Похожие презентации:
The law of mass conservation
1.
THE LAW OFMASS
CONSERVATION
2.
In the 18th centur y scientistthought that when things
bur ns a substance call
"phlogiston" came out of
them.
3.
T hen exper iments with closed vessels wher esubstances could be accur ately weighed,help
scientist such as A ntoine Lavoiser under stand that
when things bur n ,Oxygen is added
He rea lized t ha t ma t t er c ould be c ha nged
but not dest royed
4. LAW OF MASS
CONSERVATION(1789)
In chemical r eactions no matter is lost
or gr ained
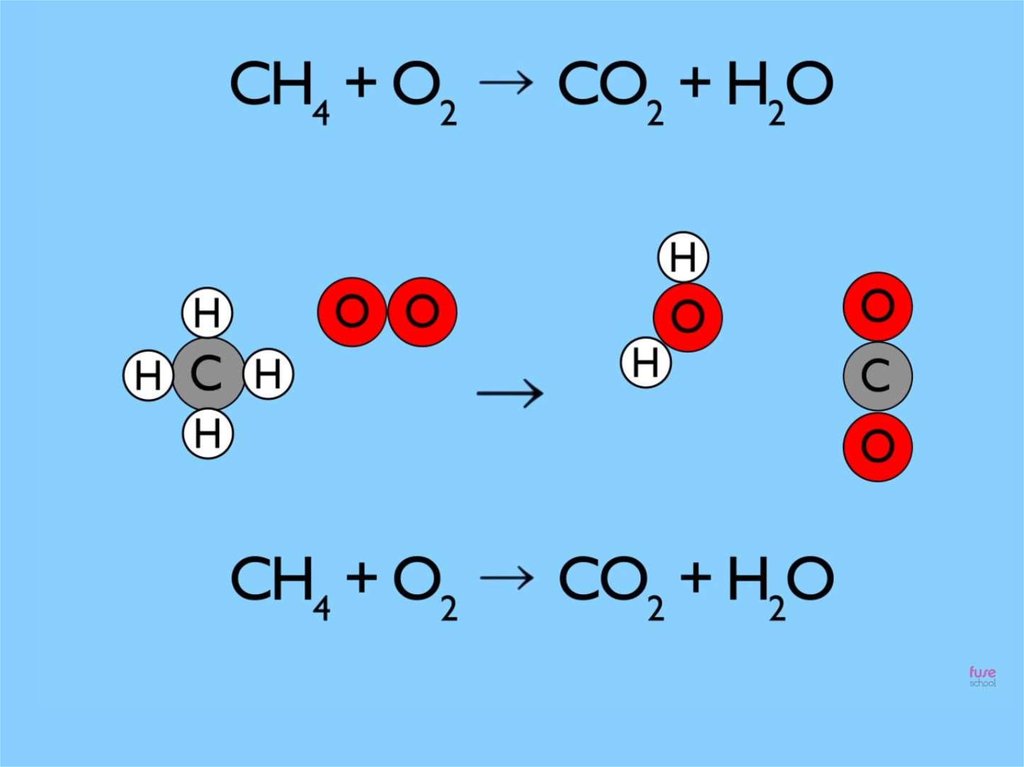
5.
Reaction that we see when we using thegas cooker which uses natural gas
6.
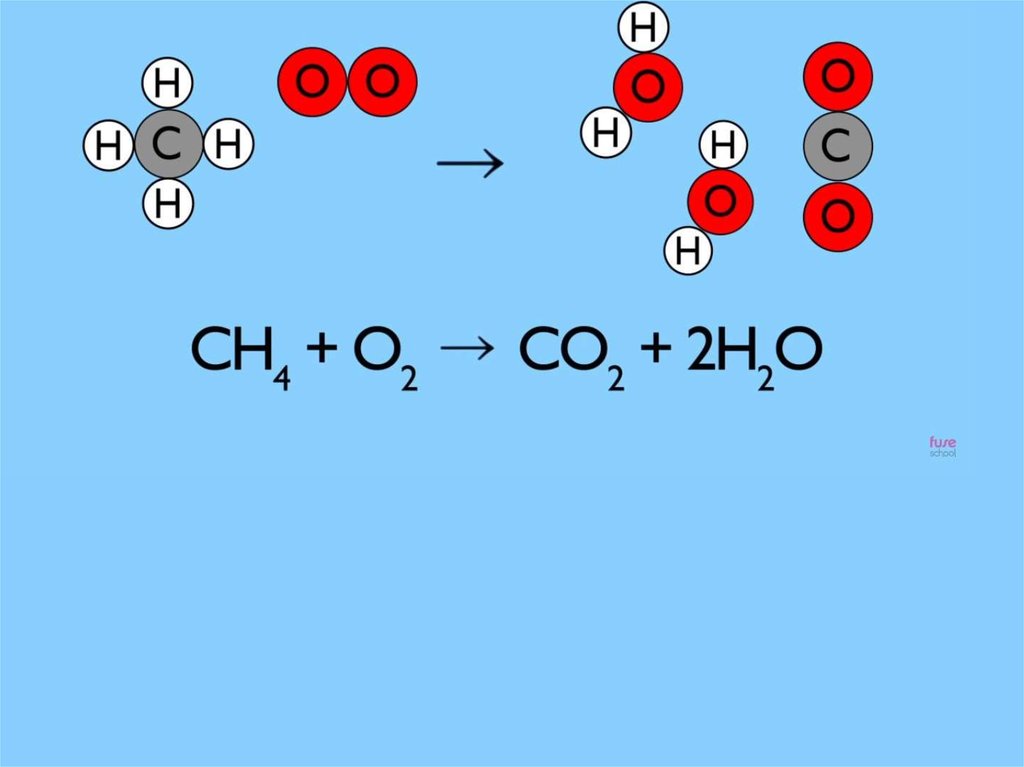
7.
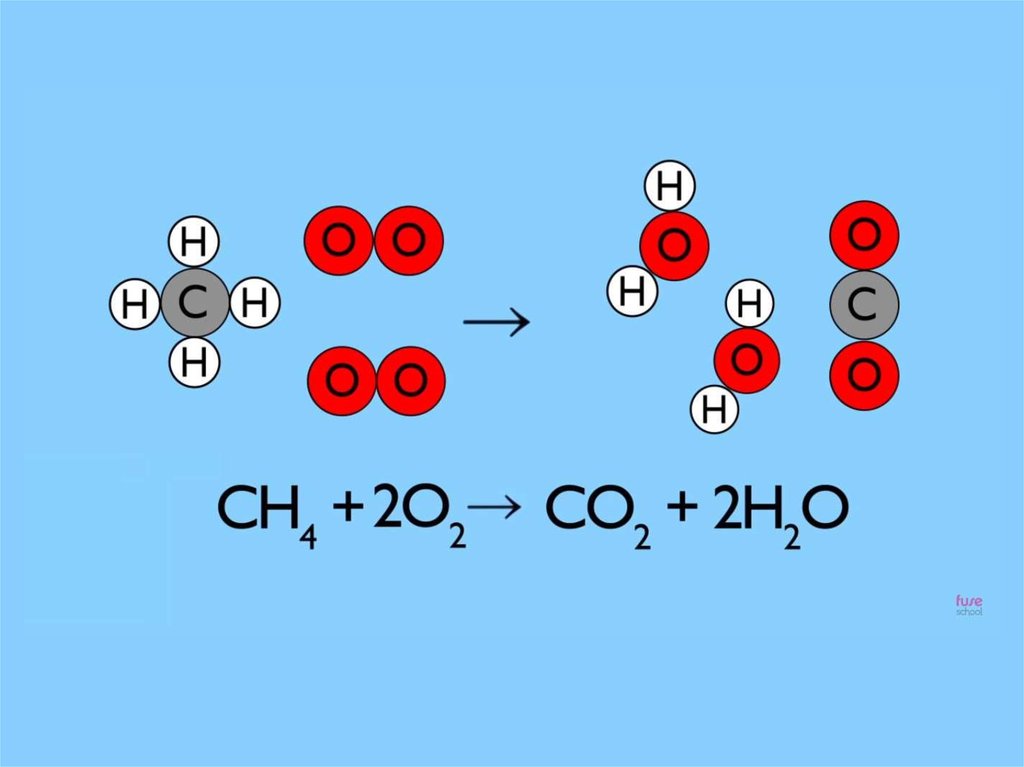
8.
9.
The law of conservat ion mass is simplysaying t hat during chemical change
t here is no loss or gain of at oms
It is for this reason that
we always balance
chemical equations






 Химия
Химия








