Похожие презентации:
Solution
1.
SolutionsProblems
2.
3.
4.
5.
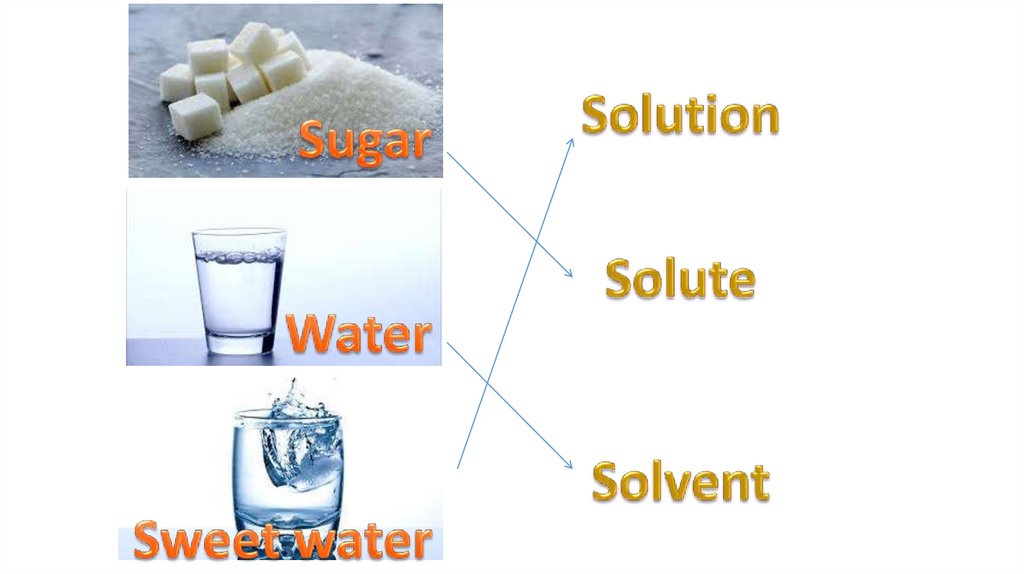
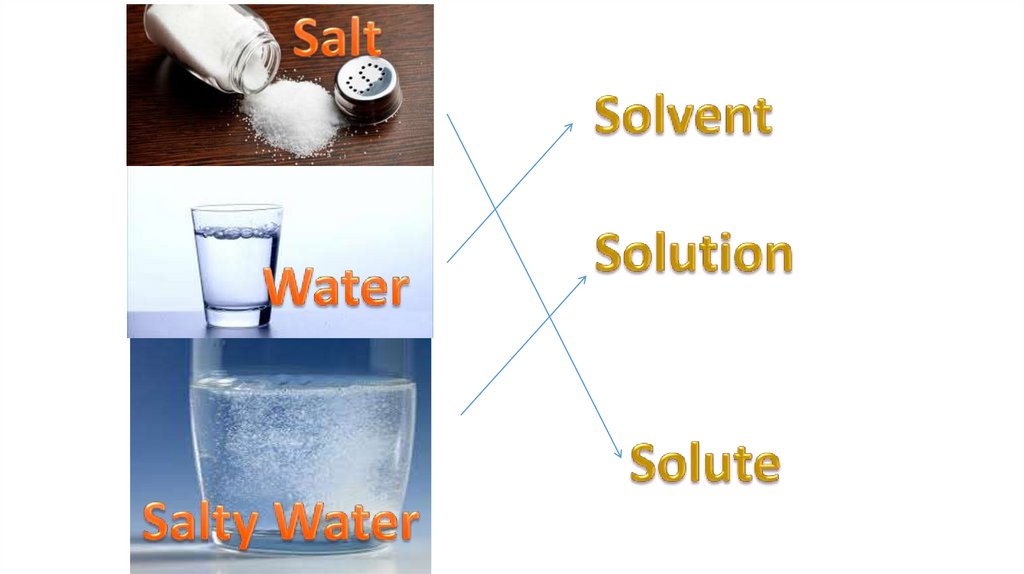
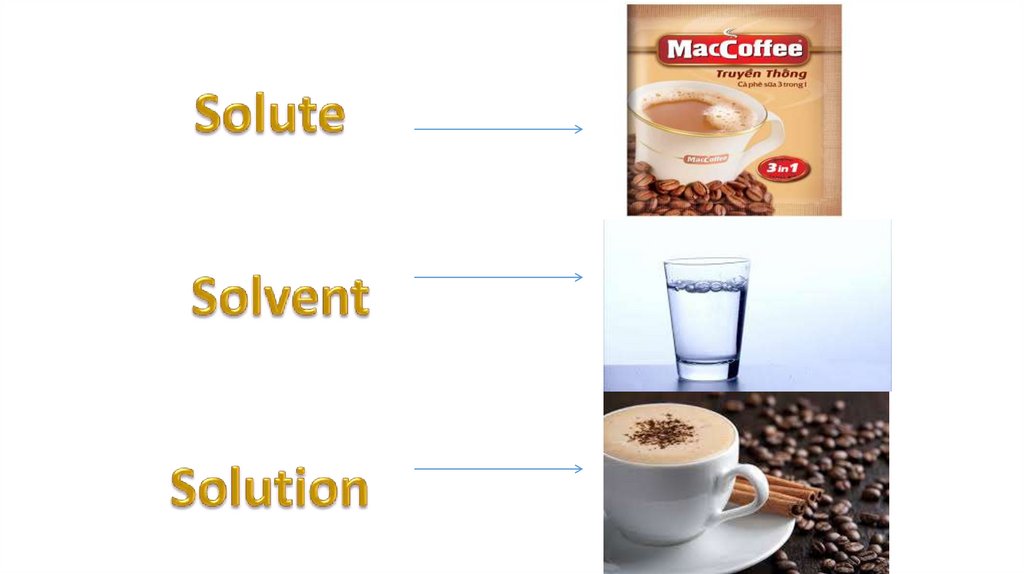
Solution = Solvent + Solute(s)*Solvent is the component of solution in
the largest amount or that determines
the state of solution.
*Solute is the part of solution dissolved
in a solvent.
*Solutions in which water is the solvent
are called aqueous solutions.
6.
Dilute solutionsConcentrated solutions
7.
Dilute and Concentrated Solutions• Solutions that contain relatively large amount of
solute are called concentrated, and relatively small
amount of solute are called dilute.
• Dilution is simply adding water to more concentrated
solution to make it dilute.
8.
• If the solubility of NaCl is 36 g NaCl in 100 g water at 20 0C . Howmany g NaCl is precipitated when 150 g NaCl is added to 400 g water
at the same temperature?
9.
Calculate the percentages of followingsolutions.
• 2 mol NaOH and 120 g water
• 100 g KCl and 300 ml water (dH2O:1 g/ml)
• 100 ml alcohol (d:0.78 g/ml) and 122 g water :
10.
Calculate the mass of solutes in the followingsolutuions.
• 500 g of 25 % X solution :
• 400 ml of 25 % X solution (d:2 g/ml)
• 800 g of 80 % NaCl solution :
• 600 ml of 0,5 M Mg(NO3)2 solution:
• 500 g of 0,5 M NaOH solution(d: 1,2g/ml):
11.
• Calculate the volume of H2 at STP released from 400 g of 36,5 % HClsolution by the following reaction. Mg + 2HCl MgCl2+H2
• (H=1, Cl=35,5)
12.
• 200 g of 40 % solution and 300 g of 40% solution:• 200g of 40% solution and 200 g of 80 % solution:
13.
• 300 ml of 0,4 M solution and 200 ml of 1,2M solution:• 400ml of 0,5M solution and 600ml of 1M solution:
14.
• 40g of CaX2 are used to prepare 500mL of 0.4M solutions. What is theatomic mass of X? (Ca = 40g/mol) (80g/mol)
15.
• There are 2 different solutions which are 20% and 40% NaCl by mass.How many grams of these solutions have to be mixed to obtain 280 g
of solution that is 32% NaCl by mass?
16.
• If 200 g of a solution that is 10% KNO3 by mass and 600 g of anothersolution that is 5% KNO3 by mass are mixed, what is the percent
concentration of KNO3 by mass in the new solution?













 Химия
Химия








