Похожие презентации:
Bohr Model of the Atom Line Spectra
1.
Bohr Model of the AtomLine Spectra
NIS Aktobe
06-02-2022
MCA
2.
Learning ObjectivesMCA
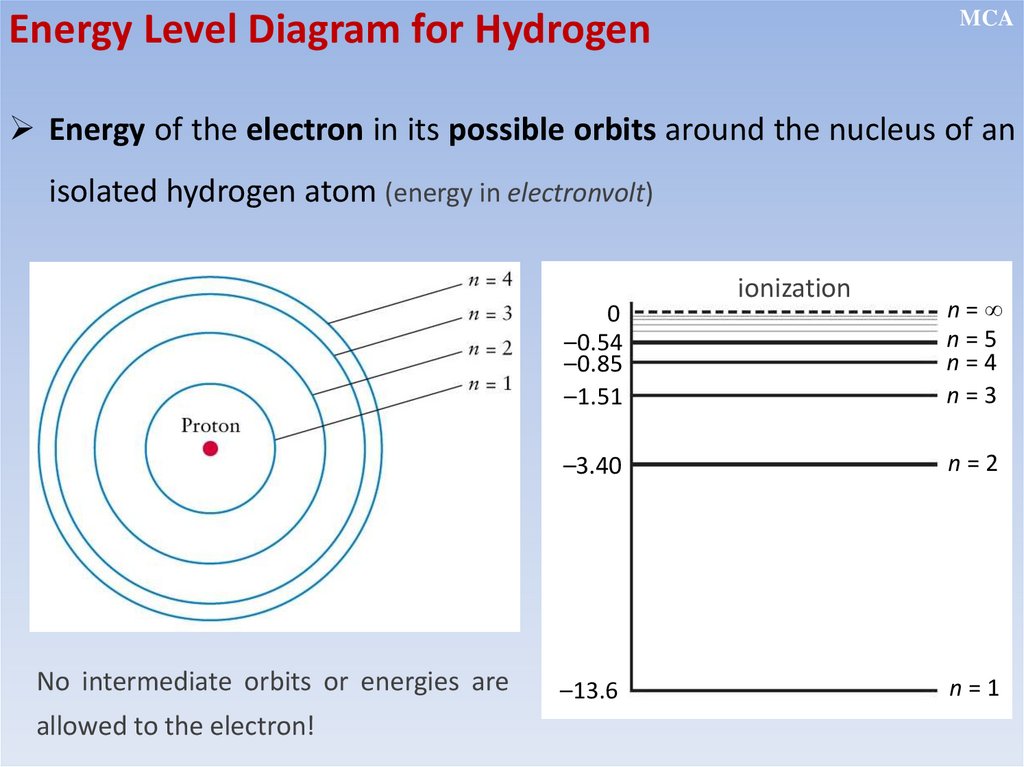
12.2.2.11 - show an understanding of the existence of discrete electron
energy levels in isolated atoms (e.g. atomic hydrogen) and deduce how
this leads to spectral lines through energy levels;
12.2.2.12 - distinguish between emission and absorption line spectra;
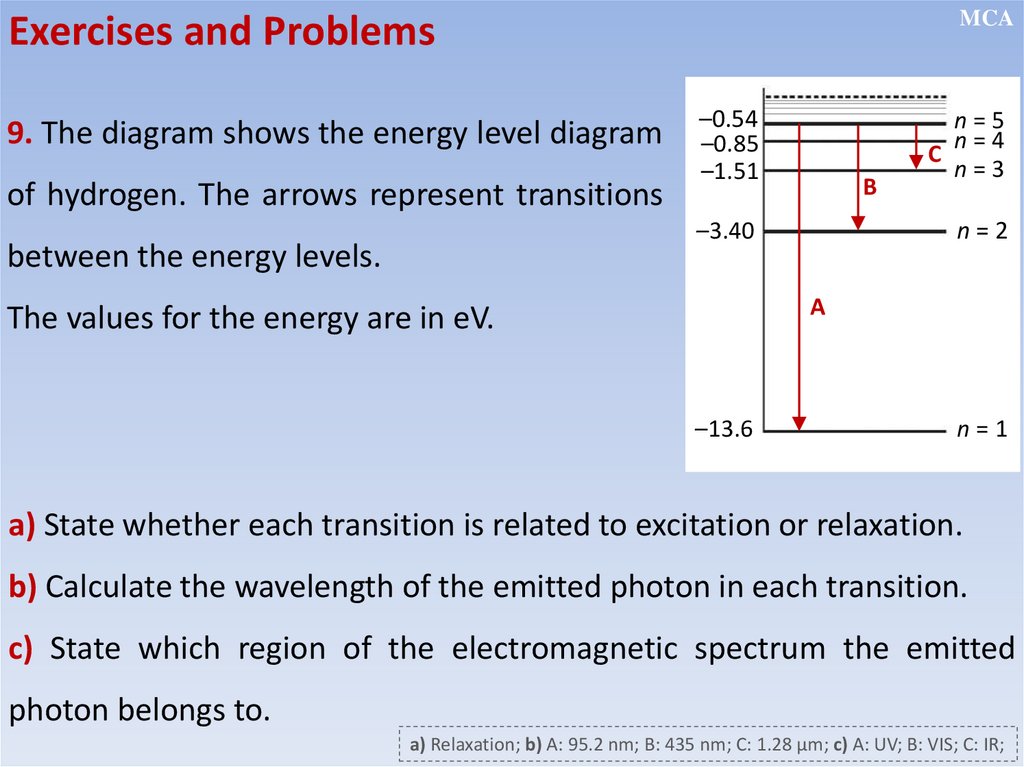
12.2.2.13 - recall and solve problems using the relation hf = E1 - E2;
12.2.2.14 - understand and explain the stability conditions of existence
of the atom using Bohr's postulates;
3.
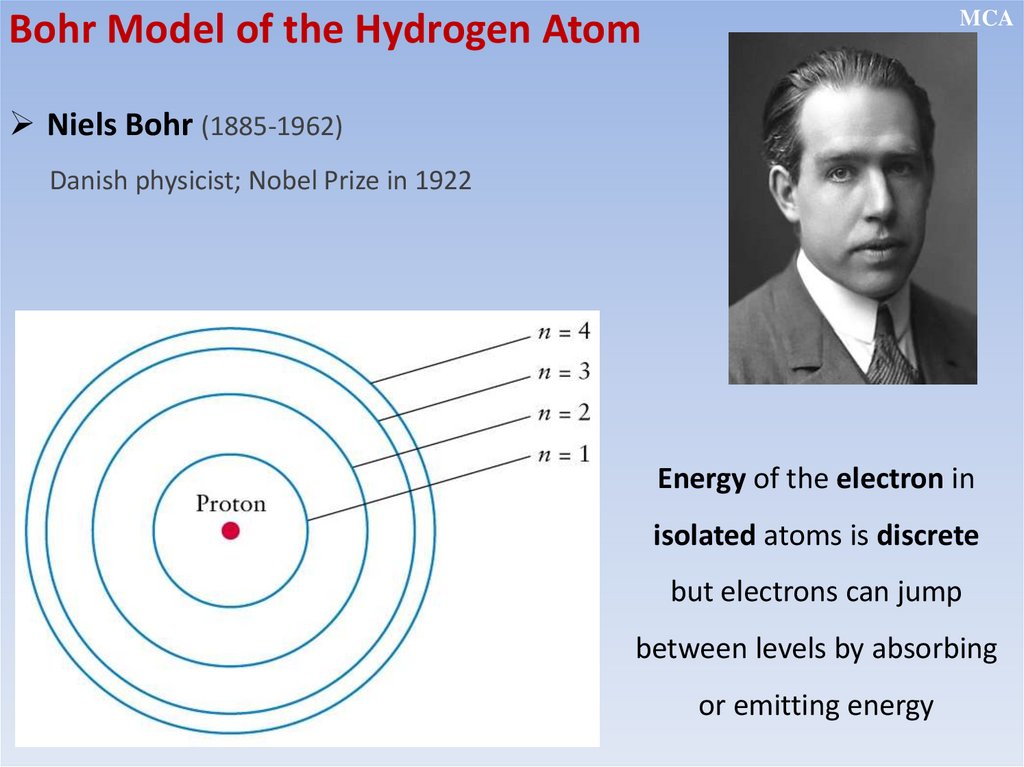
MCABohr Model of the Hydrogen Atom
Niels Bohr (1885-1962)
Danish physicist; Nobel Prize in 1922
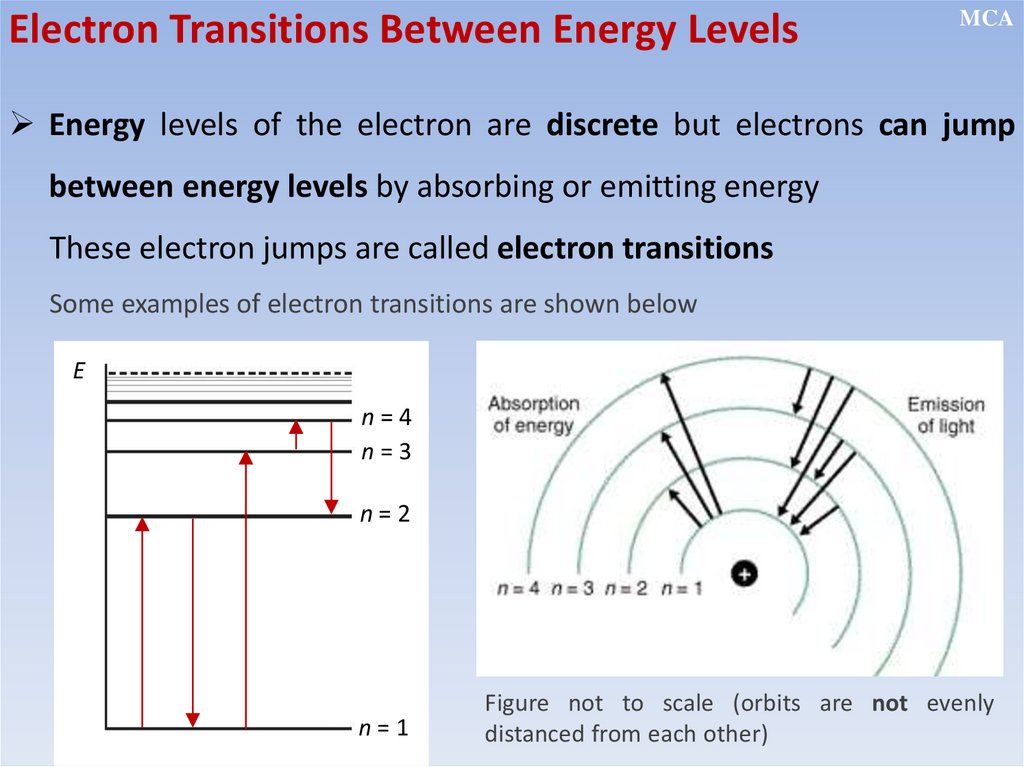
Energy of the electron in
isolated atoms is discrete
but electrons can jump
between levels by absorbing
or emitting energy
4.
Bohr´s PostulatesMCA
Bohr Postulates
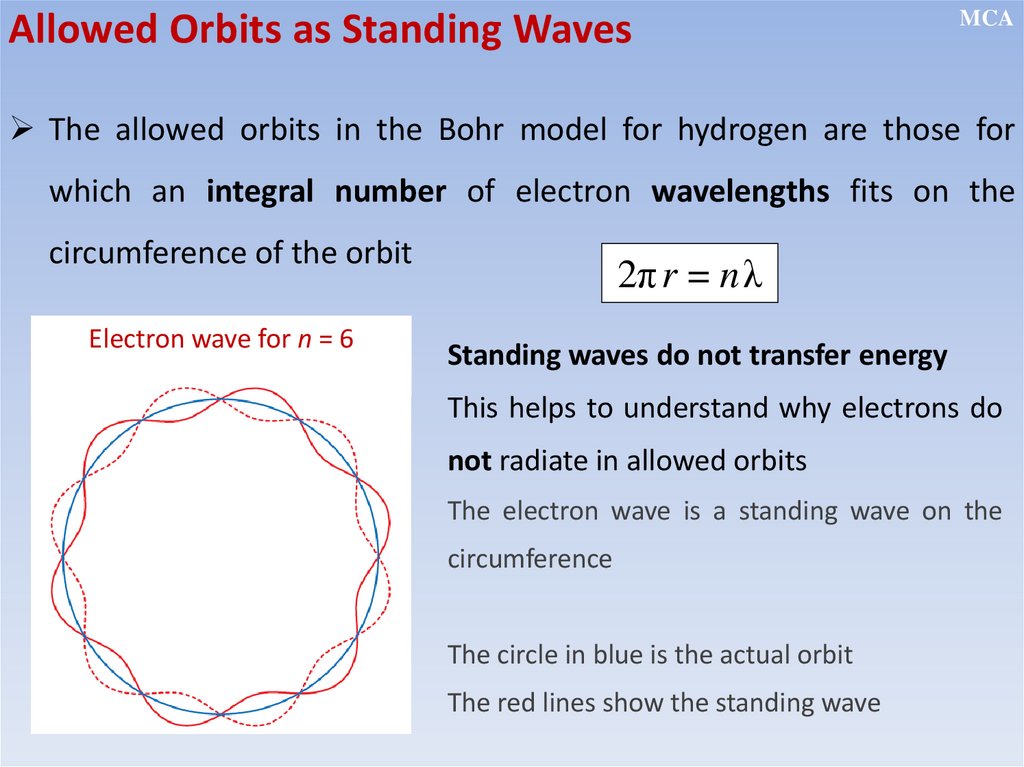
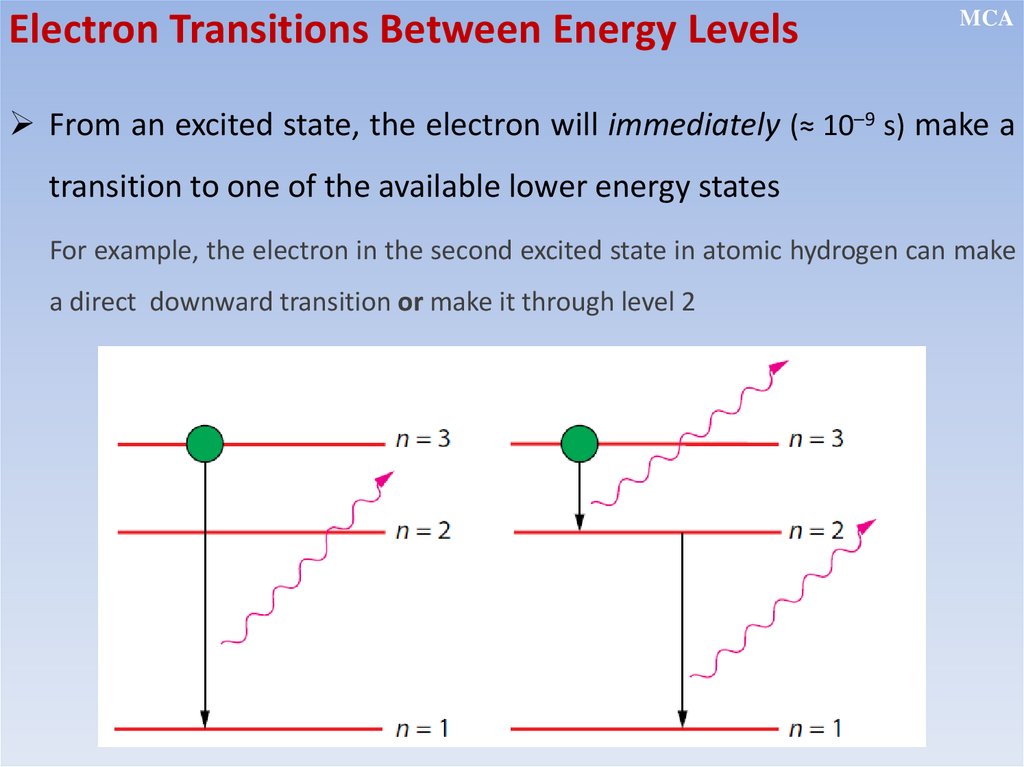
• Electrons revolve around the nucleus in certain fixed orbits of definite
energy without emission of any radiant energy (stationary orbits)
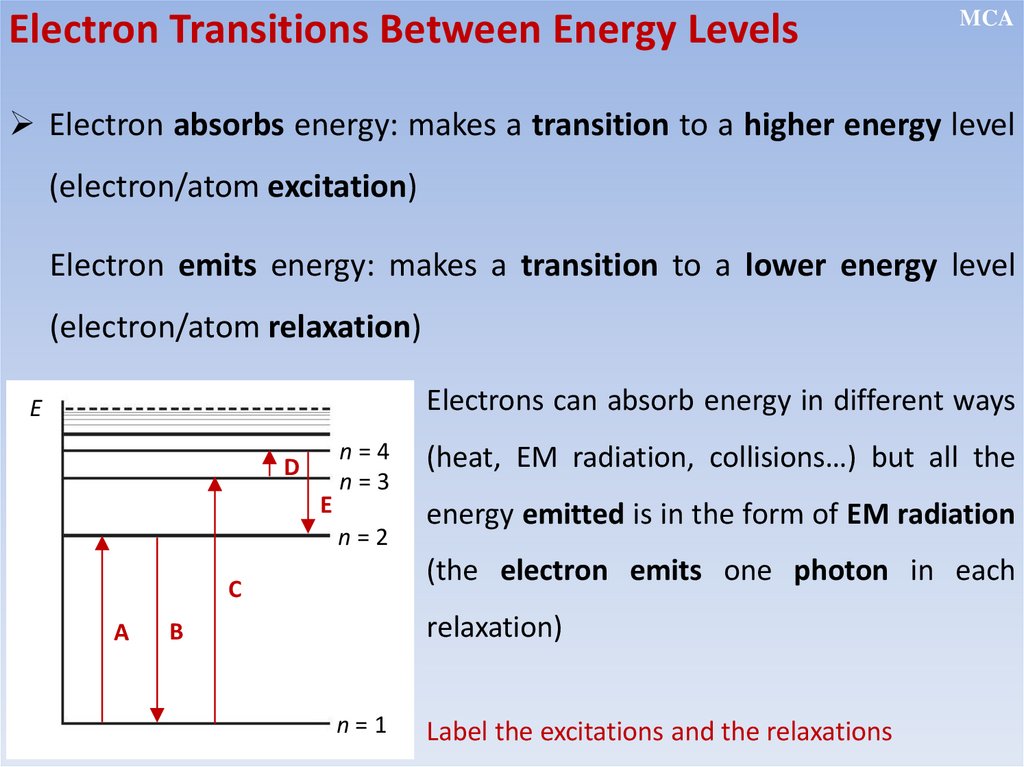
• Electrons can make a transition from a stationary state of higher
energy to a state of lower energy by emitting a single photon, and
make a transition from a lower to a higher state by absorbing energy
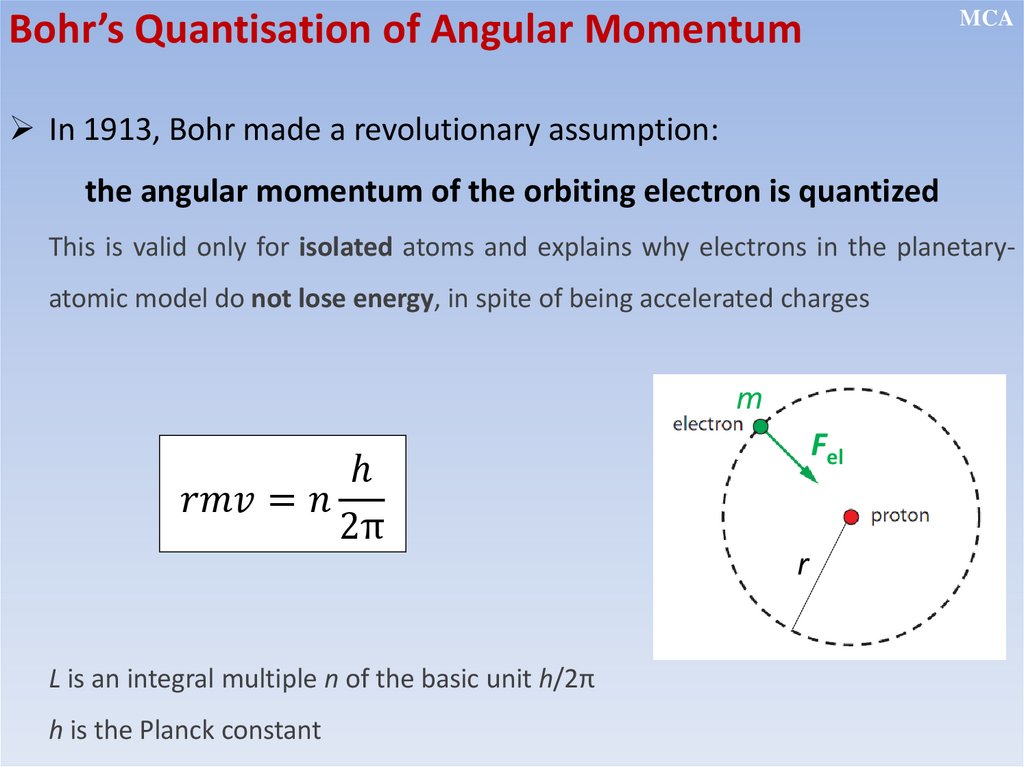
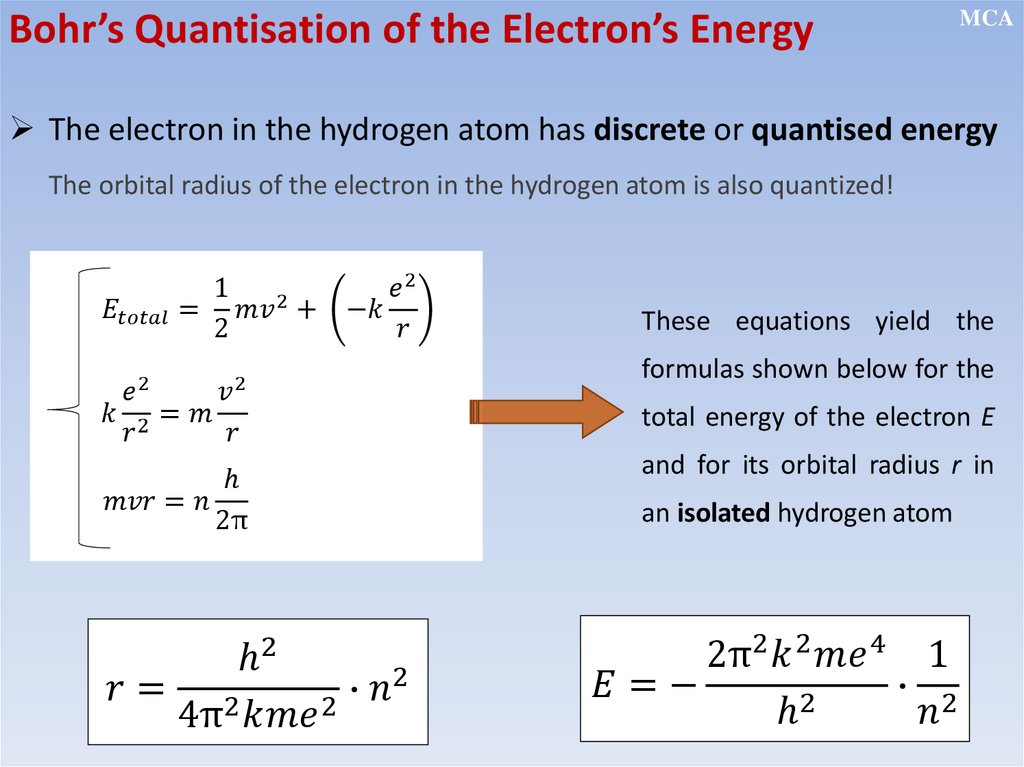
• The electron’s angular momentum in an atom is quantized (this leads
to quantization of energy)
5.
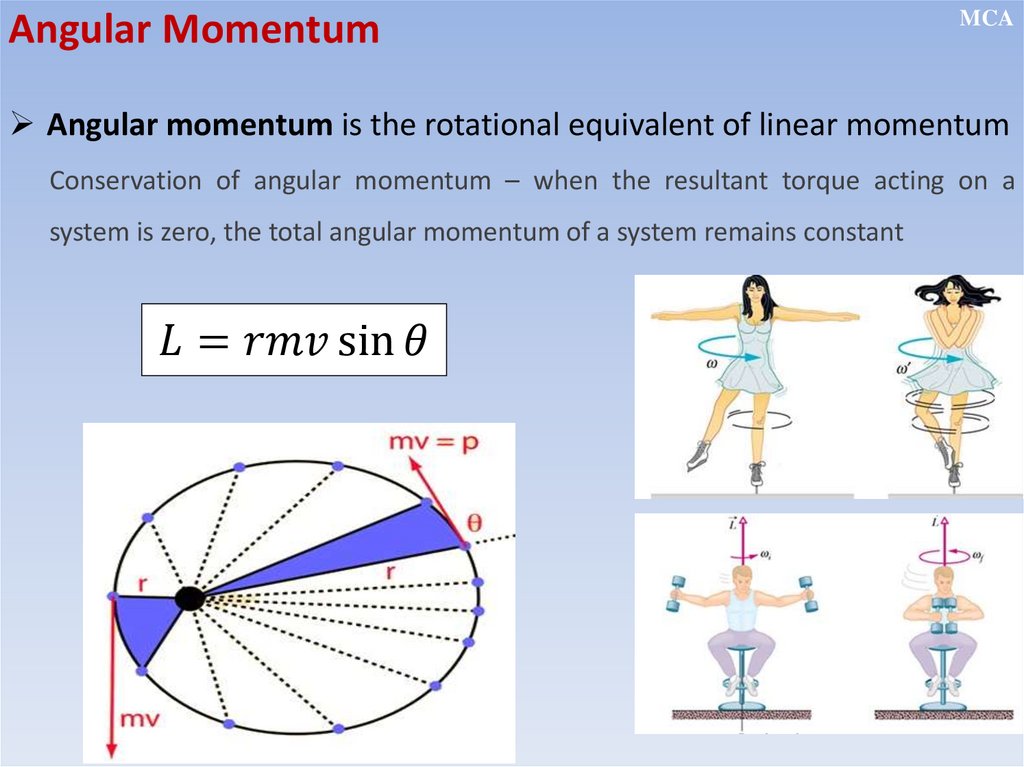
Angular MomentumMCA
Angular momentum is the rotational equivalent of linear momentum
Conservation of angular momentum – when the resultant torque acting on a
system is zero, the total angular momentum of a system remains constant




















 Физика
Физика








