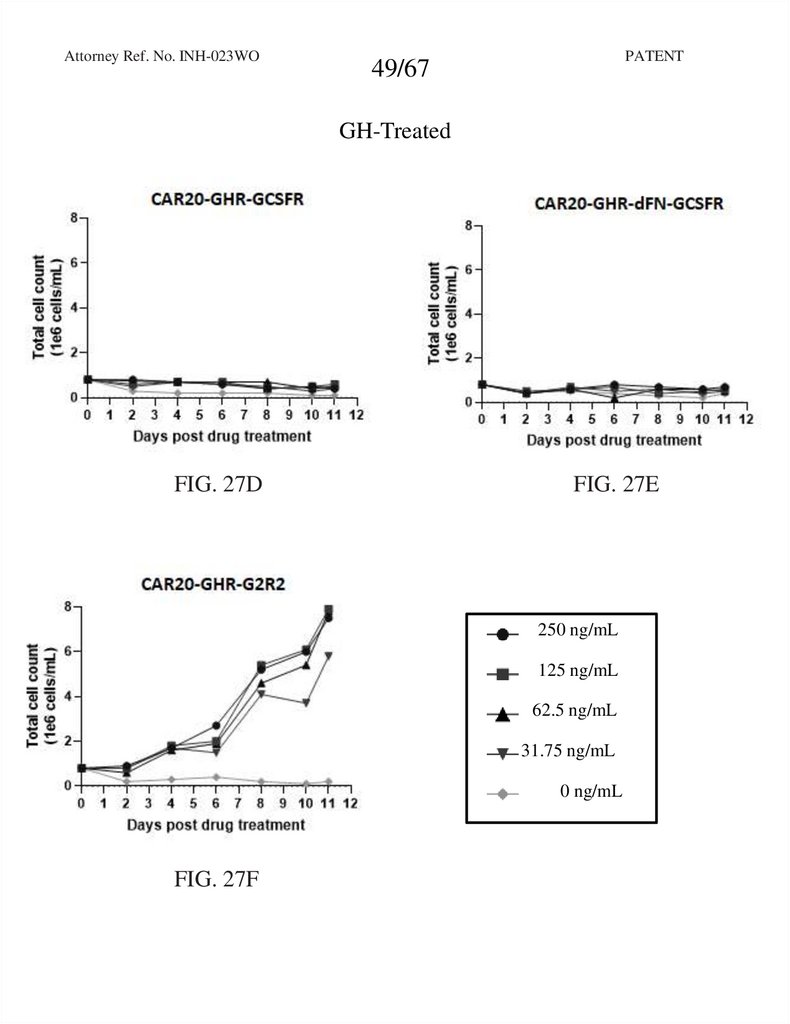
49.
Attorney Ref. No. INH-023WOPATENT
49/67
GH-Treated
FIG. 27D
FIG. 27E
250 ng/mL
125 ng/mL
62.5 ng/mL
31.75 ng/mL
0 ng/mL
FIG. 27F
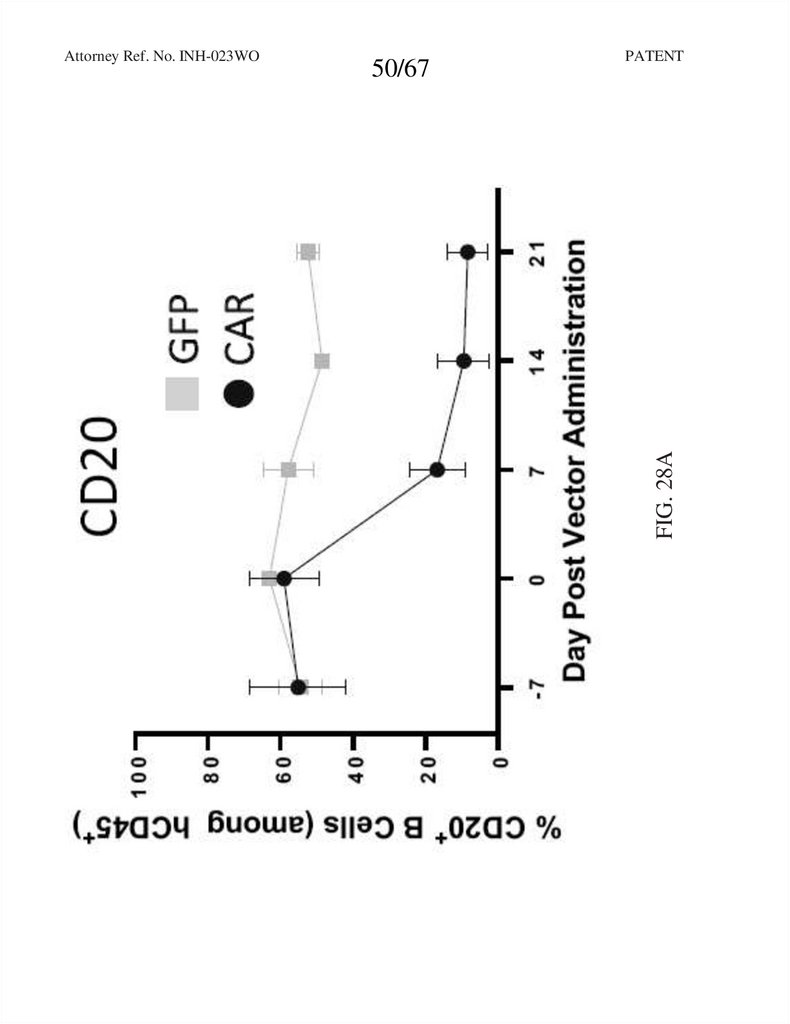
50.
50/67PATENT
FIG. 28A
Attorney Ref. No. INH-023WO
51.
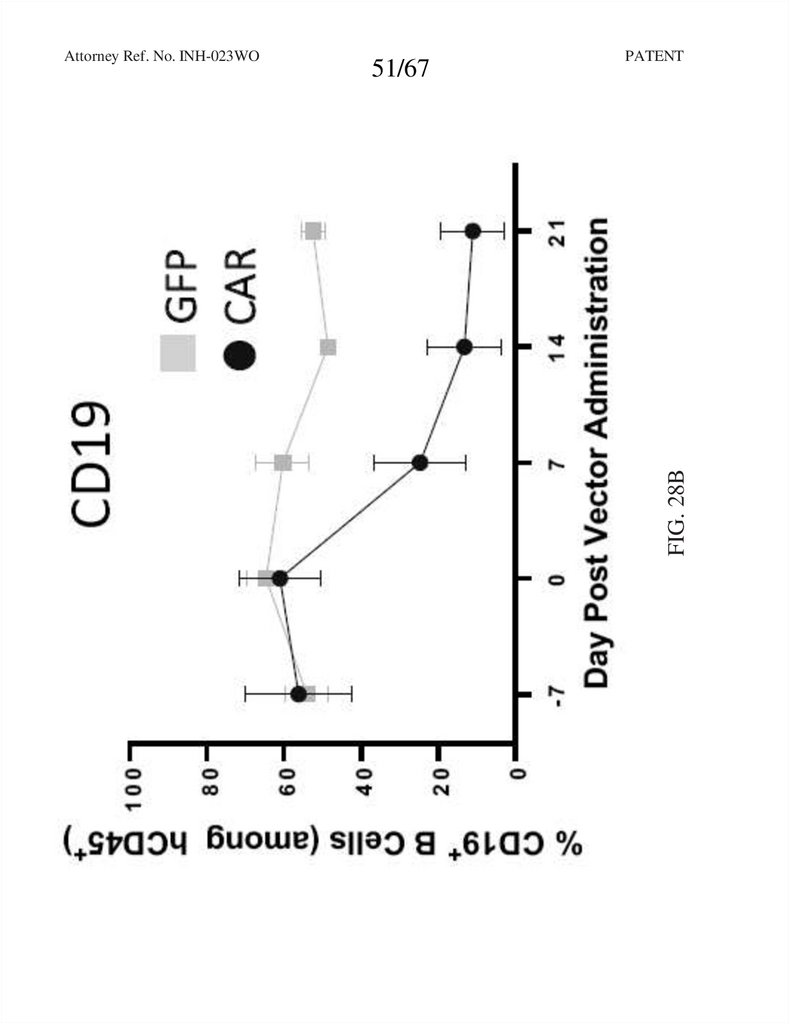
51/67PATENT
FIG. 28B
Attorney Ref. No. INH-023WO
52.
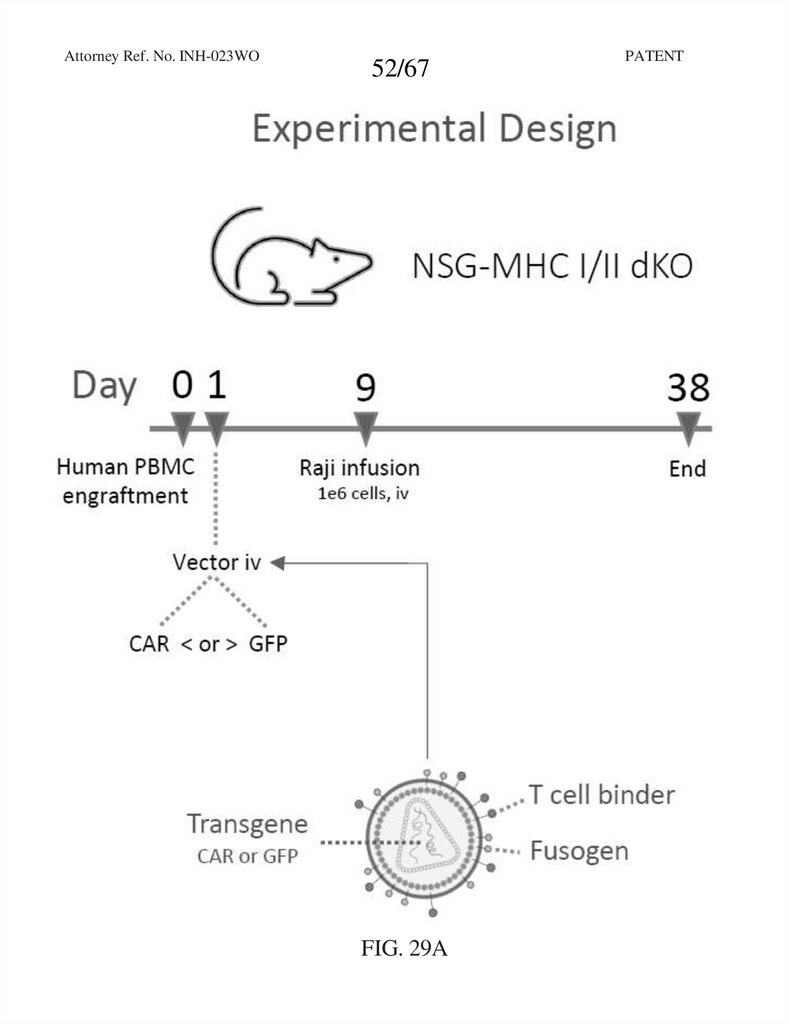
Attorney Ref. No. INH-023WO52/67
FIG. 29A
PATENT
53.
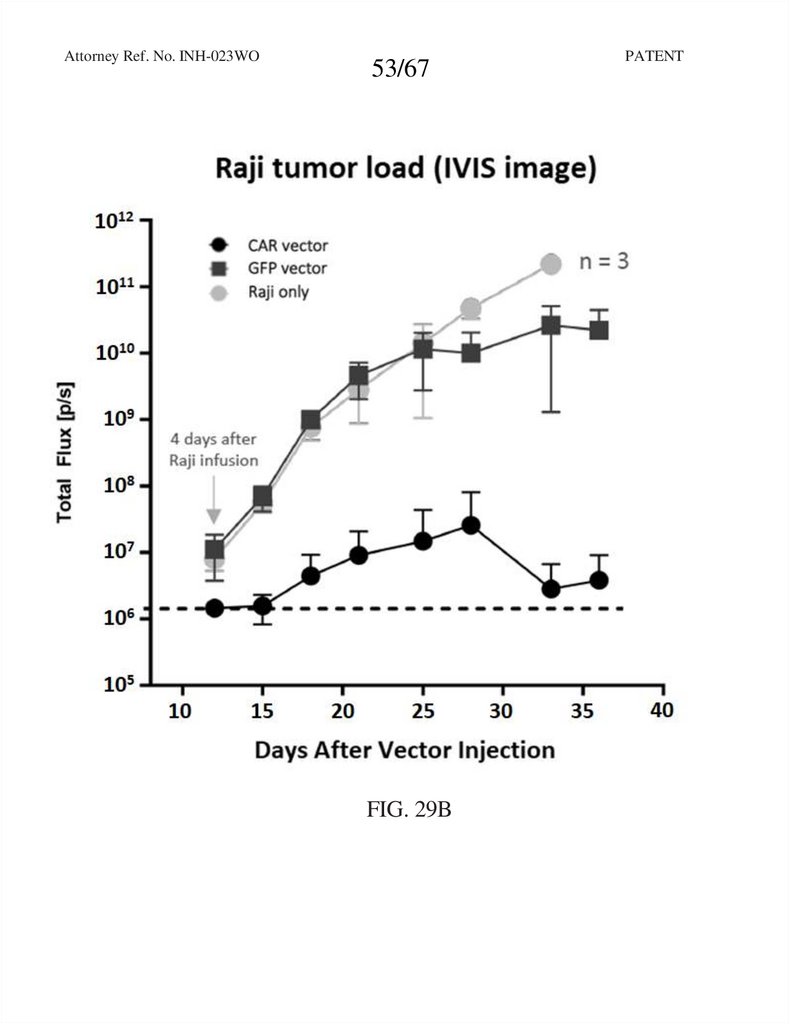
Attorney Ref. No. INH-023WO53/67
FIG. 29B
PATENT
54.
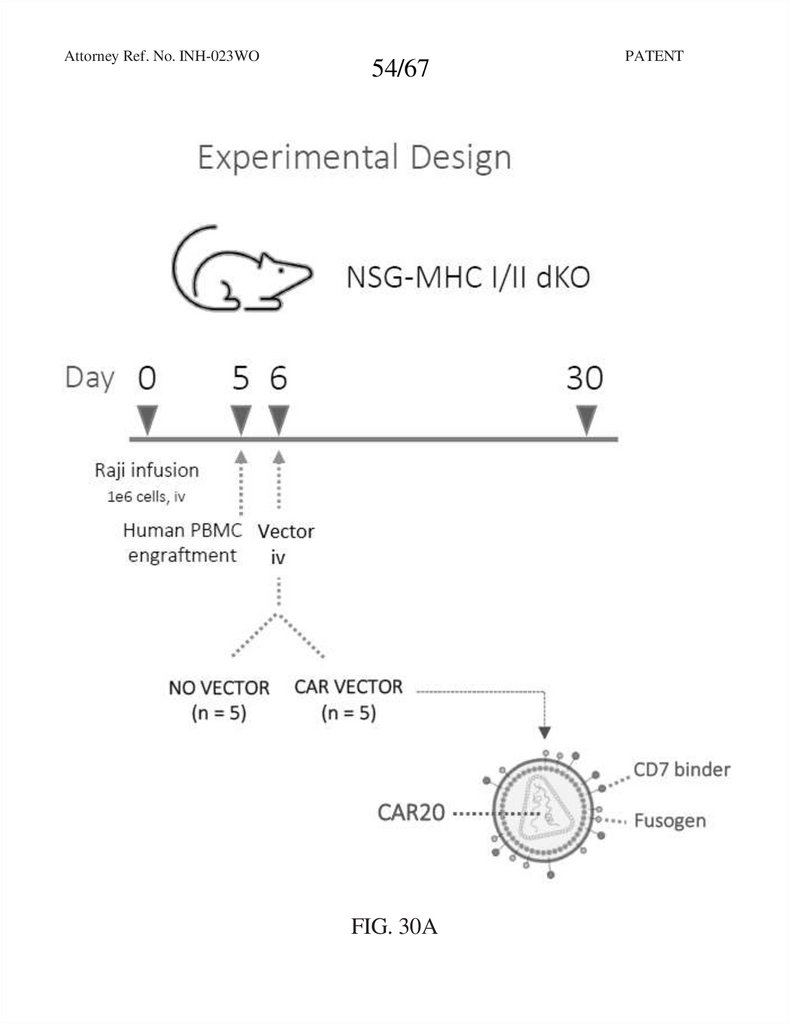
Attorney Ref. No. INH-023WO54/67
FIG. 30A
PATENT
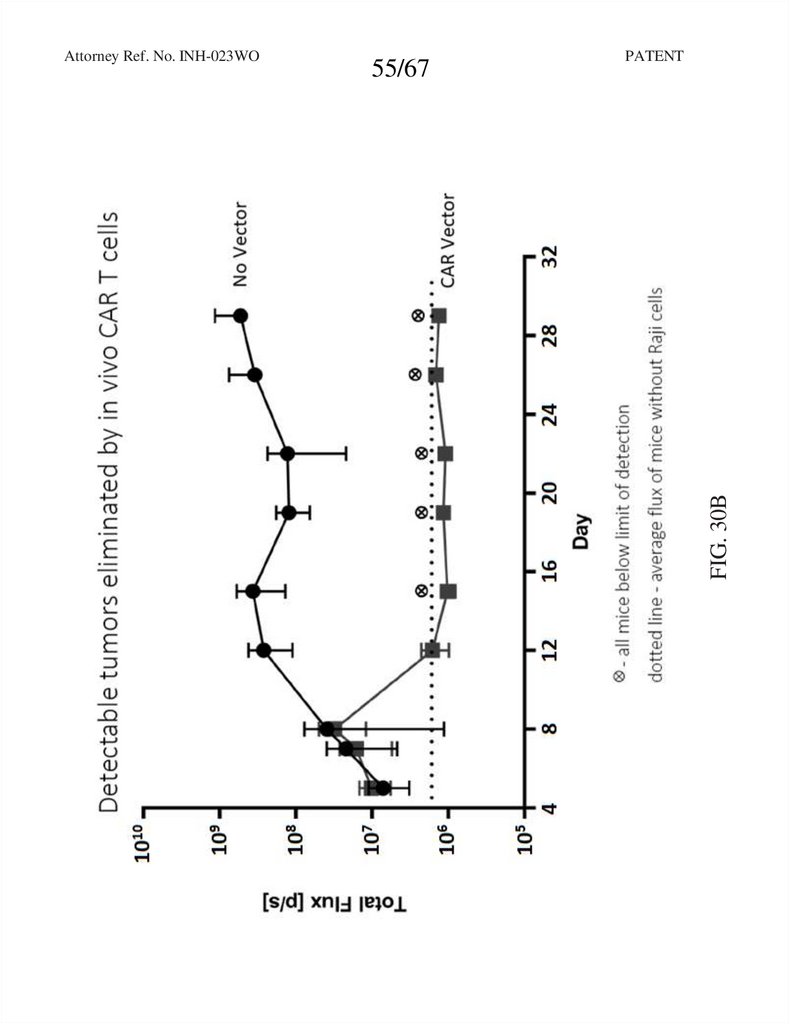
55.
55/67PATENT
FIG. 30B
Attorney Ref. No. INH-023WO
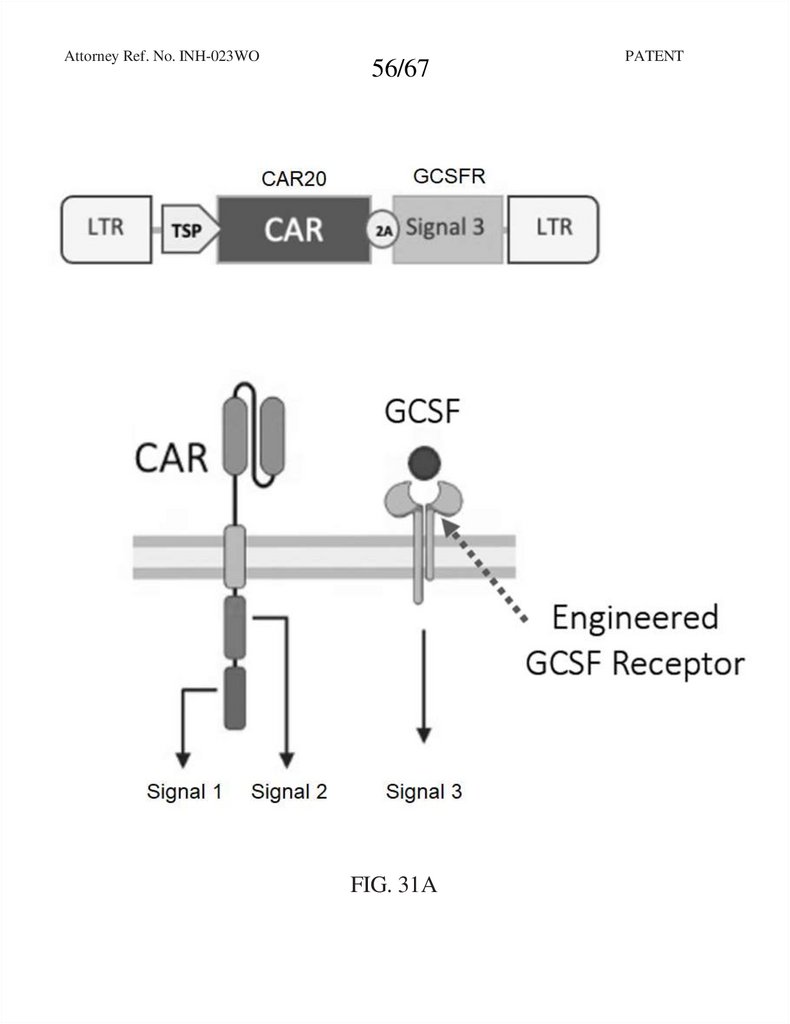
56.
Attorney Ref. No. INH-023WO56/67
FIG. 31A
PATENT
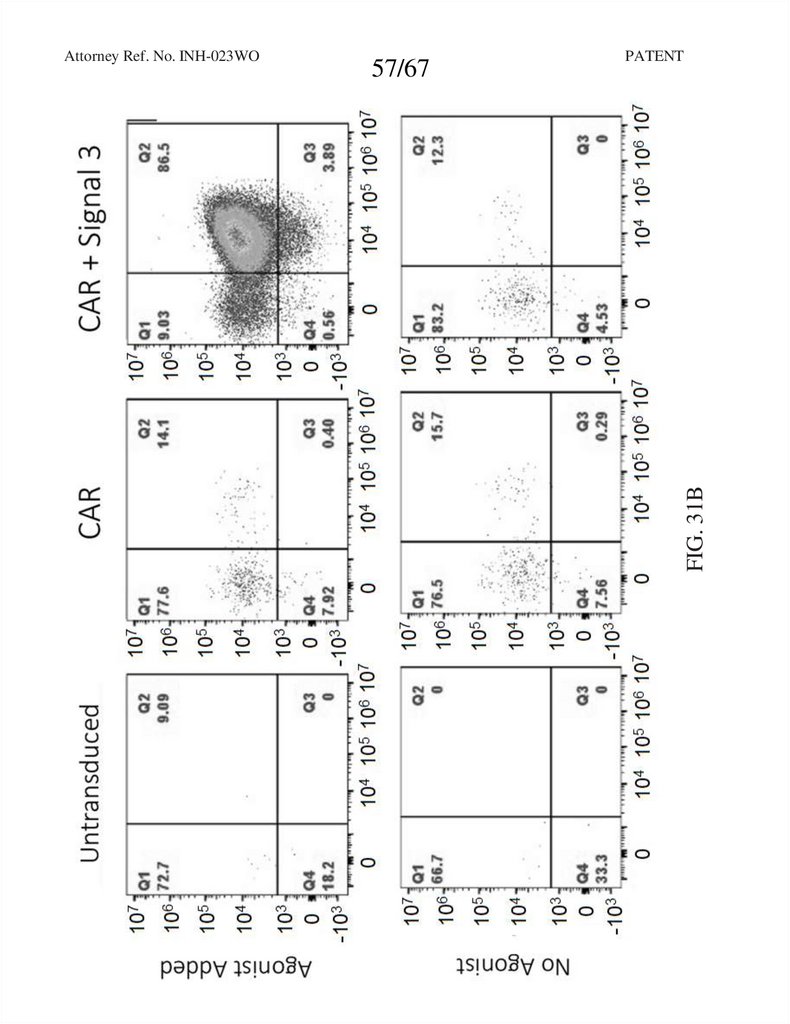
57.
57/67PATENT
FIG. 31B
Attorney Ref. No. INH-023WO
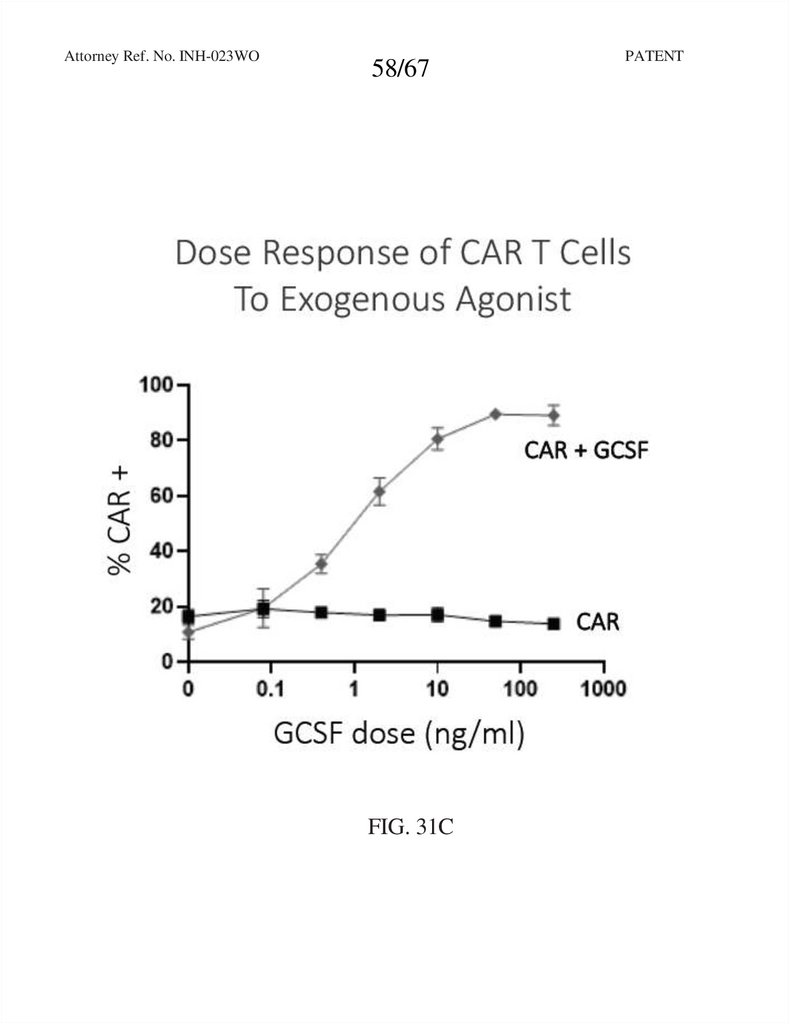
58.
Attorney Ref. No. INH-023WO58/67
FIG. 31C
PATENT
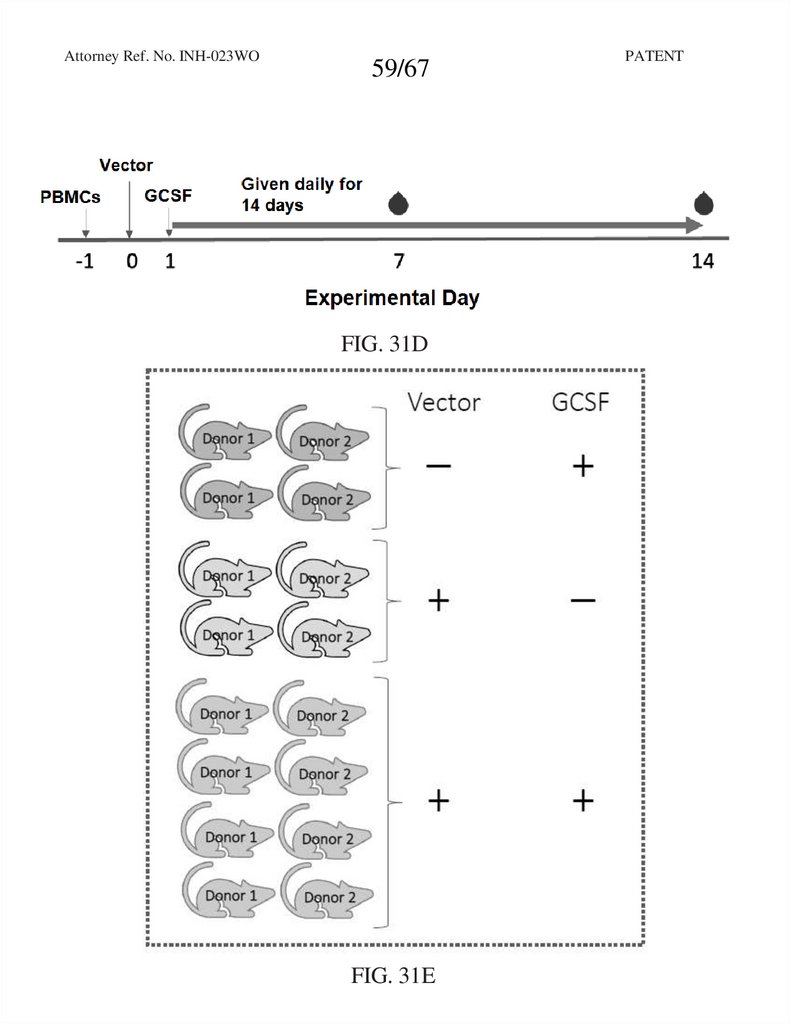
59.
Attorney Ref. No. INH-023WO59/67
FIG. 31D
FIG. 31E
PATENT
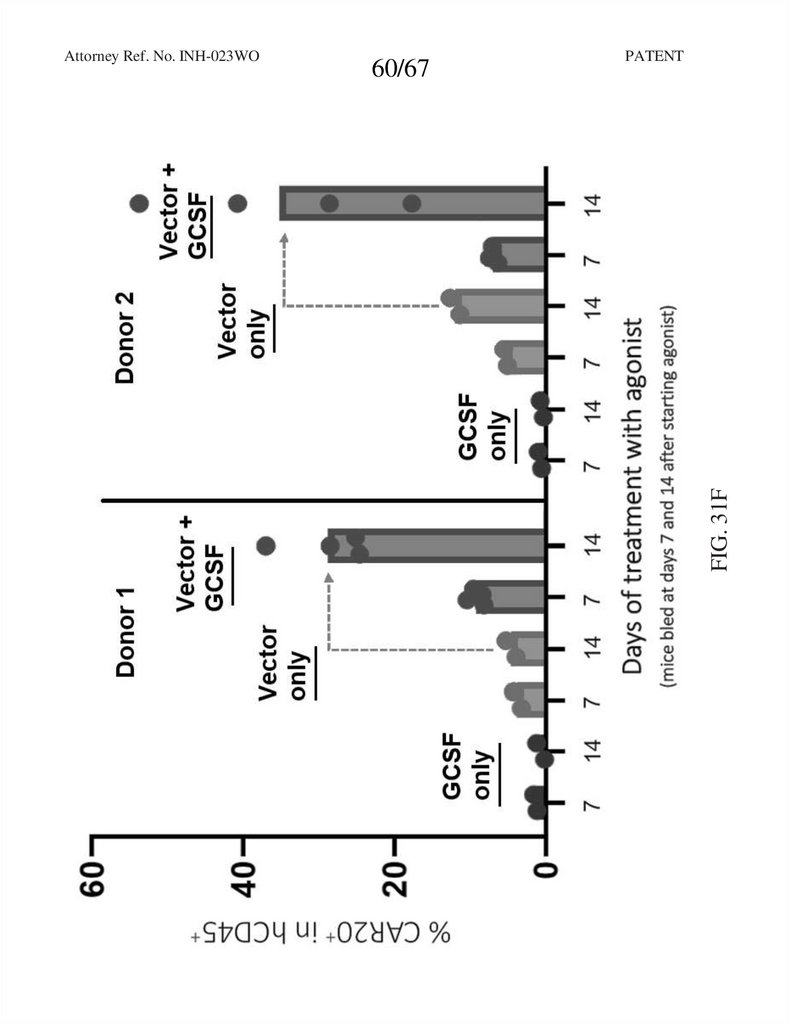
60.
60/67PATENT
FIG. 31F
Attorney Ref. No. INH-023WO
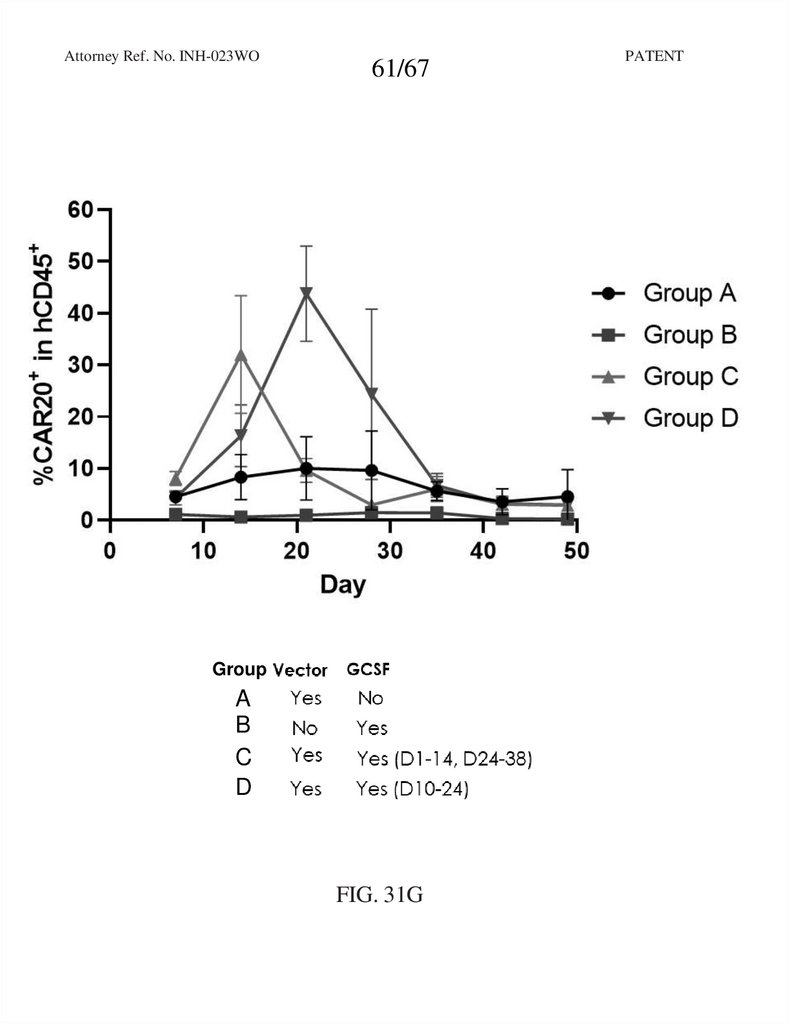
61.
Attorney Ref. No. INH-023WO61/67
Group
A
B
C
D
FIG. 31G
PATENT
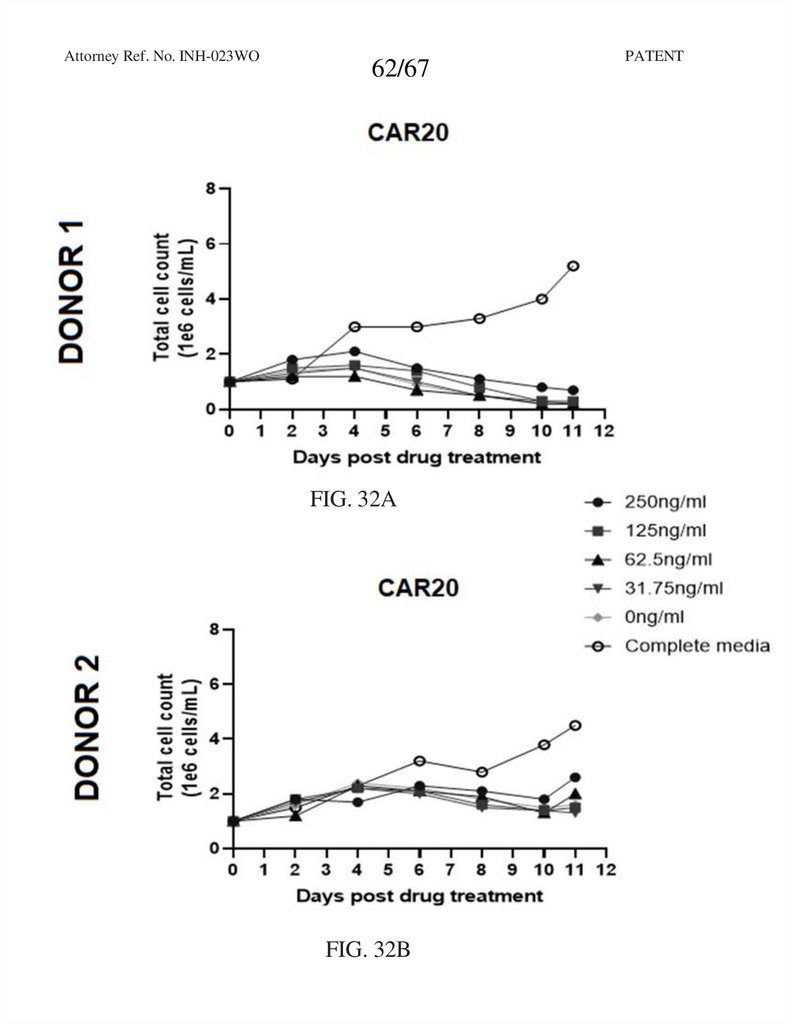
62.
Attorney Ref. No. INH-023WO62/67
FIG. 32A
FIG. 32B
PATENT
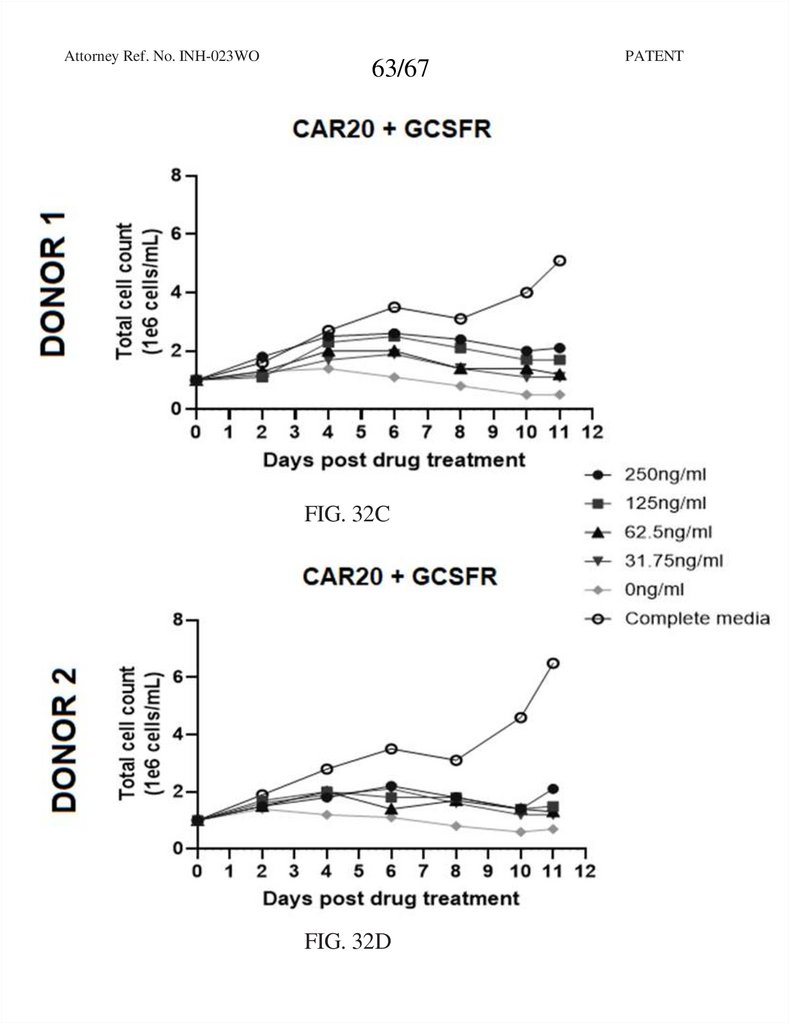
63.
Attorney Ref. No. INH-023WO63/67
FIG. 32C
FIG. 32D
PATENT
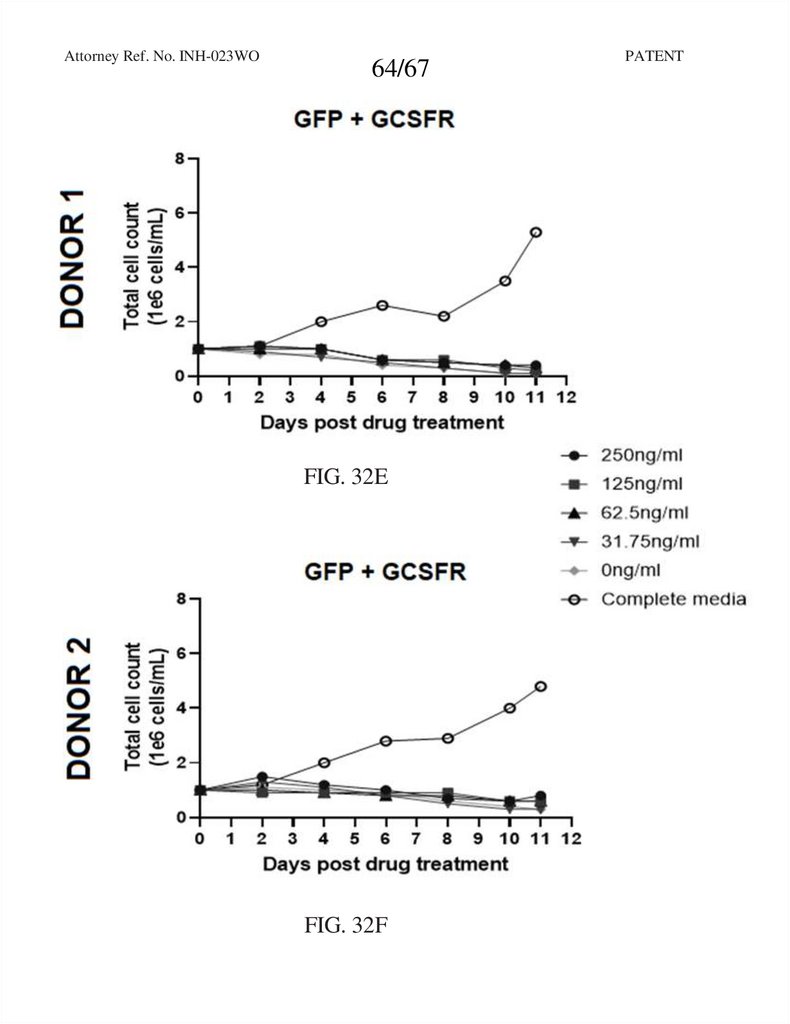
64.
Attorney Ref. No. INH-023WO64/67
FIG. 32E
FIG. 32F
PATENT
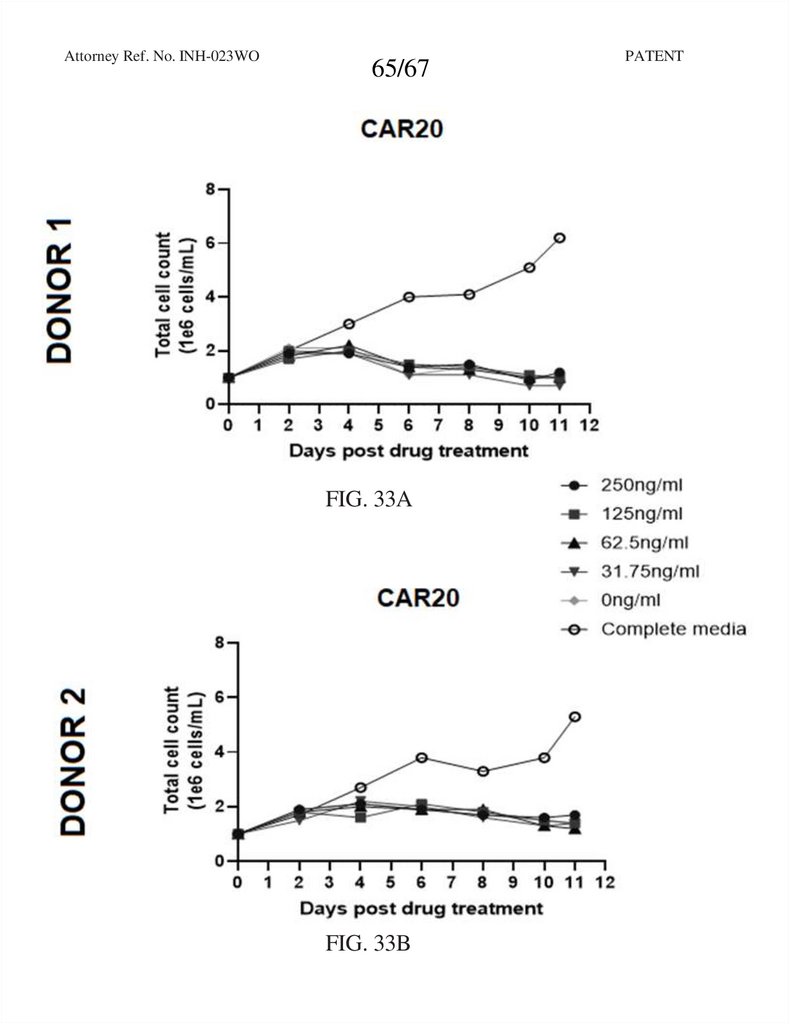
65.
Attorney Ref. No. INH-023WO65/67
FIG. 33A
FIG. 33B
PATENT
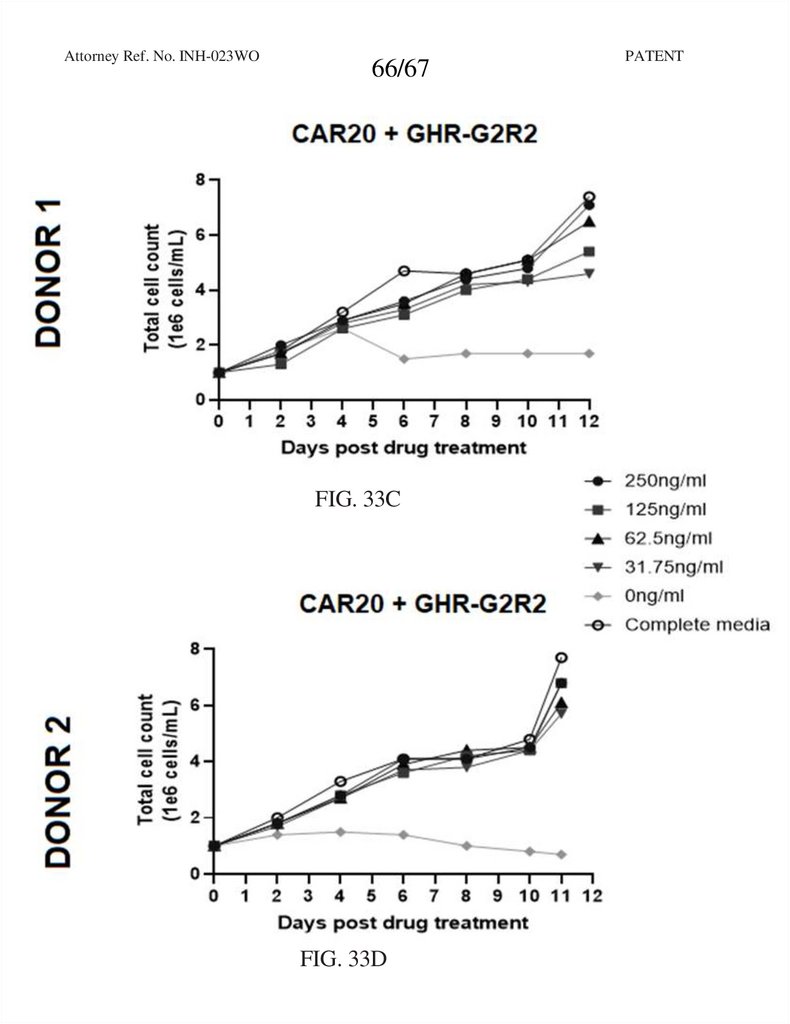
66.
Attorney Ref. No. INH-023WO66/67
FIG. 33C
FIG. 33D
PATENT
67.
Attorney Ref. No. INH-023WO67/67
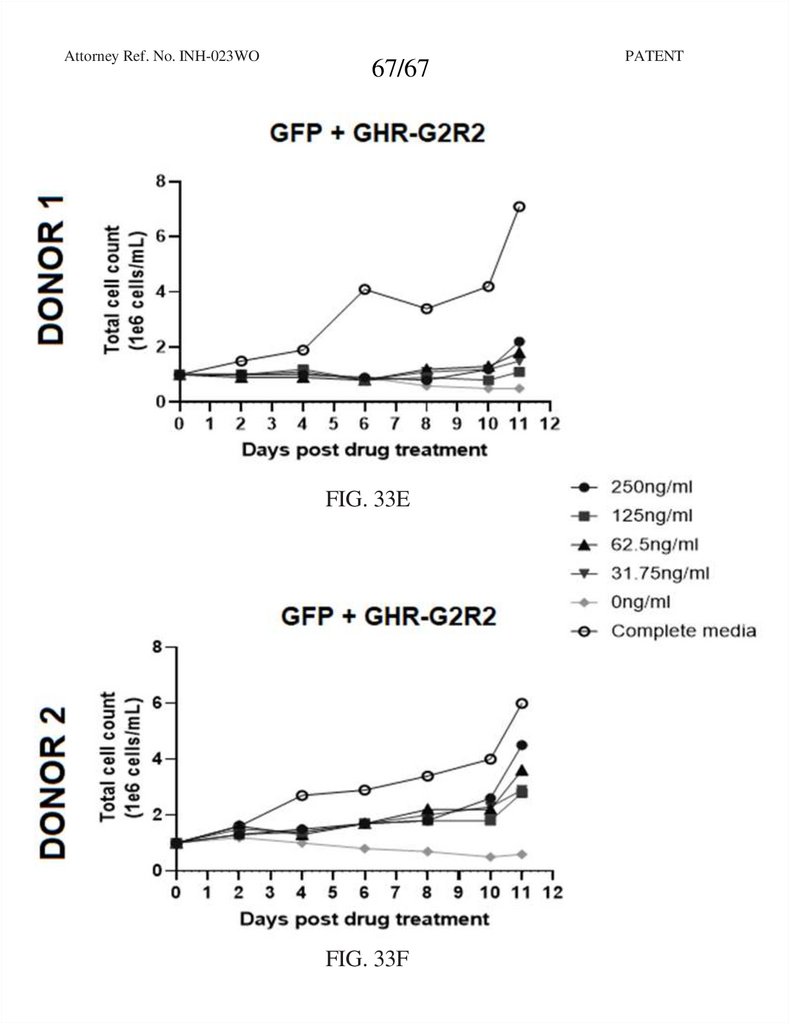
FIG. 33E
FIG. 33F
PATENT