Похожие презентации:
Introduction to histology. Histology as a science research methods
1.
Inna A.Demianenko Histology department MA CFU2.
3.
Histology-a science that studies the laws of development,
structure and function of tissues, as well as interstitial
interactions, in the historical and individual
development of Human and multicellular organisms.
4.
Histology object - tissue- they are phylogenetically formed, topographically
and functionally connected cellular systems and their
derivatives, from which organs are formed.
5.
Histology sections:1) Cytology - the doctrine of the cell.
2) Embryology is the science of the embryo
development, the laws of the laying and formation of
tissues and organs.
3) General histology - the doctrine of the
development, structure and functions of tissues.
4) Special histology - the science of the microscopic
structure of organs and organ systems.
6.
The levels of organization of thehuman body
1) Organism
2) Organ
3) Tissue
4) Cell
5) Molecule
7.
THEORETICAL AND APPLIED PROBLEMS OFHISTOLOGY:
1) STUDYING THE REGULARITIES OF CYTO,
HISTO-AND ORGANOGENESIS
2) STUDY OF MECHANISMS OF MOLECULAR
GENETIC REGULATION OF CELL
DIFFERENTIATION AND REGENERATION,
DEVELOPMENT OF METHODS OF GENE
THERAPY AND STEM CELL TRANSPLANTATION.
8.
3) STUDY OF HUMAN EMBRIOGENESIS, CRITICALPERIODS OF DEVELOPMENT, “MOTHER-FETUS”
SYSTEM AND THE REASONS FOR INFERTILITY
4) EXPLANATION OF THE ROLE OF THE NERVOUS,
ENDOCRINE AND IMMUNE SYSTEMS IN THE
REGULATION OF MORPHOGENESIS AND
FUNCTIONS OF CELLS, TISSUES AND ORGANS
5) RESEARCH OF AGE CHANGES AND ADAPTATION
OF CELLS, TISSUES AND BODIES UNDER THE
ACTION OF ADVERSE ENVIRONMENTAL FACTORS
AND DURING TRANSPLANTATION
9.
Research Methods in HistologyMicroscopy - the study of objects using a
microscope.
It is divided into several types:
optical (light) microscopy,
electron microscopy X-ray microscopy x-ray laser
microscopy and is intended for observation and
registration of enlarged images of the sample.
10.
Modern optical luminescent trinocularmicroscope
The device of an optical microscope:
A - eyepiece; B is the lens; C is the object;
D- is a condenser; E - subject table; F- is a
mirror
11.
The principle of themicroscope:
A beam of light rays is directed by a condenser lens
through the sample, and the resulting image is then
enlarged using lenses.
To measure structures in light microscopy,
micrometers are mainly used:
1 micron is 10-6 m;
In electron microscopy, nanometers are used:
1 nm is 10-9 m.
12.
Resolution- this is the minimum distance at which two adjacent
points are visible as separate.
The naked human eye has a resolution of about 1/10
mm, or 100 microns.
This means that two points that are at a distance of
less than 100 microns from each other merge into one.
The best light microscope has a resolution of about
0.2 microns, i.e. about 500 times improves the Human
eye.
13.
Modern light microscopeThe device of an optical microscope: A eyepiece; B is the lens; C is the object; D
is a condenser; E - subject table; F is a
mirror.
14.
Light microscopy methodsUltraviolet
Fluorescent
Dark field
Phase contrast
Interference
Polarizing
Confocal
15.
Ultraviolet microscopyUltraviolet rays are used in microscopy to increase the
resolution of an optical microscope.
An increase in resolution is achieved by the use of UV
rays which are invisible to the eye.
The average wavelength becomes equal to 0.2 μm, i.e.,
two times less than the wavelength of the visible part
of the spectrum.
16.
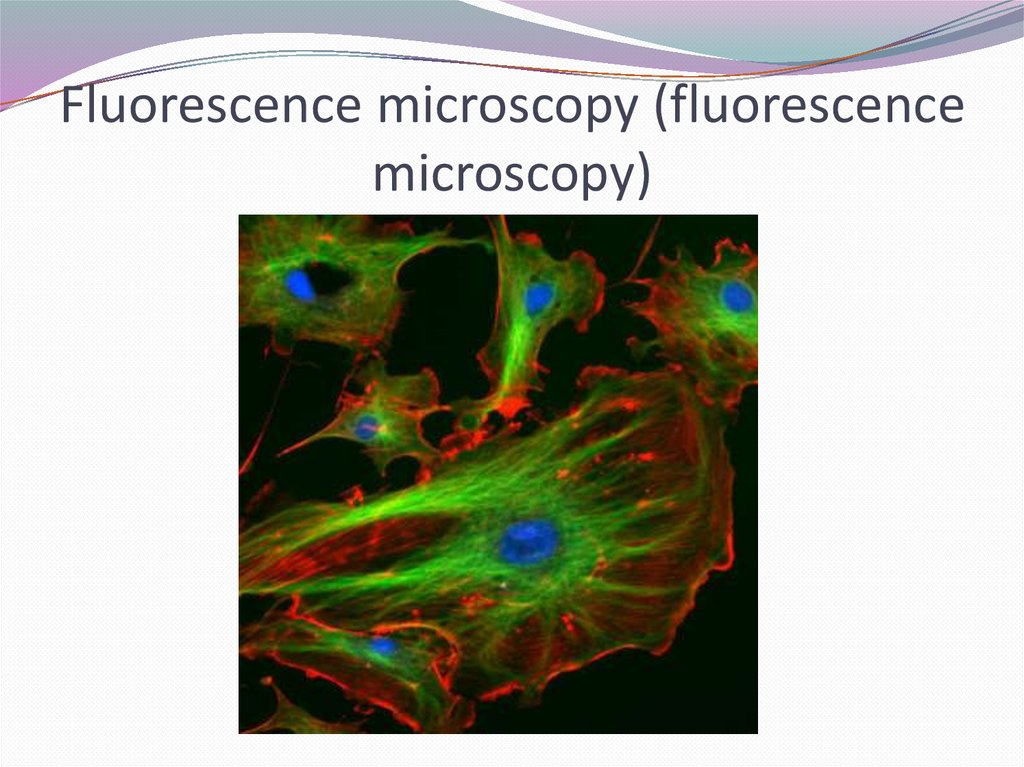
Fluorescence microscopy (fluorescencemicroscopy)
- a special type of microscopy based on the use of intrinsic
(primary) or induced (secondary) photoluminescence of
microscopic objects.
The visible luminescence of the drug is excited either by
blue-violet light or by ultraviolet rays.
Light sources are ultrahigh-pressure mercury-quartz
lamps.
17.
Fluorescence microscopy (fluorescencemicroscopy)
18.
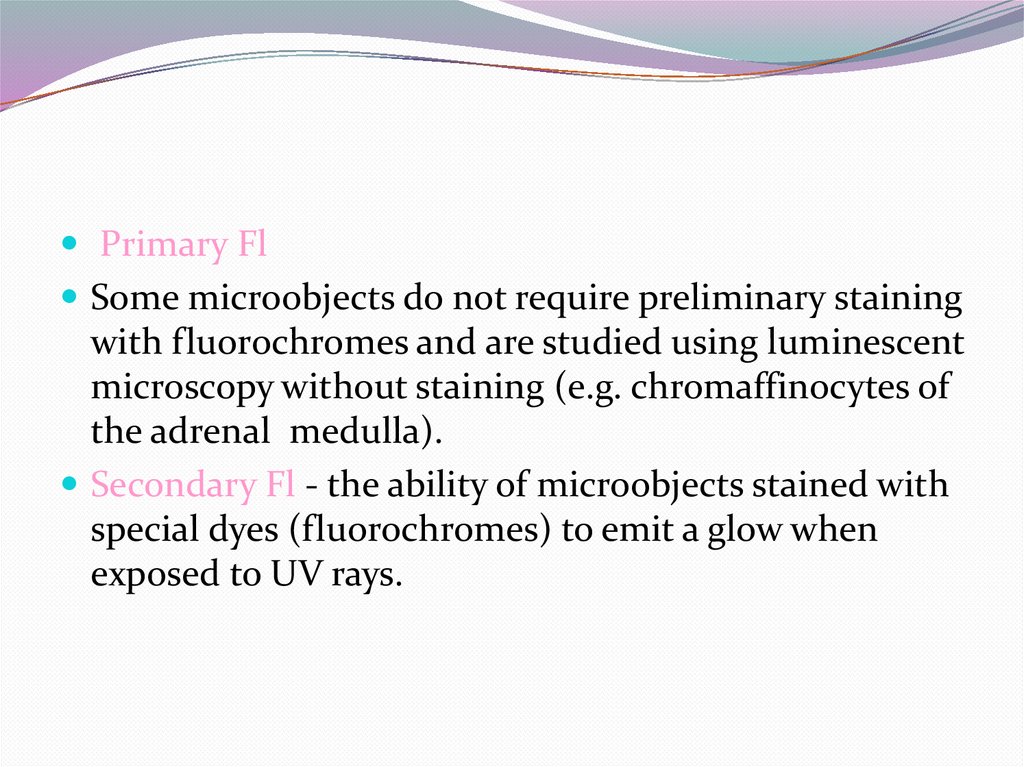
Primary FlSome microobjects do not require preliminary staining
with fluorochromes and are studied using luminescent
microscopy without staining (e.g. chromaffinocytes of
the adrenal medulla).
Secondary Fl - the ability of microobjects stained with
special dyes (fluorochromes) to emit a glow when
exposed to UV rays.
19.
Fluorochromesas a rule, fluoresce differently depending on the
chemical composition of the structures with which
they interact.
Fluorochromes: fluorescein, acridine orange,
coryphosphine.
When stained with acridine orange, DNA gives a redgreen glow, and RNA gives an orange.
20.
Dark - field microscopeFor dark-field microscopy, ordinary lenses and special
dark-field capacitors are used.
The object is illuminated by oblique side rays and only
rays scattered by particles in the preparation enter the
microscope objective.
Dark-field microscopy is based on the Tyndall effect, a
well-known example of which is the detection of dust
particles in air when illuminated by a narrow ray of
sunlight.
21.
Dark field microscopy in incident lightThe sample is illuminated from the side
(green line).
The image is created by light scattering on
the inhomogeneities of the sample.
22.
Dark Field MicroscopyGives a luminous image
of an object on a dark
background Used to
observe living unpainted
objects.
23.
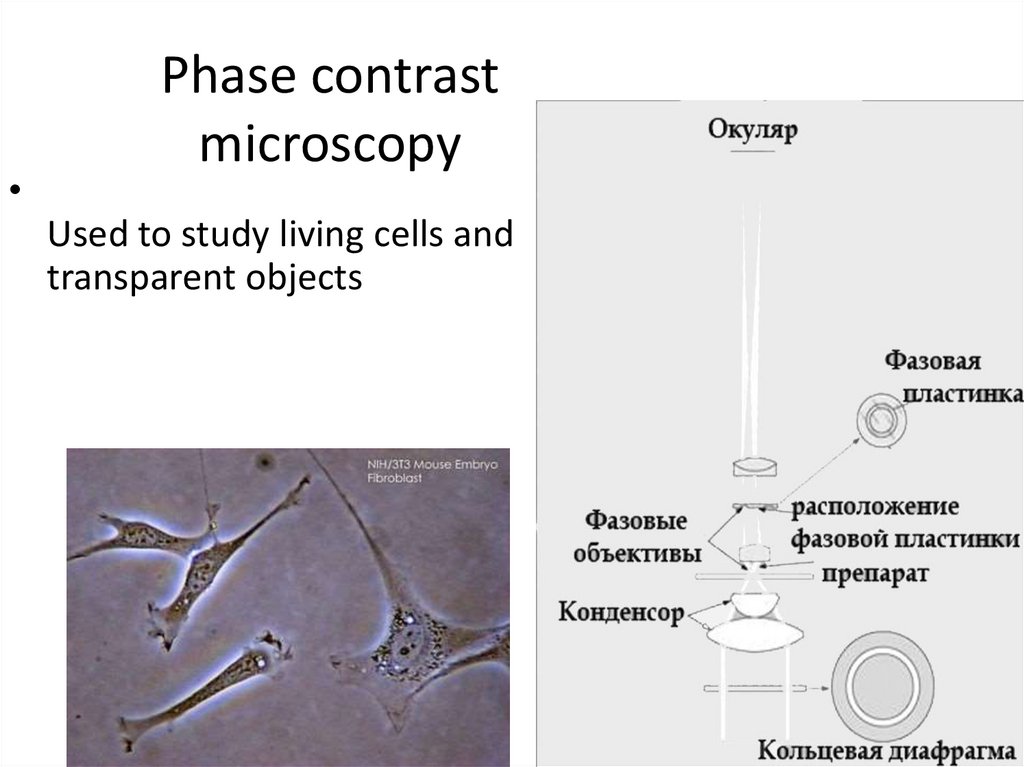
Phase contrast microscopy— a method for studying cells in a light
microscope equipped with a phase-contrast
device, which consists of an annular diaphragm
in the condenser and a phase plate in the
lens.
Due to the phase shift of the light wave, the
contrast of the structures of the studied object
increases, which is associated with a different
refractive index.
24.
Phase contrast
microscopy
Used to study living cells and
transparent objects
25.
Interference microscopy• In an interference microscope, light beams incident
on an object bifurcate: one beam passes through
the object, the other goes past it.
• In the ocular part of the microscope, both beams
are again connected and interfere with each other.
• By the phase shift of one beam relative to another,
one can judge the concentrations of various
substances in the object.
26.
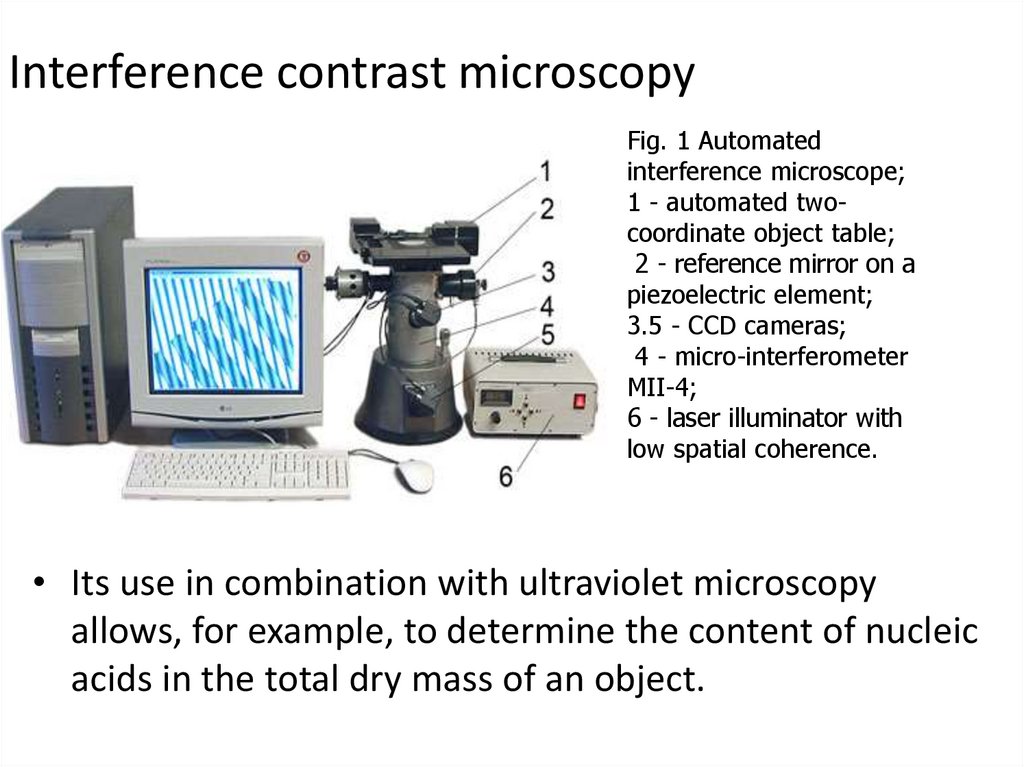
Interference contrast microscopyFig. 1 Automated
interference microscope;
1 - automated twocoordinate object table;
2 - reference mirror on a
piezoelectric element;
3.5 - CCD cameras;
4 - micro-interferometer
MII-4;
6 - laser illuminator with
low spatial coherence.
• Its use in combination with ultraviolet microscopy
allows, for example, to determine the content of nucleic
acids in the total dry mass of an object.
27.
POLARIZING MICROSCOPY• The principle of the method is based on the study of an
object in the light formed by two rays polarized in mutually
perpendicular planes.
• Passing through structures with a strict orientation of
molecules, the rays are delayed relative to each other due
to their unequal refraction.
• The resulting phase shift is an indicator of birefringence of
cell structures.
28.
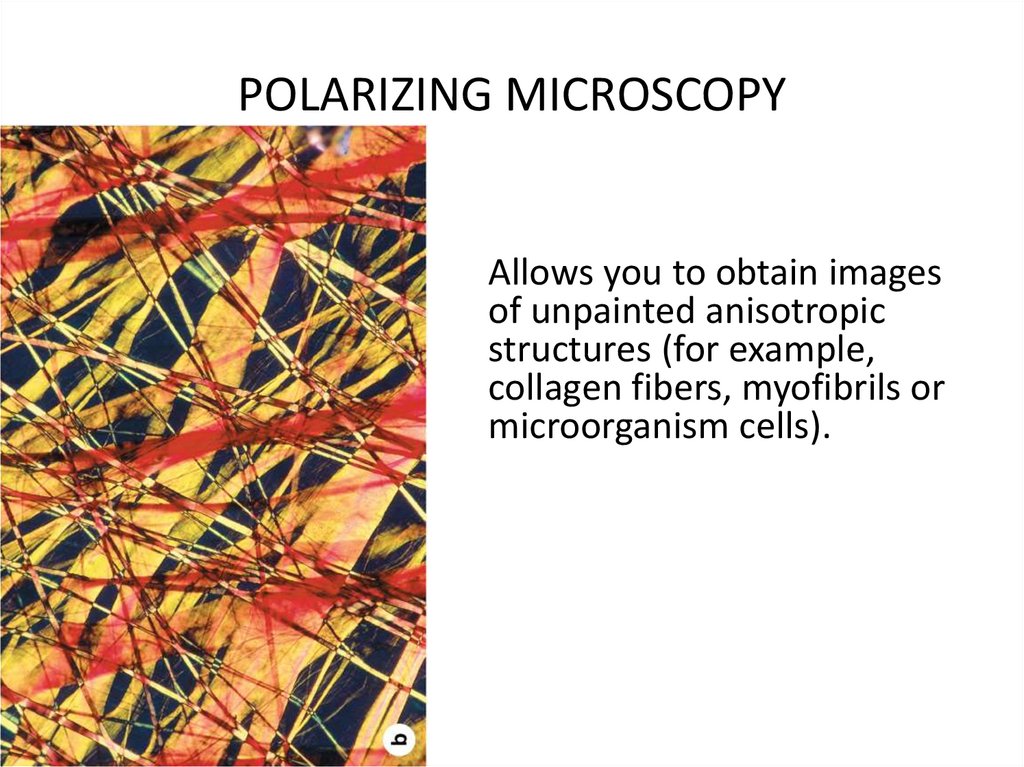
POLARIZING MICROSCOPYAllows you to obtain images
of unpainted anisotropic
structures (for example,
collagen fibers, myofibrils or
microorganism cells).
29.
Confocal microscopy- a method using a laser beam as a illuminator,
which sequentially scans the entire thickness
of the preparation.
• Information about the density of the object
for each scan line is transmitted to the
computer, where special program provides
three-dimensional reconstruction of the
investigated object.
30.
CONFOCAL SCANNING LASER MICROSCOPE31.
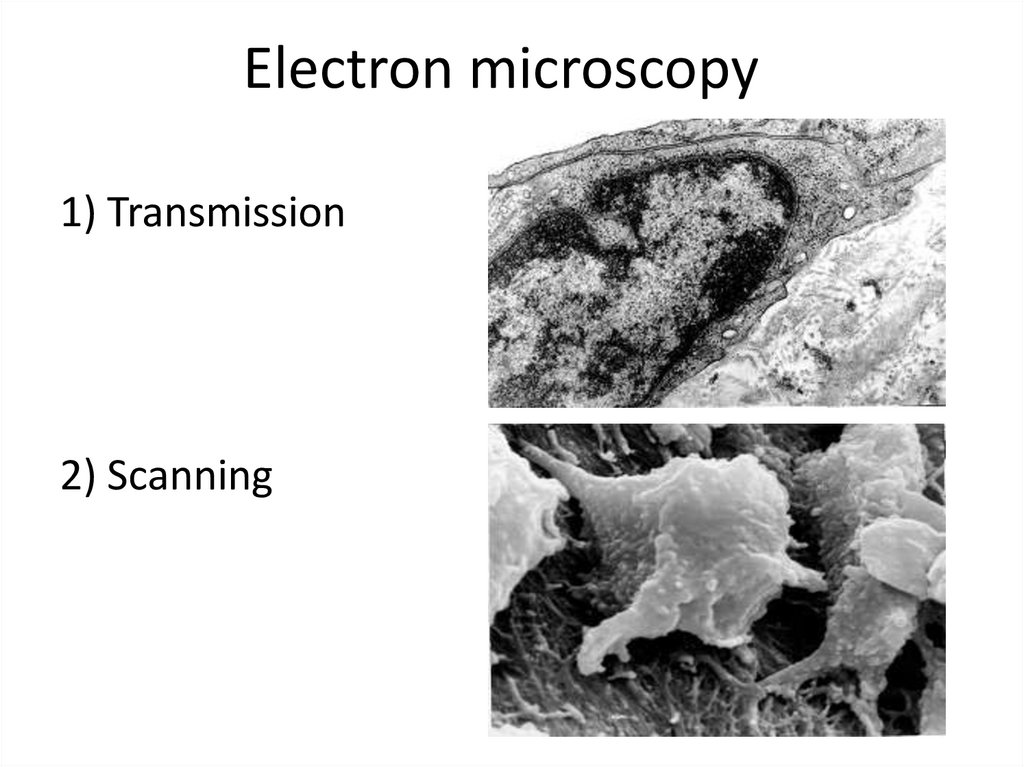
Electron microscopy• Electron microscopes (EM) use an electron
beam whose electromagnetic wavelength is
100 000 times shorter than the wavelength of
visible light.
• The resolving power of EMs is hundreds and
thousands of times greater than a light
microscope and is 0.5-10 nm. Modern EMs
increase the object up to 1 000 000 times.
32.
Electron microscopy1) Transmission
2) Scanning
33.
Transmission Electron Microscopy(TEM)
34.
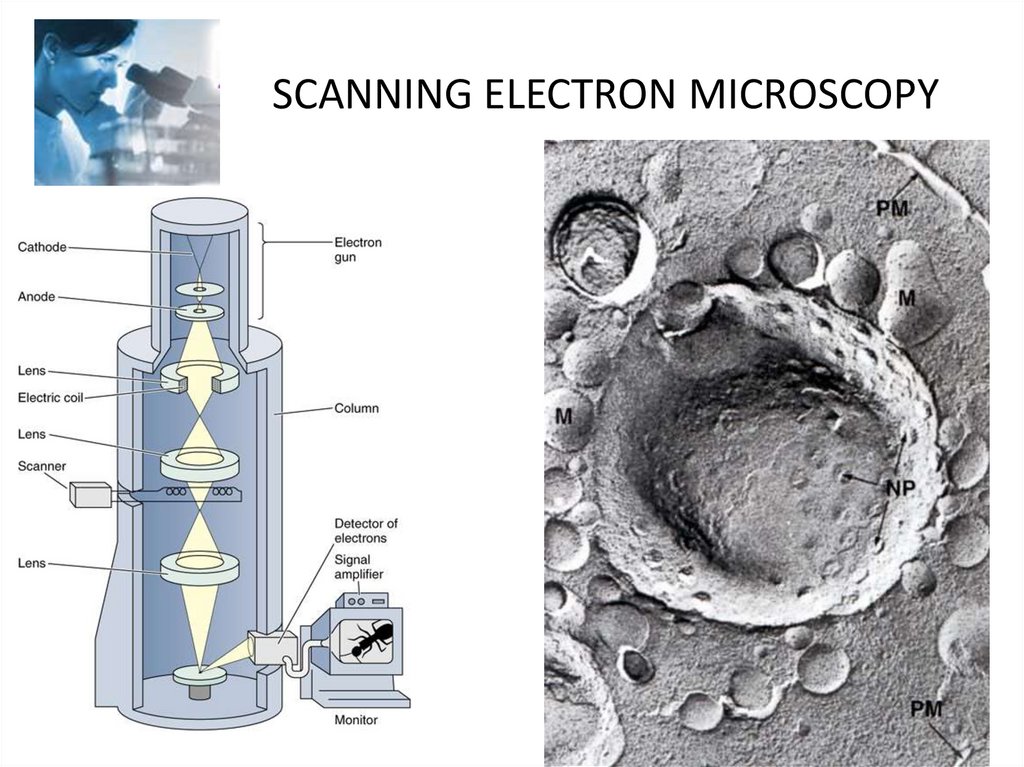
SCANNING ELECTRON MICROSCOPY35.
FREEZING METHOD• In a special device,
cells are frozen and
cleaved along the
line of cell
membranes At the
same time, it
became possible to
study the structure
of plasmolemma
and karyolemma
36.
RADIOAUTOGRAPHY METHOD• - allows you to study the distribution in cells and tissues of
substances into which radioactive isotopes are artificially
introduced (3Н, 14С, 32Р, etc.). The isotope introduced into the
animal’s body is included in the corresponding structures (for
example, 3H- thymidine — in the nuclei of cells synthesizing
DNA).
• The method is based on the ability of isotopes incorporated
into cells to restore Ag bromide photoemulsions, which cover
sections of tissue or cells. Ag grains (tracks) formed after the
manifestation of a photoemulsion serve as a kind of
autograph, by the location of which they judge the inclusion
of the substances used in the cell.
37.
RADIOAUTOGRAPHY METHODThe method determines:
• the rate of incorporation of
labeled amino acids into
proteins, the formation of
nucleic acids, the exchange of
iodine in thyroid cells, etc
38.
Histochemical methodsThey are based on the use of chemical reactions to
detect the distribution of chemicals in the structures
of cells, tissues and organs. Modern histochemical
methods make it possible to detect amino acids,
proteins, nucleic acids, various types of
carbohydrates, lipids, etc.
• E.g. : PAS-reaction for glycogen
39.
Feulgen stainis a staining technique discovered by Robert Feulgen and used in
histology to identify chromosomal material or DNA in cell
specimens. It is darkly stained. It depends on acid hydrolysis
of DNA, therefore fixating agents using strong acids should be
avoided.
40.
Cytospectrophotometry• - a method for studying the chemical
composition of a cell, based on the selective
absorption by certain substances of rays with
a certain wavelength.
• The intensity of light absorption, which
depends on the concentration of the
substance, is a quantitative determination of
its content in the cell.
41.
IMMUNOHISTOCHEMISTRY
Immunocytochemical reactions are used to
identify specific proteins.
• For this, specific serums containing antibodies
are obtained (for example, against microtubule
protein - tubulin).
• Further, these antibodies are chemically
combined with a fluorochrome (or other
marker).
42.
• If labeled antibodies are applied to ahistological section, they come into conjunction
with the corresponding proteins of the cell and
a specific glow appears, visible in a luminescent
microscope.
43.
IMMUNOHISTOCHEMISTRYThe method is based on the
visualization of the antigenantibody reaction.
This method can detect cells of
certain types (B and T lymphocytes) hormone
receptors, glycocalyx structures
and cytoskeletal proteins in
cells.
44.
Light microscope45.
The main stages of the preparation ofhistological preparations for light microscopy:
1. taking material (specimen);
2. chem. fixation (formalin);
3. washing in water;
4. 1-st dehydration (ethyl alcohol from 70% to
100%);
• 5. cliaring (xylene);
• 6. infiltration (xylene / paraffin);
• 7. embedding ( in paraffin);
46.
Taking material (specimen)47.
Chem. fixation (formalin)48.
Dehydration49.
• 8. sectioning (with microtome) - slice 2-10microns;
• 9. installation of slices on a glass slide;
• 10. removal of paraffin (with xylene);
• 11. rehydration (ethyl alcohol from 100% to 70%)
• 12. staning;
• 13. 2nd dehydration (ethyl alcohol from 70% to
100%);
• 14. The conclusion of the cuts in the resin of
conifers ("Siberian Balsam")
50.
Microtome51.
sectioning52.
StainingDyes
Basic
Hematoxylin
(Basophylic structures of cell)
Acid
EOSIN (Acidophylic
structures cell)
53.
STAINING: hematoxylin-eosin54.
DyesSpecial
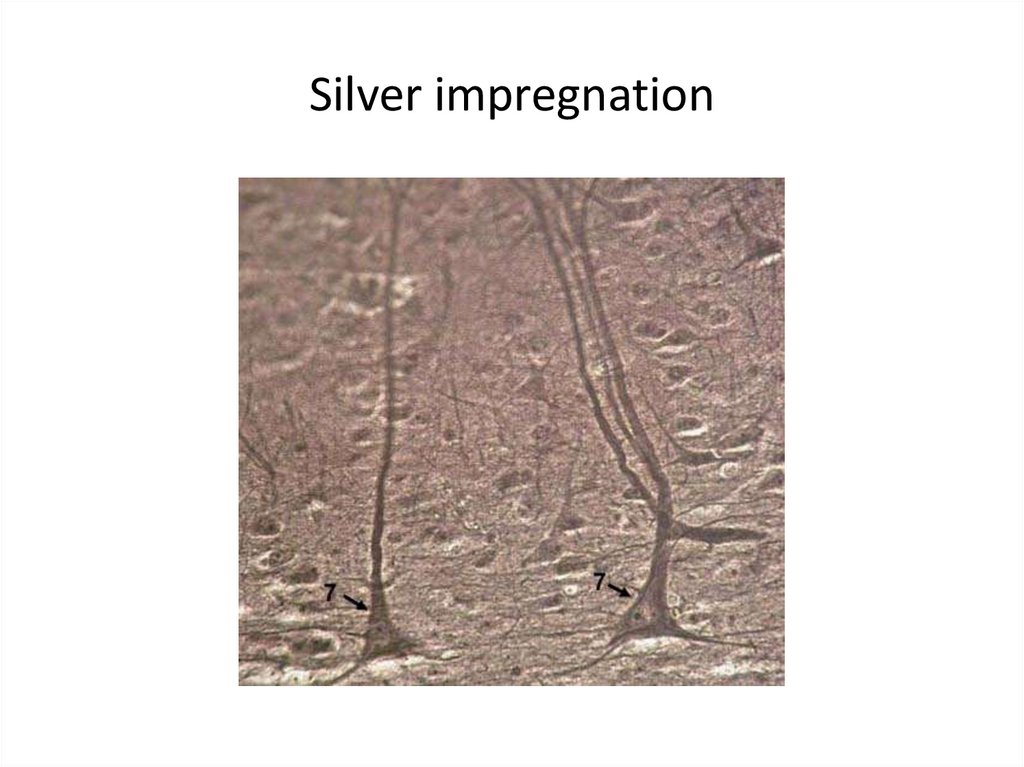
(iron hematoxylin,
salts of Silver,
orsein)
Histochemical
(Sudan III,
PAS-reaction)
55.
Silver impregnation56.
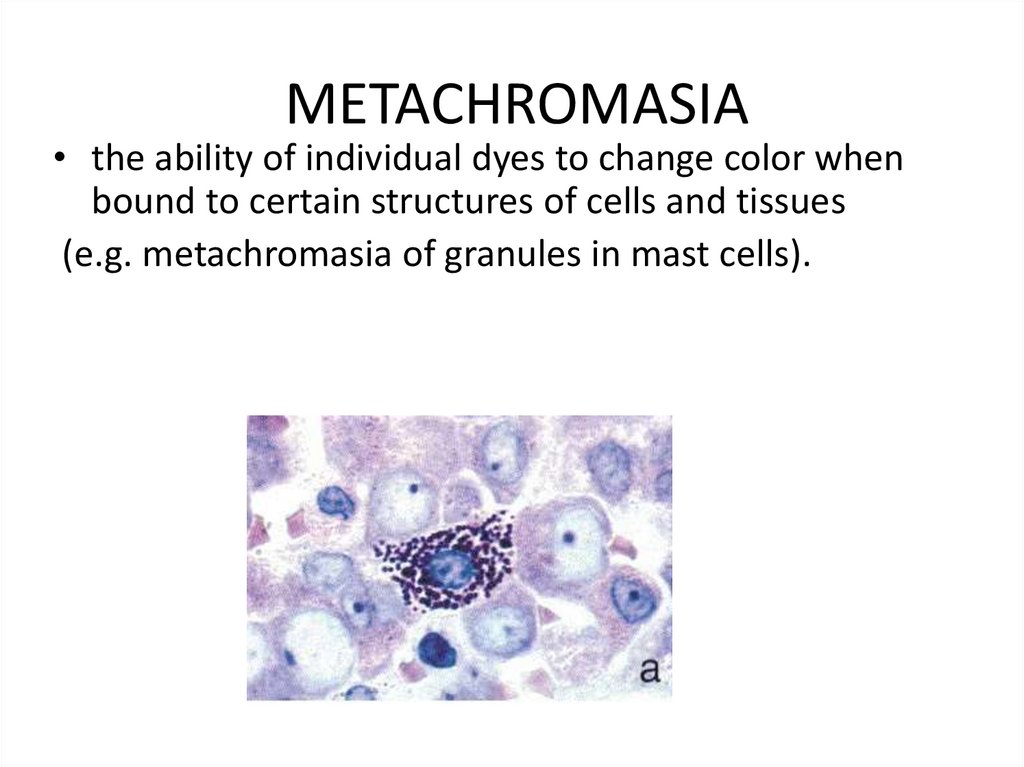
METACHROMASIA• the ability of individual dyes to change color when
bound to certain structures of cells and tissues
(e.g. metachromasia of granules in mast cells).
57.
Electron microscopy1) Transmission
2) Scanning
58.
Transmission Electron Microscope59.
The main stages of the preparation of histologicslides s for electron transmission microscopy:
• 1. taking material (specimen);
• 2. fixation (glutaraldehyde, Os2O4 -osmium
acid);
• 3. washing in phosphate buffer;
• 4. dehydration (ethyl alcohol from 25% to
100%)
• 5. clearing (propylene oxide);
• 6. infiltration (propylene oxide / plastic);
60.
• 7. embedding (plastic);• 8. preparation of slices (ultratom)
- semi-thin sections (0.5-1 microns)
ultrathin sections (30-50 nm)
• 9. installation of slices on a cooper grid
• 10. staining (contrasting) of sections with
salts of heavy metals.
61.
We wish you successful studyingof Histology course!



















































 Биология
Биология