Похожие презентации:
Experimental methods for studies of ion – molecule reaktions and of ion – electron rekombination
1.
Experimental methods forstudies of ion – molecule
reaktions and of ion – electron
rekombination
2.
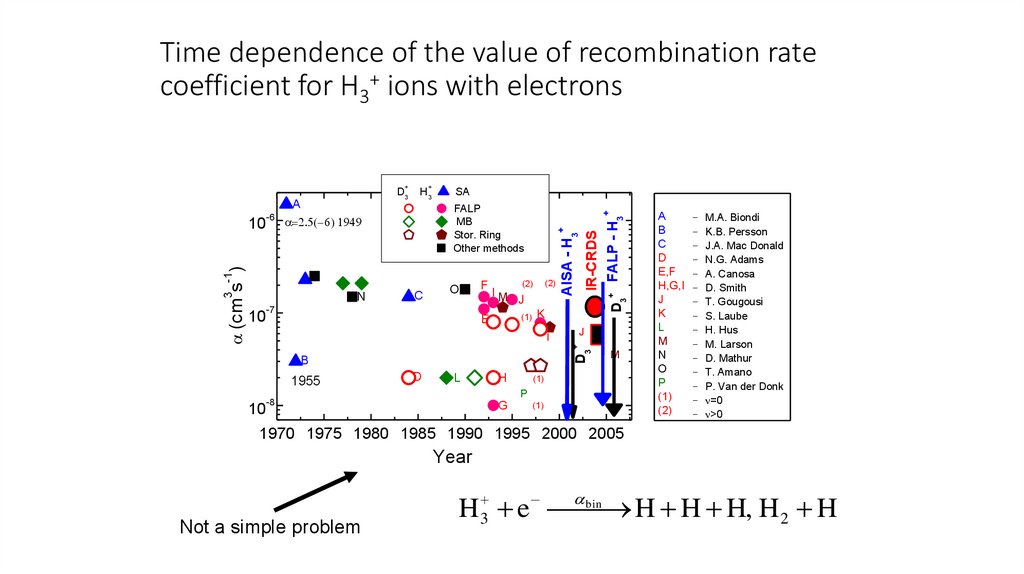
Time dependence of the value of recombination ratecoefficient for H3+ ions with electrons
3 -1
(cm s )
FALP
MB
Stor. Ring
Other methods
N
C
O
F
IM
-7
10
J
(1)
E
(2)
(2)
K
J
D3
+
I
IR-CRDS
+
+
D3 FALP - H3
-6
10
SA
+
A
+
H3
AISA - H3
+
D3
B
1955
D
L
H
M
(1)
P
-8
G
10
(1)
A
B
C
D
E,F
H,G,I
J
K
L
M
N
O
P
(1)
(2)
-
M.A. Biondi
K.B. Persson
J.A. Mac Donald
N.G. Adams
A. Canosa
D. Smith
T. Gougousi
S. Laube
H. Hus
M. Larson
D. Mathur
T. Amano
P. Van der Donk
=0
>0
1970 1975 1980 1985 1990 1995 2000 2005
Year
Not a simple problem
bin
H 3 e
H H H, H 2 H
3.
Different experimentsIon Storage Ring
+ No buffer gas
+ Excellent energy resolution
- Complicated estimation of cross
section from measured data
- Rotational temperature of ions in
the ring can be >= 300 K
Afterglow plasma
+ Many collisions of ions with buffer gas
particles – effective thermalization
+ The measured quantity is thermal rate
coefficient
- Complicated chemical kinetics
- Presence of third bodies can influence
the recombination
4.
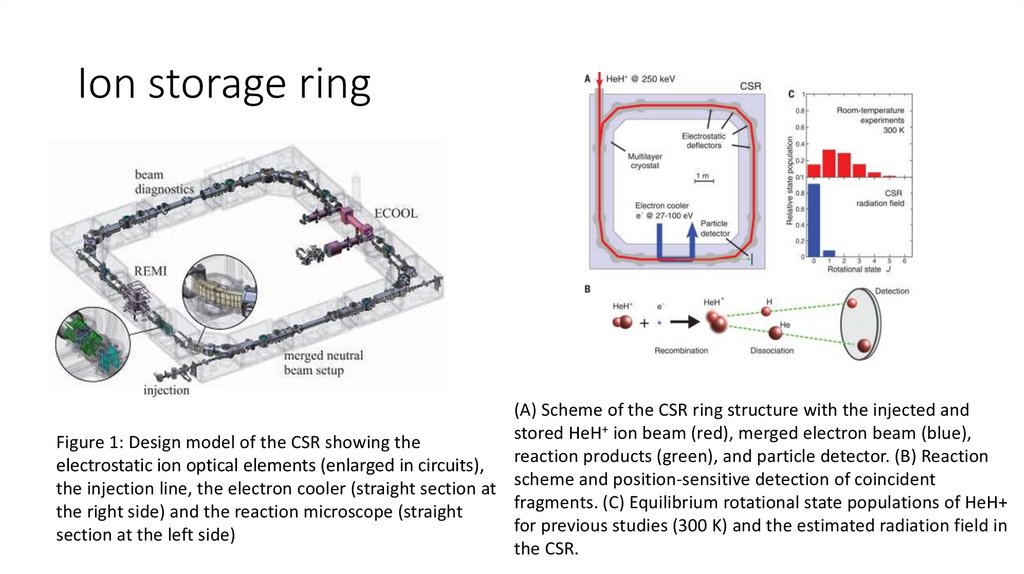
Ion storage ring(A) Scheme of the CSR ring structure with the injected and
+ ion beam (red), merged electron beam (blue),
stored
HeH
Figure 1: Design model of the CSR showing the
reaction products (green), and particle detector. (B) Reaction
electrostatic ion optical elements (enlarged in circuits),
the injection line, the electron cooler (straight section at scheme and position-sensitive detection of coincident
fragments. (C) Equilibrium rotational state populations of HeH+
the right side) and the reaction microscope (straight
for previous studies (300 K) and the estimated radiation field in
section at the left side)
the CSR.
5.
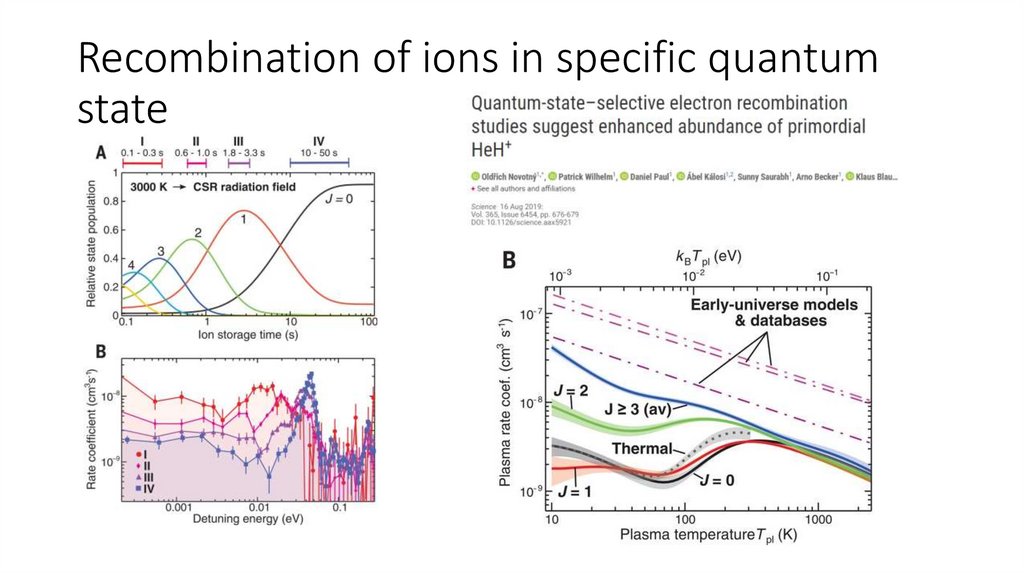
Recombination of ions in specific quantumstate
6.
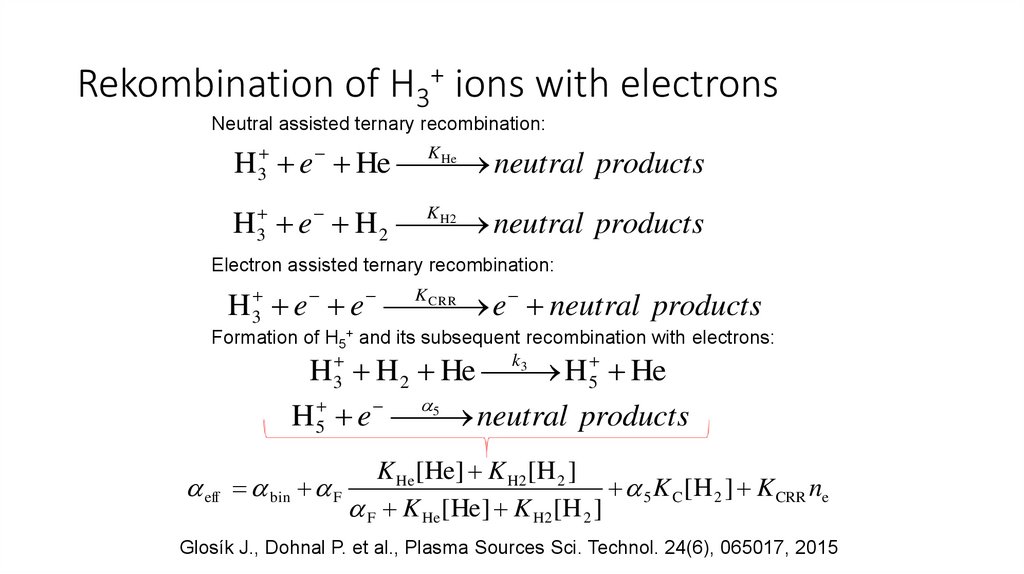
Rekombination of H3+ ions with electronsNeutral assisted ternary recombination:
He
H 3 e He K
neutral products
H2
H 3 e H 2 K
neutral products
Electron assisted ternary recombination:
3
H e e
e neutral products
K CRR
Formation of H5+ and its subsequent recombination with electrons:
3
H H 2 He H 5 He
5
H 5 e
neutral products
eff
k3
K He [He ] K H2 [H 2 ]
bin F
5 K C [H 2 ] K CRR ne
F K He [He ] K H2[H 2 ]
Glosík J., Dohnal P. et al., Plasma Sources Sci. Technol. 24(6), 065017, 2015
7.
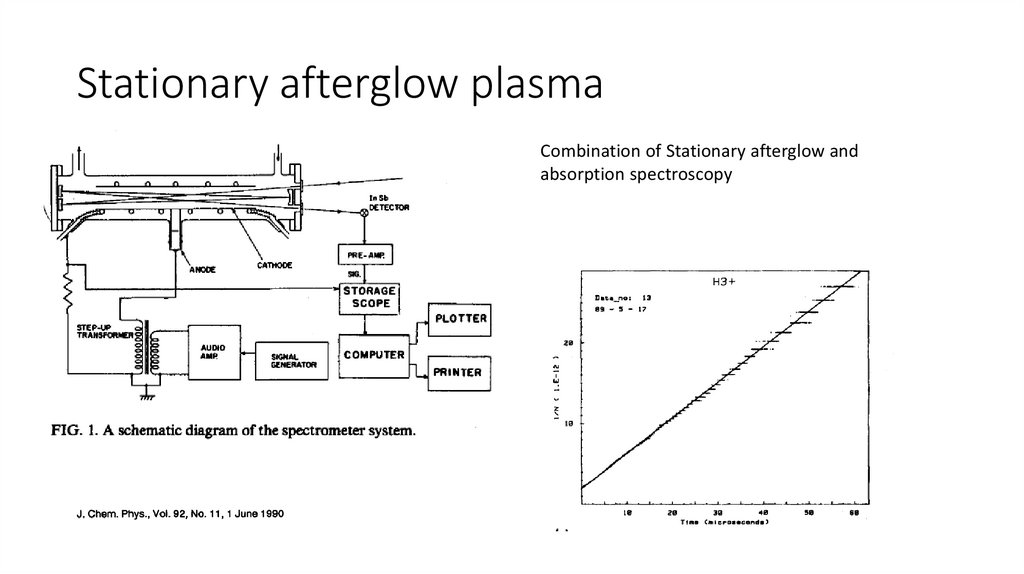
Stationary afterglow plasmaCombination of Stationary afterglow and
absorption spectroscopy
8.
Stationary afterglow plasmaAISA – Advanced Integrated Stationary Afterglow
Mass spectrometer + Langmuir probe diagnostics
Phys. Rev. Lett., 88 (4): Art. No. 044802 (4 pages), 2002.
9.
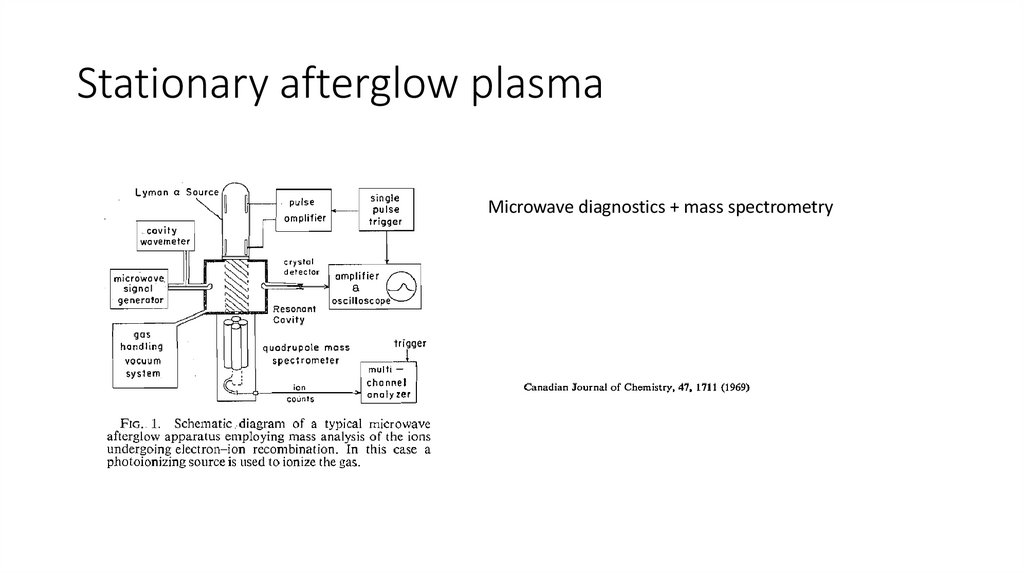
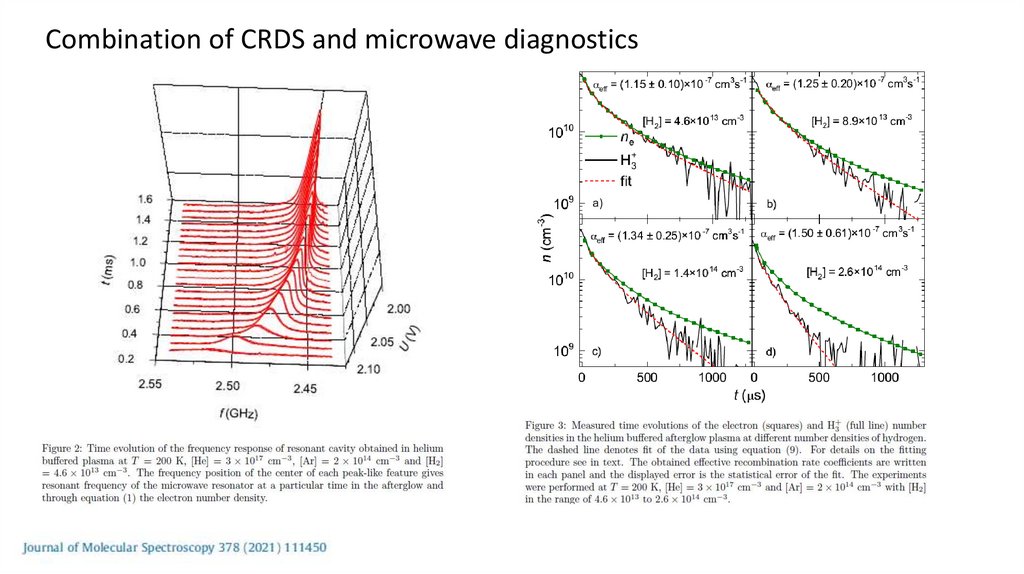
Stationary afterglow plasmaMicrowave diagnostics + mass spectrometry
10.
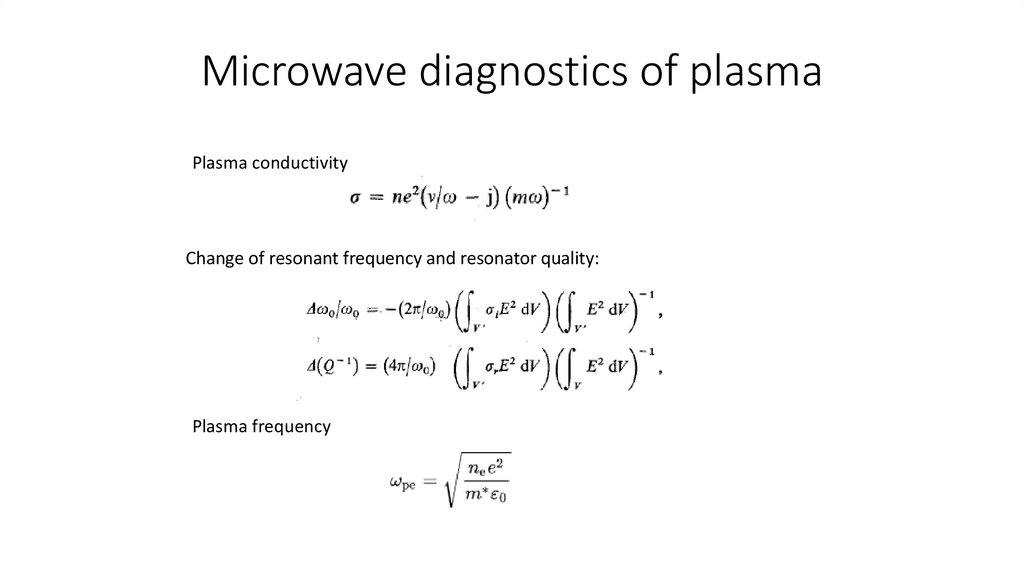
Microwave diagnostics of plasmaPlasma conductivity
Change of resonant frequency and resonator quality:
Plasma frequency
11.
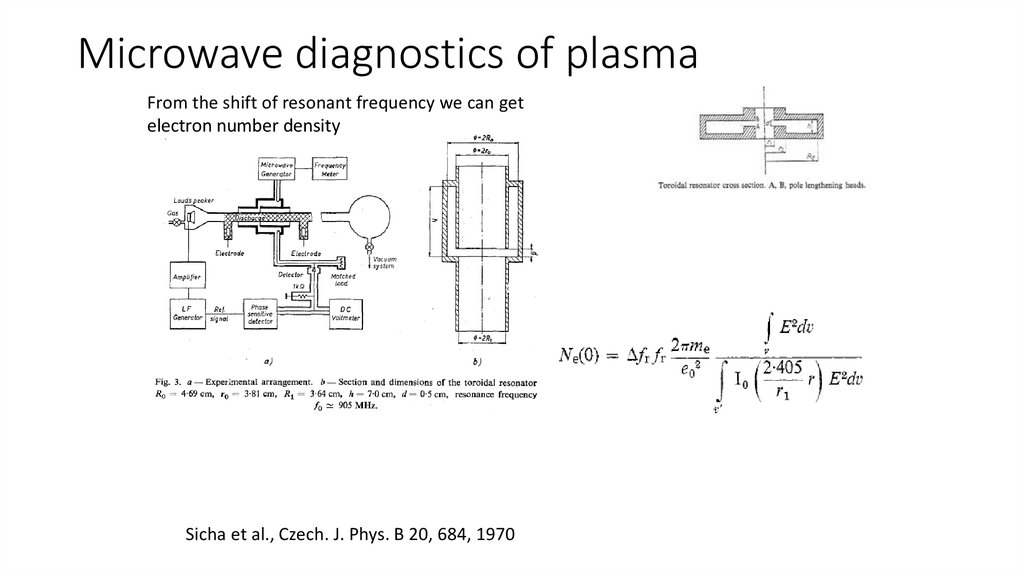
Microwave diagnostics of plasmaFrom the shift of resonant frequency we can get
electron number density
Sicha et al., Czech. J. Phys. B 20, 684, 1970
12.
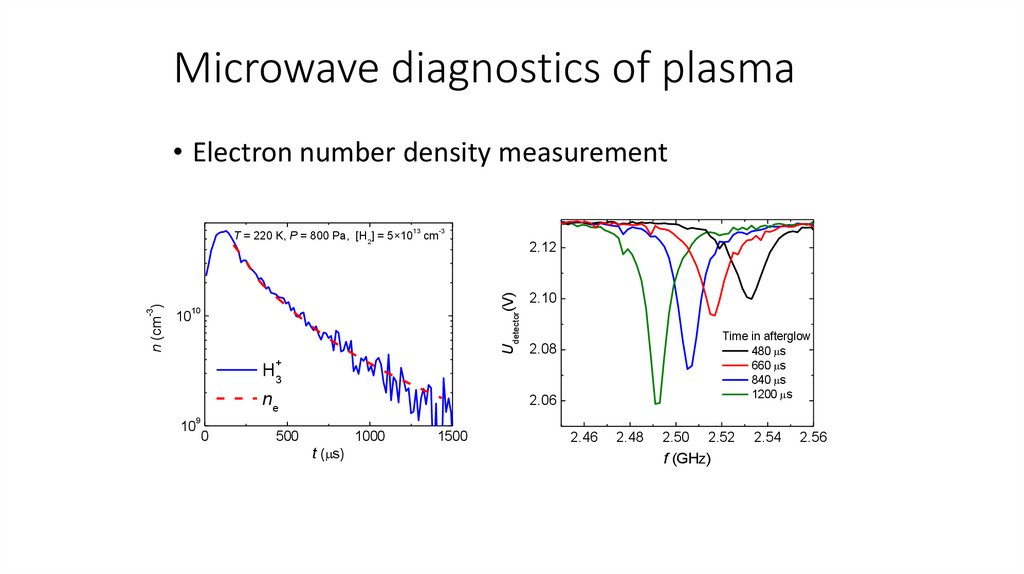
Microwave diagnostics of plasma• Electron number density measurement
13
10
-3
2.12
Udetector(V)
-3
n (cm )
T = 220 K, P = 800 Pa, [H2] = 5×10 cm
10
2.10
Time in afterglow
480 s
660 s
840 s
1200 s
2.08
+
H3
ne
10
2.06
9
0
500
1000
t ( s)
1500
2.46
2.48
2.50
2.52
f (GHz)
2.54
2.56
13.
SA-CRDS apparatus• CRDS – Cavity Ring Down Spectroscopy
• SA – Stationary Afterglow
Highly reflective mirror
–
–
–
–
Discharge tube diameter– 1.5 cm
He buffer gas flow ~ 400 – 1600 sccm
Pressure ~ 200 – 1500 Pa
Temperature range ~ 77 – 300 K
14.
Cavity ringdown spectroscopyFirst used for mirror reflectivity determination (Herbelin et al. 1980).
Later, the dependence of ring-down time on absorption between the mirrors was
observed (O‘Keefe et al. 1988)
Highly reflective mirrors by Layertec
(diameter 6.3 mm, reflectivity R =
99.99 %)
Herbelin et al., Appl. Opt. 19, 144, 1980.
O‘Keefe et al., Rev. Sci. Instrum. 59, 2544, 1988.
15.
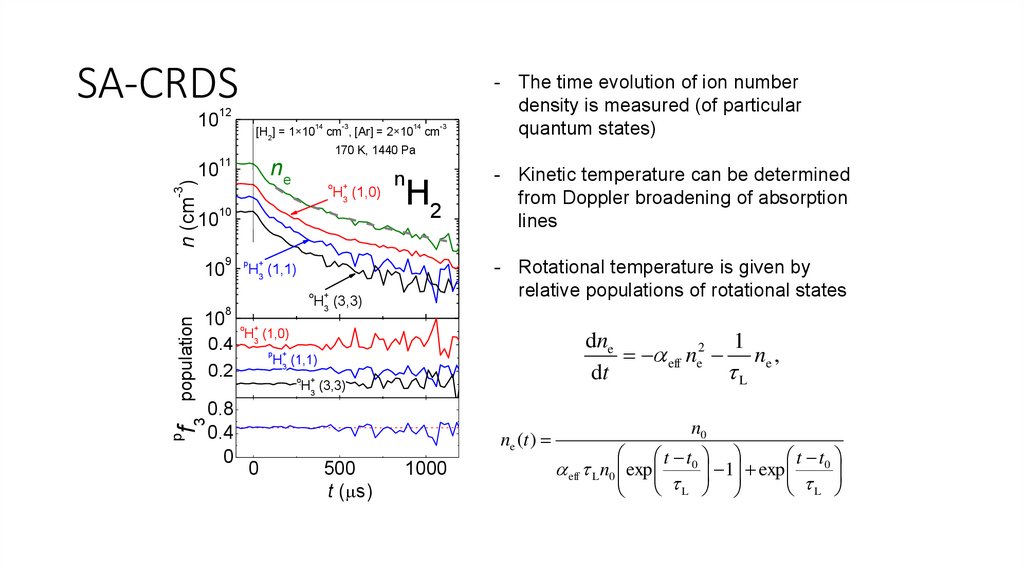
SA-CRDS10
12
10
11
14
-3
14
-3
[H2] = 1×10 cm , [Ar] = 2×10 cm
- The time evolution of ion number
density is measured (of particular
quantum states)
ne
-3
n (cm )
170 K, 1440 Pa
10
o
H3 (1,0)
10
10
9
p
10
H2
+
H3 (3,3)
o
+
H3 (1,0)
p
dne
1
eff ne2 ne ,
dt
L
+
H3 (1,1)
o
+
H3 (3,3)
0.8
0.4
0
f3
p
- Kinetic temperature can be determined
from Doppler broadening of absorption
lines
- Rotational temperature is given by
relative populations of rotational states
+
8
0.4
0.2
n
H3 (1,1)
o
population
+
ne (t )
0
500
t ( s)
1000
n0
t t0
t t0
1 exp
L
L
eff L n0 exp
16.
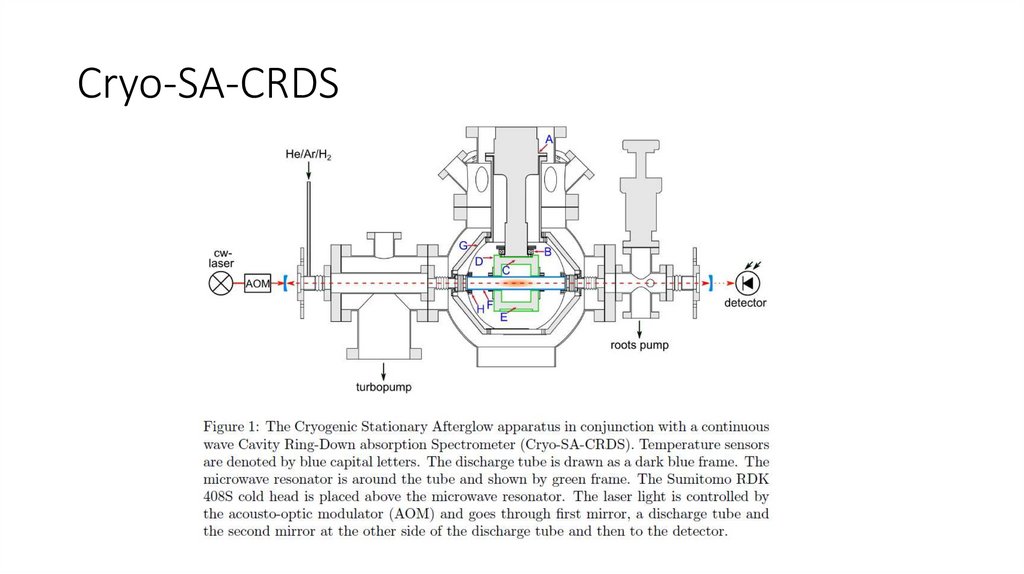
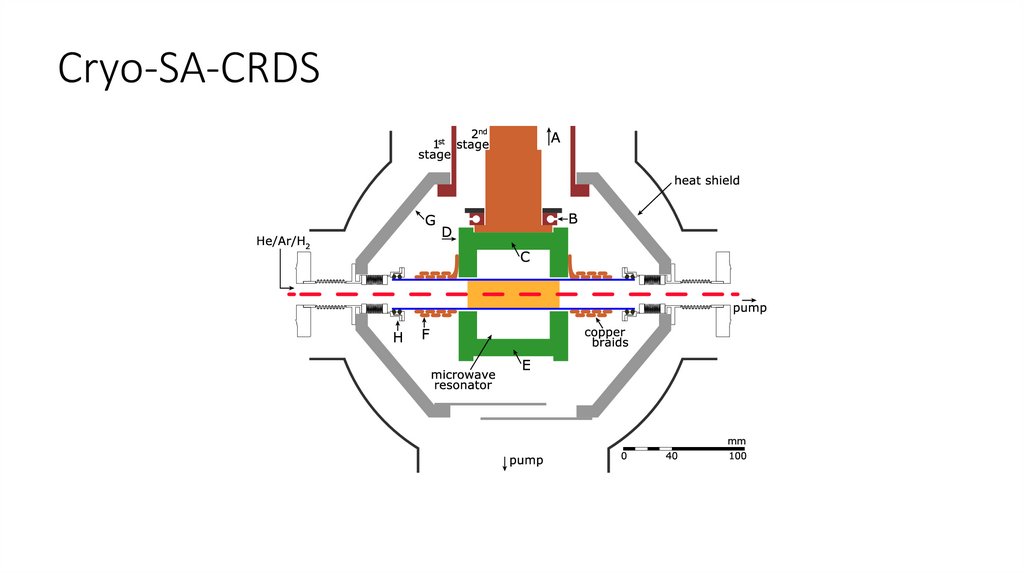
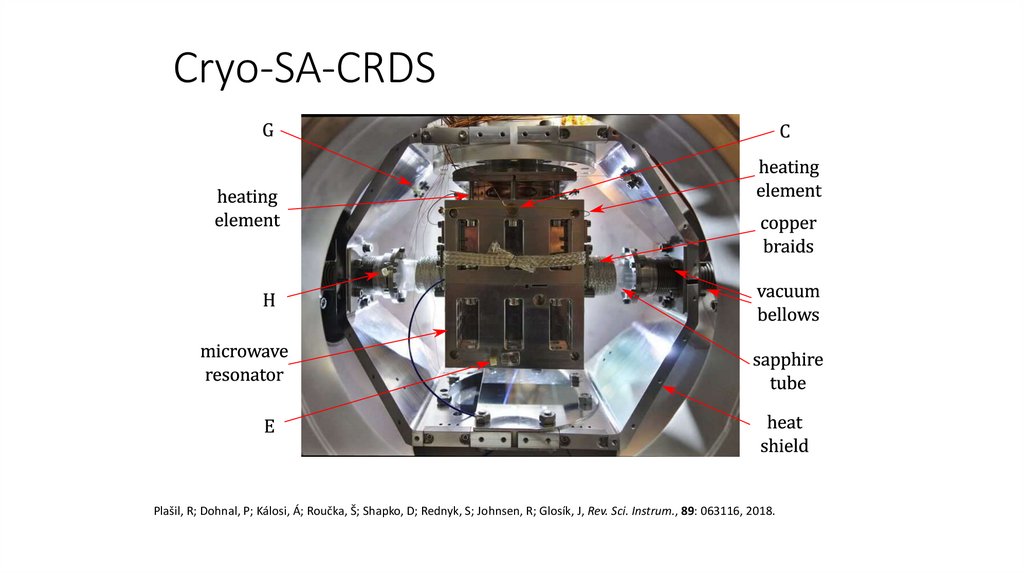
Cryo-SA-CRDS17.
Cryo-SA-CRDS18.
Cryo-SA-CRDSPlašil, R; Dohnal, P; Kálosi, Á; Roučka, Š; Shapko, D; Rednyk, S; Johnsen, R; Glosík, J, Rev. Sci. Instrum., 89: 063116, 2018.
19.
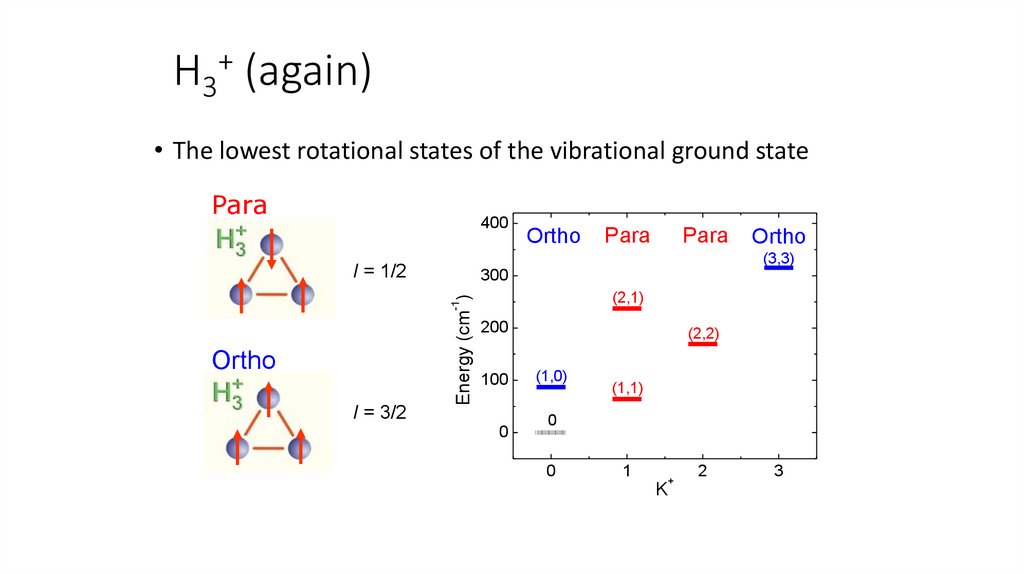
+H3 (again)
• The lowest rotational states of the vibrational ground state
Para
400
Ortho
Ortho
300
(2,1)
-1
Energy (cm )
I = 3/2
Para
(3,3)
I = 1/2
Ortho
Para
200
100
0
(2,2)
(1,0)
(1,1)
0
0
1
+
K
2
3
20.
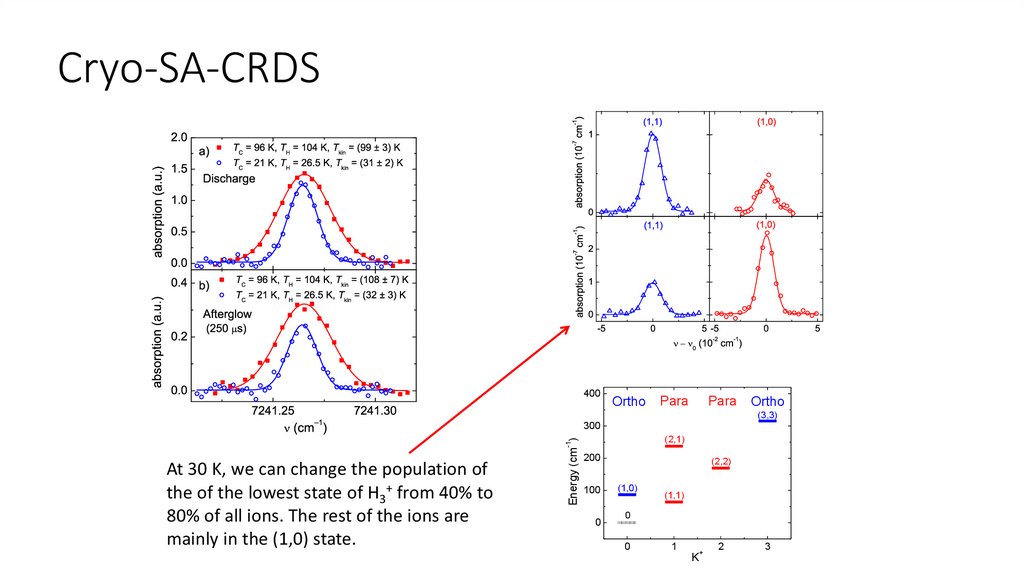
Cryo-SA-CRDS400
Ortho
Para
Para
Ortho
(3,3)
(2,1)
-1
Energy (cm )
300
At 30 K, we can change the population of
the of the lowest state of H3+ from 40% to
80% of all ions. The rest of the ions are
mainly in the (1,0) state.
200
100
0
(2,2)
(1,0)
(1,1)
0
0
1
+
K
2
3
21.
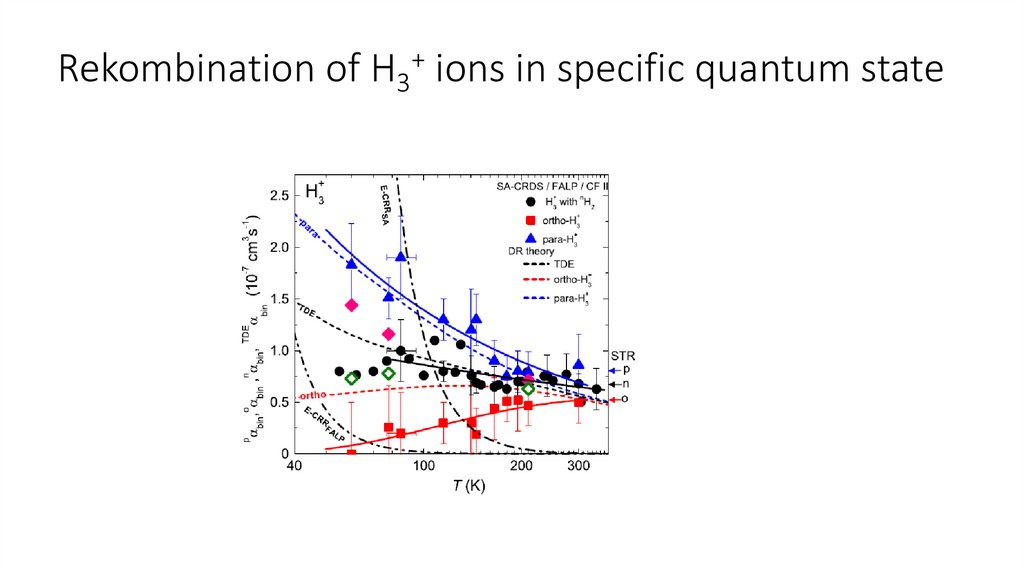
Rekombination of H3+ ions in specific quantum state22.
Combination of CRDS and microwave diagnostics23.
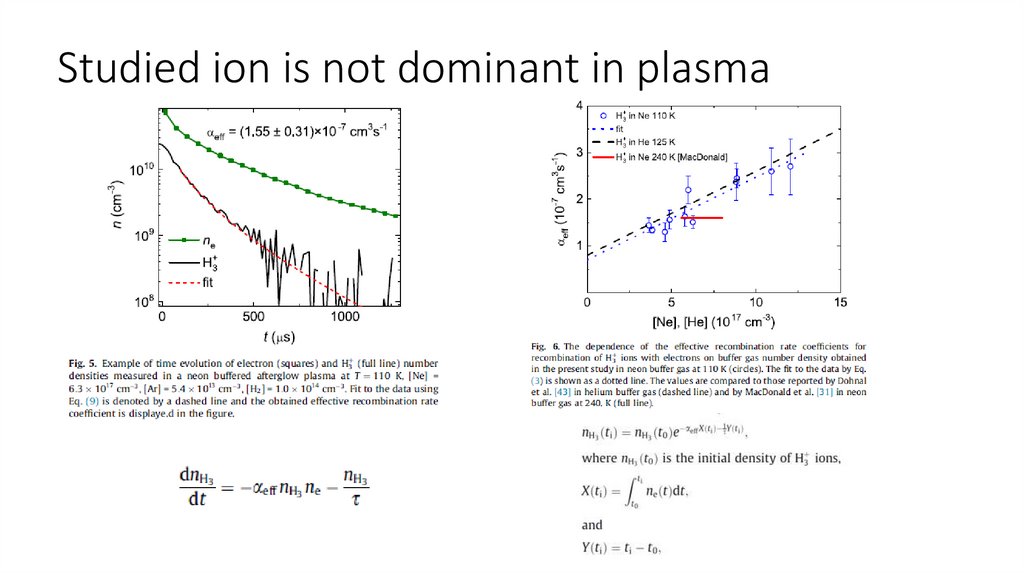
Studied ion is not dominant in plasma24.
Flowing afterglow plasma25.
Flowing afterglow plasma26.
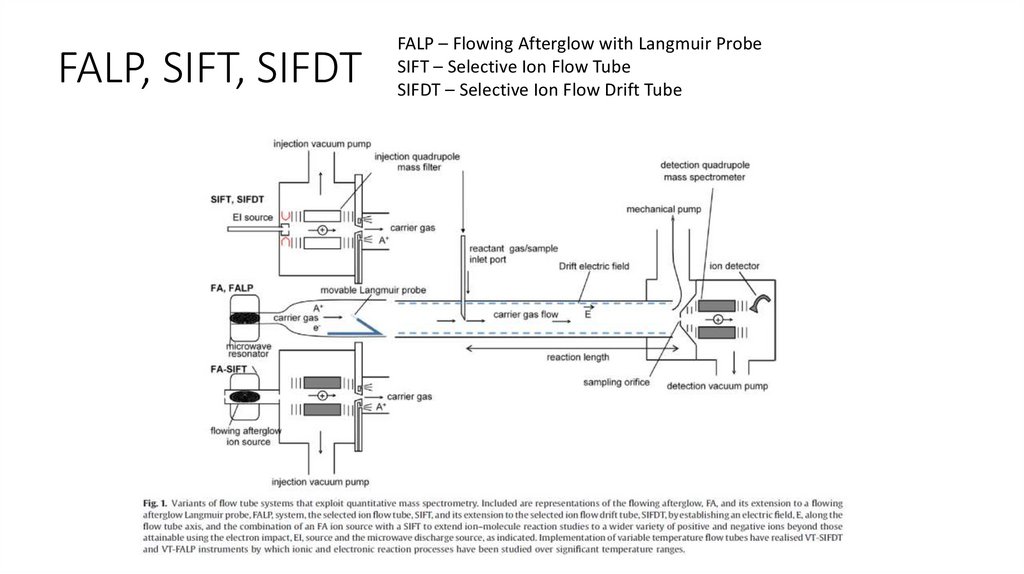
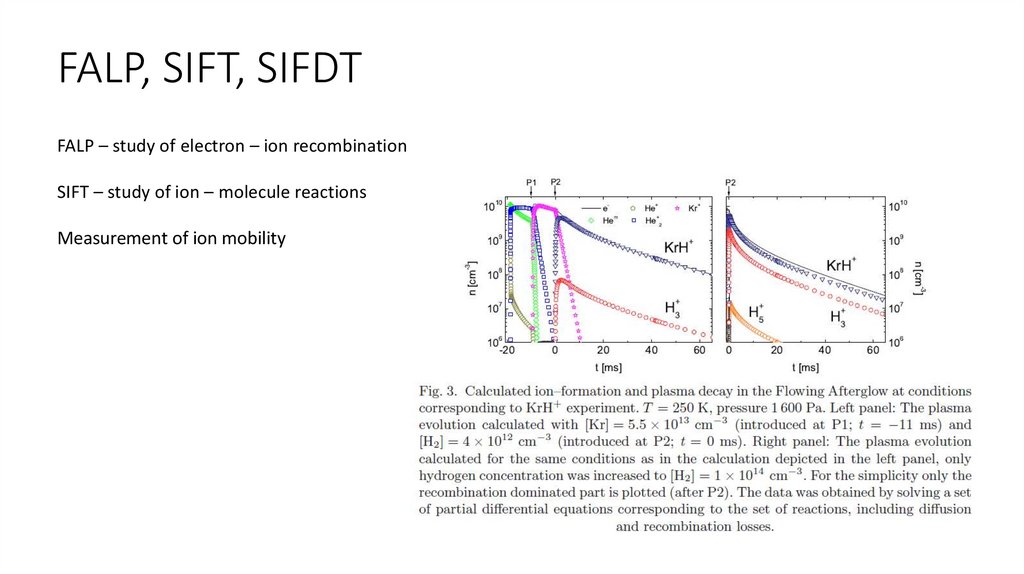
FALP, SIFT, SIFDTFALP – Flowing Afterglow with Langmuir Probe
SIFT – Selective Ion Flow Tube
SIFDT – Selective Ion Flow Drift Tube
27.
FALP, SIFT, SIFDTFALP – study of electron – ion recombination
SIFT – study of ion – molecule reactions
Measurement of ion mobility
28.
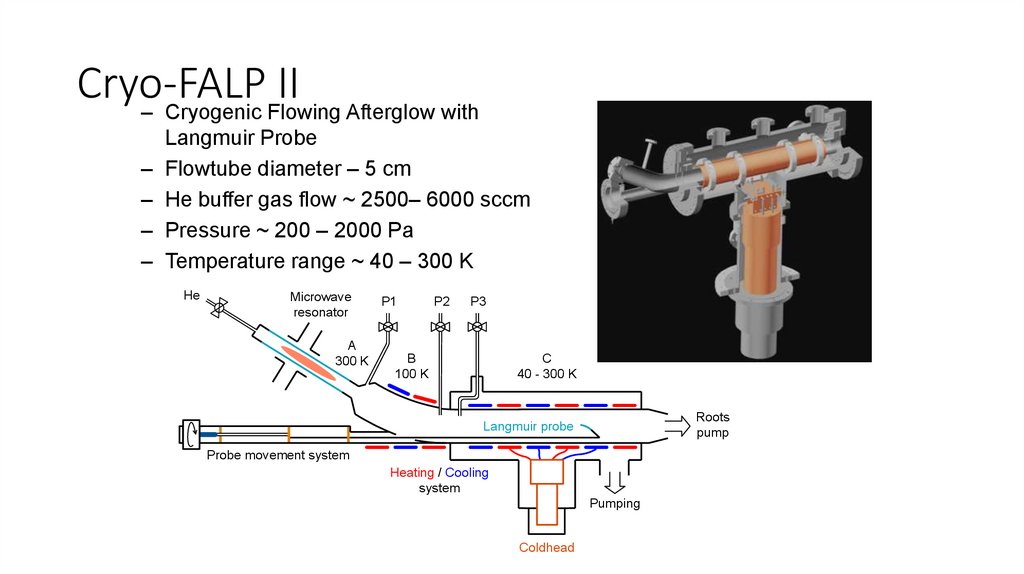
Cryo-FALPII
– Cryogenic Flowing Afterglow with
–
–
–
–
Langmuir Probe
Flowtube diameter – 5 cm
He buffer gas flow ~ 2500– 6000 sccm
Pressure ~ 200 – 2000 Pa
Temperature range ~ 40 – 300 K
He
Microwave
resonator
A
300 K
P1
P2
P3
B
100 K
C
40 - 300 K
Roots
pump
Langmuir probe
Probe movement system
Heating / Cooling
system
Pumping
Coldhead
29.
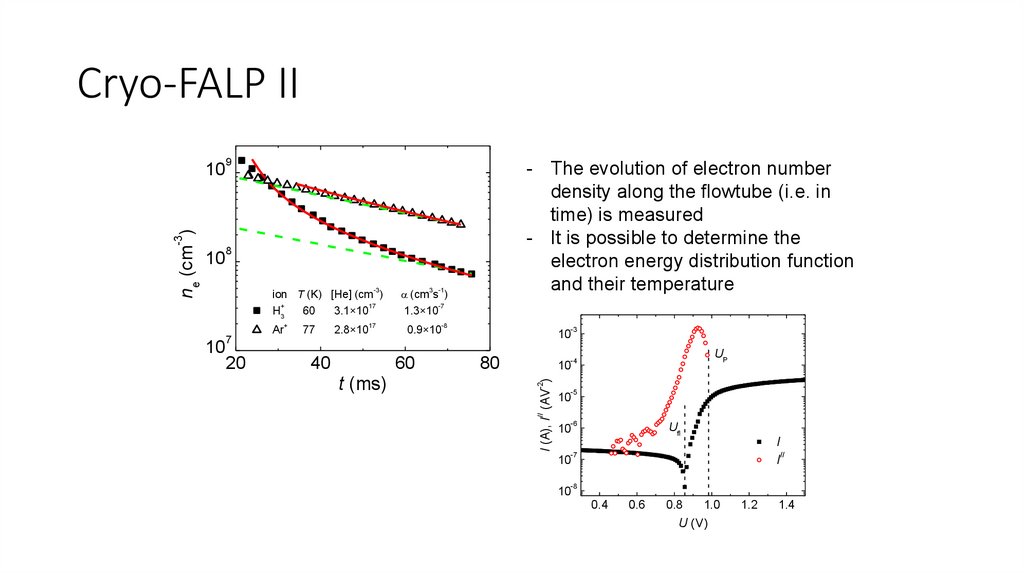
Cryo-FALP II9
- The evolution of electron number
density along the flowtube (i.e. in
time) is measured
- It is possible to determine the
electron energy distribution function
and their temperature
-3
8
10
10
20
+
77
2.8×10
17
40
-7
1.3×10
-8
0.9×10
60
t (ms)
80
10
-3
10
-4
10
-5
10
-6
10
-7
10
-8
UP
-2
7
Ar
3 -1
(cm s )
//
-3
ion T (K) [He] (cm )
+
17
H3
60
3.1×10
I (A), I (AV )
ne (cm )
10
Ufl
0.4
0.6
0.8
I
//
I
1.0
U (V)
1.2
1.4
30.
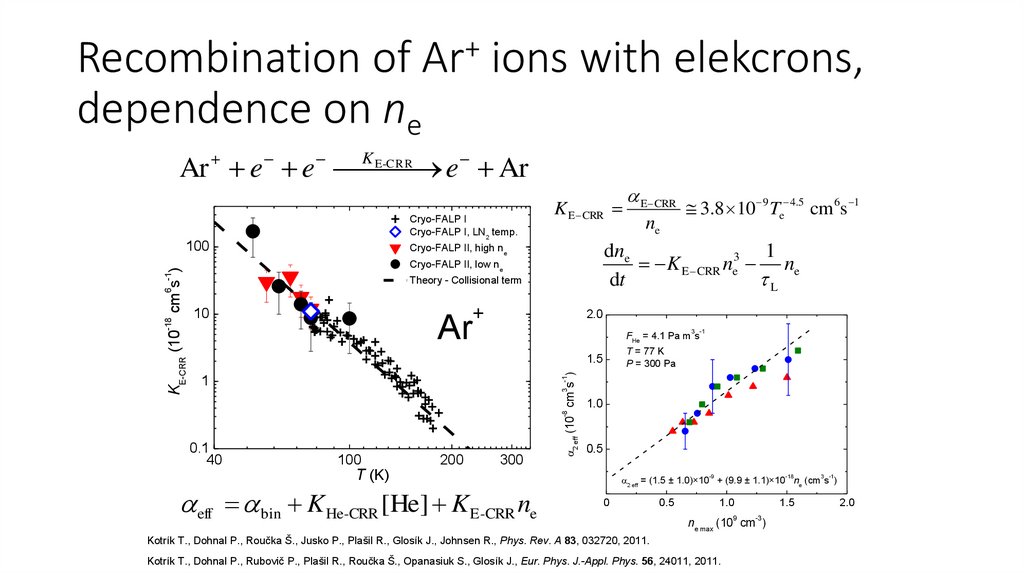
Recombination of Ar+ ions with elekcrons,dependence on ne
E-CRR
Ar e e K
e Ar
Cryo-FALP I
Cryo-FALP I, LN2 temp.
Cryo-FALP II, low ne
+
ne
3.8 10 9 Te 4.5 cm 6s 1
2.0
Ar
3 -1
FHe = 4.1 Pa m s
T = 77 K
P = 300 Pa
1.5
3 -1
1
2 eff (10 cm s )
-18
1.0
-8
KE-CRR (10
Theory - Collisional term
10
E CRR
dne
1
K E CRR ne3 ne
dt
L
Cryo-FALP II, high ne
6 -1
cm s )
100
K E CRR
0.1
40
100
200
300
0.5
T (K)
eff bin K He-CRR [He] K E-CRR ne
-9
-18
3 -1
2 eff = (1.5 ± 1.0)×10 + (9.9 ± 1.1)×10 ne (cm s )
0
0.5
1.0
9
1.5
-3
ne max (10 cm )
Kotrík T., Dohnal P., Roučka Š., Jusko P., Plašil R., Glosík J., Johnsen R., Phys. Rev. A 83, 032720, 2011.
Kotrík T., Dohnal P., Rubovič P., Plašil R., Roučka Š., Opanasiuk S., Glosík J., Eur. Phys. J.-Appl. Phys. 56, 24011, 2011.
2.0
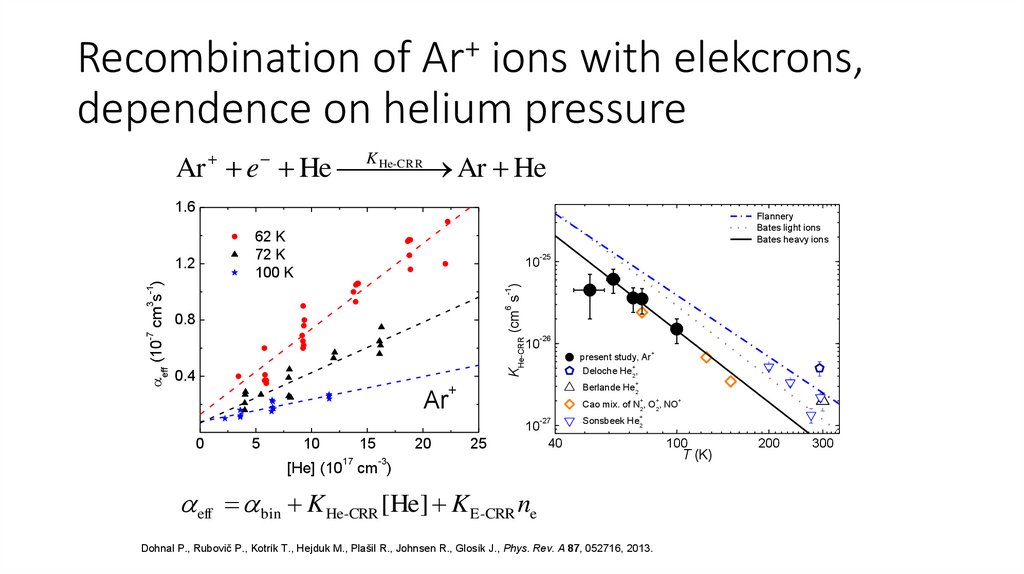
31.
Recombination of Ar+ ions with elekcrons,dependence on helium pressure
He-CRR
Ar e He K
Ar He
1.6
62 K
72 K
100 K
10
-25
6
-1
3 -1
KHe-CRR (cm s )
1.2
eff (10 cm s )
Flannery
Bates light ions
Bates heavy ions
-7
0.8
10
0.4
-26
present study, Ar
+
Deloche He2,
+
Berlande He2
+
Ar
+
5
10
15
17
20
+
+
Cao mix. of N2, O2, NO
10
0
+
25
-27
+
Sonsbeek He2
40
-3
[He] (10 cm )
eff bin K He-CRR [He] K E-CRR ne
Dohnal P., Rubovič P., Kotrík T., Hejduk M., Plašil R., Johnsen R., Glosík J., Phys. Rev. A 87, 052716, 2013.
100
T (K)
200
300
32.
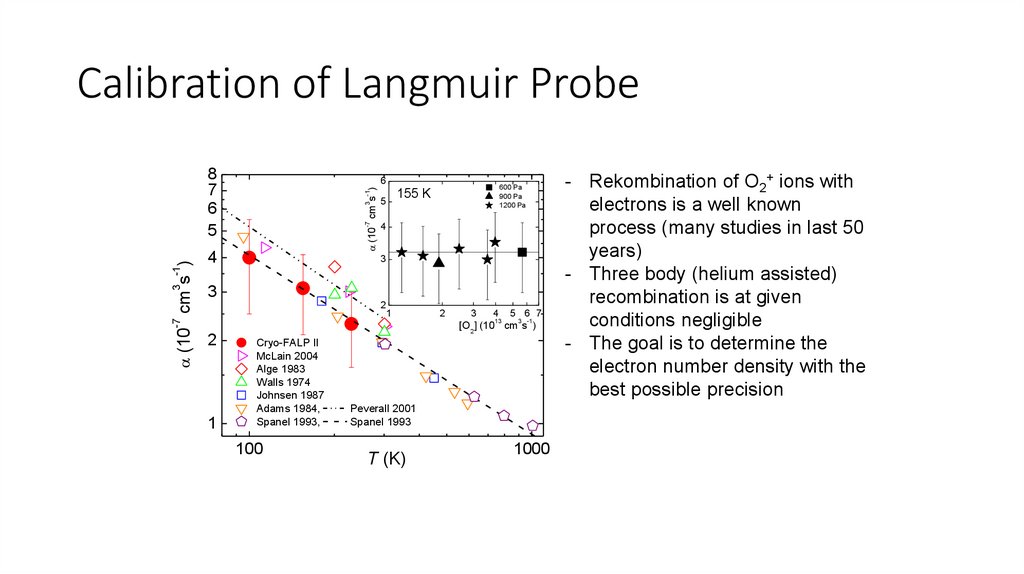
Calibration of Langmuir Probe-7
3 -1
(10 cm s )
6
4
600 Pa
900 Pa
1200 Pa
155 K
5
4
3
3
2
-7
3 -1
(10 cm s )
8
7
6
5
1
2
3
4
13
2
1
5 6 7
3 -1
[O2] (10 cm s )
Cryo-FALP II
McLain 2004
Alge 1983
Walls 1974
Johnsen 1987
Adams 1984,
Spanel 1993,
100
Peverall 2001
Spanel 1993
T (K)
1000
- Rekombination of O2+ ions with
electrons is a well known
process (many studies in last 50
years)
- Three body (helium assisted)
recombination is at given
conditions negligible
- The goal is to determine the
electron number density with the
best possible precision
33.
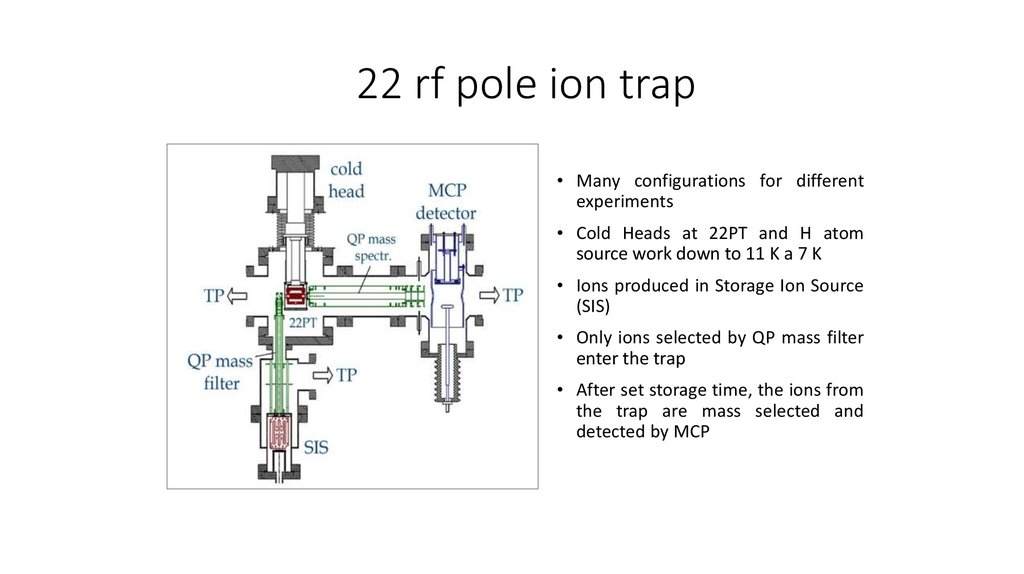
22 rf pole ion trap• Many configurations for different
experiments
• Cold Heads at 22PT and H atom
source work down to 11 K a 7 K
• Ions produced in Storage Ion Source
(SIS)
• Only ions selected by QP mass filter
enter the trap
• After set storage time, the ions from
the trap are mass selected and
detected by MCP
34.
22 rf pole ion trap35.
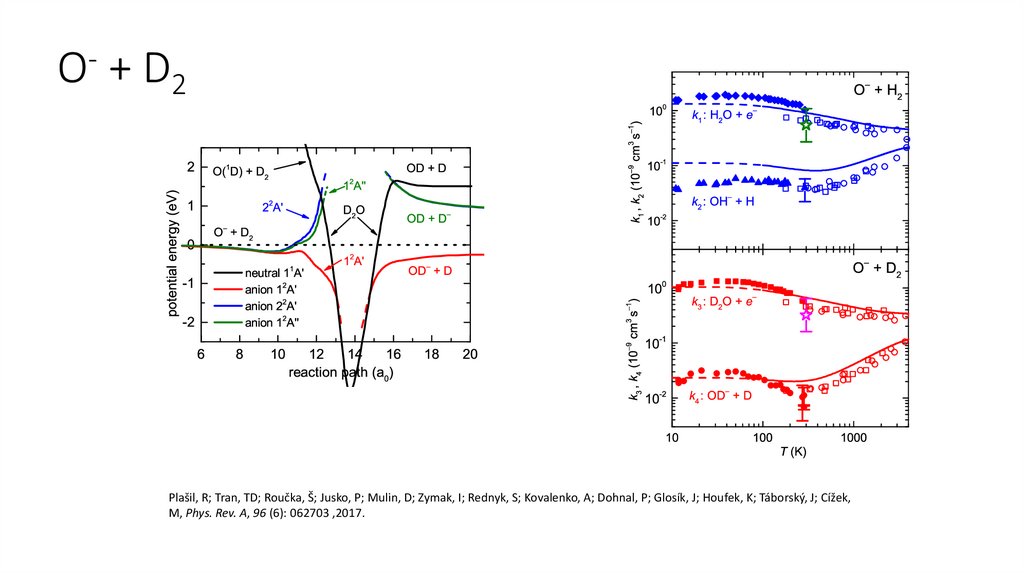
O- + D2Plašil, R; Tran, TD; Roučka, Š; Jusko, P; Mulin, D; Zymak, I; Rednyk, S; Kovalenko, A; Dohnal, P; Glosík, J; Houfek, K; Táborský, J; Cížek,
M, Phys. Rev. A, 96 (6): 062703 ,2017.







 Механика
Механика








