Похожие презентации:
Solutions. Equilibrium in solutions of strong and weak electrolytes
1.
Lecture № 5Solutions. Equilibrium in
solutions of strong and weak
electrolytes
2.
Context1. Classification of solutions
2. Solutions and concentration
3. Strong and weak electrolytes
4. Colligative properties: osmosis
5. Osmosis
6. The pH concept
7. Strong acids and bases
8. Acids and bases: different approaches
9. Conjugate acids and bases
10. Weak acids and bases
11. pH in solutions of strong and weak acids
12. Solutions of weak acids, weak bases and salts
3.
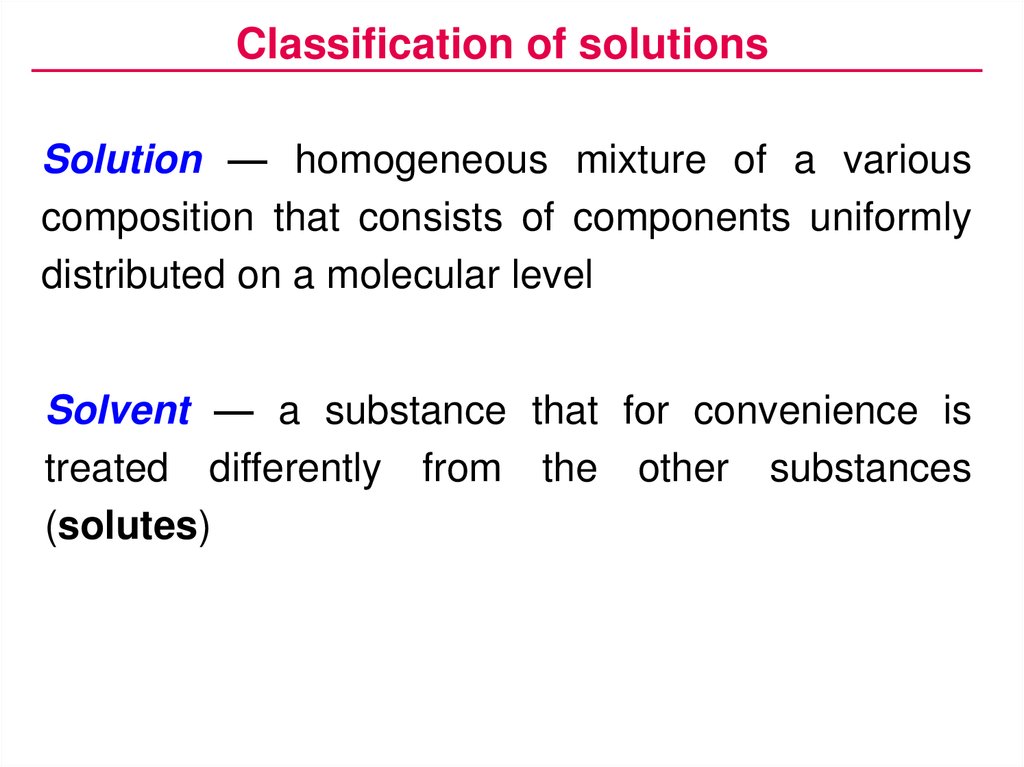
Classification of solutionsSolution — homogeneous mixture of a various
composition that consists of components uniformly
distributed on a molecular level
Solvent — a substance that for convenience is
treated differently from the other substances
(solutes)
4.
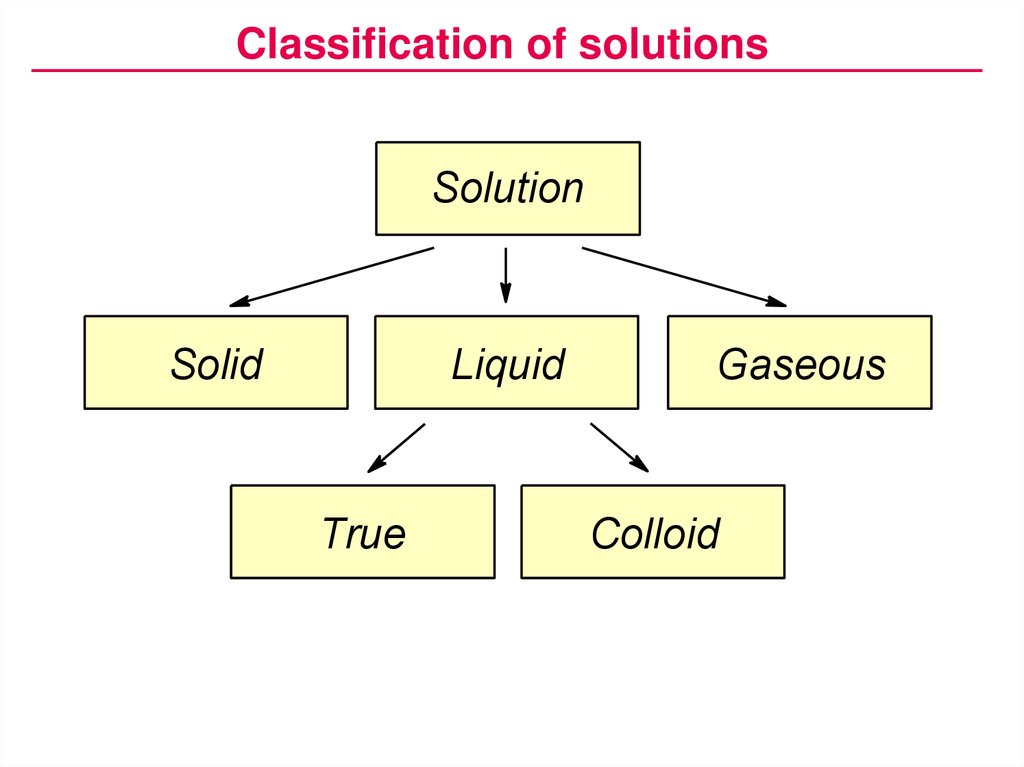
Classification of solutionsSolution
Solid
Liquid
True
Gaseous
Colloid
5.
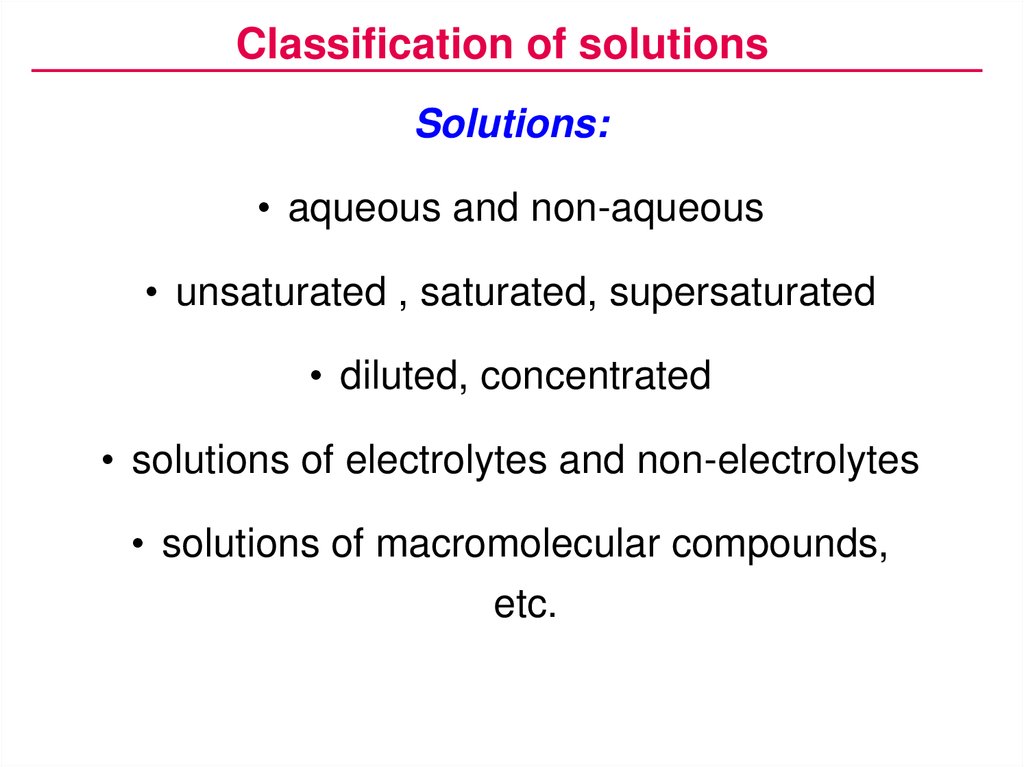
Classification of solutionsSolutions:
• aqueous and non-aqueous
• unsaturated , saturated, supersaturated
• diluted, concentrated
• solutions of electrolytes and non-electrolytes
• solutions of macromolecular compounds,
etc.
6.
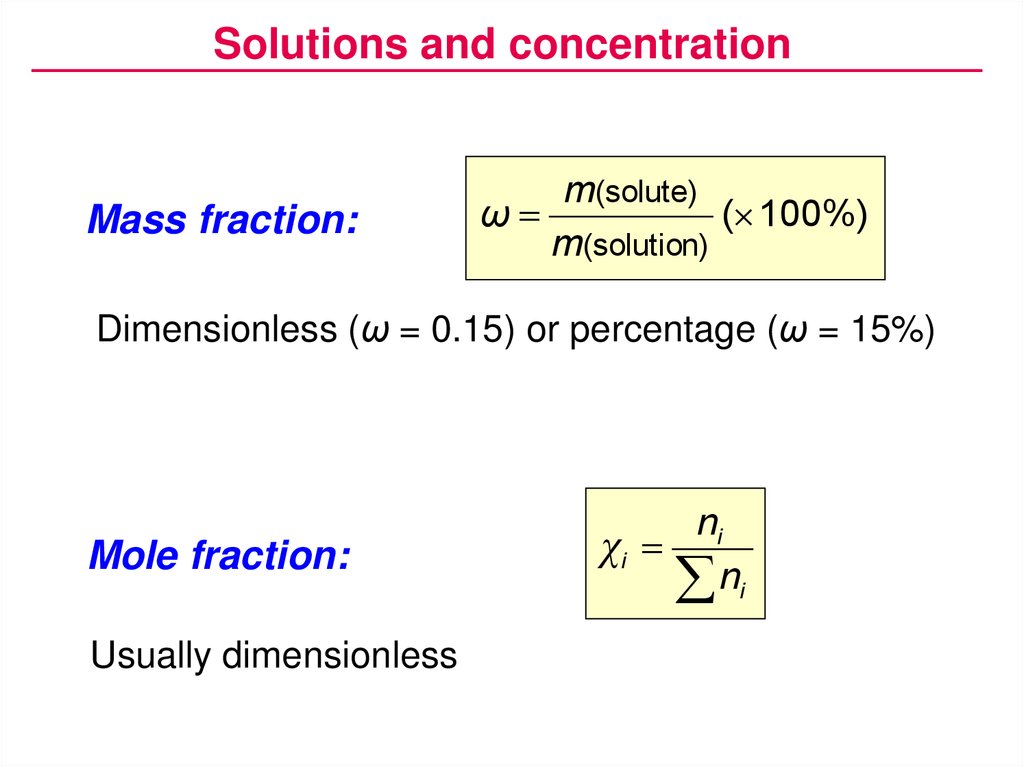
Solutions and concentrationMass fraction:
m(solute)
ω
( 100%)
m(solution)
Dimensionless (ω = 0.15) or percentage (ω = 15%)
Mole fraction:
Usually dimensionless
ni
χi
ni
7.
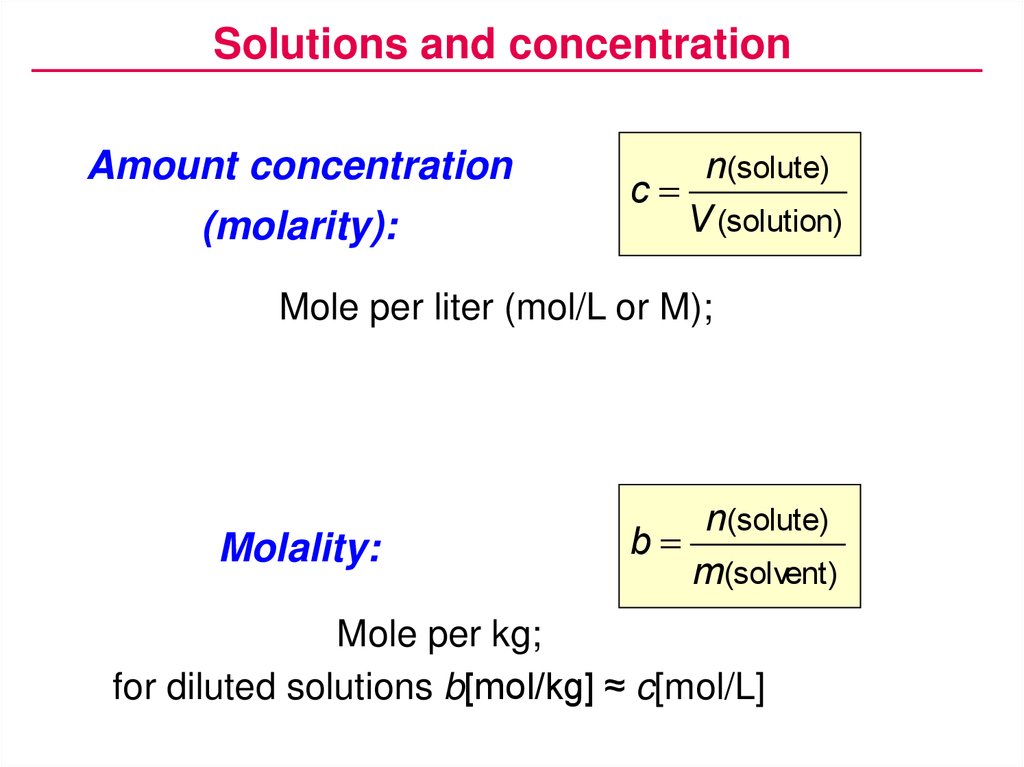
Solutions and concentrationAmount concentration
(molarity):
n(solute)
c
V (solution)
Mole per liter (mol/L or M);
Molality:
n(solute)
b
m(solvent)
Mole per kg;
for diluted solutions b[mol/kg] ≈ c[mol/L]
8.
Solutions and concentrationExample. Glucose (5.4 g) and sodium hydroxide
(0.6 g) were dissolved in water to give 100 ml of
solution with ρ = 1.05 g/ml. Calculate mass fractions,
mole fractions and amount concentrations of all
substances in the solution.
Name
Formula
Molar mass
Glucose
C6H12O6
180
Sodium
hydroxide
NaOH
40
Water
H2O
18
Mass
Amount
9.
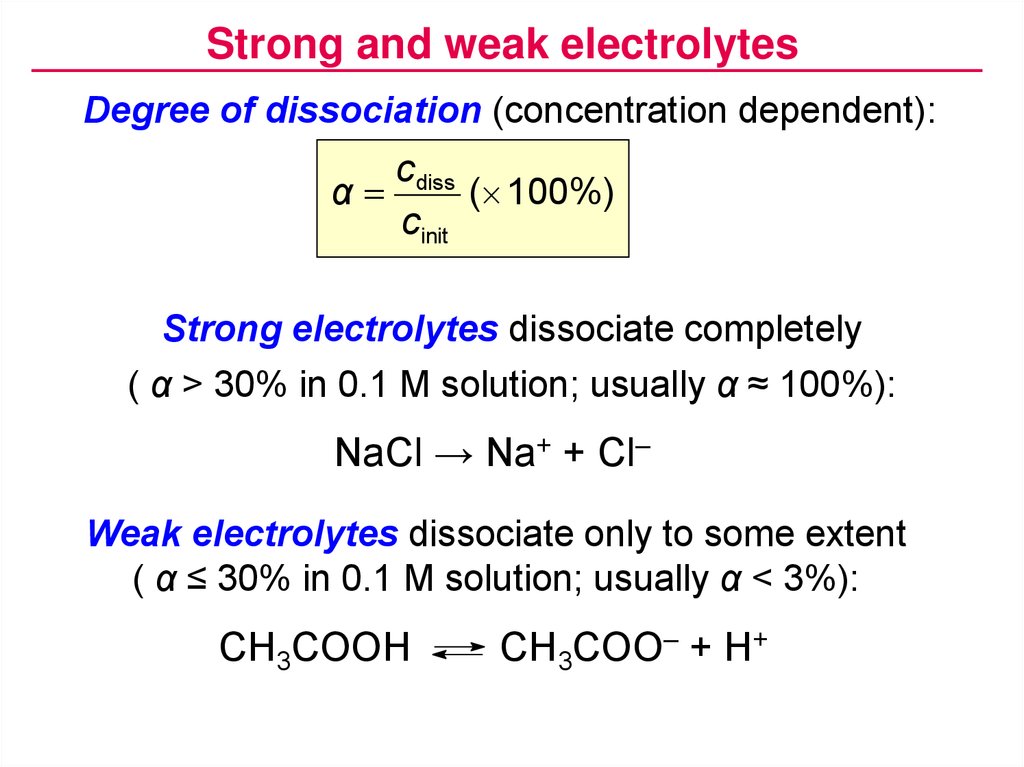
Strong and weak electrolytesDegree of dissociation (concentration dependent):
c diss
α
( 100%)
cinit
Strong electrolytes dissociate completely
( α > 30% in 0.1 M solution; usually α ≈ 100%):
NaCl → Na+ + Cl–
Weak electrolytes dissociate only to some extent
( α ≤ 30% in 0.1 M solution; usually α < 3%):
CH3COOH
CH3COO– + H+
10.
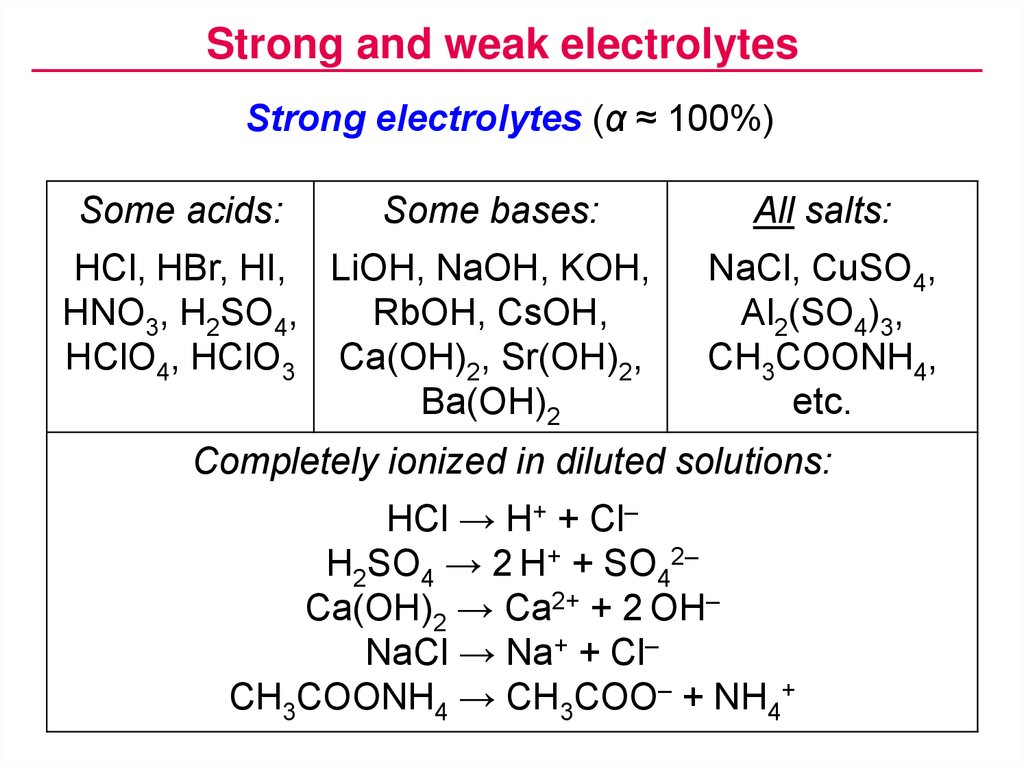
Strong and weak electrolytesStrong electrolytes (α ≈ 100%)
Some acids:
Some bases:
HCl, HBr, HI, LiOH, NaOH, KOH,
HNO3, H2SO4,
RbOH, CsOH,
HClO4, HClO3 Ca(OH)2, Sr(OH)2,
Ba(OH)2
All salts:
NaCl, CuSO4,
Al2(SO4)3,
CH3COONH4,
etc.
Completely ionized in diluted solutions:
HCl → H+ + Cl–
H2SO4 → 2 H+ + SO42–
Ca(OH)2 → Ca2+ + 2 OH–
NaCl → Na+ + Cl–
CH3COONH4 → CH3COO– + NH4+
11.
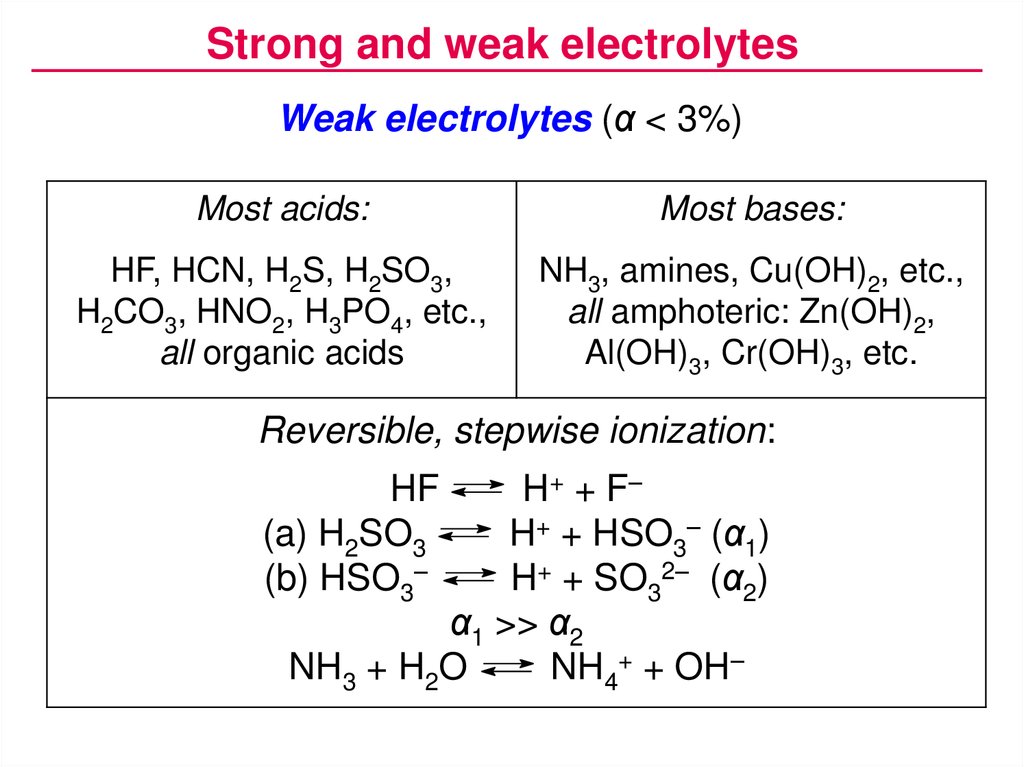
Strong and weak electrolytesWeak electrolytes (α < 3%)
Most acids:
Most bases:
HF, HCN, H2S, H2SO3,
H2CO3, HNO2, H3PO4, etc.,
all organic acids
NH3, amines, Cu(OH)2, etc.,
all amphoteric: Zn(OH)2,
Al(OH)3, Cr(OH)3, etc.
Reversible, stepwise ionization:
H + + F–
H+ + HSO3– (α1)
H+ + SO32– (α2)
α1 >> α2
NH3 + H2O
NH4+ + OH–
HF
(a) H2SO3
(b) HSO3–
12.
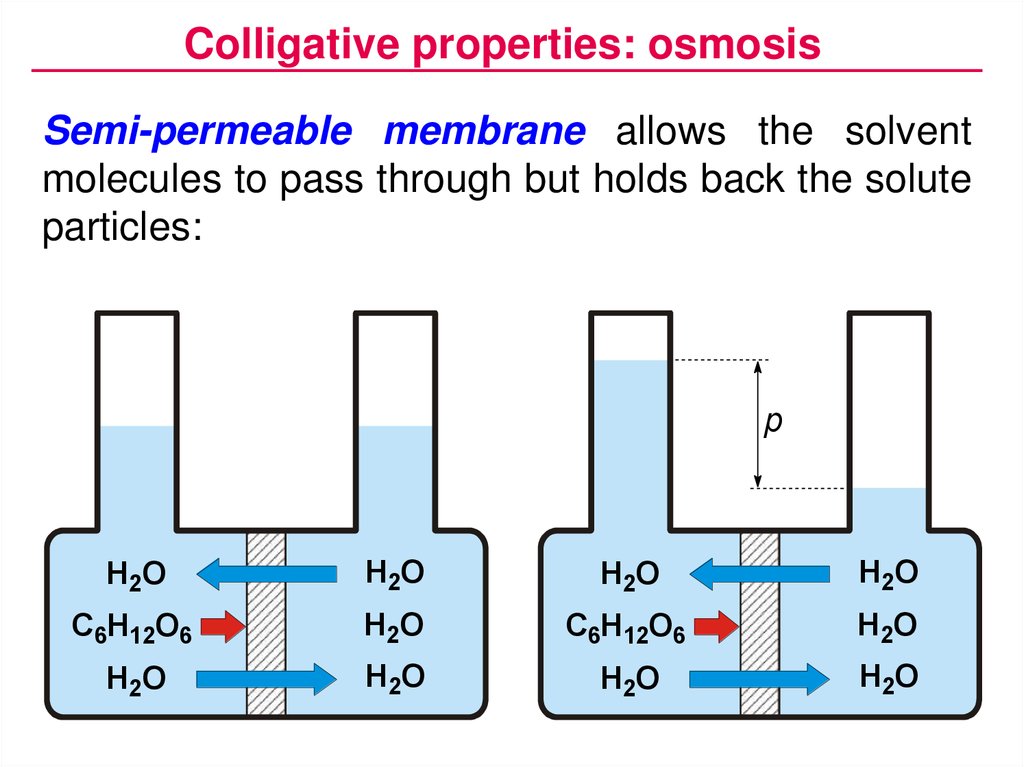
Colligative properties: osmosisSemi-permeable membrane allows the solvent
molecules to pass through but holds back the solute
particles:
p
H2O
H2O
H2O
H2O
C6H12O6
H2O
C6H12O6
H2O
H2O
H2O
H2O
H2O
13.
OsmosisOsmotic pressure — an excess pressure required
to maintain osmotic equilibrium between a solution
and the pure solvent separated by a membrane
permeable only to the solvent:
π = bosm RT ≈ cosm RT
Osmolarity (cosm) — the total molar concentration
of all solvated particles of the solutes in the
solution:
cosm = Σ(cn • in)
14.
OsmosisIsotonic coefficient, or van't Hoff factor (i) — the
number of moles of particles (ions and/or
undissociated molecules) per mole of solute:
Solute type
Example
Non-electrolyte C6H12O6
Weak
electrolyte
Strong
electrolyte
CH3COOH
i
i=1
CH3COO– + H+
1<i<2
NaCl → Na+ + Cl–
i=2
CaCl2 → Ca2+ + 2 Cl–
i=3
Na3PO4 → 3 Na+ + PO43–
i=4
15.
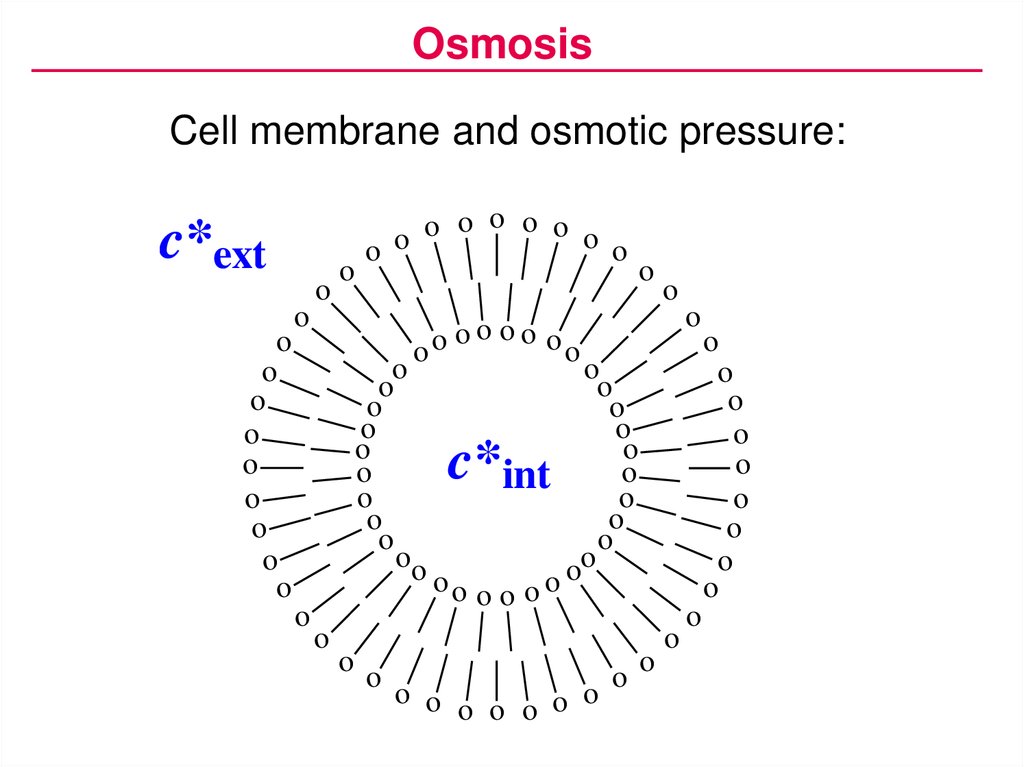
OsmosisCell membrane and osmotic pressure:
c*ext
o
o
o
o
o
o
o
o
o
o
o
o
o o o o o o
o
o
o
o
oo oo o
o
o
o
o
o
o
o
o
o
o
o
o
oo
o
o
o
o
o
o
o
o
o
o
o
int
o
o
o
o
oo o o o o o
o
c*
o o
o
o o o o
o
o
o
o
o
o
o
o
o
o
o
o
o
16.
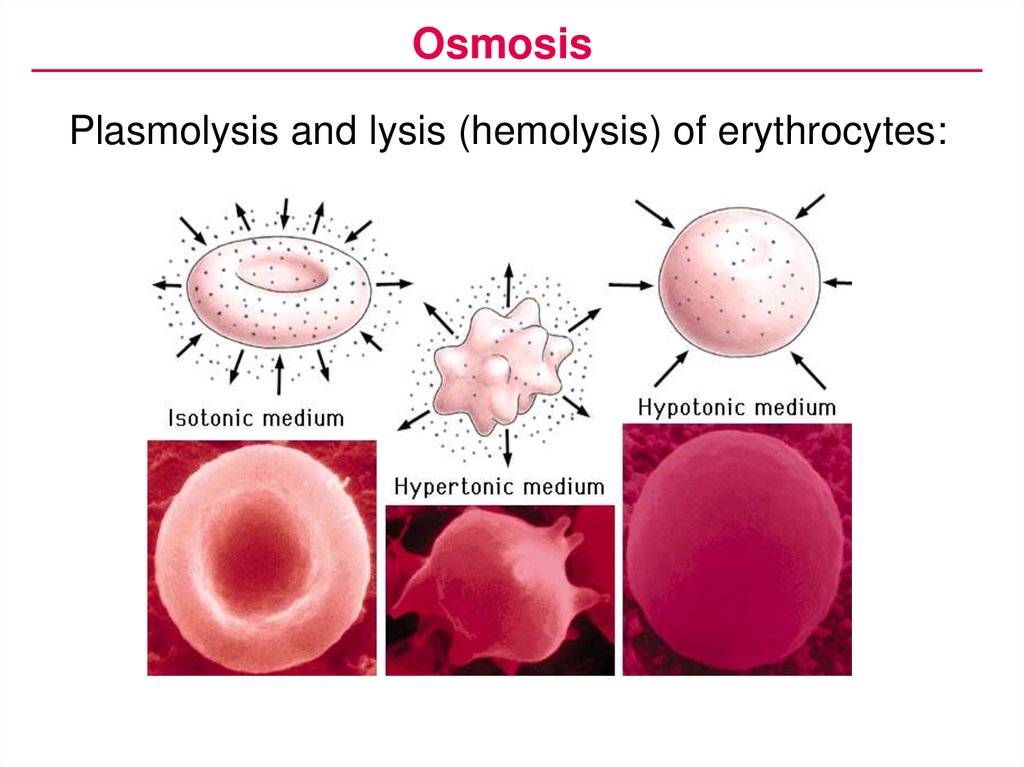
OsmosisPlasmolysis and lysis (hemolysis) of erythrocytes:
17.
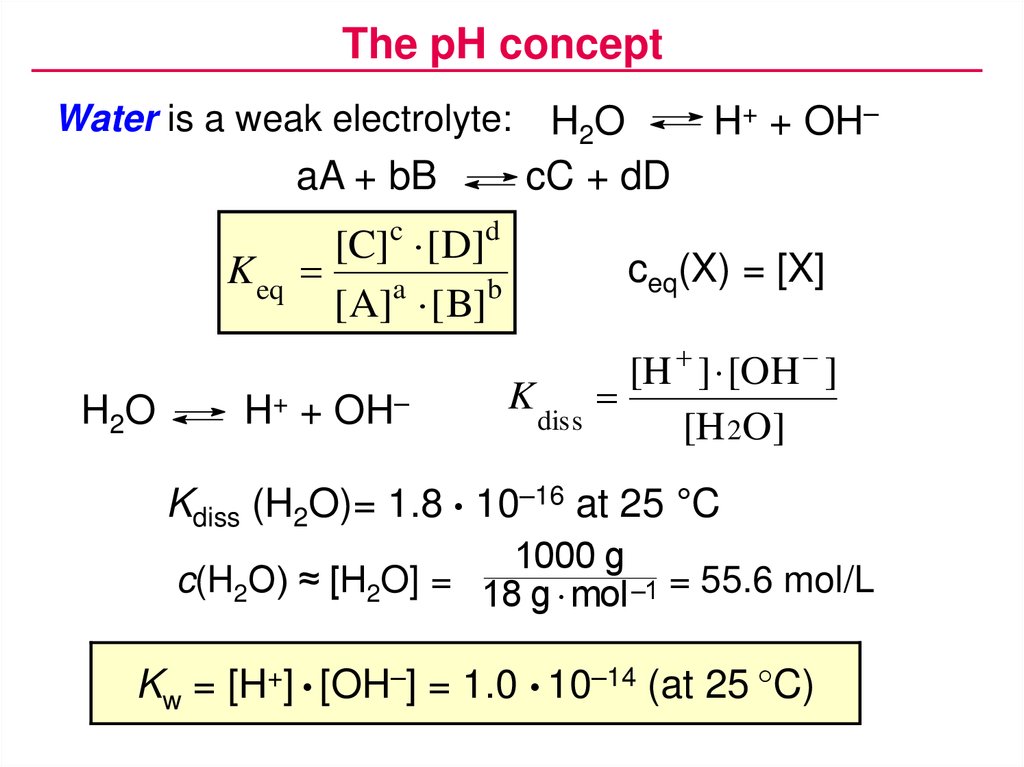
The pH conceptWater is a weak electrolyte:
aA + bB
K eq
H2O
H2O
cC + dD
[C]c [ D]d
[A]a [ B]b
H+ + OH–
H+ + OH–
ceq(X) = [X]
K
diss
[H ] [OH ]
[H 2O]
Kdiss (H2O)= 1.8 • 10–16 at 25 °C
1000 g
c(H2O) ≈ [H2O] = 18 g mol –1 = 55.6 mol/L
Kw = [H+] • [OH–] = 1.0 • 10–14 (at 25 °C)
18.
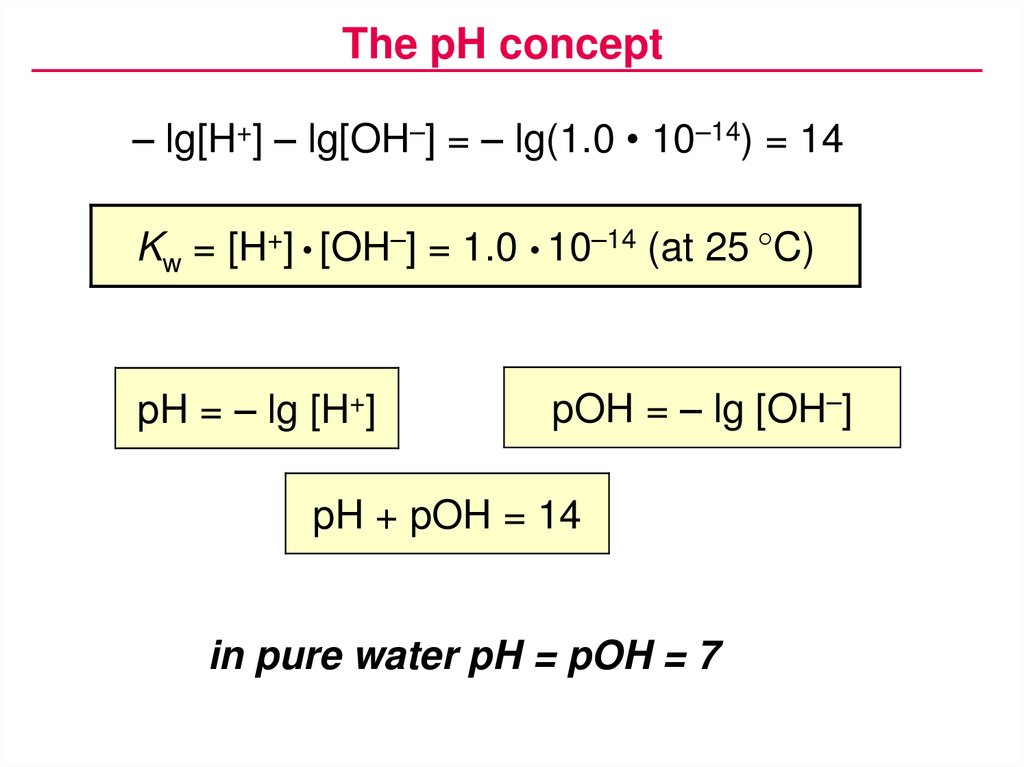
The pH concept– lg[H+] – lg[OH–] = – lg(1.0 • 10–14) = 14
Kw = [H+] • [OH–] = 1.0 • 10–14 (at 25 °C)
pH = – lg [H+]
pOH = – lg [OH–]
pH + pOH = 14
in pure water pH = pOH = 7
19.
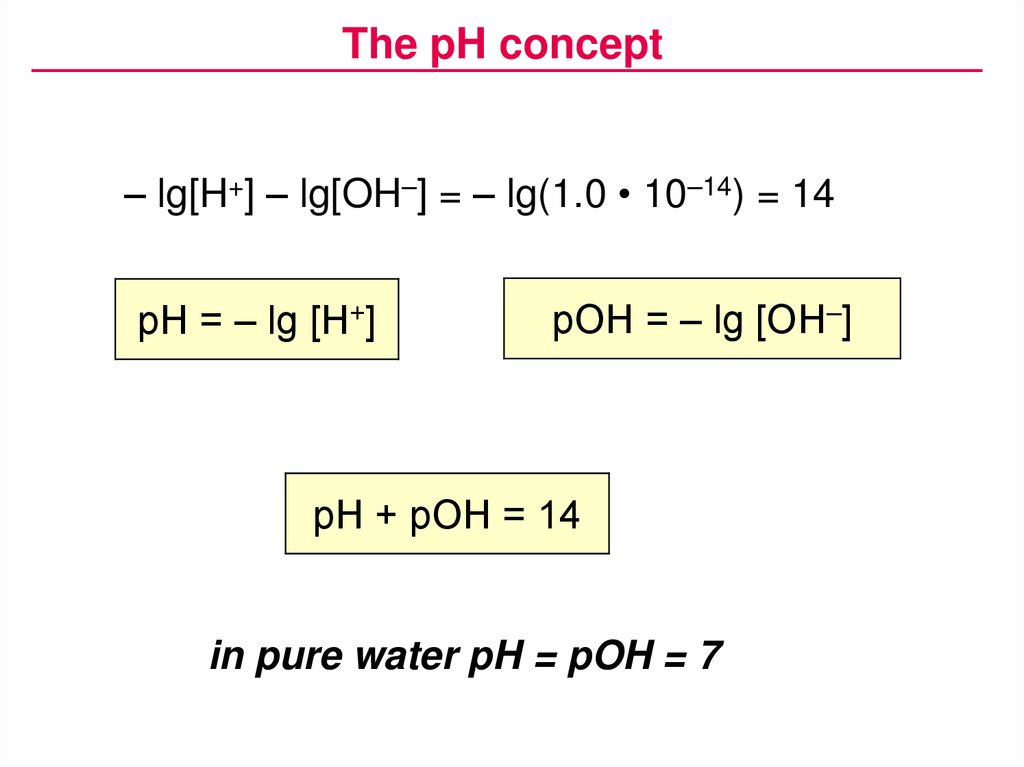
The pH concept– lg[H+] – lg[OH–] = – lg(1.0 • 10–14) = 14
pH = – lg [H+]
pOH = – lg [OH–]
pH + pOH = 14
in pure water pH = pOH = 7
20.
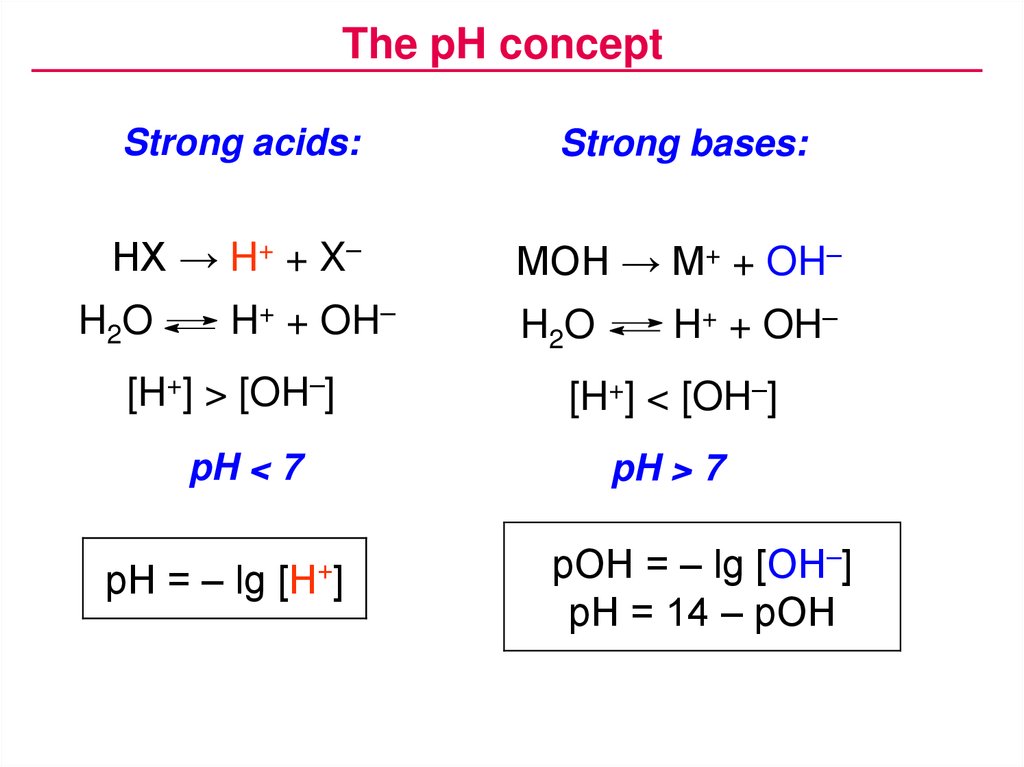
The pH conceptStrong acids:
Strong bases:
HX → H+ + X–
MOH → M+ + OH–
H2O
H+ + OH–
[H+] > [OH–]
pH < 7
pH = – lg
[H+]
H2O
H+ + OH–
[H+] < [OH–]
pH > 7
pOH = – lg [OH–]
pH = 14 – pOH
21.
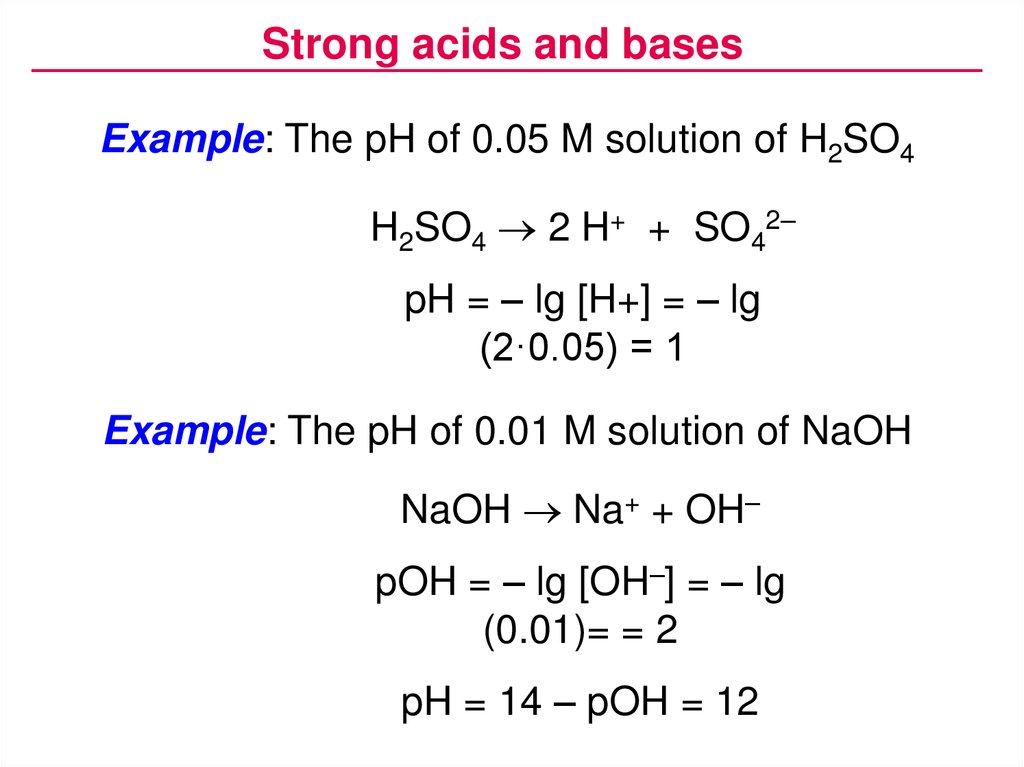
Strong acids and basesExample: The pH of 0.05 M solution of H2SO4
H2SO4 2 H+ + SO42–
pH = – lg [H+] = – lg
(2·0.05) = 1
Example: The pH of 0.01 M solution of NaOH
NaOH Na+ + OH–
pOH = – lg [OH–] = – lg
(0.01)= = 2
pH = 14 – pOH = 12
22.
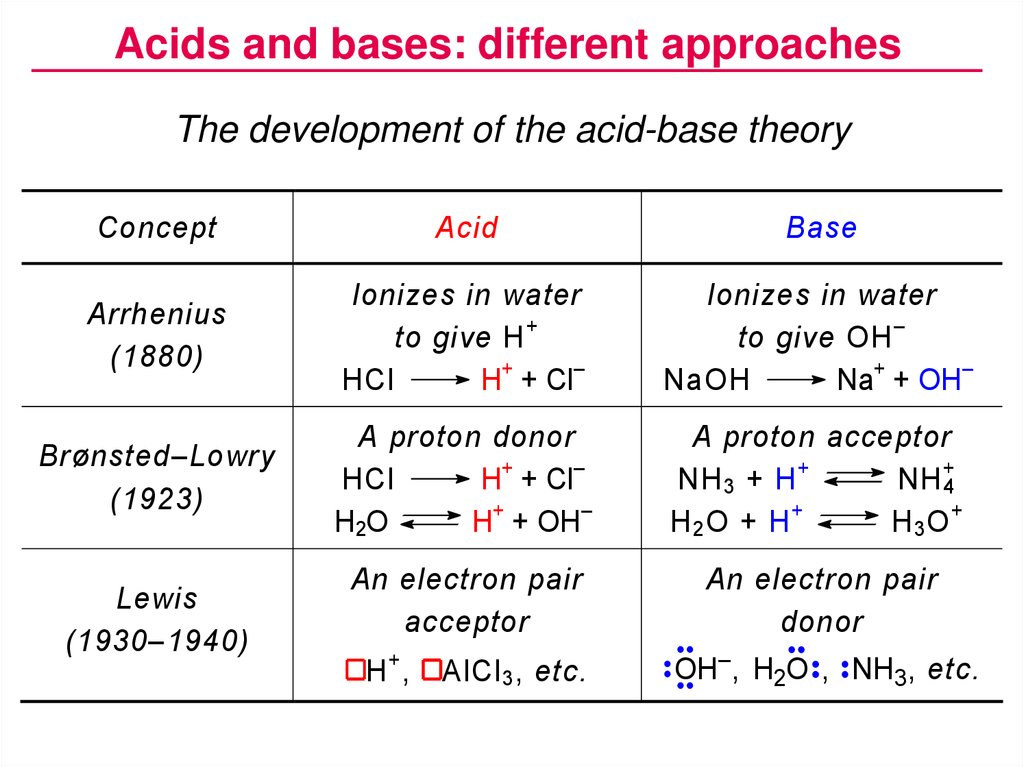
Acids and bases: different approachesThe development of the acid-base theory
Concept
Acid
Base
Arrhenius
(1880)
Ionizes in water
to give H +
HCl
H+ + Cl–
Ionizes in water
to give OH –
NaOH
Na+ + OH–
Brønsted–Lowry
(1923)
A proton donor
HCl
H+ + Cl–
H 2O
H+ + OH–
A proton acceptor
NH 3 + H +
NH 4+
H2O + H+
H3O+
An electron pair
acceptor
An electron pair
donor
Lewis
(1930–1940)
H+,
AlCl 3 , etc.
OH– , H2O , NH3 , etc.
23.
Conjugate acids and basesConjugate acid-base pairs:
Conjugate acid ⇄ H+ + conjugate base
Conjugate base + H+ ⇄ conjugate acid
Water, the most important electrolyte:
conjugate
acid
H3O+
– H+
conjugate
base
H2O
– H+
+ H+ conjugate + H+
acid
OH–
conjugate
base
Example: Write conjugate acids and/or bases for the
following particles: CO32–, NH3, NH4+, HS–, H2S.
24.
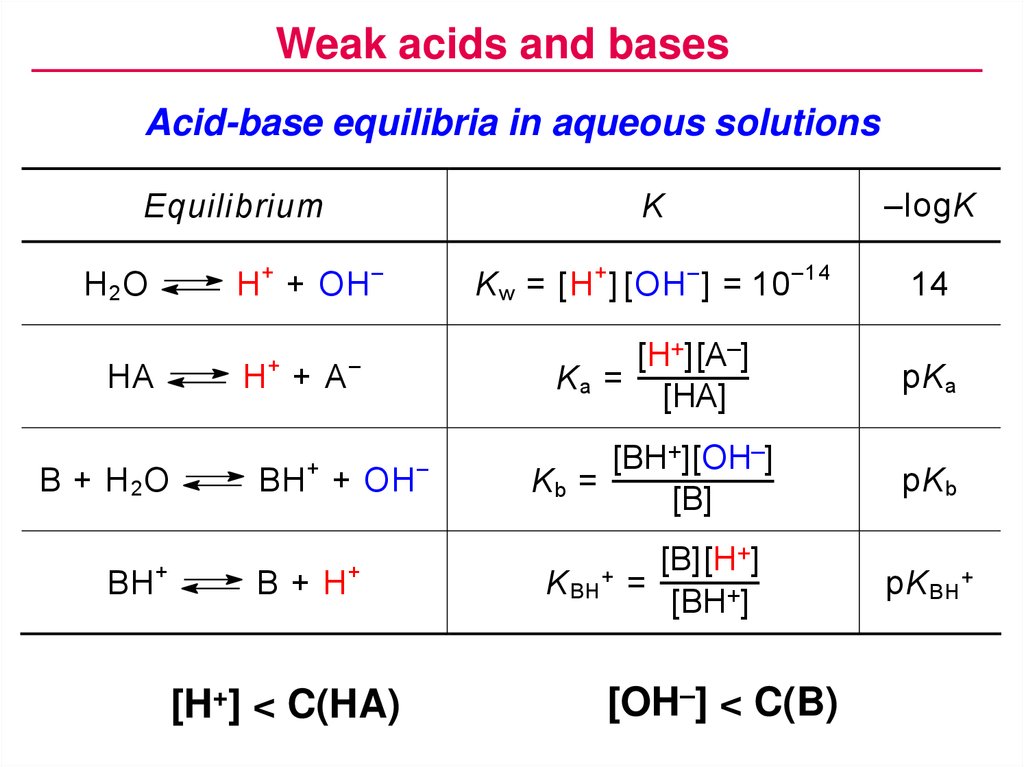
Weak acids and basesAcid-base equilibria in aqueous solutions
K
–logK
K w = [H + ] [OH – ] = 10 –14
14
[H+][A–]
Ka =
[HA]
pK a
[BH+][OH–]
Kb =
[B]
pK b
Equilibrium
H + + OH –
H2O
+
HA
H +A
B + H2O
BH
+
–
+
BH + OH
B+H
+
[H+] < C(HA)
–
K BH +
[B][H+]
=
[BH+]
[OH–] < C(B)
pK BH +
25.
pH in solutions of strong and weak acidsStrong acid:
HCl →
H2O
H+
+
Cl–
H+ + OH–
[H+] = c (HCl)
pH = – lg [H+]
= – lg c(HCl)
Weak acid:
HNO2
H2O
H+ + NO2–
H+ + OH–
[H+] < c (HNO2)
[H ] [A ]
Ka
[HA]
[H+] = [A–]; ca [HA]
[H ]2
Ka
ca
or pKa = 2pH + lgca
pH = ½[pKa – lg c(HNO2)]
26.
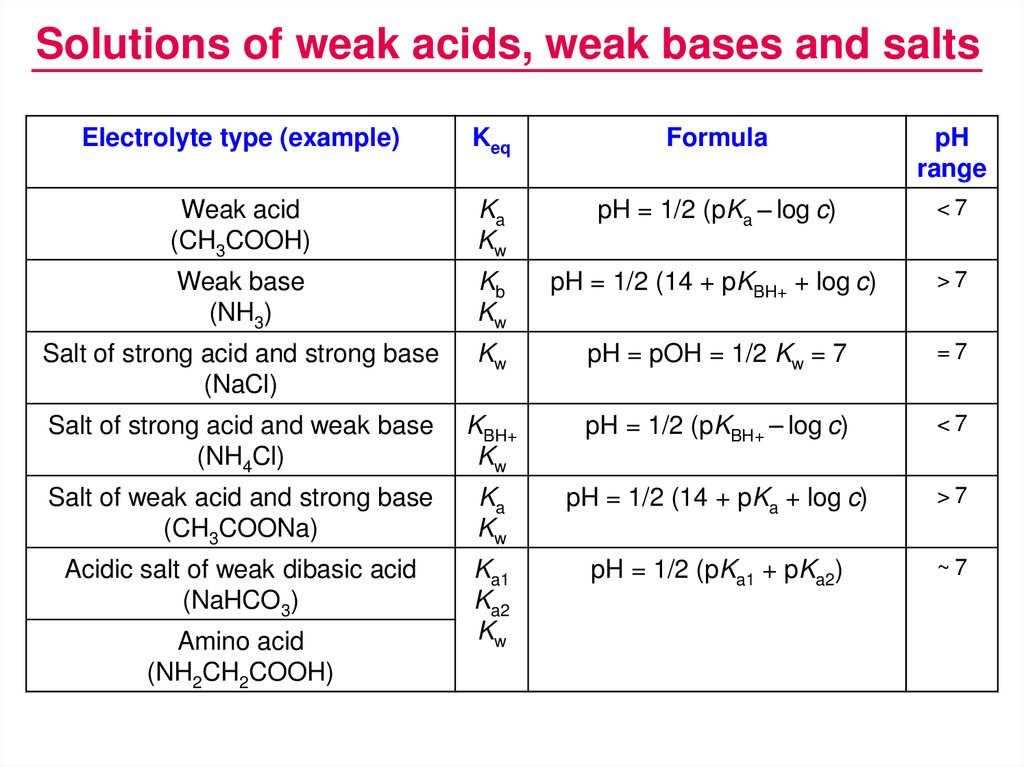
Solutions of weak acids, weak bases and saltsElectrolyte type (example)
Keq
Formula
pH
range
Weak acid
(CH3COOH)
Ka
Kw
pH = 1/2 (pKa – log c)
<7
Weak base
(NH3)
Kb
Kw
pH = 1/2 (14 + pKBH+ + log c)
>7
Salt of strong acid and strong base
(NaCl)
Kw
pH = pOH = 1/2 Kw = 7
=7
Salt of strong acid and weak base
(NH4Cl)
KBH+
Kw
pH = 1/2 (pKBH+ – log c)
<7
Salt of weak acid and strong base
(CH3COONa)
Ka
Kw
pH = 1/2 (14 + pKa + log c)
>7
Acidic salt of weak dibasic acid
(NaHCO3)
Ka1
Ka2
Kw
pH = 1/2 (pKa1 + pKa2)
~7
Amino acid
(NH2CH2COOH)








 Химия
Химия








