Похожие презентации:
Магнитные изотопные эффекты в металлзависимом ферментативном катализе
1.
МАГНИТНЫЕ ИЗОТОПНЫЕ ЭФФЕКТЫВ МЕТАЛЛ-ЗАВИСИМОМ
ФЕРМЕНТАТИВНОМ КАТАЛИЗЕ.
История вопроса, достижения и перспективы
практического применения.
Кузнецов Д.А.
Кафедра медицинских нанобиотехнологий
МБФ РНИМУ им. Н.И. Пирогова,
Отдел строения вещества Института химической
физики им. Н.Н. Семёнова РАН.
www.rsmu.ru
2015
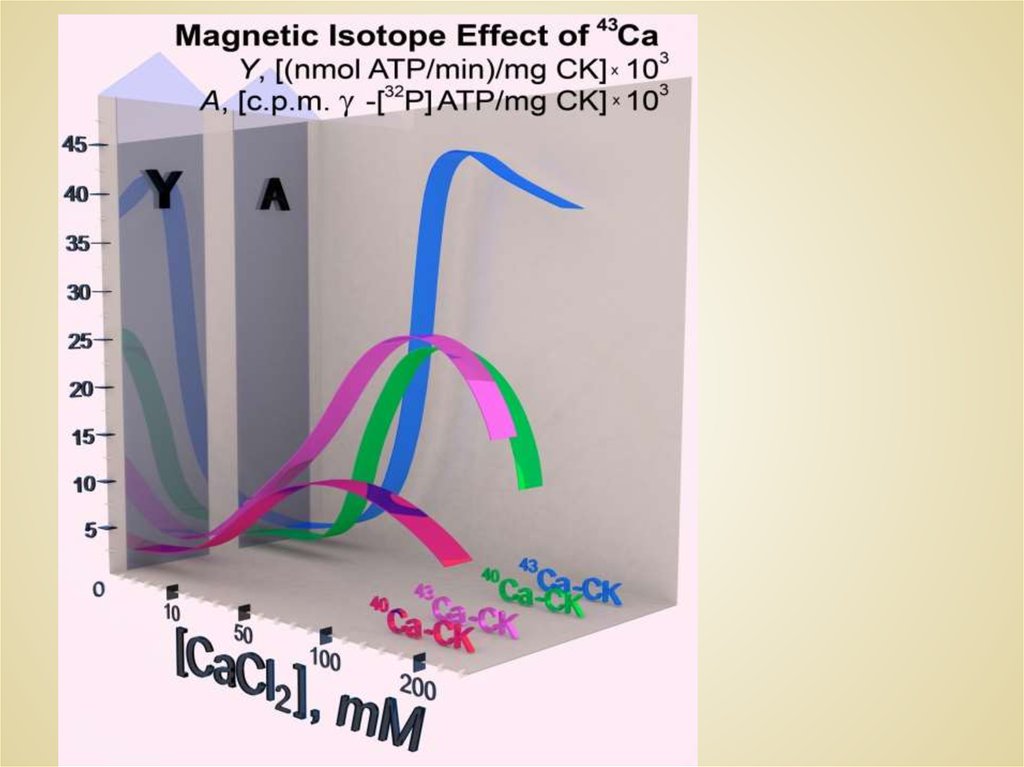
2. Mg and Ca Isotopes Natural Abundance
Abundance, %Nuclei
Nuclear spin
24Mg
78,99
0
25Mg
10,00
+5/2
26Mg
11,01
0
40Ca
96.94
0
43Ca
1.317
-7/2
Nuclear magnetic
moment, μ
-0,85545
+0,87515
3. Mg and Zn Isotopes Natural Abundance
Abundance, %Nuclei
Nuclear spin
24Mg
78,99
0
25Mg
10,00
+5/2
26Mg
11,01
0
64Zn
48,6
0
66Zn
27,9
0
67Zn
4,1
-5/2
68Zn
18,8
0
70Zn
0,6
0
Nuclear magnetic
moment, μ
-0,85545
+0,87515
4.
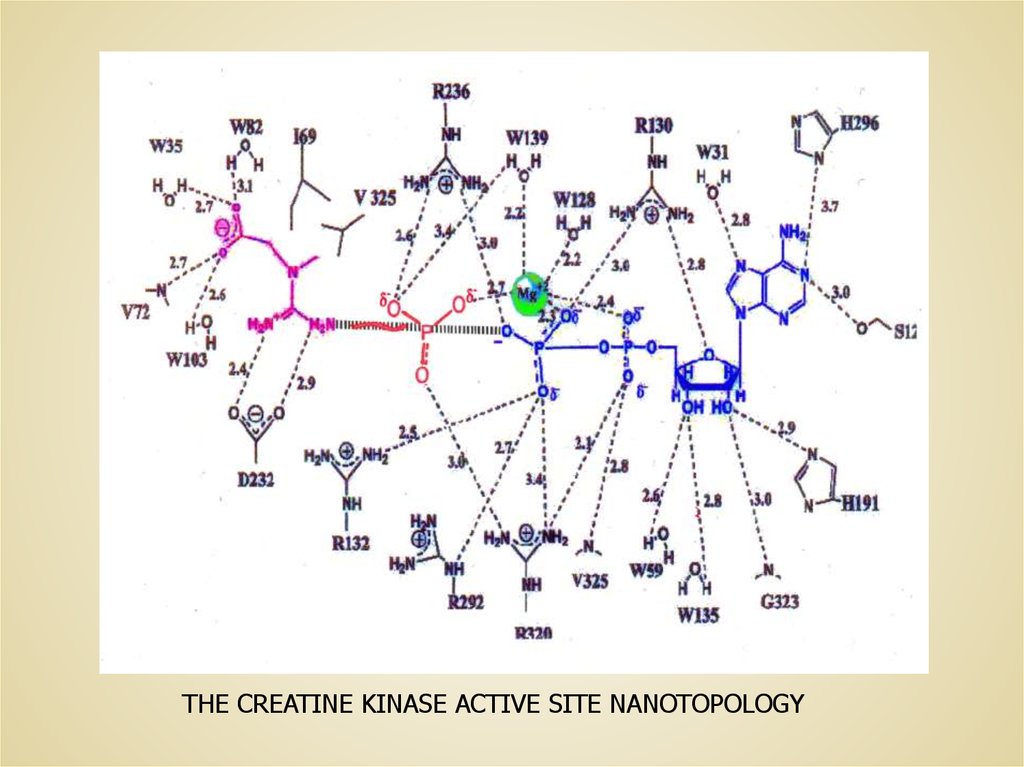
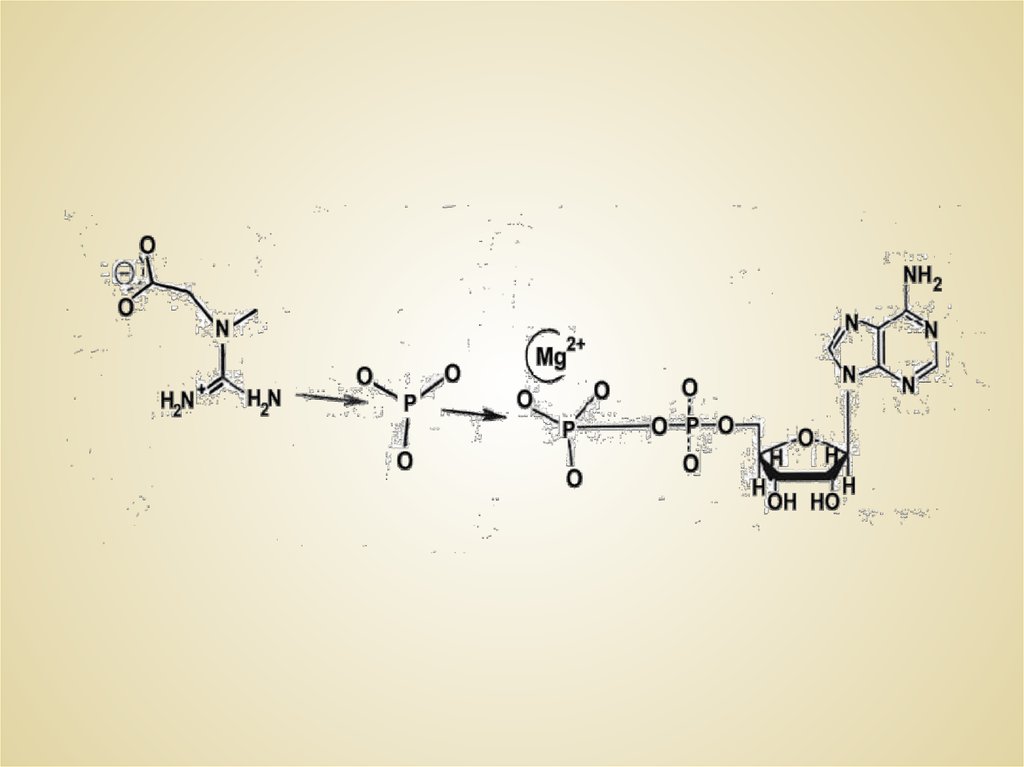
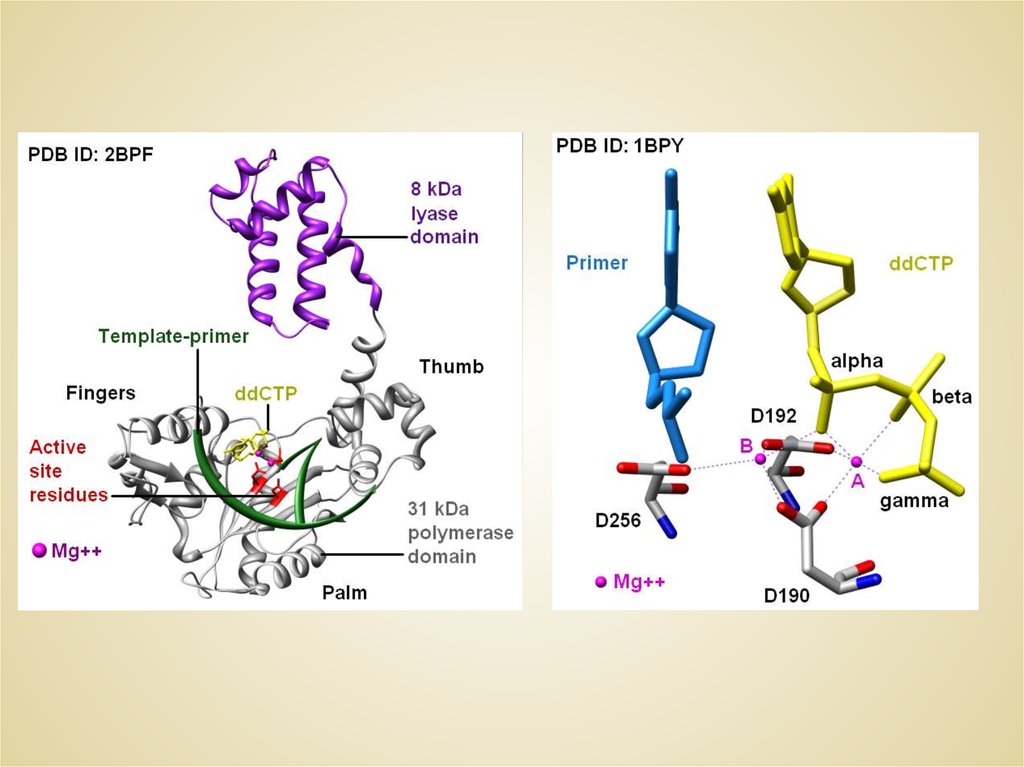
THE CREATINE KINASE ACTIVE SITE NANOTOPOLOGY5.
6.
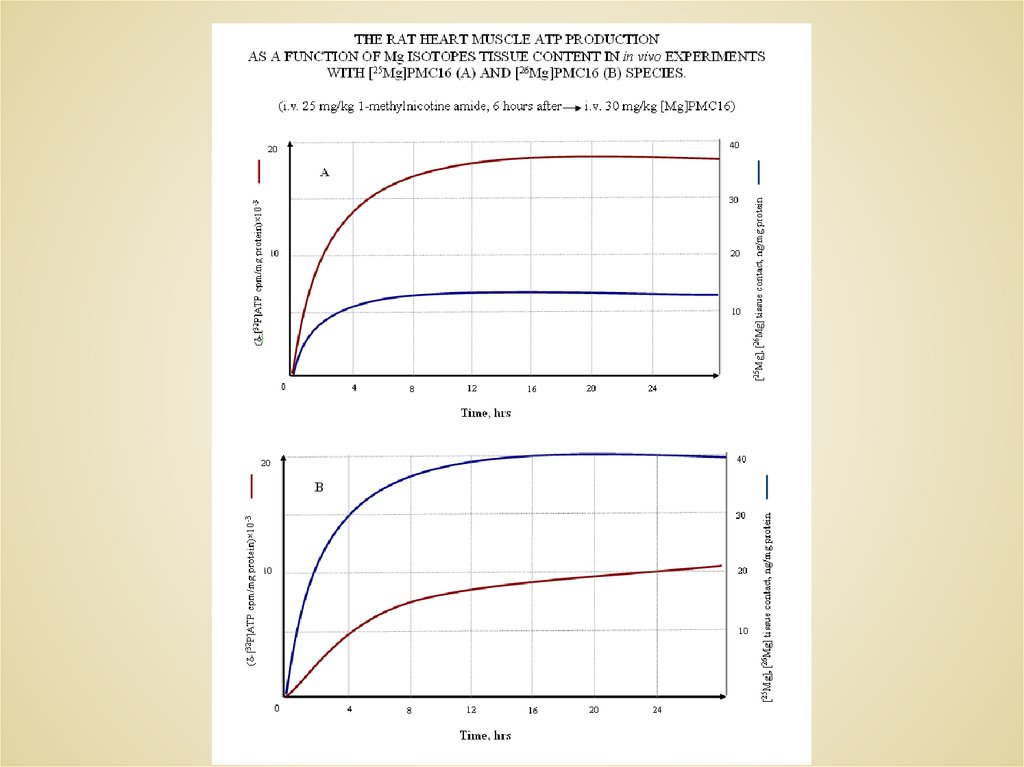
The rate of ATP formation by mitochondria (A) and by creatinekinase (B) as a function of magnesium isotope
intact mitochondria
mitochondria subjected to a selective blockade of oxidative
phosphorylation by 1-methylnicotine amide.
A
B
25
1.0
24
26
The yield of ATP is given in mmole/g total protein
7.
8.
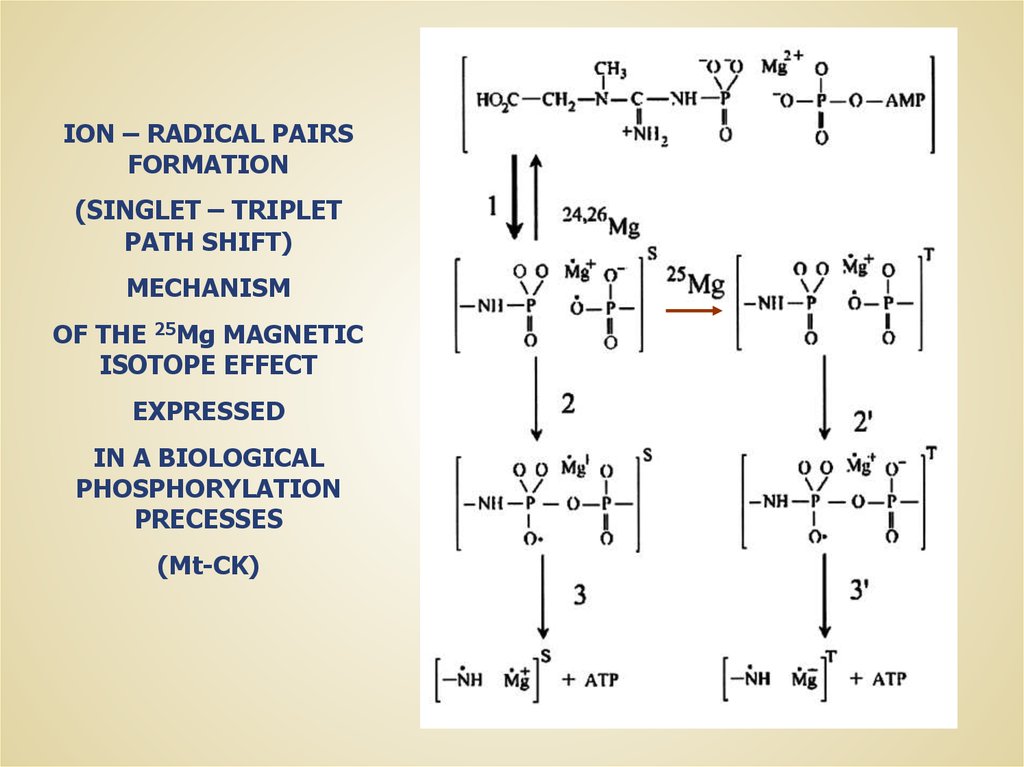
ION – RADICAL PAIRSFORMATION
(SINGLET – TRIPLET
PATH SHIFT)
MECHANISM
OF THE 25Mg MAGNETIC
ISOTOPE EFFECT
EXPRESSED
IN A BIOLOGICAL
PHOSPHORYLATION
PRECESSES
(Mt-CK)
9. Phosphoglycerate kinase
A 10330
6
5
25
Mg
4
3
2
20
1
10
0
0
10
20
30
MgCl2, mM
24
Mg
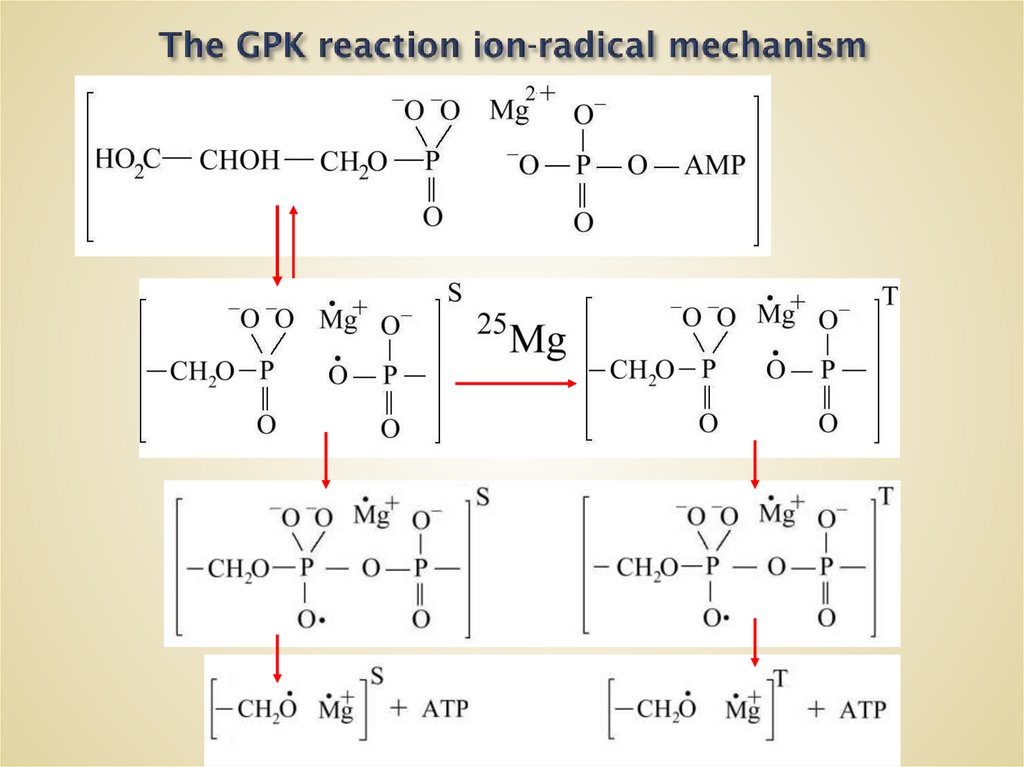
10. The GPK reaction ion-radical mechanism
11.
R RT
RR
R R
Zeeman interaction
.
Fermi interaction
Microwaves
P = f [H,ai,mn, In, mI, HI,w , J]
S
12.
13.
-OOCCH2
2+ H N
2 Mg -OOC
2
COO-
CH
COO-
CH2
N
N
Fe
N
-OOC
COO-
-OOC
COO-
2+
2 Mg -OOC
Fe
N
N
N
N
COO-
COO-
HC
-OOC
NH2
2+
2 Mg
CH2
-OOC
-OOC
COO-
CH2
N
CH2
CH2
CH2
COO-
NH2
CH
H2N
-OOC
COO-
CH2
HC
2+
2 Mg
CH2
CH
H2N
COO-
CH2
-OOC
-OOC
COO-
2+
2 Mg -OOC
COO-
2+
2 Mg -OOC
-OOC
COO-
-OOC
COO-
N
N
Fe
N
N
Fe
N
N
N
N
COO-
-OOC
COOCOO-
-OOC
2+
2 Mg
-OOC
CH2
H2N
CH
CH2
-OOC
COO-
-OOC
2+
2 Mg
14.
-OOCCOO-
CH2
CH2
HC
NH2
H2N
H2C
CH
CH2
N
N
Fe
N
Fe
N
N
N
N
N
CH2
CH2
CH2
CH2
HC
H2N
NH2
HC
CH
COO-
COO-
-OOC
-OOC
N
N
Fe
N
N
N
Fe
N
N
CH2
H2N
CH
CH2
-OOC
CH
CH2
CH2
CH2
CH2
H2N
NH2
N
15.
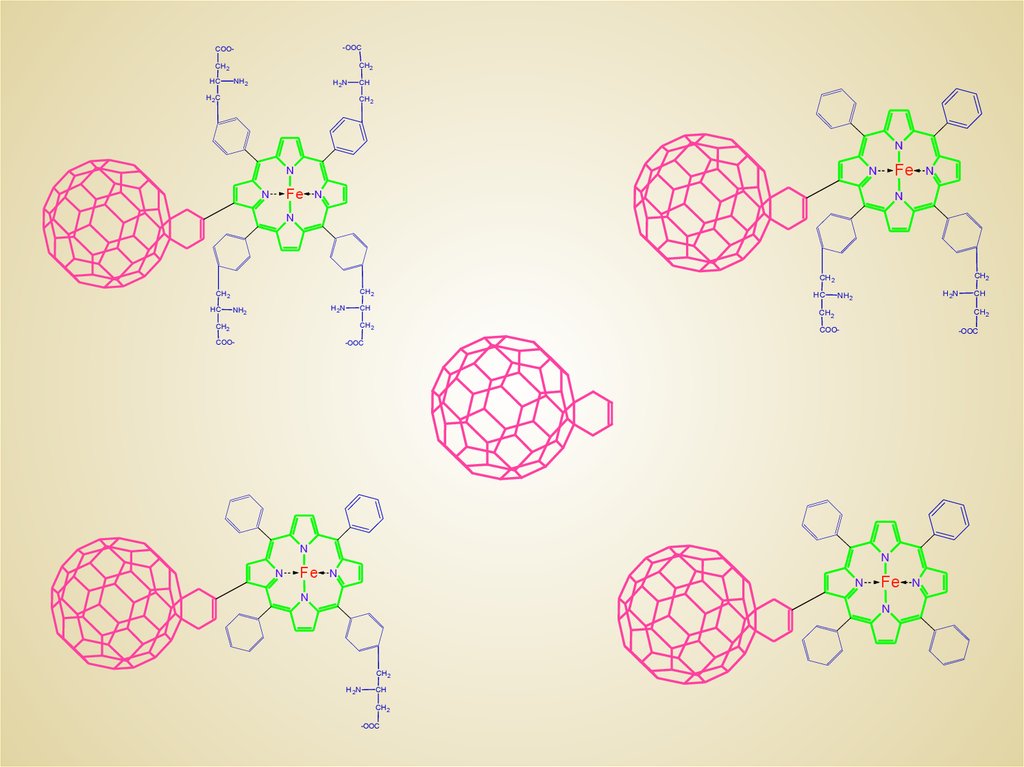
Buckminsterfullerene(C60)-2-(butadiene-1-yl)-tetra(o- -aminobutyryl-o-phtalyl)porphyrinPORPHYLLERENE – MC16
-OOC
COO-
CH2
CH2
HC
NH2
H2C
COO-
2+ H N
2 Mg -OOC
2
CH
CH2
-OOC
COO-
N
Fe
N
N
N
COO-
COO-
CH2
HC
NH2
CH2
COO-
-OOC
-OOC
2+
2 Mg
H2N
CH2
CH
CH2
-OOC
16.
17. PMC16 CATIONITE PROPERTIES AND THE NANOCLUSTERS FORMATION AS A FUNCTION OF pH
14.8nm10.2nm
6.4nm
4.7nm
3.2nm
1.15nm
pH
Blue arrow shows the iron-dextrane sphere exclusion limit
Blue arrow shows the iron-dextrane sphere exclusion limit
, portion of the total PMC16 magnesium
18.
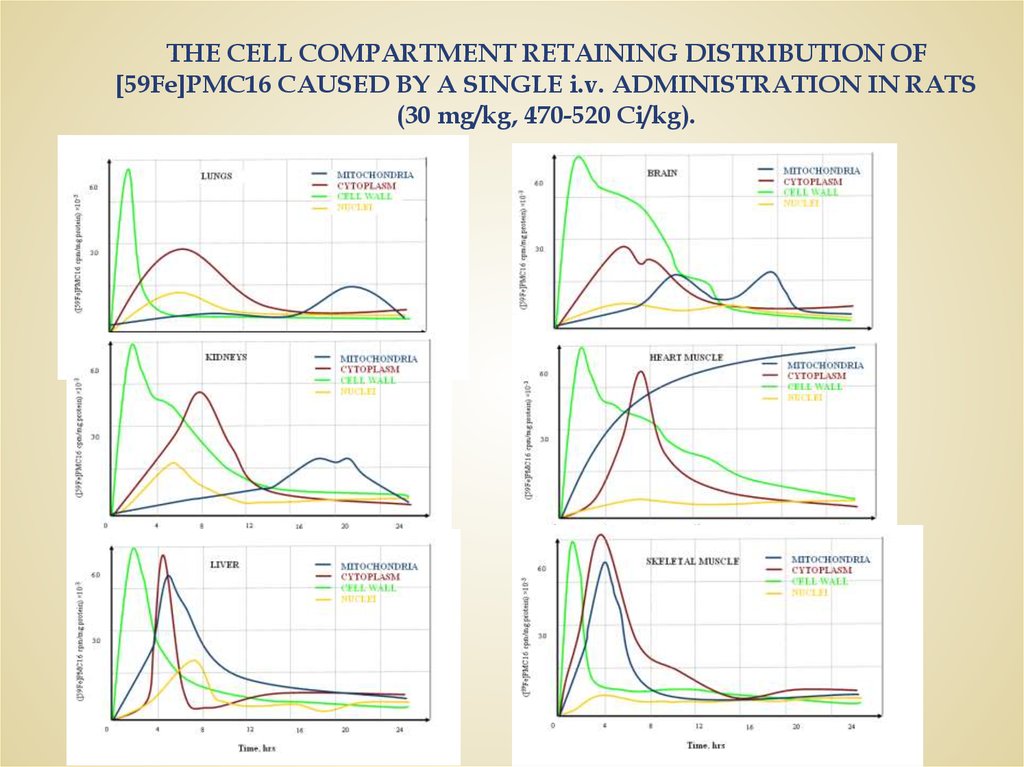
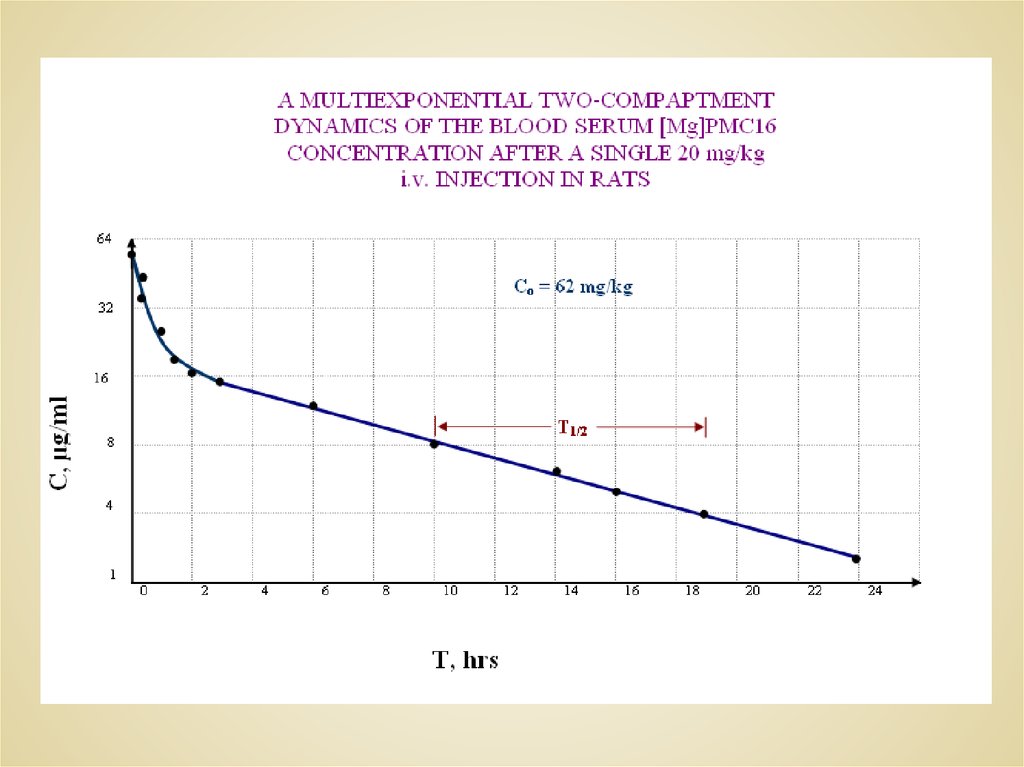
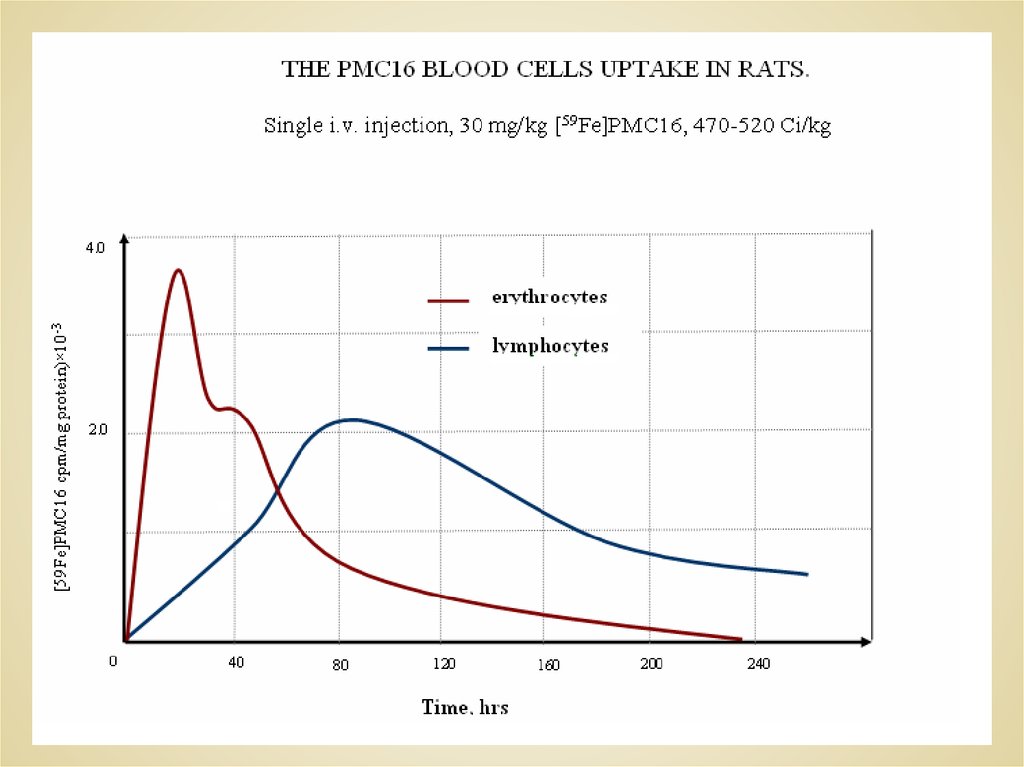
THE CELL COMPARTMENT RETAINING DISTRIBUTION OF[59Fe]PMC16 CAUSED BY A SINGLE i.v. ADMINISTRATION IN RATS
(30 mg/kg, 470-520 Ci/kg).
19.
epoxy spacerPorphyrin domain
Fe2+
cyclohexyl
interface
The CL-Agarose
40-60 μ bead
C60 – fullerene
(“buckminster ball”)
nucleus
Me2+- loading
“cavity”
20. AN AFFINITY CHEROMATOGRAPHY OF THE HUMAN MYOCARDIAL MITOCHONDRIA MEMBRANE PROTEINS ON THE COLUMN WITH AGAROSE-6B-CL-[C17]-PMC16
21.
22.
23.
24.
25.
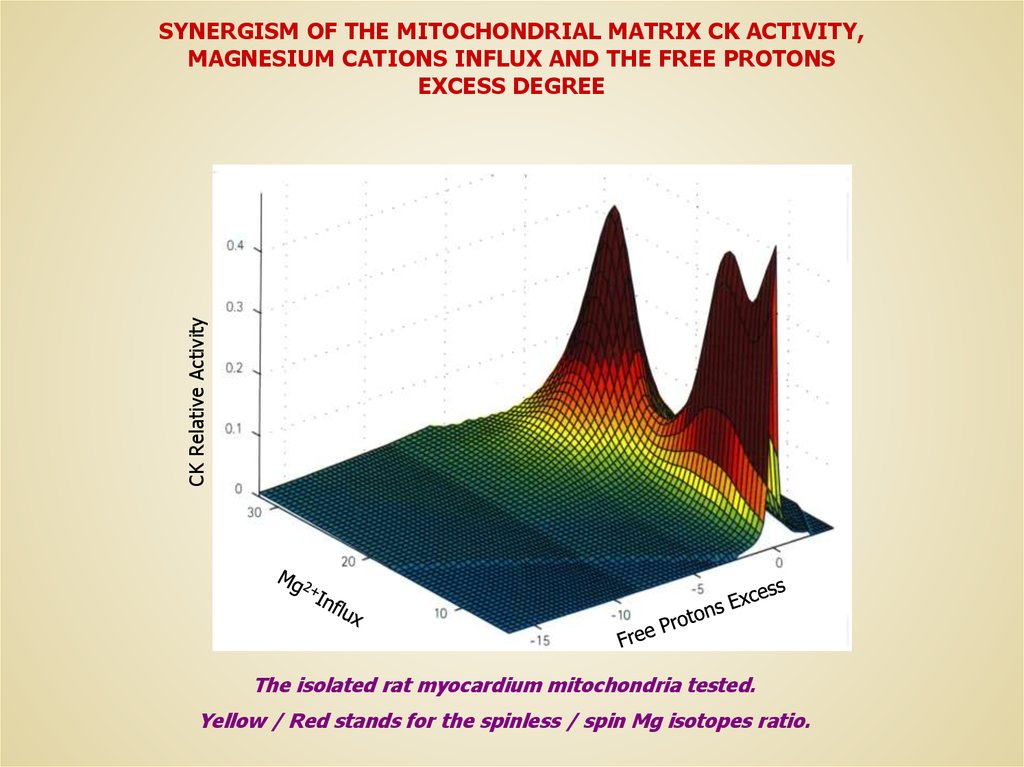
CK Relative ActivitySYNERGISM OF THE MITOCHONDRIAL MATRIX CK ACTIVITY,
MAGNESIUM CATIONS INFLUX AND THE FREE PROTONS
EXCESS DEGREE
The isolated rat myocardium mitochondria tested.
Yellow / Red stands for the spinless / spin Mg isotopes ratio.
26.
SYNERGISM OF THE ATP YIELD, OXYGEN CONSUMPTION ANDTHE Mg2+ INFLUX IN THE PERFUSED ISOLATED RABBIT HEART
MUSCLE TISSUE
A
ATP yield, Y/Yo
ATP yield, Y/Yo
B
A – Zero spin magnesium test
B – Magnetic magnesium test
27.
28.
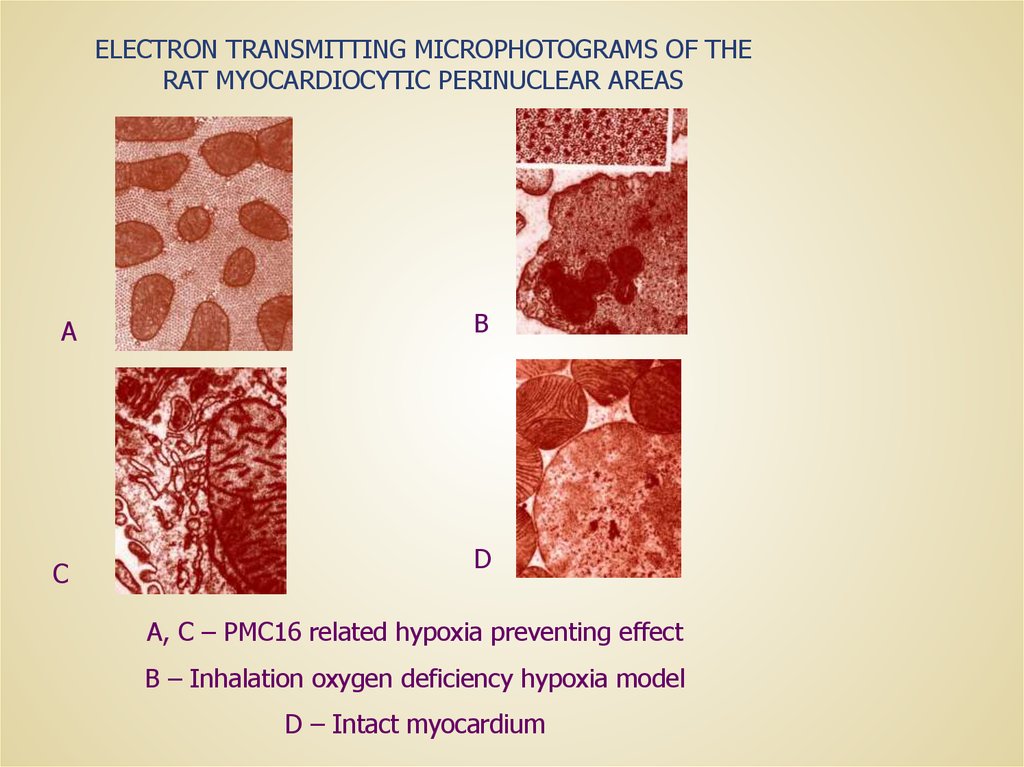
ELECTRON TRANSMITTING MICROPHOTOGRAMS OF THERAT MYOCARDIOCYTIC PERINUCLEAR AREAS
A
C
B
D
A, C – PMC16 related hypoxia preventing effect
B – Inhalation oxygen deficiency hypoxia model
D – Intact myocardium
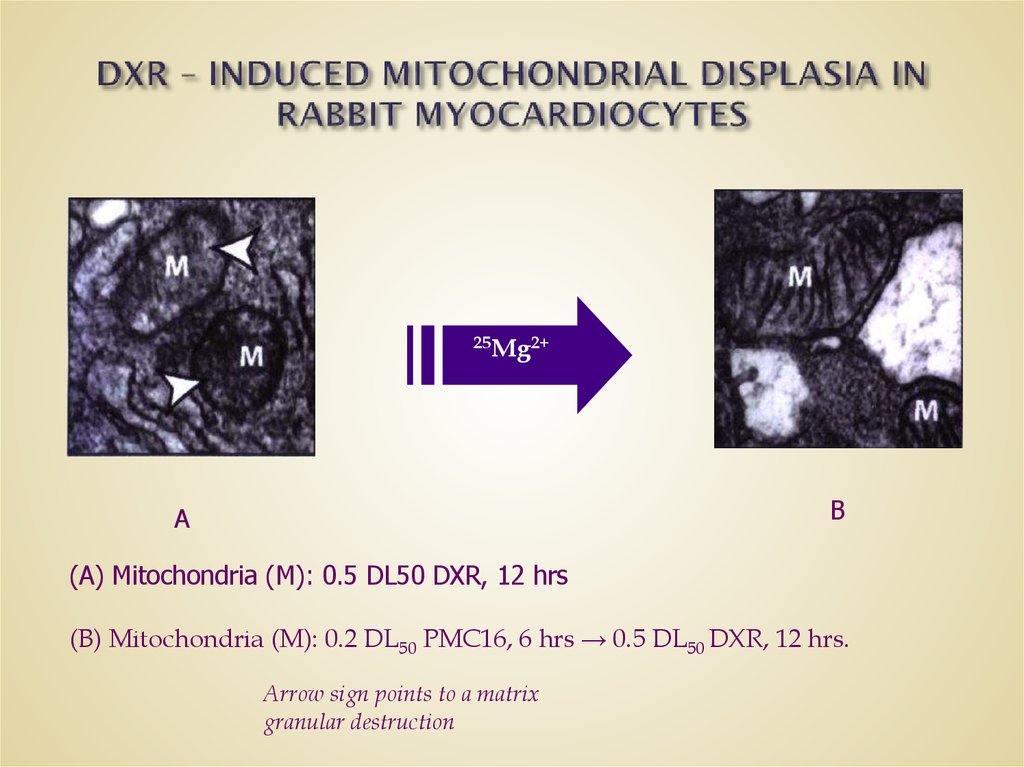
29. DXR – INDUCED MITOCHONDRIAL DISPLASIA IN RABBIT MYOCARDIOCYTES
25Mg2+B
A
(A) Mitochondria (M): 0.5 DL50 DXR, 12 hrs
(B) Mitochondria (M): 0.2 DL50 PMC16, 6 hrs → 0.5 DL50 DXR, 12 hrs.
Arrow sign points to a matrix
granular destruction
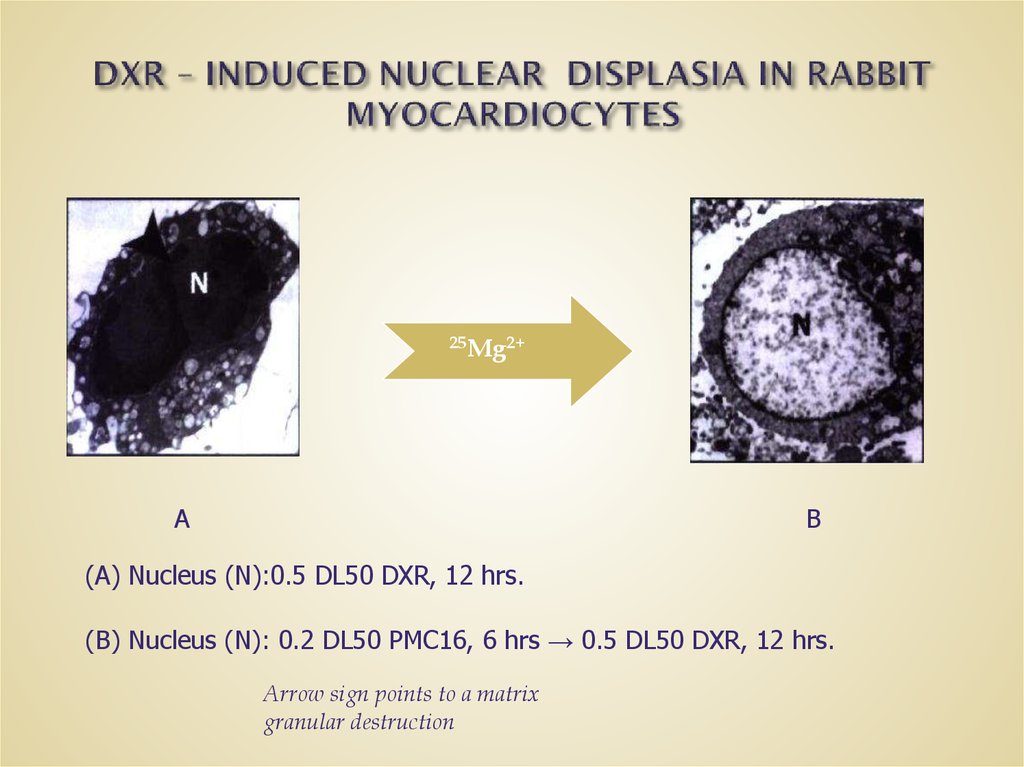
30. DXR – INDUCED NUCLEAR DISPLASIA IN RABBIT MYOCARDIOCYTES
25Mg2+A
B
(A) Nucleus (N):0.5 DL50 DXR, 12 hrs.
(B) Nucleus (N): 0.2 DL50 PMC16, 6 hrs → 0.5 DL50 DXR, 12 hrs.
Arrow sign points to a matrix
granular destruction
31. FRAGMENTATION OF THE RABBIT MYOCARDIOCYTES MITOCHONDRIA IN THE DXR-INDUCED ACUTE HYPOXIA
(a) 0.8 DL50 DXR, 20 min (i.v.)(b) 0.8 DL50 DXR, 4 hrs (i.v.)
(c) 0.8 DL50 DXR, 12 hrs (i.v.)
(d) 0.2 DL50 PMC16, 10 hrs (i.v.)→ DL50 DXR, 12 hrs (i.v.)
32. FRAGMENTATION OF THE RABBIT MYOCARDIOCYTES MITOCHONDRIA IN THE 1-METHYLNICOTINE AMIDE (MNA) – INDUCED ACUTE HYPOXIA
(a) 1.0 DL50 MNA, 6 HRS (i.v.)(b) 1.0 DL50 MNA, 12 hrs (i.v.)
(c) 1.0 DL50 MNA, 24 hrs (i.v.)
33.
34. THE EFFECT OF A PMC16 – TARGETED DELIVERY OF Mg2+ ON THE DOXORUBICIN (DXR) PRE – SUPPRESSED ATP PRODUCTION IN RAT MYOCARDIUM
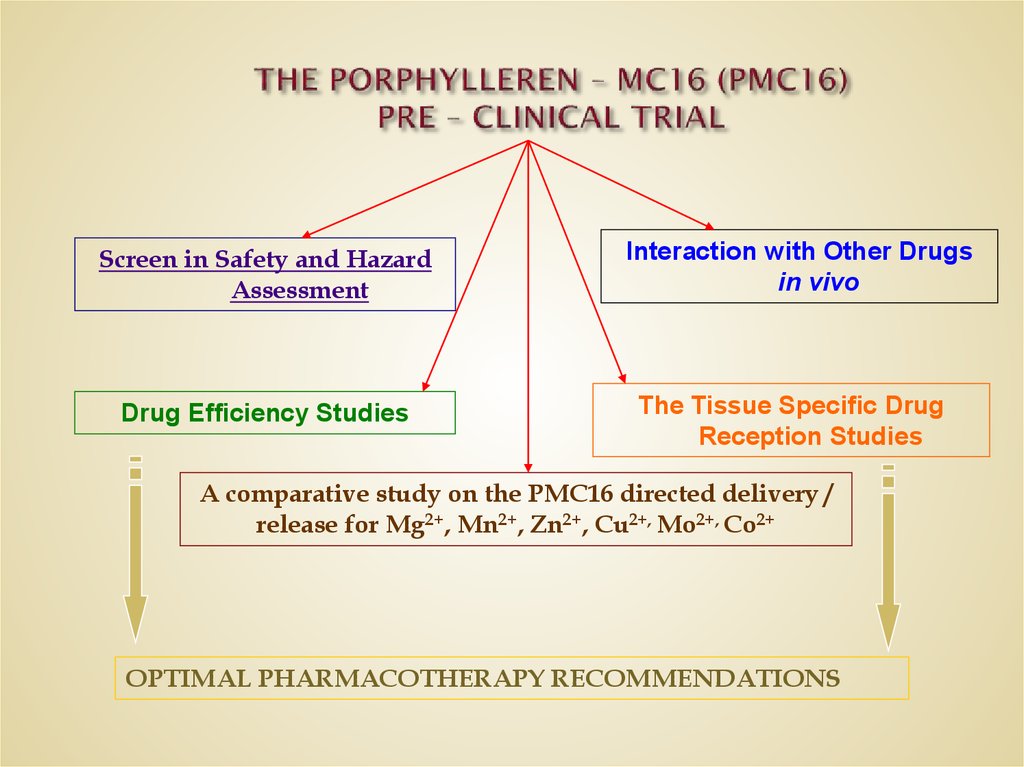
0.8 DL50 DXR, i.v., 6 hrs → PMC16, i.v., 6 hrs35. THE PORPHYLLEREN – MC16 (PMC16) PRE – CLINICAL TRIAL
Screen in Safety and HazardAssessment
Drug Efficiency Studies
Interaction with Other Drugs
in vivo
The Tissue Specific Drug
Reception Studies
A comparative study on the PMC16 directed delivery /
release for Mg2+, Mn2+, Zn2+, Cu2+, Mo2+, Co2+
OPTIMAL PHARMACOTHERAPY RECOMMENDATIONS
36.
PMC16 CLUSTER POSITIONING INSIDE THE RATMYOCARDIOCYTIC MITOCHONDRIAL MEMBRANE IN METABOLIC
ACIDOSIS (a, c) AND IN NORMAL CONDITIONS (b, d)
a, b – Laser contrast (Nanofinder-S-6A) images
C, d – Confocal scanning microscopy
37.
38.
39.
40.
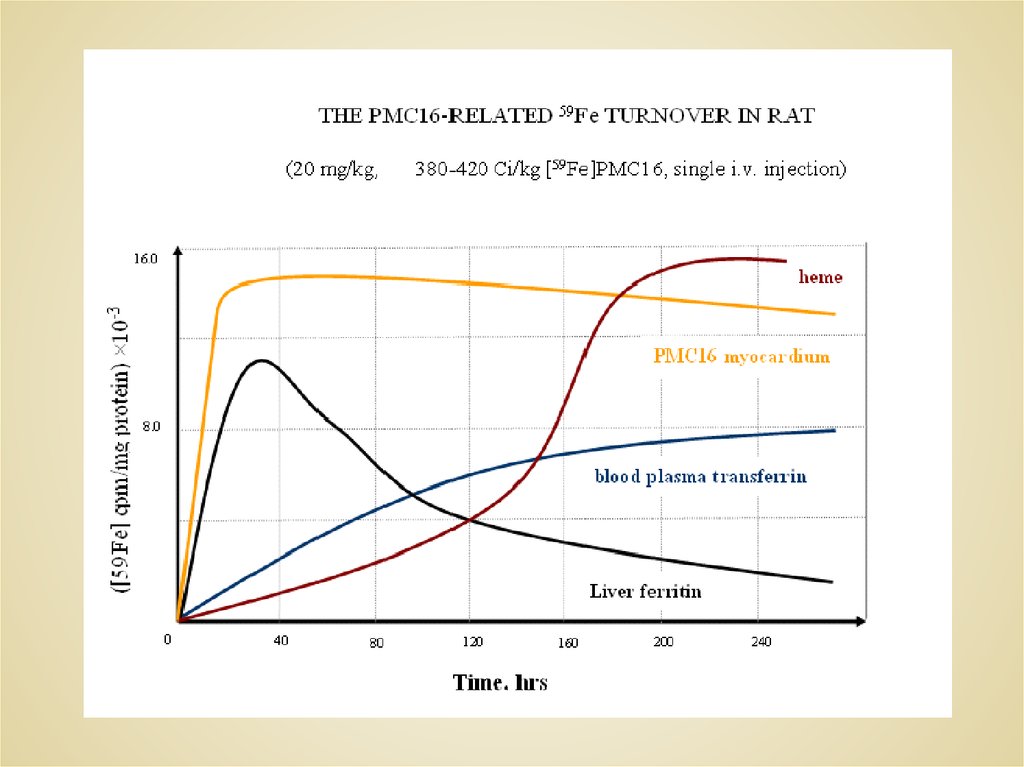
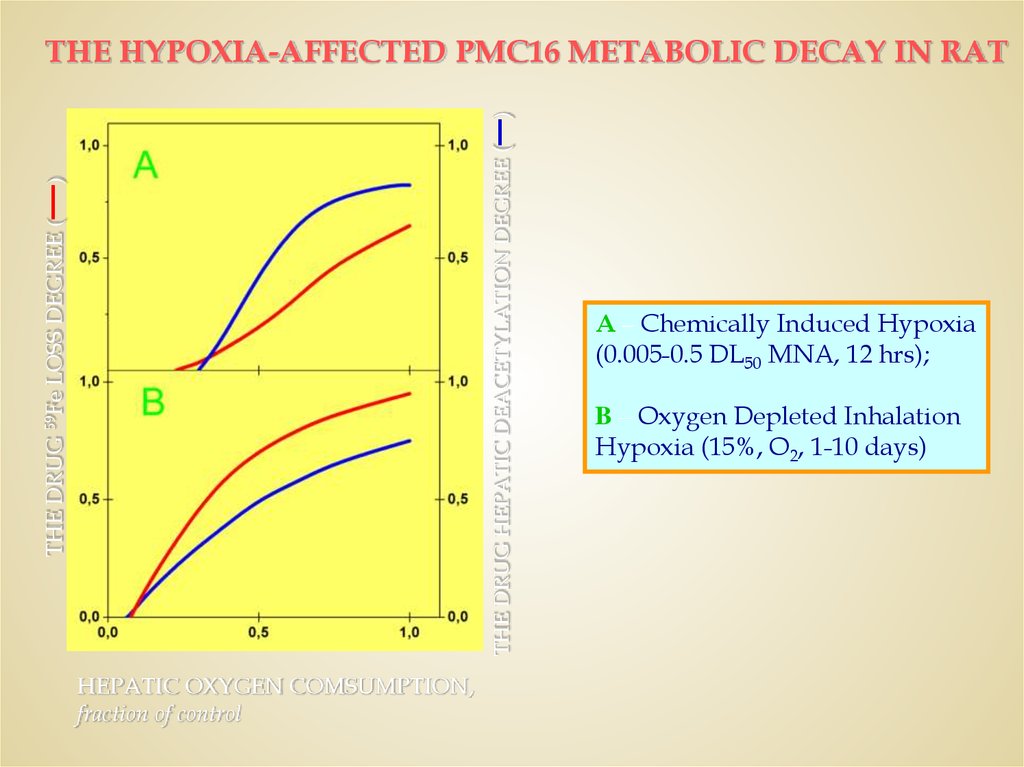
THE DRUG 59Fe LOSS DEGREE (—)THE DRUG HEPATIC DEACETYLATION DEGREE (
―)
THE HYPOXIA-AFFECTED PMC16 METABOLIC DECAY IN RAT
HEPATIC OXYGEN COMSUMPTION,
fraction of control
A – Chemically Induced Hypoxia
(0.005-0.5 DL50 MNA, 12 hrs);
B – Oxygen Depleted Inhalation
Hypoxia (15%, O2, 1-10 days)
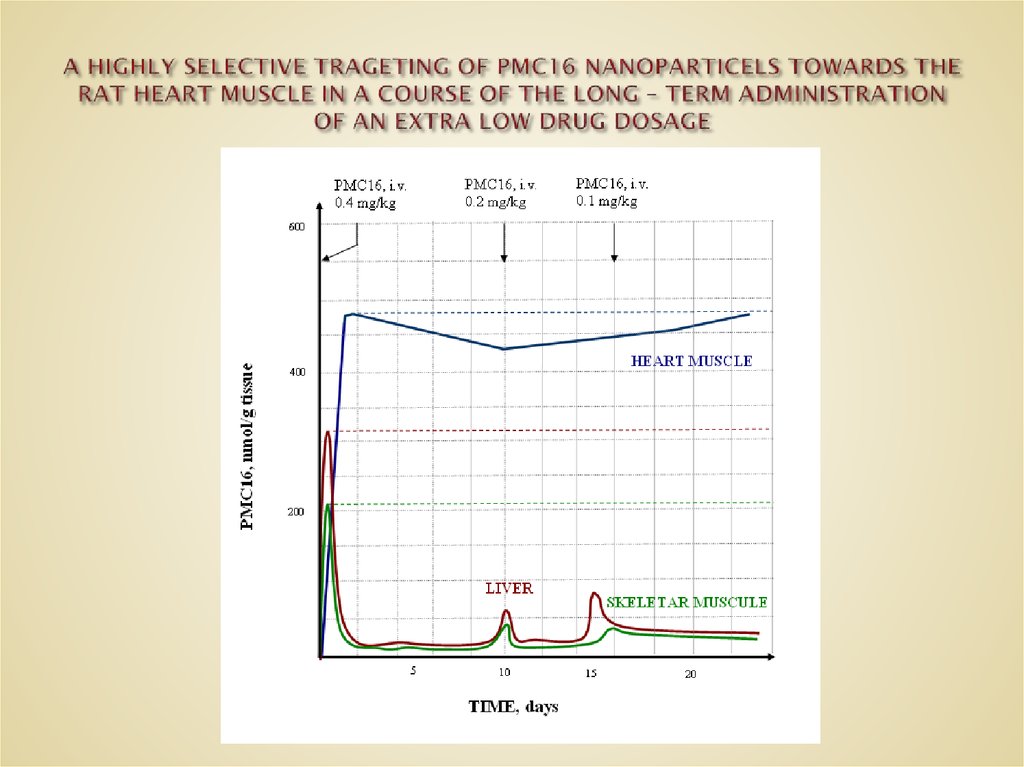
41. A HIGHLY SELECTIVE TRAGETING OF PMC16 NANOPARTICELS TOWARDS THE RAT HEART MUSCLE IN A COURSE OF THE LONG – TERM ADMINISTRATION
42.
NOTE: DXR, 20 mg/kg/24 hrs, i.v.:MNA, 10 mg/kg/24 hrs, i.v.:
43.
44.
45.
46.
47.
48.
49.
50.
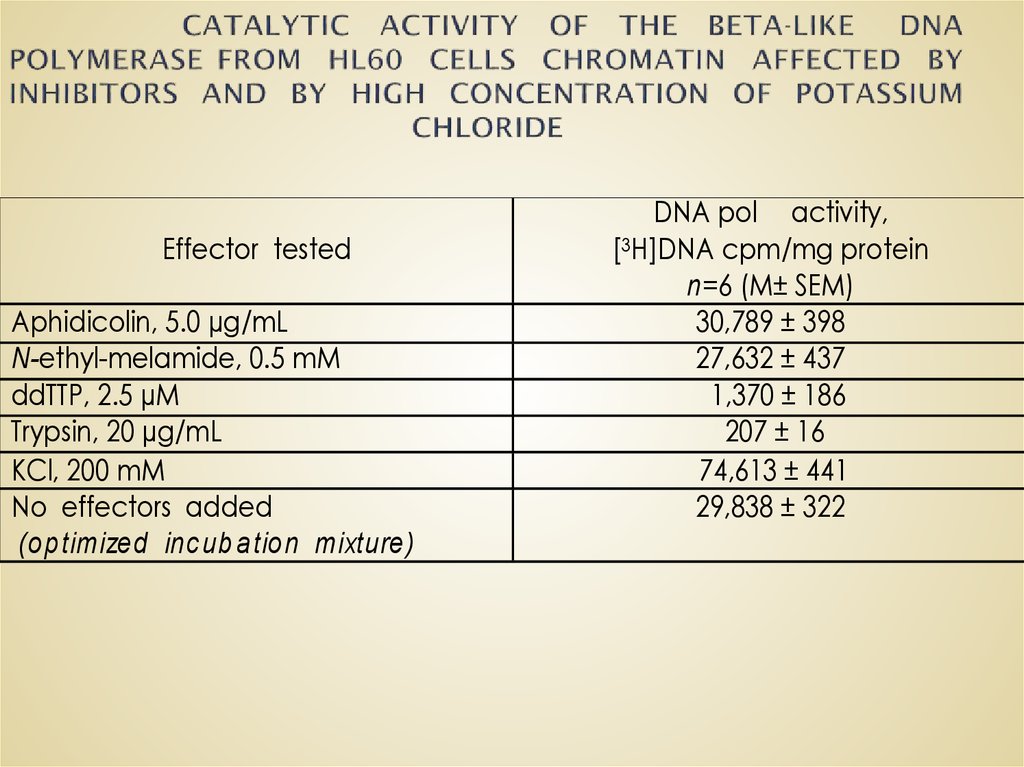
51. CATALYTIC ACTIVITY OF THE BETA-LIKE DNA POLYMERASE FROM HL60 CELLS CHROMATIN AFFECTED BY INHIBITORS AND BY HIGH CONCENTRATION
Effector testedAphidicolin, 5.0 µg/mL
N-ethyl-melamide, 0.5 mM
ddTTP, 2.5 µM
Trypsin, 20 µg/mL
KCl, 200 mM
No effectors added
(op timized inc ub a tion mixture)
DNA pol activity,
[3H]DNA cpm/mg protein
n=6 (M± SEM)
30,789 ± 398
27,632 ± 437
1,370 ± 186
207 ± 16
74,613 ± 441
29,838 ± 322
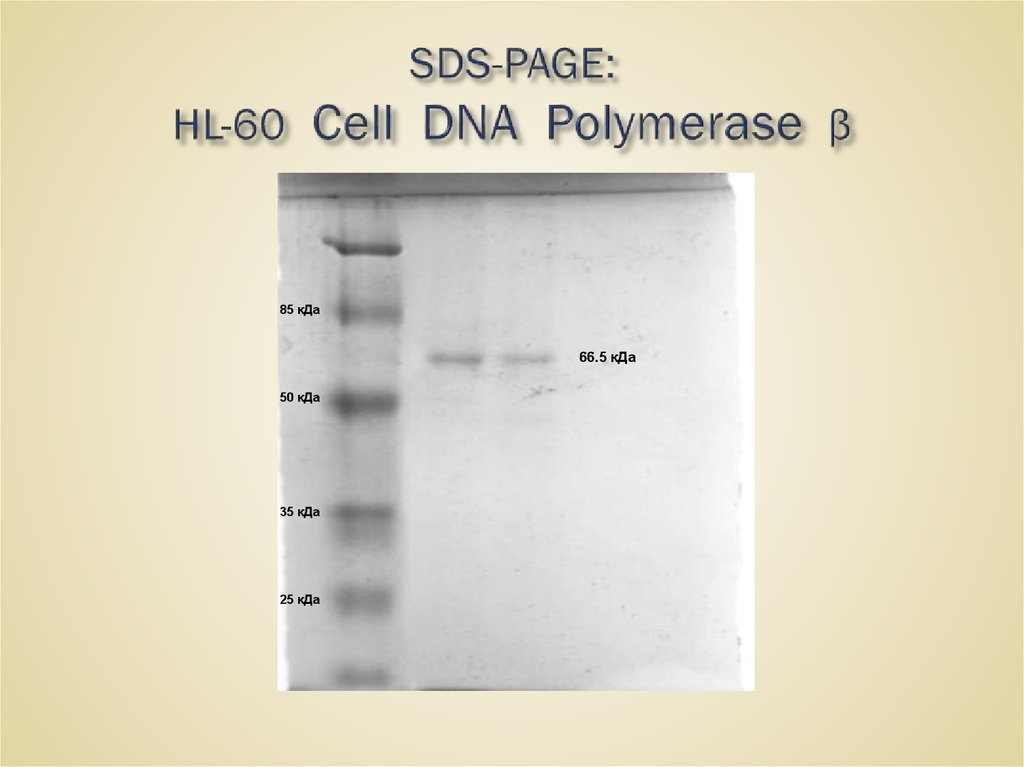
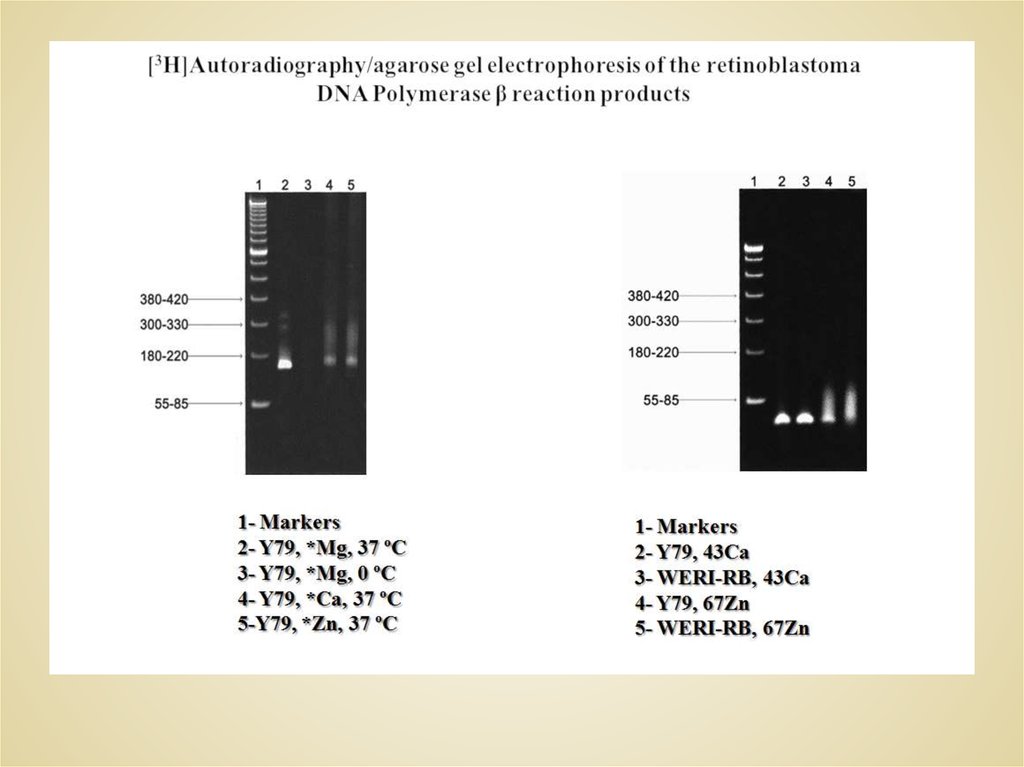
52. SDS-PAGE: HL-60 Cell DNA Polymerase β
85 кДа66.5 кДа
50 кДа
35 кДа
25 кДа
53.
n54.
55.
56.
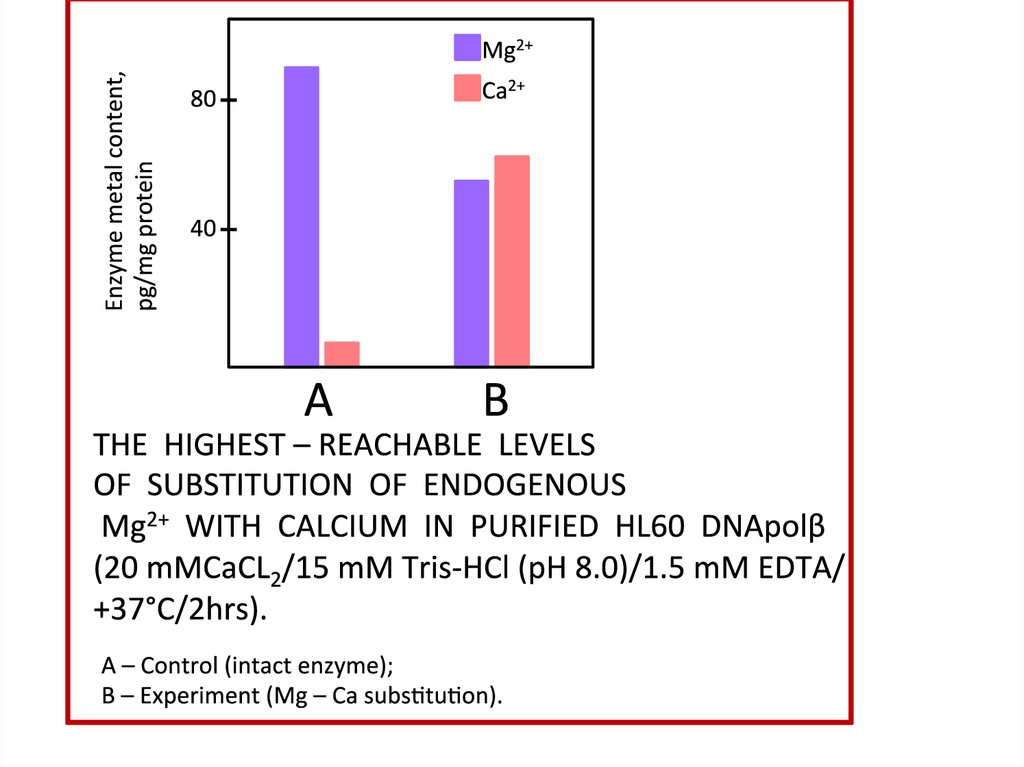
Структура диссертационного исследованияИСП – МС: Распределение изотопов Me2+
*,25 MgCl
*,43 СаCl
2
2
*,67
ZnCl2
PMC 16
PMC 16 - *Mg
PMC 16 - *Ca
PMC 16 - *Zn
PMC 16 – 25 Mg
PMC 16 – 43Ca
PMC 16 – 67Zn
Клетки
ОМЛ и РБ
Параметры
цитометрии,
апоптоз
Цитозоль,
Митохондрии,
Нуклеоплазма,
Хроматин
ПОИСК И АНАЛИЗ
КОРРЕЛЯЦИЙ
Хроматин
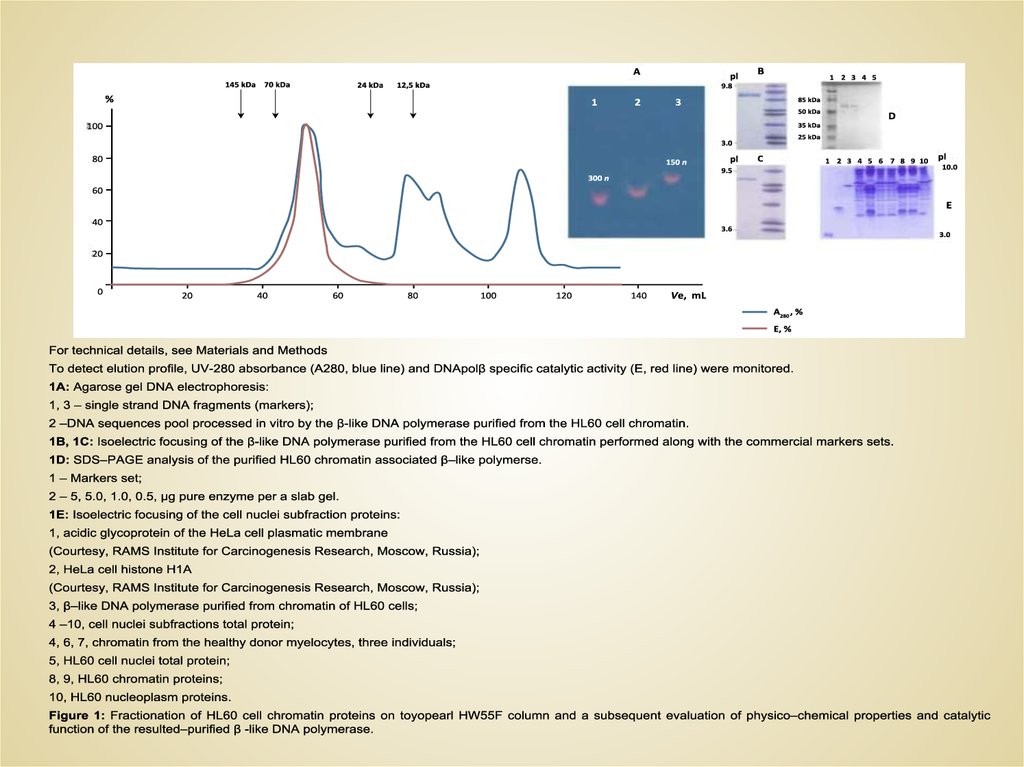
Выделение ДНК- пол β
Определение β - критериев:
• MW, ИЭТ, Км, Кcat
• 200 mM KCl
• Ингибиторы
• 3',5'-ДНКазная активность
• Предельный
размер
процессируемых
фрагментов ДНК
МИЭ:
25Mg2+
43Ca2+
67Zn2+
Изотермы
аффиности:
Me2+/DNApolβ
57.
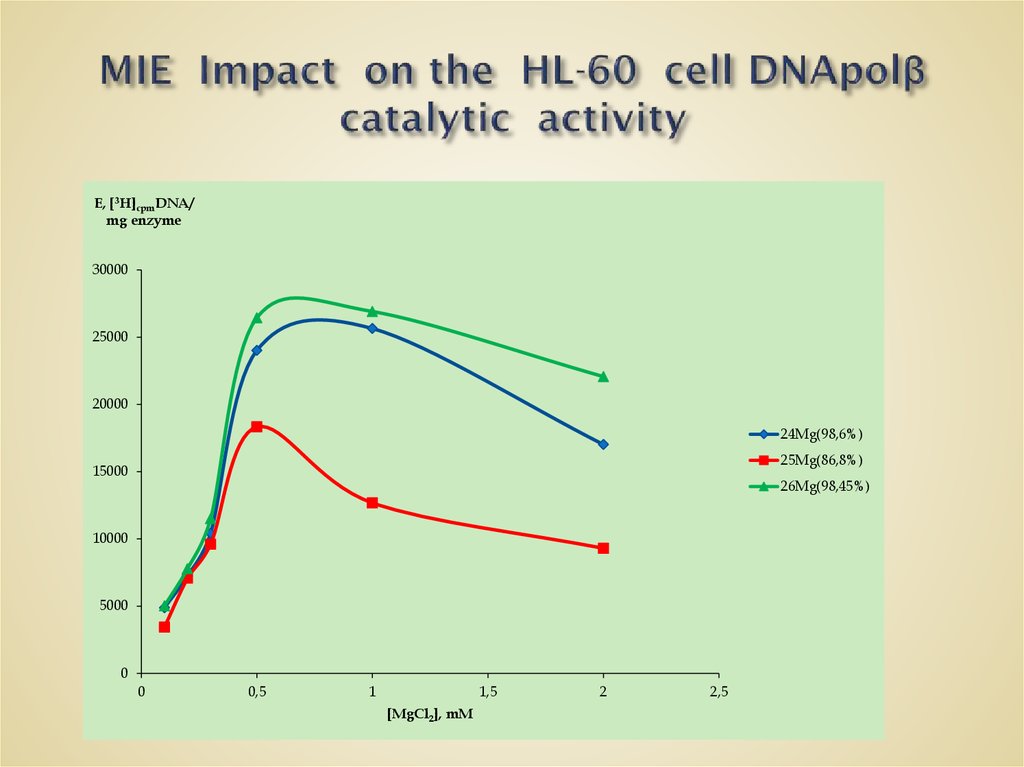
58. MIE Impact on the HL-60 cell DNApolβ catalytic activity
E, [3H]cpmDNA/mg enzyme
80000
70000
60000
50000
24Mg(98,6%)
25Mg(86,8%)
40000
26Mg(98,45%)
30000
20000
10000
0
0
20
40
60
[MgCl2], mM
80
100
120
59. MIE Impact on the HL-60 cell DNApolβ catalytic activity
E, [3H]cpmDNA/mg enzyme
30000
25000
20000
24Mg(98,6%)
25Mg(86,8%)
15000
26Mg(98,45%)
10000
5000
0
0
0,5
1
1,5
[MgCl2], mM
2
2,5
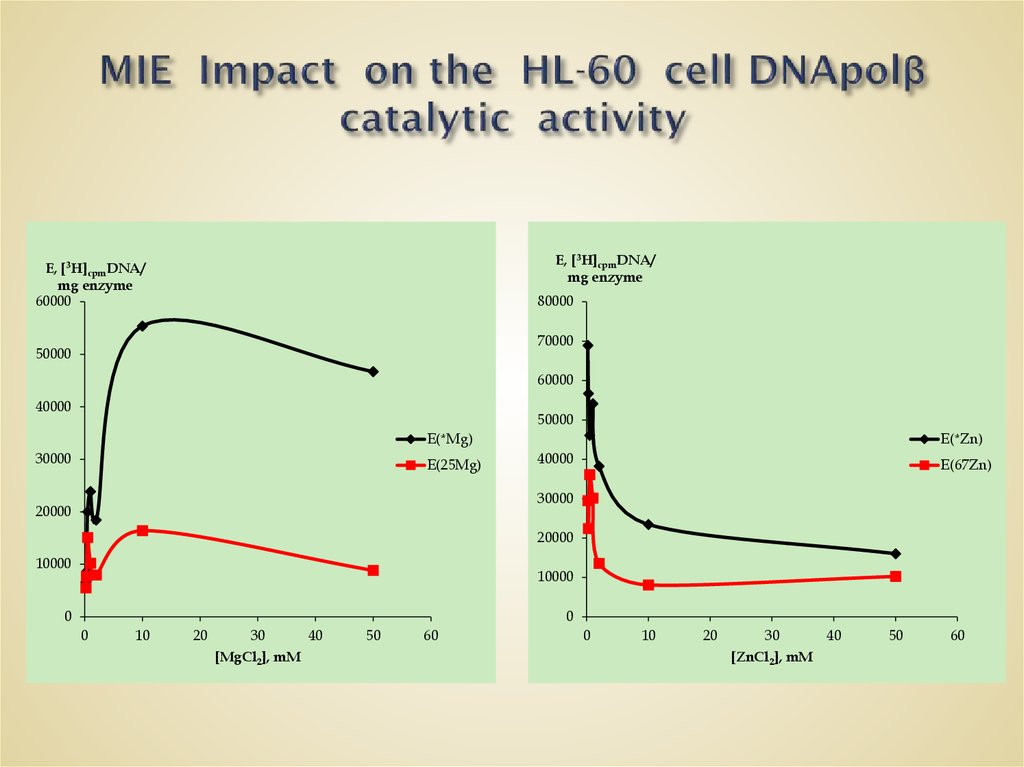
60. MIE Impact on the HL-60 cell DNApolβ catalytic activity
E, [3H]cpmDNA/mg enzyme
E, [3H]cpmDNA/
mg enzyme
60000
80000
70000
50000
60000
40000
50000
E(*Mg)
30000
E(25Mg)
E(*Zn)
40000
E(67Zn)
30000
20000
20000
10000
10000
0
0
0
10
20
30
[MgCl2], mM
40
50
60
0
10
20
30
[ZnCl2], mM
40
50
60
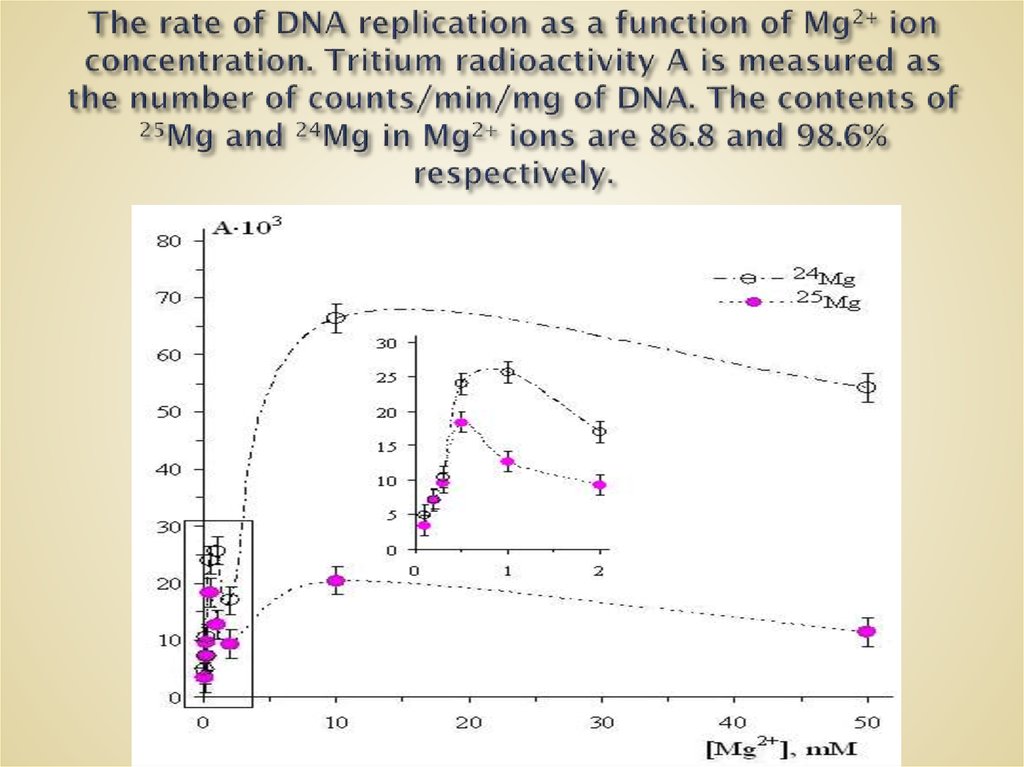
61. The rate of DNA replication as a function of Mg2+ ion concentration. Tritium radioactivity A is measured as the number of
62. The rate of DNA replication as a function of Mg2+ ion concentration. Tritium radioactivity A is measured as the number of
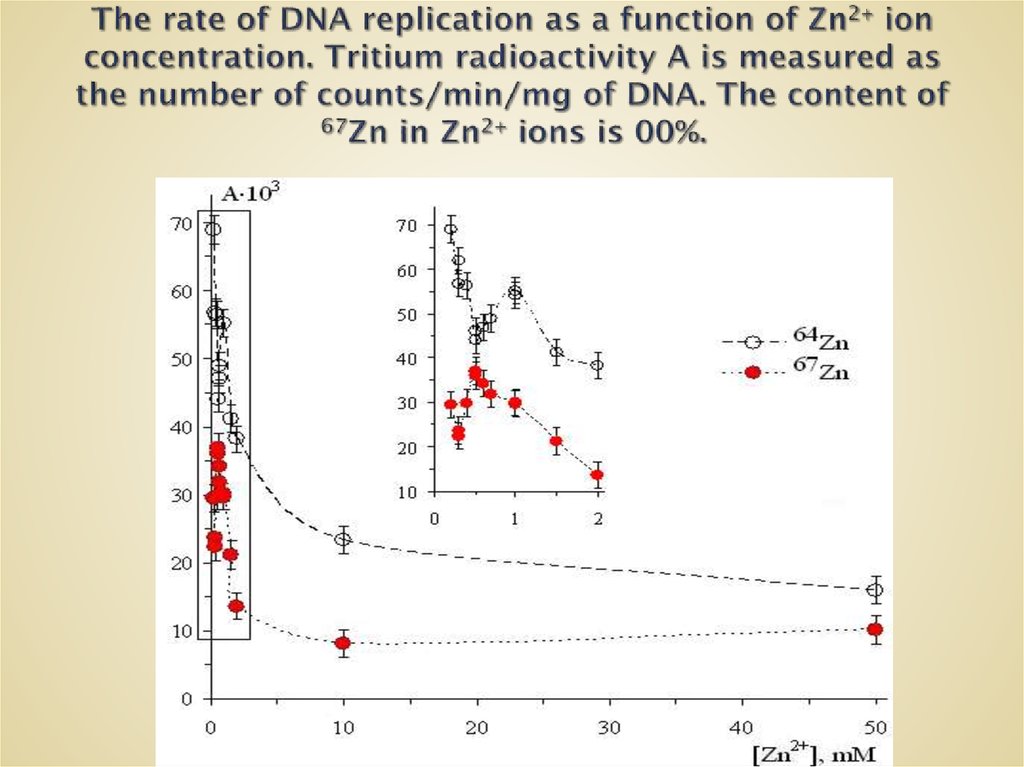
63. The rate of DNA replication as a function of Zn2+ ion concentration. Tritium radioactivity A is measured as the number of
64.
43Ca+$$$$$$$$$$$
DNA$
>$
43Ca2+$
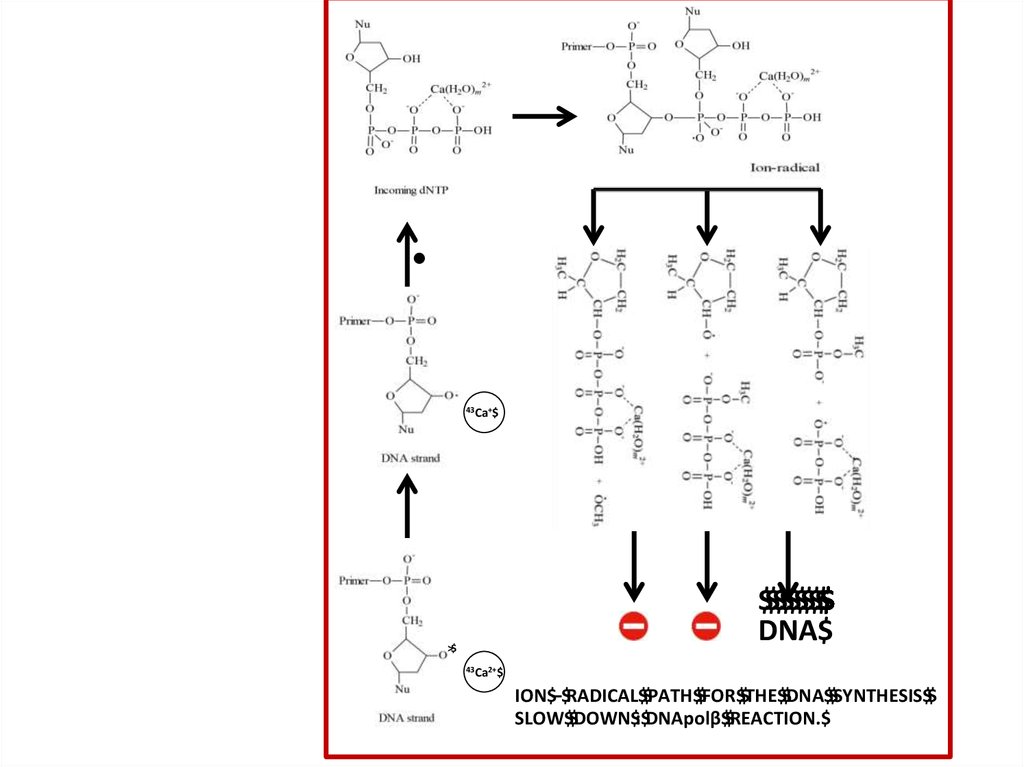
ION$–$RADICAL$$PATH$$FOR$$THE$$DNA$$SYNTHESIS$$
SLOW$$DOWN$:$DNApolβ$$REACTION.$
65.
Diphosphate#to##remove#by#the#
#2nd#enzyme’s#
#Mg2+#ion,unless#
#replaced#
INSERTION#
to#the#nascent#
DNA#chain#
#
THE##dNTP#–#OXYRADICAL#THREE#CHANNEL##SPREADING##
DECAY.##DNApolβ##REACTION.#
66.
67.
68.
69.
70.
71.
72.
73.
74.
КОНЦЕПЦИЯ БУЧАЧЕНКО – КУЗНЕЦОВА :Синергизм цитоплазматических и внутриядерных событий,
конвертирующих МИЭ 25Mg в цитостатическое воздействие на клетку опухоли
EXTRACELLULAR ENVIRONMENT
PMC16
PMC 16
25Mg2+
PMC 16
Invalid
DNA
Repair
25Mg2+
25Mg2+
DNApolβ
25Mg2+
CK, αPGK, PK,
ATPsynthase
Anabolism
Support
CYTOPLASM
∆[dNTP]↑
NUCLEUS
Oversaturation
of
dNTP nuclear
pool
75.
1.Jorg Pedersen, South Denmark University, Biophisical enzymology
department, Denmark, Odense
2.
Nikita Lukzen, Duke University, laboratory of magnetic biology, USA
3.
William Robinson, Nantes University, Isotopic research center, France
4.
Nicolas Turro (+), Ron Barthels, Columbia University, USA
5.
Nima Amirshahi, Teheran Medical University, Iran
6.
Xeng Wu, Nankin State University, China
7.
S.A. Roumyantsev, M.A. Orlova, State Research center of gematology,
oncology and immunology, Russia
8.
Wolfgang Maret, King’s college of London, UK













![AN AFFINITY CHEROMATOGRAPHY OF THE HUMAN MYOCARDIAL MITOCHONDRIA MEMBRANE PROTEINS ON THE COLUMN WITH AGAROSE-6B-CL-[C17]-PMC16 AN AFFINITY CHEROMATOGRAPHY OF THE HUMAN MYOCARDIAL MITOCHONDRIA MEMBRANE PROTEINS ON THE COLUMN WITH AGAROSE-6B-CL-[C17]-PMC16](https://cf2.ppt-online.org/files2/slide/r/rfPmKUQIk9zJloghFcE5vtqMDA6ndBpXRjH40b7i3/slide-19.jpg)



































 Промышленность
Промышленность