Похожие презентации:
The development of nanoporous hydrogen storages
1. THE DEVELOPMENT OF NANOPOROUS HYDROGEN STORAGES
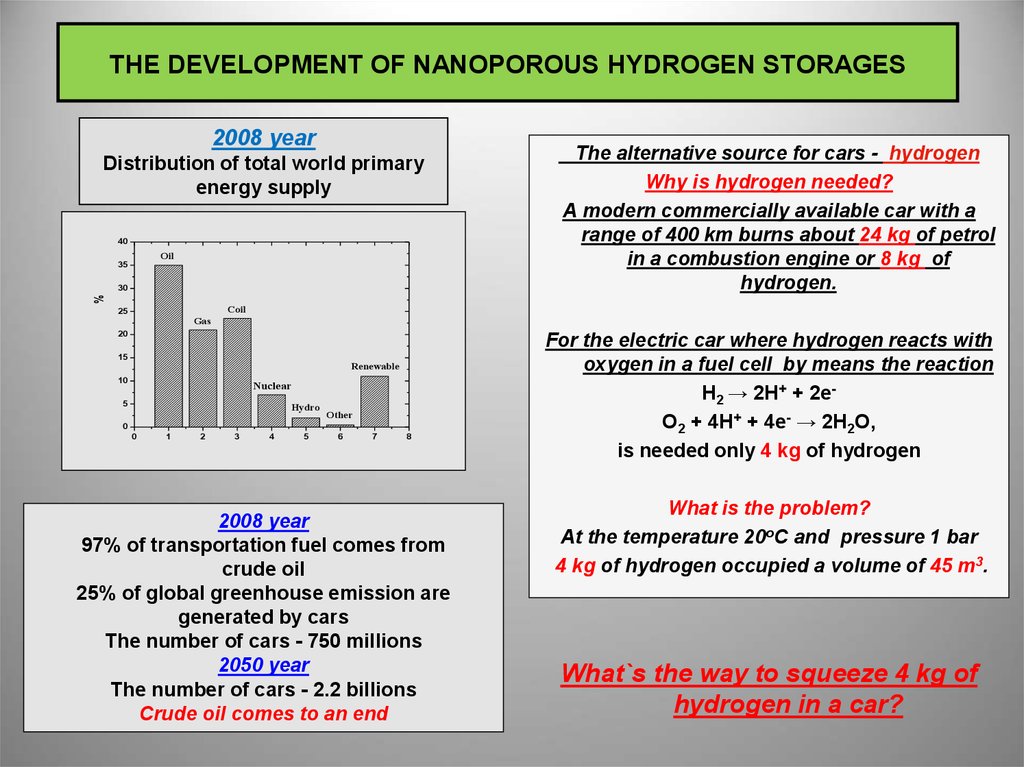
2008 yearDistribution of total world primary
energy supply
40
Oil
35
%
30
The alternative source for cars - hydrogen
Why is hydrogen needed?
A modern commercially available car with a
range of 400 km burns about 24 kg of petrol
in a combustion engine or 8 kg of
hydrogen.
Coil
25
Gas
20
15
Renewable
10
Nuclear
5
Hydro
Other
0
0
1
2
3
4
5
6
7
8
2008 year
97% of transportation fuel comes from
crude oil
25% of global greenhouse emission are
generated by cars
The number of cars - 750 millions
2050 year
The number of cars - 2.2 billions
Crude oil comes to an end
For the electric car where hydrogen reacts with
oxygen in a fuel cell by means the reaction
Н2 → 2Н+ + 2eO2 + 4H+ + 4e- → 2H2O,
is needed only 4 kg of hydrogen
What is the problem?
At the temperature 20oC and pressure 1 bar
4 kg of hydrogen occupied a volume of 45 m3.
What`s the way to squeeze 4 kg of
hydrogen in a car?
2. Conventional and nonconventional hydrogen storages.
Storage in high-pressure tanks – up to 700 atmospheres.Disadvantages – spontaneous leak of hydrogen and high risk of depressurization.
Storage in liquid state– (-252°С).
Disadvantages – thigh cost of equipment for hydrogen storage and cooling, evaporation and high risk of
depressurization.
Hydrogen storage in solid state.
Requirements.
Gravimetric capacitance- > 6 weight % H2, Hydrogen pressure at its saturation - < 3 МPа,
Hydrogenation time - < 5 minutes, Temperature of hydrogen desorption - < 85°С
Porous
(physical adsorption)
Compact
(chemical adsorption)
1. Carbon nanostructures
Nanotubes (single-layer, multilayer),
nanofibers, fullerene, graphene,
activated carbon.
1. Mg - based hydrides
MgH2 – (Ti, V, Ni, Cu, Fe, Mn),
MgH2 – (V2O5, Nb2O5, Fe2O3, Al2O3, TiO2)
2. Complex hydrides
NaAlH6, LiAlH4, KAlH4
3. LiN - based hydrides
LiNH2, Li2NH, Li2MgN2H2, Li3BN2H8
4. Intermetallic compounds
LaNi5, FeTi, TiVCr, TiZrNi, TiCrMn
2. Metal - organic structures
MOF-5,177 (Zn4O-[O2C-C6H4-CO2]2),
MIL-53,101(Cr,Al,O [O2C-C6H4-CO2]2),
IMOF-1,3,12 (Zn4O-CxHy(CO2)2)
So far none of the solid-state hydrogen accumulators satisfy
the necessary requirements.
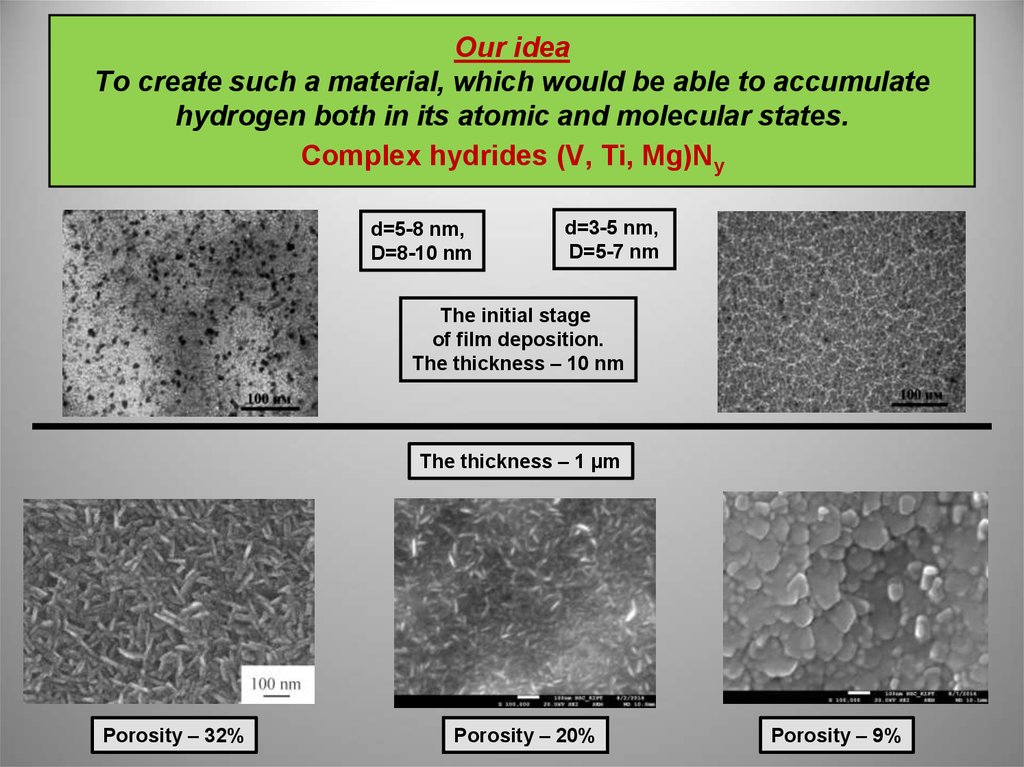
3. Our idea To create such a material, which would be able to accumulate hydrogen both in its atomic and molecular states. Complex hydrides (V, Ti, Mg)Ny
d=5-8 nm,D=8-10 nm
d=3-5 nm,
D=5-7 nm
The initial stage
of film deposition.
The thickness – 10 nm
The thickness – 1 µm
Porosity – 32%
Porosity – 20%
Porosity – 9%
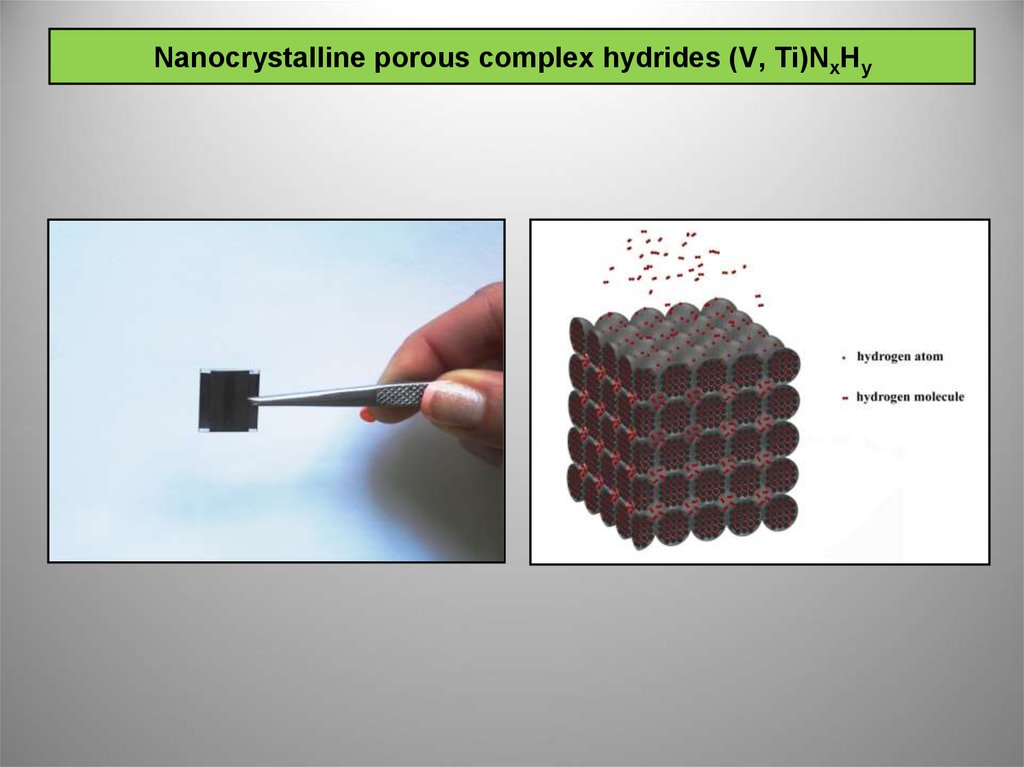
4. Nanocrystalline porous complex hydrides (V, Ti)NxHy
5. Structural changes in VNx films by absorption and desorption of hydrogen. Scanning and transmission microscopy.
Initial stateH2, 0,3 MPa, 1 hour, 20oC
Annealing 250oC
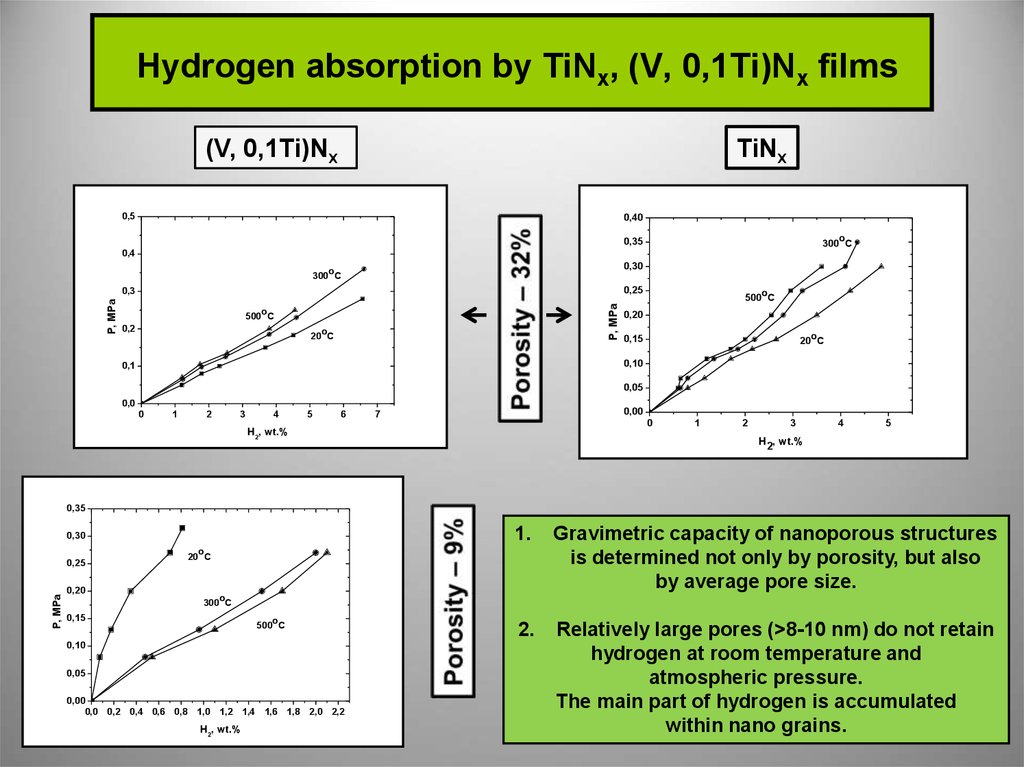
6. Hydrogen absorption by TiNx, (V, 0,1Ti)Nx films
(V, 0,1Ti)NxTiNx
0,5
0,40
o
300 C
0,35
0,4
0,30
o
300 C
0,25
P, MPa
P, MPa
0,3
o
500 C
0,2
o
20 C
o
500 C
0,20
o
20 C
0,15
0,10
0,1
0,05
0,0
0
1
2
3
4
5
6
0,00
7
0
H2, wt.%
1
2
3
4
5
H2, wt.%
0,35
0,30
1.
Gravimetric capacity of nanoporous structures
is determined not only by porosity, but also
by average pore size.
2.
Relatively large pores (>8-10 nm) do not retain
hydrogen at room temperature and
atmospheric pressure.
The main part of hydrogen is accumulated
within nano grains.
o
P, MPa
0,25
0,20
20 C
o
300 C
0,15
o
500 C
0,10
0,05
0,00
0,0 0,2 0,4 0,6 0,8 1,0 1,2 1,4 1,6 1,8 2,0 2,2
H2, wt.%
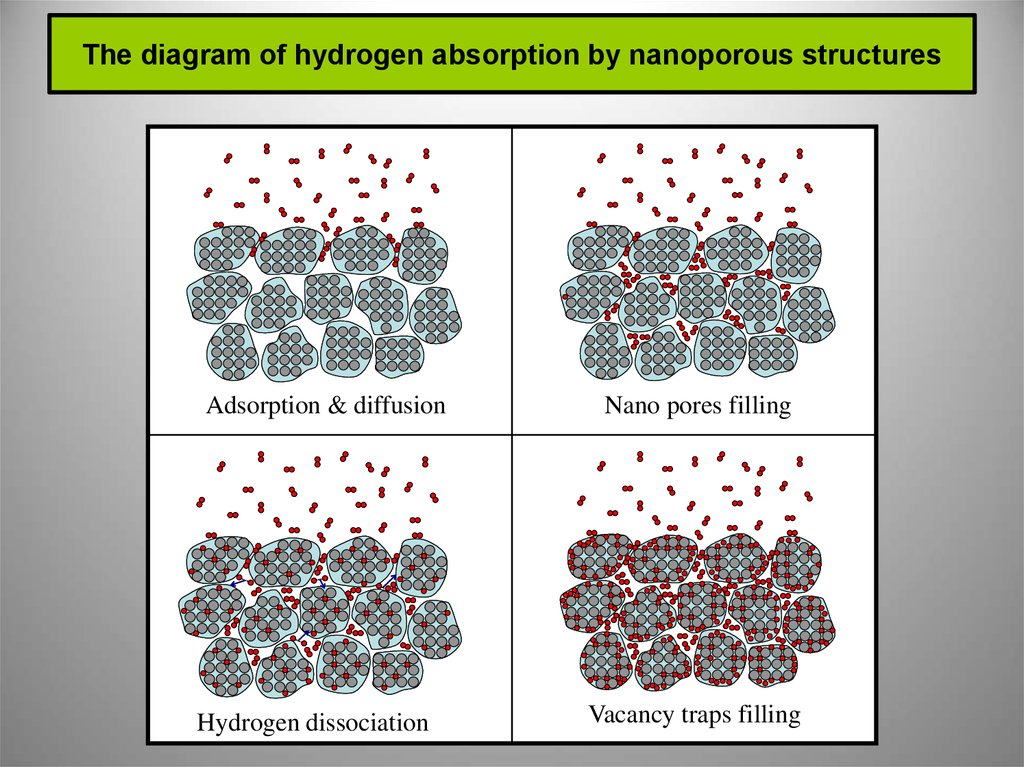
7. The diagram of hydrogen absorption by nanoporous structures
Adsorption & diffusionHydrogen dissociation
Nano pores filling
Vacancy traps filling
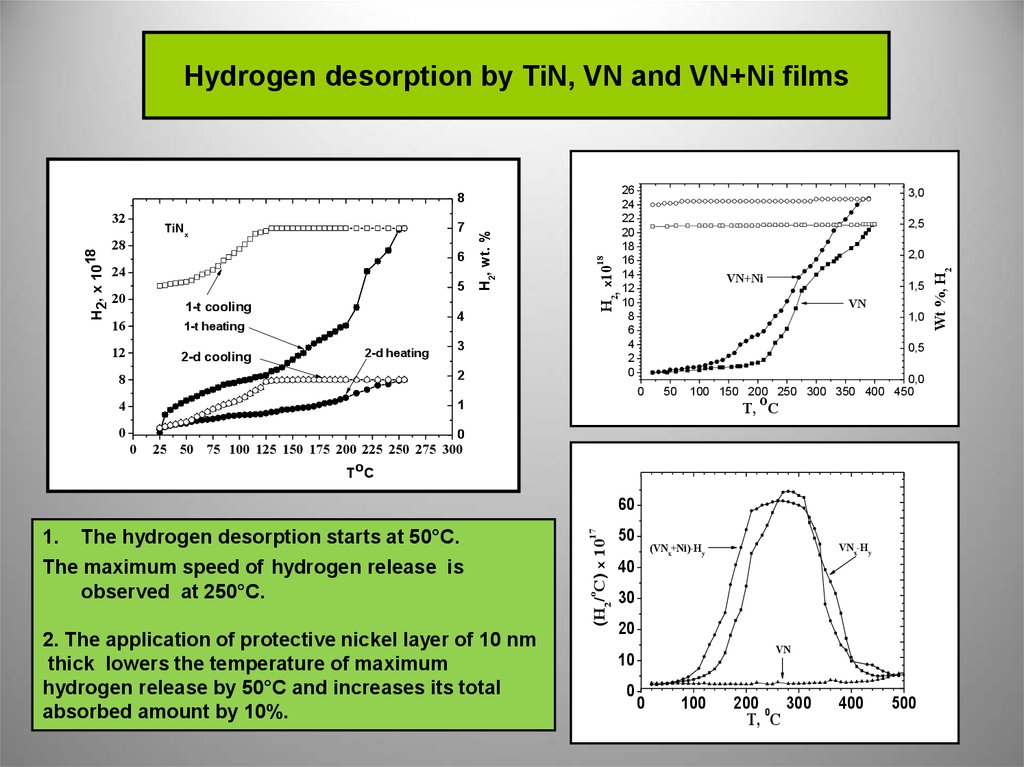
8. Hydrogen desorption by TiN, VN and VN+Ni films
624
5
20
1-t cooling
16
1-t heating
12
2-d cooling
4
2-d heating
3
8
2
4
1
0
0
26
24
22
20
18
16
14
12
10
8
6
4
2
0
3,0
2,5
2,0
VN+Ni
1,5
VN
1,0
0,5
0
50
0,0
100 150 200 250 300 350 400 450
о
Т, С
0
25 50 75 100 125 150 175 200 225 250 275 300
To C
2. The application of protective nickel layer of 10 nm
thick lowers the temperature of maximum
hydrogen release by 50°С and increases its total
absorbed amount by 10%.
o
(H2/ C) x 10
1. The hydrogen desorption starts at 50°С.
The maximum speed of hydrogen release is
observed at 250°С.
17
60
50
VNx-Hy
(VNx+Ni)-Hy
40
30
20
VN
10
0
0
100
200 0 300
Т, С
400
500
Wt %, Н2
28
18
7
TiNx
Н2, x10
H2, x 1018
32
H2, wt. %
8
9. CONCLUSIONS
Nanocrystalline thin film structures based on vanadium, titanium andmagnesium (VN, TiN, Mg3N2) can be used as a solid-state hydrogen storages
successfully.
Ion-beam assisted technology is an effective method of such thin film
nanocrystalline materials preparation.
High degree of non equilibrium of current method in aggregate with varying
its basic parameters component make possible to produce nanocrystalline
structures (5-10 nm), in which the intergranular joints can contain nanopores
(3-5 nm). Such structures are capable to accumulate more than 7 wt. % of
hydrogen.
The important role of open nanoporosity (pore ensemble joined with grain
boundaries) is in branched network creation in which hydrogen penetrates
into storage volume at a low pressure (< 0,5 МPа) and short time interval
(~2-5 min).


 Химия
Химия








