Похожие презентации:
Gas hydrates
1.
Gas Hydrates2
1
2. Gas-Hydrate resources
«There to the North of USSR wide territories existwhere layers have temperature lower than 0 C at 400
m and even 600 m depth and where gas-hydrates
fields may exist.
- I.N.Strijev, I.E.Khodanovitch
“Dobitcha gaza", 1946 , p. 349
The possibility of gas-hydrate existenceat the natural
conditions was shown in the experimental works
that were carried out in Gubkin State University in
1969.
It was a scientific discovery
The Authors are Yu.F.Makagon, F.A.Trebin,
V.G.Vasiliev, N.V.Charskyi, A.A.Trofimuk
2
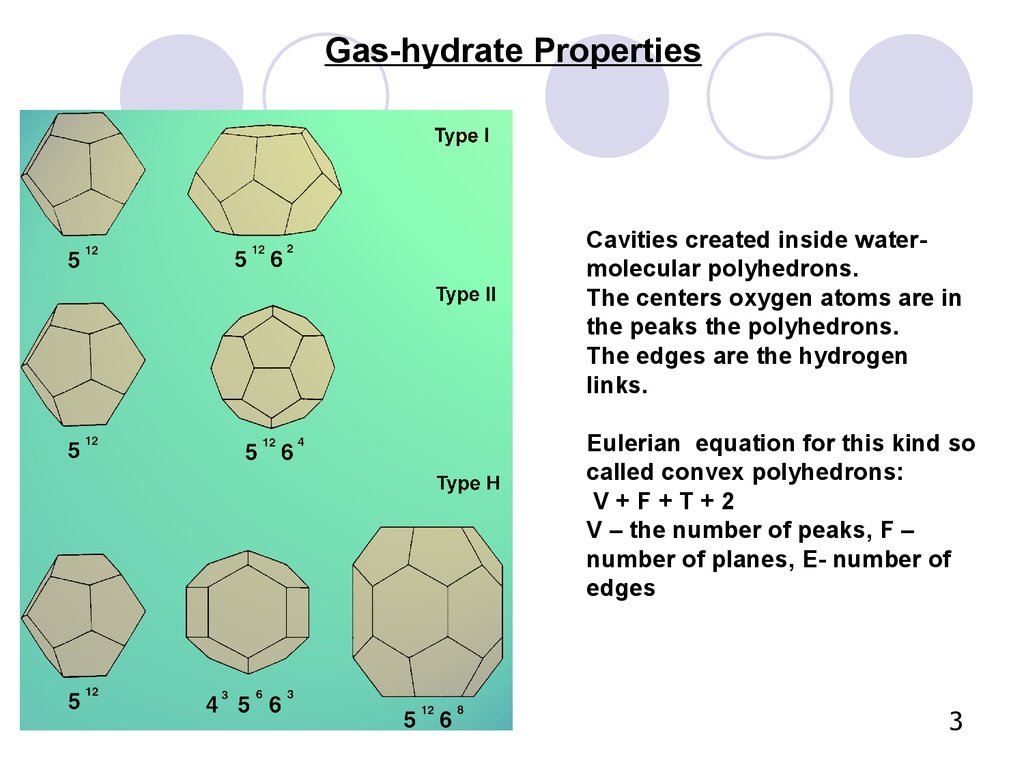
3. Cavities created inside water-molecular polyhedrons. The centers oxygen atoms are in the peaks the polyhedrons. The edges are the hydrogen links. Eulerian equation for this kind so called convex polyhedrons: V + F + T + 2 V – the number of peaks, F –
Gas-hydrate PropertiesCavities created inside watermolecular polyhedrons.
The centers oxygen atoms are in
the peaks the polyhedrons.
The edges are the hydrogen
links.
Eulerian equation for this kind so
called convex polyhedrons:
V+F+T+2
V – the number of peaks, F –
number of planes, Е- number of
edges
3
4.
Gas-hydrate PropertiesCavity
(number of
sides)
Volume of the
polyhedron
V
E
F*
D (12)
20
30
12(512)
5,2
168
Т (14)
24
36
12(51262)
5,32**
6,4
230
P(15)
26
39
15(51263)
6,1**
7,0
260
Р(16)
28
42
16(51264)
6,6
290
Е(20)
36
54
20(51268)
9,6**
7,3
Cavity diameter
4
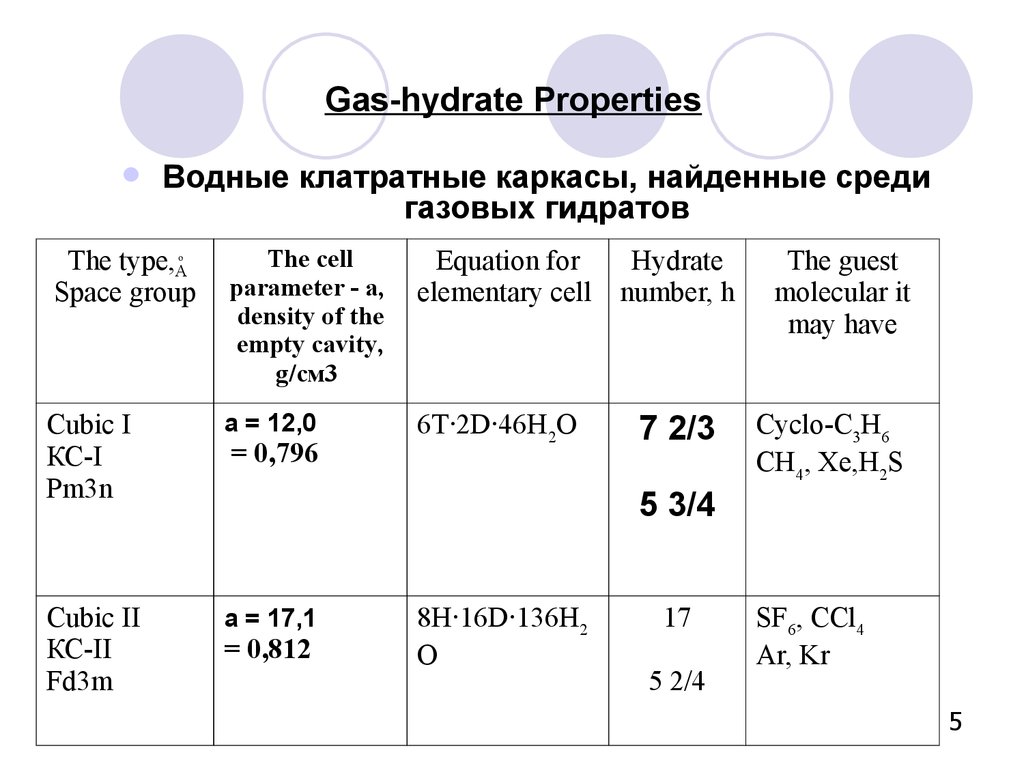
5. Gas-hydrate Properties
Водные клатратные каркасы, найденные средигазовых гидратов
The type,A
Space group
The cell
parameter - а,
density of the
empty cavity,
g/см3
Equation for
elementary cell
Hydrate
number, h
Cubic I
КС-I
Pm3n
а = 12,0
6Т∙2D∙46Н2О
7 2/3
Cubic II
КС-II
Fd3m
а = 17,1
o
= 0,796
The guest
molecular it
may have
Cyclo-С3Н6
СН4, Хе,Н2S
5 3/4
= 0,812
8Н∙16D∙136Н2
О
17
5 2/4
SF6, CCl4
Ar, Kr
5
6.
In contrast to conventional natural gas,methane hydrates occur only in sediments
characterized by well-known pressure and
temperature conditions, meaning that
exploration activities can be strictly limited
to specific zones
6
7.
The stability of an idealized methane hydrate in nature (area to the left ofthe red phase boundary) in nominal marine (A) and permafrost (B) cases
7
8.
89.
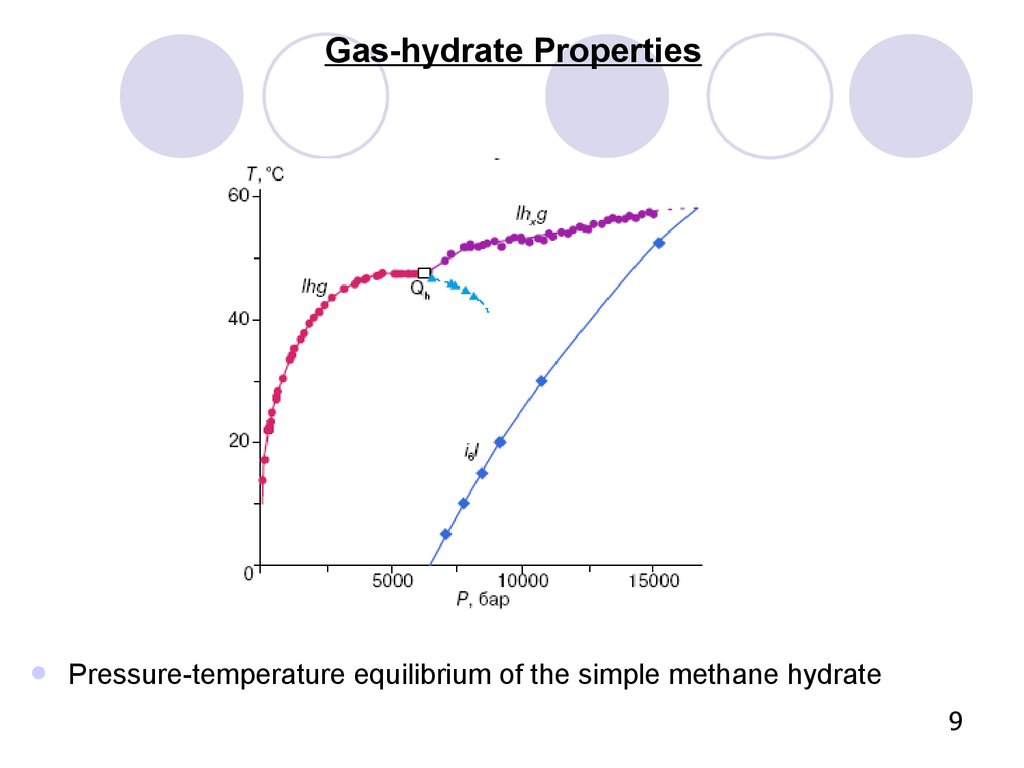
Gas-hydrate PropertiesPressure-temperature equilibrium of the simple methane hydrate
9
10.
1011.
1112.
1213.
Our knowledge on gas hydrate production from naturalreservoirs comes primarily from laboratory studies and
modeling using numerical simulators. However, these
simulations and modeling efforts utilize real data from
the field or production test data, such as that obtained
from the Mt. Elbert project on the North Slope of Alaska,
the Mallik production test in Canada, and borehole
information from suspected hydrate accumulations in the
deepwater GOM. The short-term tests at Mt. Elbert and
Mallik have answered many questions, while raising
many more that can only be addressed by a series of
long-term production tests in a variety of settings.
13
14.
PHYSICAL PROPERTIES OF SILICA GEL SAMPLESSample Name
6 nm SG 30 nm SG 100 nm SG
mean particle diameter (μm) 150 to 250 40 to 75
40 to 75
mean pore diameter (nm)
5.51
30.1
94.5
specific pore volume (m3/kg) 8.4×10-4 8.4×10-4
8.3×10-4
specific surface area (m2/kg) 586×103 94.9×103 42.4×103
As a porous material, spherical
silica gels of nominal pore diameter 6 nm, 30 nm, and 100 nm
were selected and purchased from Aldrich (6 nm) and Silicycle
(30 nm and 100 nm), respectively. All the materials were used
without further treatment. The properties of silica gels having 6
and 30 nm pore diameters were measured by nitrogen
adsorption/desorption experiments with ASAP 2400
(Micrometrics), and those of 100 nm pore diameter by mercury
intrusion
14
15.
Probabilistic Nature of Resource AssessmentIn order to capture the uncertainty in the evaluation process,
the estimate of undiscovered in-place gas hydrate is
expressed as a cumulative probability distribution, where a
specified volume or more of resources corresponds to a
probability of occurrence. The low estimate corresponds to the
95th percentile value of the distribution, the mean estimate
corresponds to the statistical average of all values in the
distribution, and the high estimate corresponds to the 5th
percentile value of the distribution. As in most stochastic
resource assessments, and certainly in one where a new
methodology has been developed and deployed, the reader is
encouraged to view the mean estimate as the expected value.
15
16.
(1) Analytic methods employed are based on massbalance – an input-output analysis. Inputs are the mass
of organic carbon available for conversion to methane,
the volume of rock that possesses the physical and
chemical conditions required to contain hydrates, and
the fraction of that rock volume that constitutes effective
void space into which hydrates can concentrate. While
there are other possible methodologies, mass balance
has two important advantages: it is transparent and
allows extreme variable disaggregation. Therefore, as
new and better information about total organic carbon in
sediments, or heat flow or an improved formulation of
hydrate phase relationships becomes available, the
system can be easily updated.
16
17.
(2) It is cell-based. The study area is 457,933 km2 and isdivided into a grid of 202,079 cells, each 2.32 km2. The mass
balance analysis is applied to each cell, providing a level of
spatial resolution that supports detailed mapping. The spatial
distributions of in-place methane hydrates, both in absolute
geographic space and its relative spatial distribution (e.g.,
clustering), have a critical impact on the fraction that will be
technically and, ultimately, economically recoverable.
17
18.
(3) It is stochastic. Many input variables are treated asuncertain quantities and are assigned probability distributions.
In some cases, parameters of these distributions are also
treated as uncertain quantities. Consequently, key output
variables are also uncertain quantities with probability
distributions determined jointly by model structure and these
probability distributions.
18
19. Literature
1.2.
3.
4.
5.
Boswell, R., and T. Collett, 2006. The gas hydrates resource
pyramid, Fire in the Ice, US Department of Energy, Office of Fossil
Energy, National Energy Technology Laboratory, 6(3), p. 5-7. http://
www.netl.doe.gov/technologies/oil-gas/publications/hydrates/2009
Reports/FITI06_Pyramid.pdf
http://ru.wikipedia.org
Kang S., Ryu H., Seo Y. Phase Behavior of CO2 and CH4 Hydrate
in Porous Media. World Academy of Science, Engineering and
Technology 33 2007
http://www.waset.org/journals/waset/v33/v33-37.pdf
Preliminary Evaluation of In-Place Gas Hydrate Resources: Gulf of
Mexico Outer Continental Shelf U.S. Department of the Interior
Minerals Management Service Resource Evaluation Division
February 1, 2008
http://www.boemre.gov/revaldiv/GasHydrateFiles/MMS2008-004.pdf
Sloan, E.D., Clathrate Hydrates of Natural Gases, Marcel Dekker,
New York, 1998.
19














 Химия
Химия Промышленность
Промышленность








