Похожие презентации:
Potential Sweep Voltammetry (or Potential Scan Techniques)
1.
Potential Sweep Voltammetry(or Potential Scan Techniques)
These techniques are based on scanning
the potential through the allowed
potential window in whole or in part.
1
2.
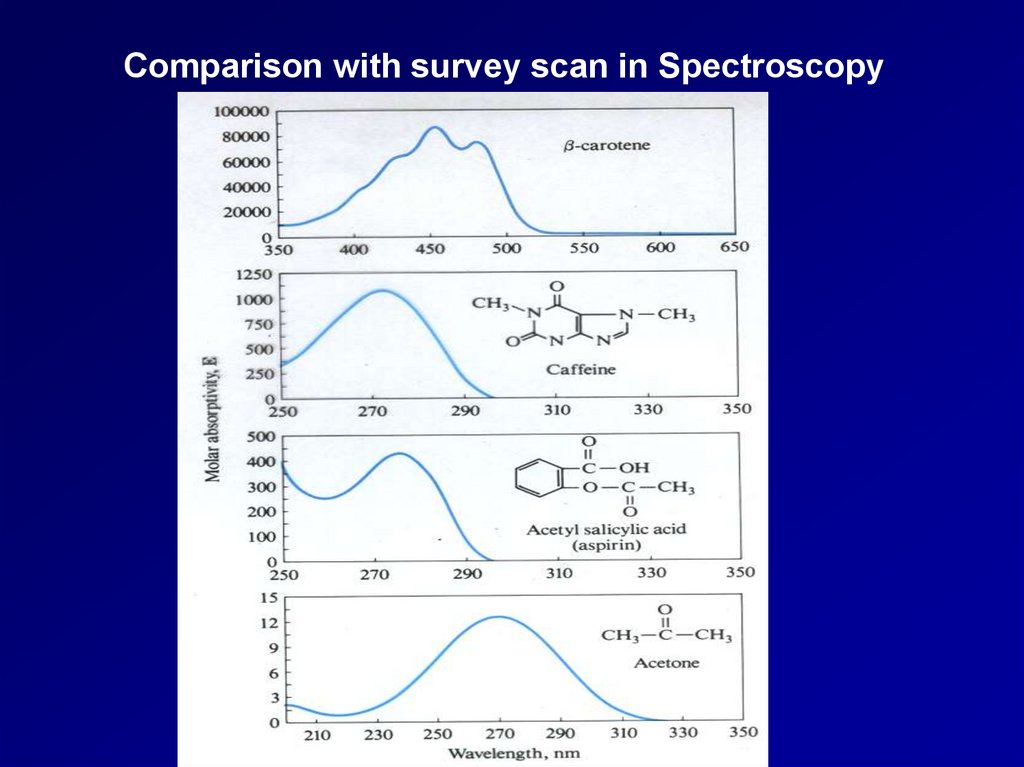
Comparison with survey scan in Spectroscopy3.
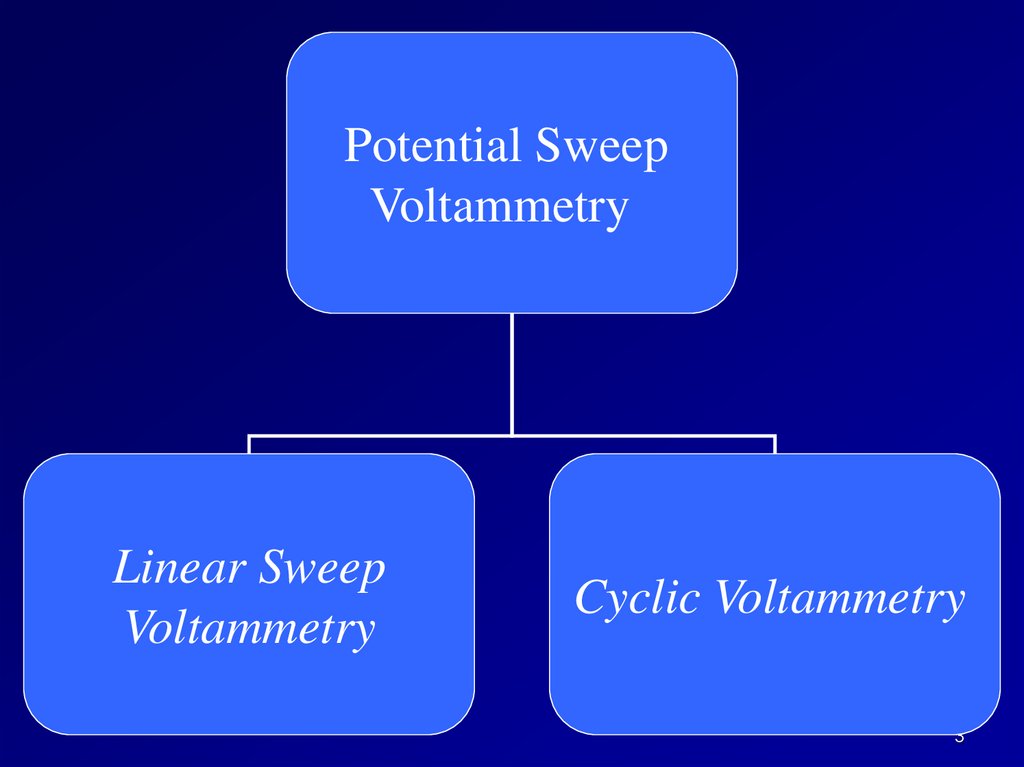
Potential SweepVoltammetry
Linear Sweep
Voltammetry
Cyclic Voltammetry
3
4.
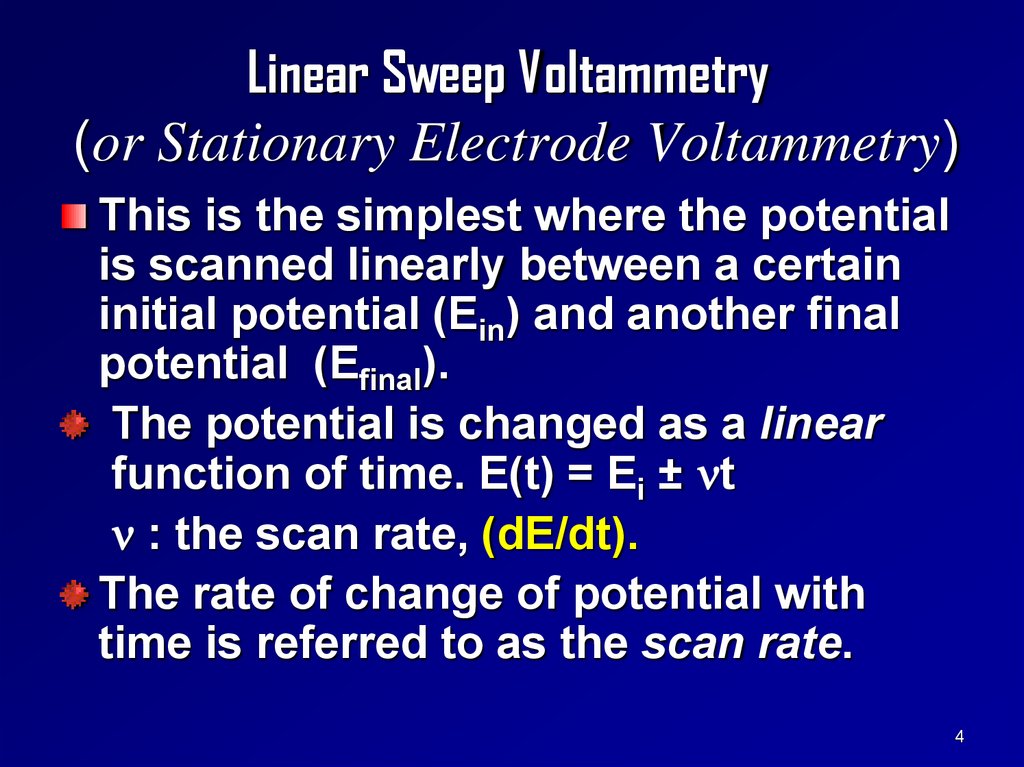
Linear Sweep Voltammetry(or Stationary Electrode Voltammetry)
This is the simplest where the potential
is scanned linearly between a certain
initial potential (Ein) and another final
potential (Efinal).
The potential is changed as a linear
function of time. E(t) = Ei ± t
: the scan rate, (dE/dt).
The rate of change of potential with
time is referred to as the scan rate.
4
5.
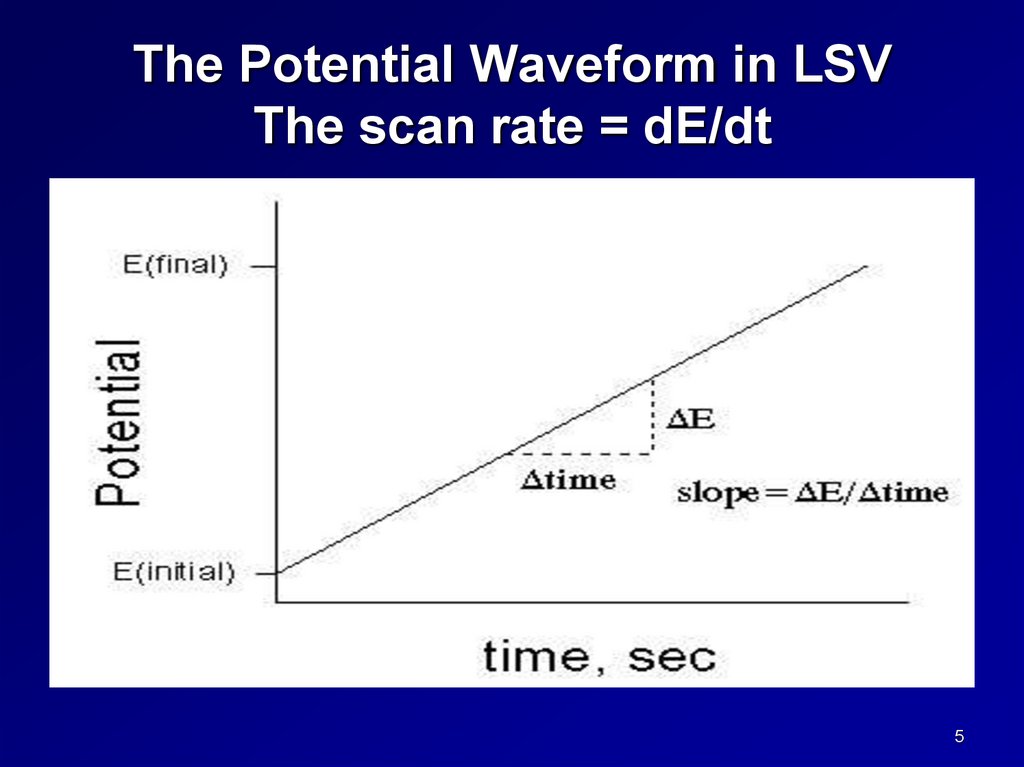
The Potential Waveform in LSVThe scan rate = dE/dt
5
6.
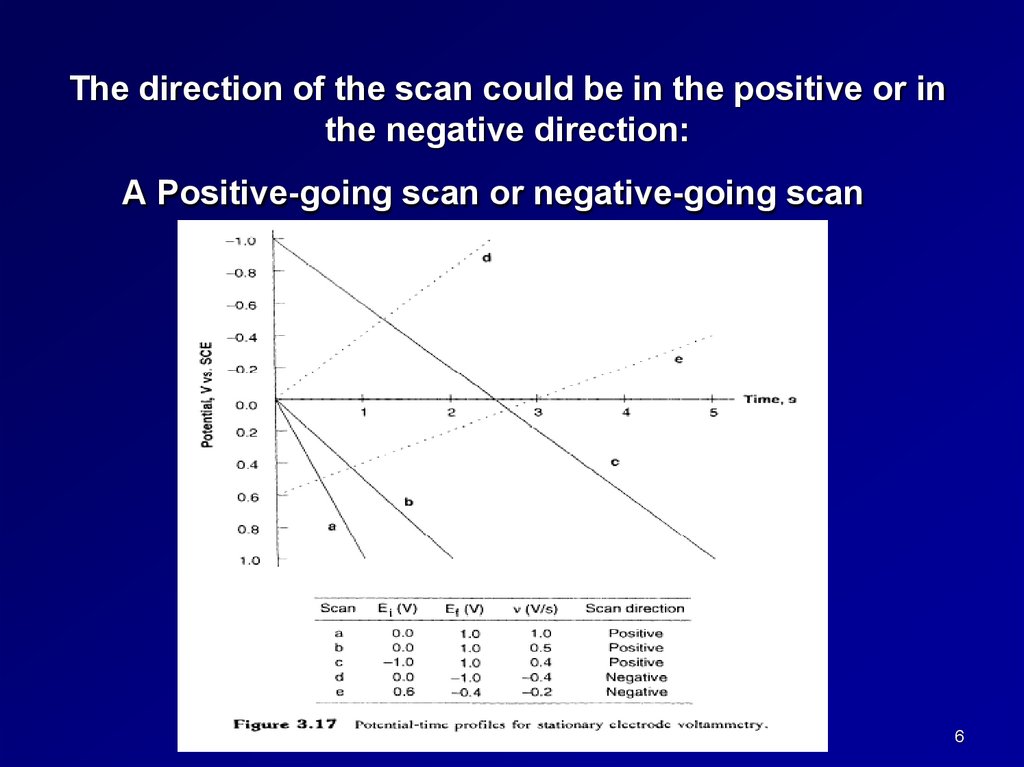
The direction of the scan could be in the positive or inthe negative direction:
A Positive-going scan or negative-going scan
6
7.
The Linear Sweep VoltammetryExperiment
- Quiescent (stagnant) solution.
- A supporting electrolyte is added.
- A stationary electrode.
- Many materials can be used as electrodes
depending on the purpose of the experiment.
- Metals and noble metals, hanging drop
mercury electrode (HDME) semiconductors,
conducting polymers, carbon electrodes,
modified metallic electrodes, …….etc.
7
8.
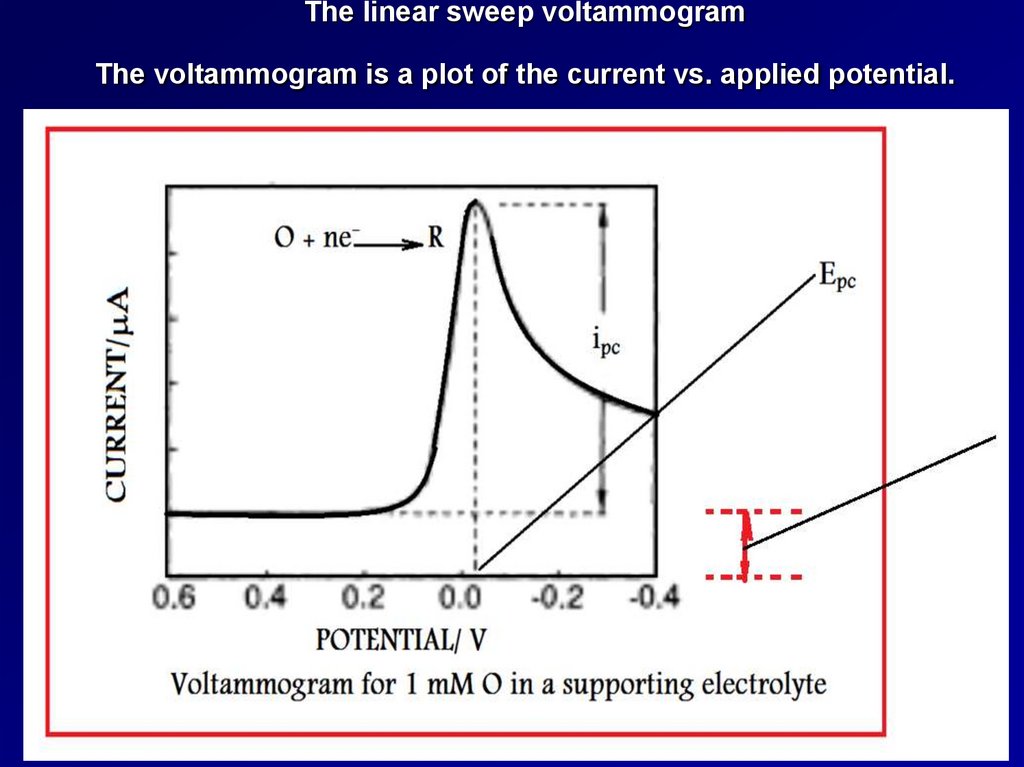
The linear sweep voltammogramThe voltammogram is a plot of the current vs. applied potential.
8
9.
Useful parameters from the LSV1. The peak potential, Ep:
It is related to the chemical species.
2. Peak current (ip) :
ip is proportional to the concentration.
3. For reversible systems, Ep, is
independent of the concentration.
4. ip is proportional to scan rate.
9
10.
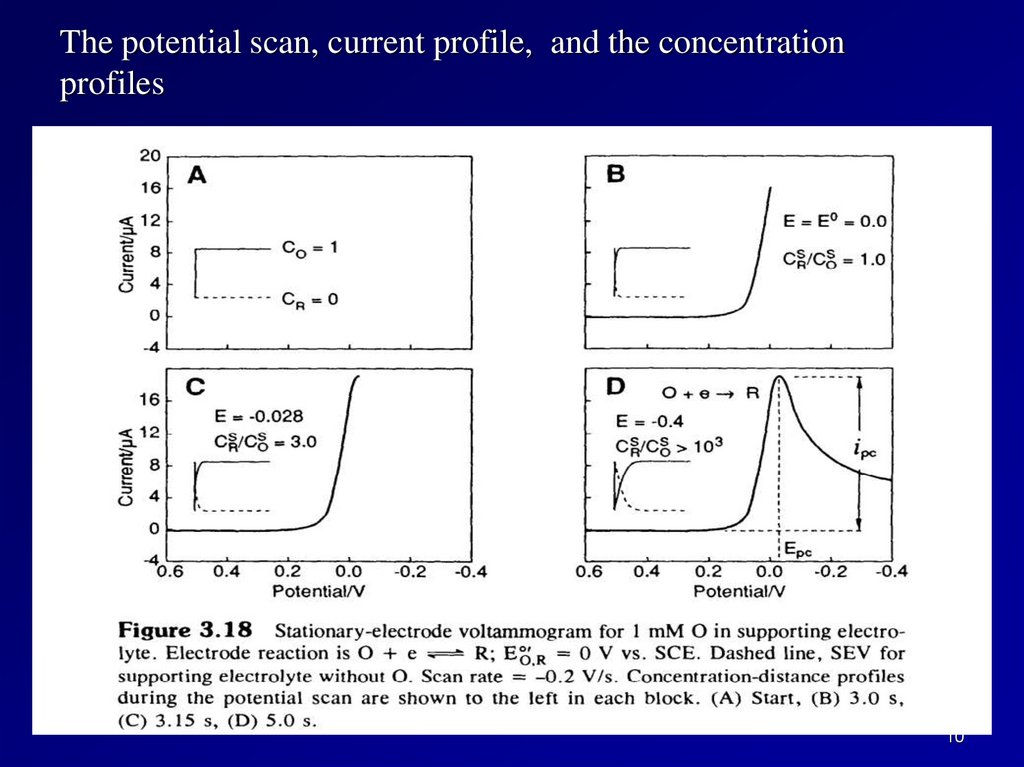
The potential scan, current profile, and the concentrationprofiles
10
11.
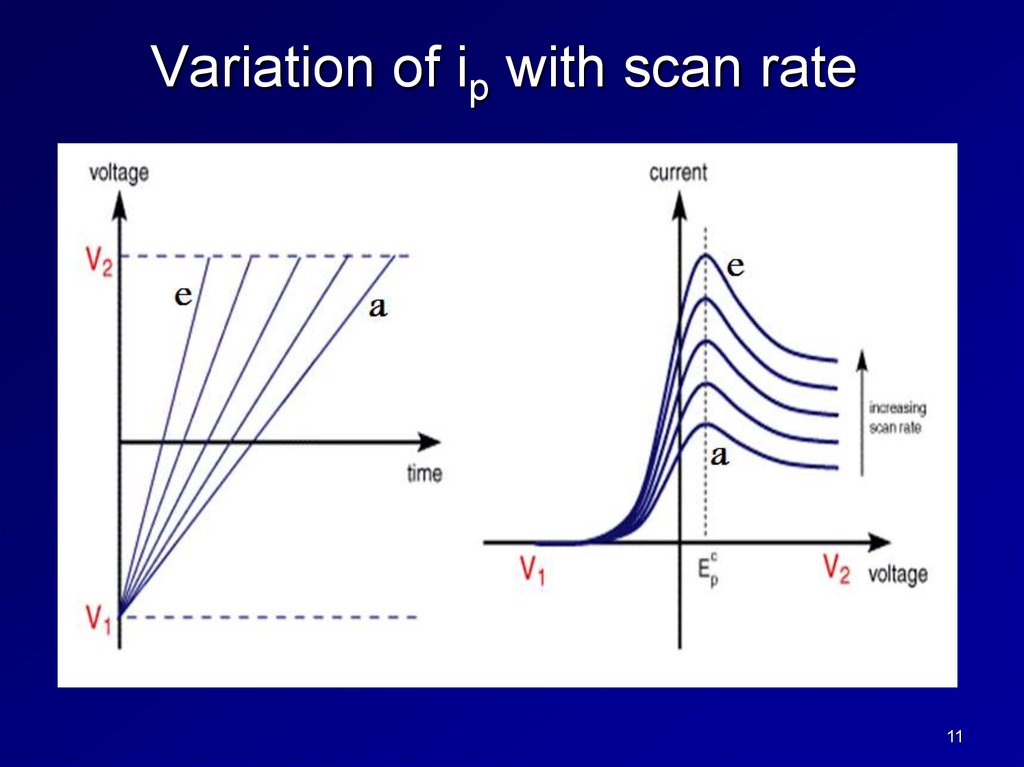
Variation of ip with scan rate11
12.
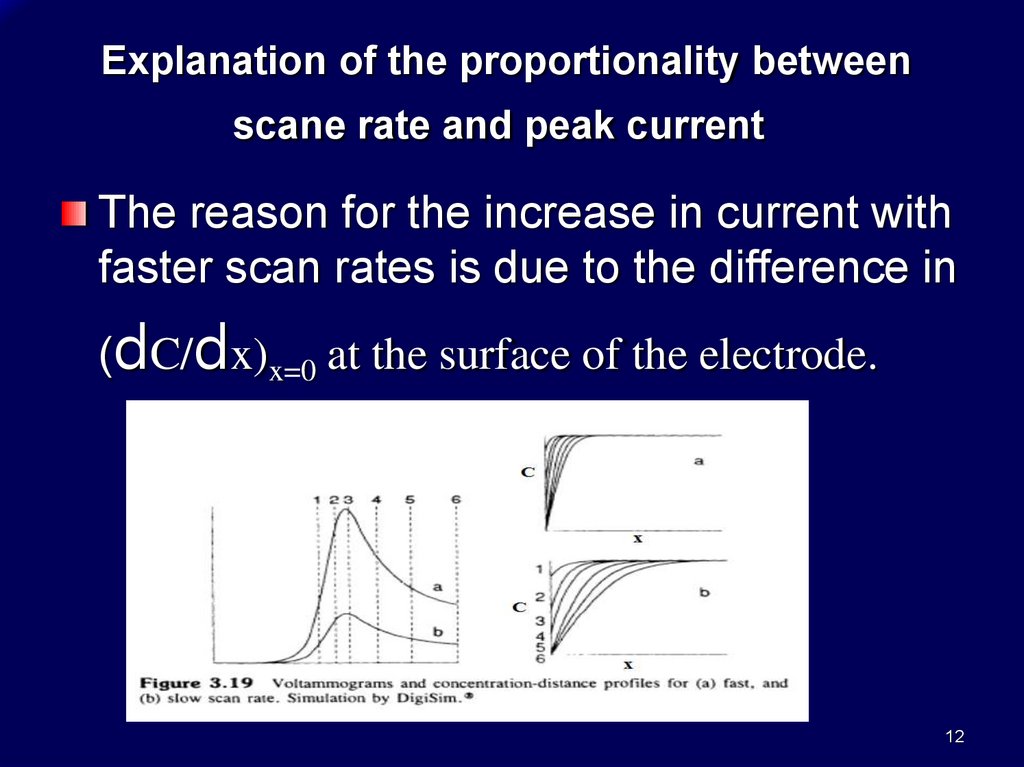
Explanation of the proportionality betweenscane rate and peak current
The reason for the increase in current with
faster scan rates is due to the difference in
(dC/dx)x=0 at the surface of the electrode.
12
13.
LSV Voltammograms for reversible systemsFor a reversible system, the peak current is given by RandelsSevcik equation
ip = (2.69x105)n3/2AD1/2Co* 1/2
where
ip = peak current (in A)
n : no. of electrons transferred per mole of
the electrolyzed species
A : electrode surface area (in cm2).
D : diffusion coefficient of the oxidized or the reduced
species.
: scan rate (V/s)
The peak potential , with the assumption that Do = DR at 25 C,
for a reduction is given by
Ep = E ’O/R – (0.029/n)
E ’O/R is the formal electrode potential.
Note that
ip 1/2
Ep is independent of the scan rate
13
14.
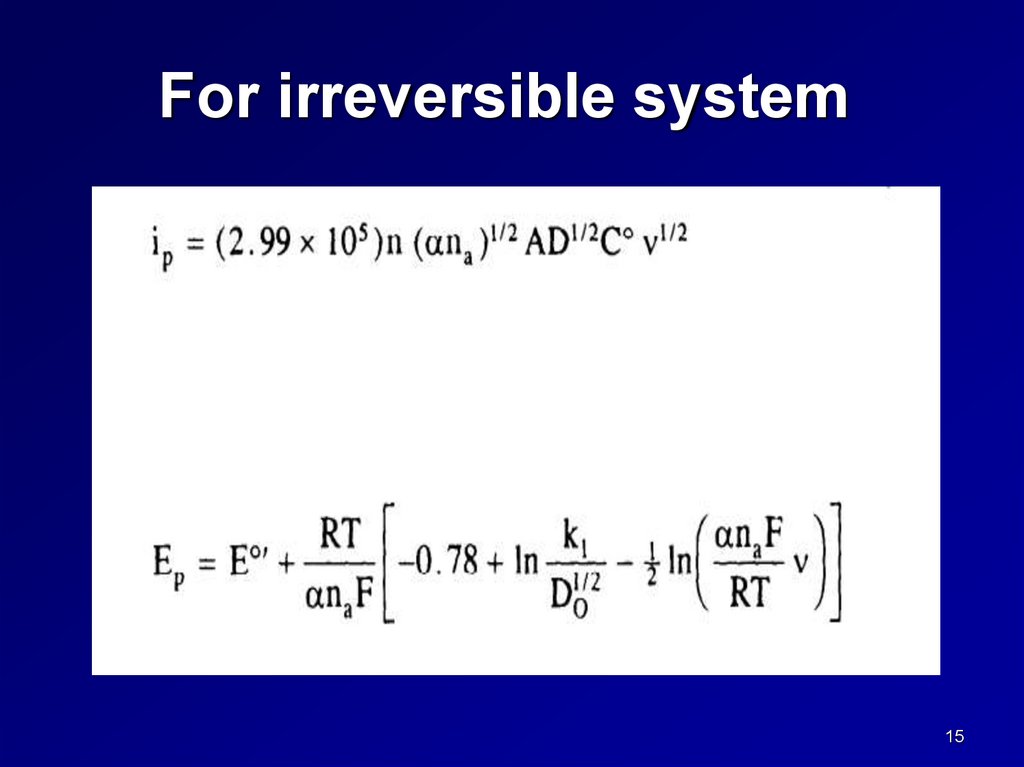
LSV voltammograms for irreversible systemsFor irreversible systems , the equation for
ip is given by
ip = (2.99x105) n ( na)1/2AD1/2Co* 1/2
where na is the number of electrons involved in the
rate determined step.
Note that
ip is still proportional to 1/2 and to the bulk
concentration.
The peak potential however is dependent on scan
rate and on the concentration.
14
15.
For irreversible system15
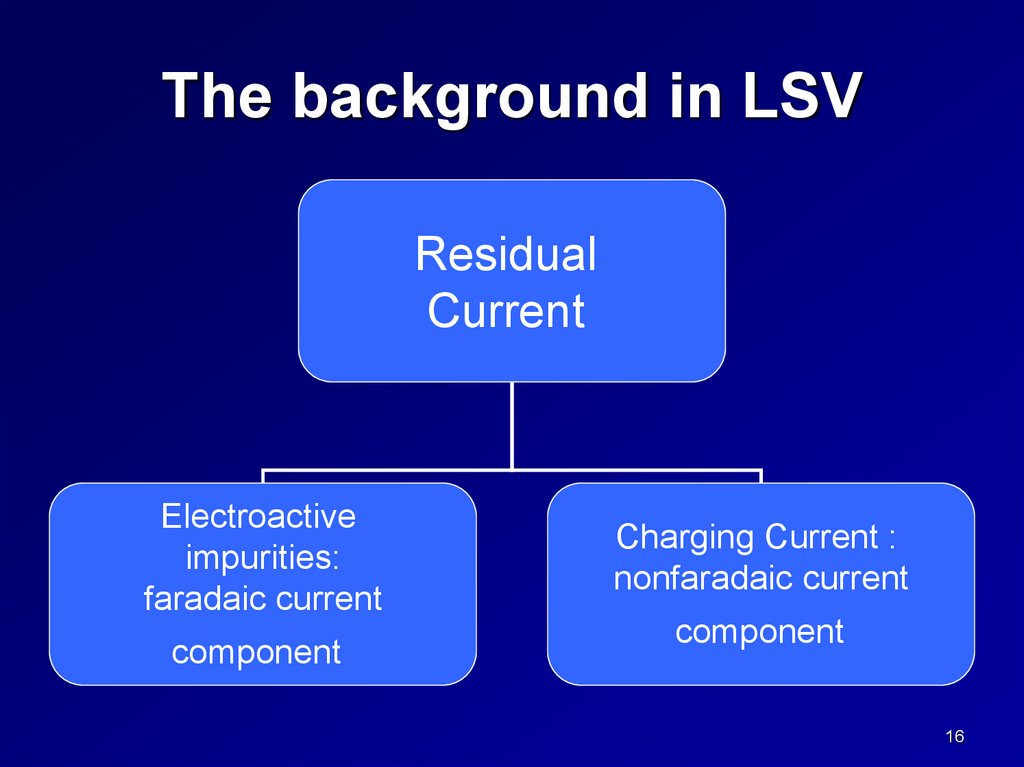
16.
The background in LSVResidual
Current
Electroactive
impurities:
faradaic current
component
Charging Current :
nonfaradaic current
component
16
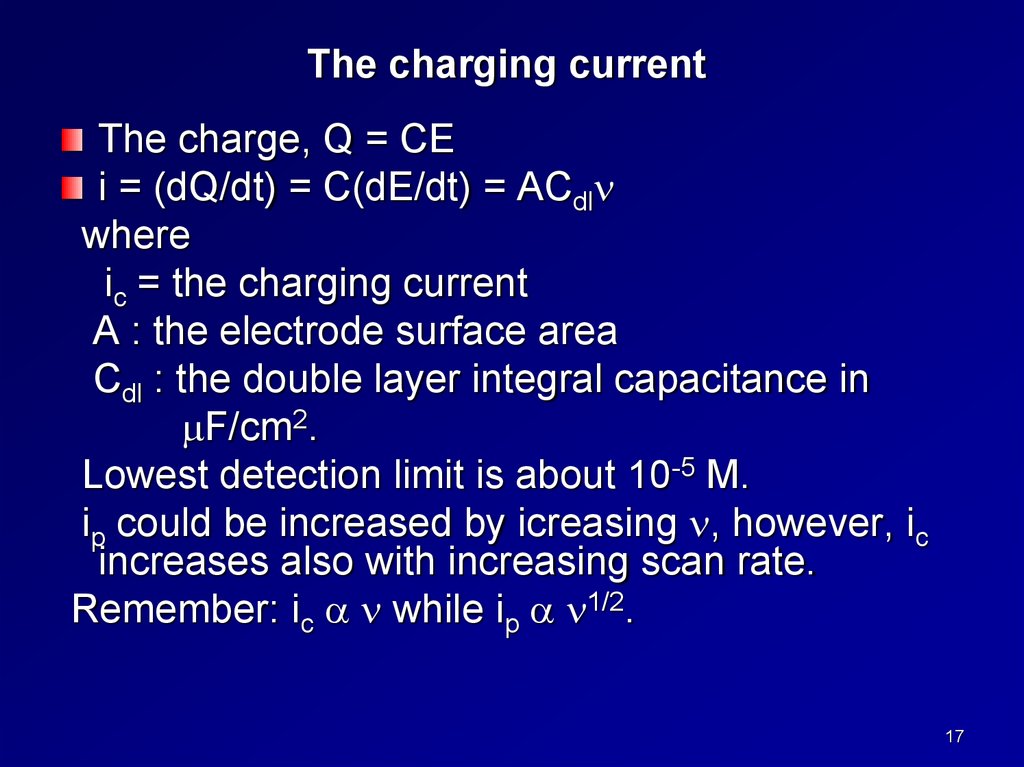
17.
The charging currentThe charge, Q = CE
i = (dQ/dt) = C(dE/dt) = ACdl
where
ic = the charging current
A : the electrode surface area
Cdl : the double layer integral capacitance in
F/cm2.
Lowest detection limit is about 10-5 M.
ip could be increased by icreasing , however, ic
increases also with increasing scan rate.
Remember: ic while ip 1/2.
17
18.
Cyclic voltammetryCyclic voltammetry is an extension of LSV.
Cyclic voltammetry is the potential sweep
technique analog of the double potential
step chronoamperometry. It combines a
forward potential scan plus a reversal at
certain potential.
18
19.
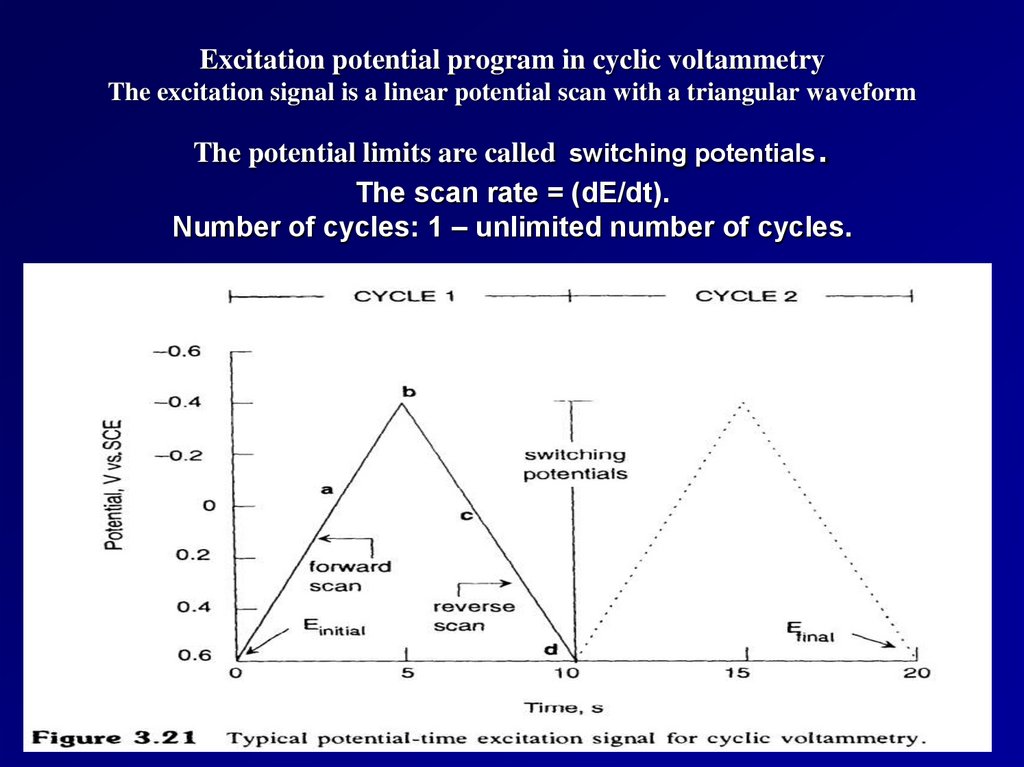
Excitation potential program in cyclic voltammetryThe excitation signal is a linear potential scan with a triangular waveform
.
The potential limits are called switching potentials
The scan rate = (dE/dt).
Number of cycles: 1 – unlimited number of cycles.
19
20.
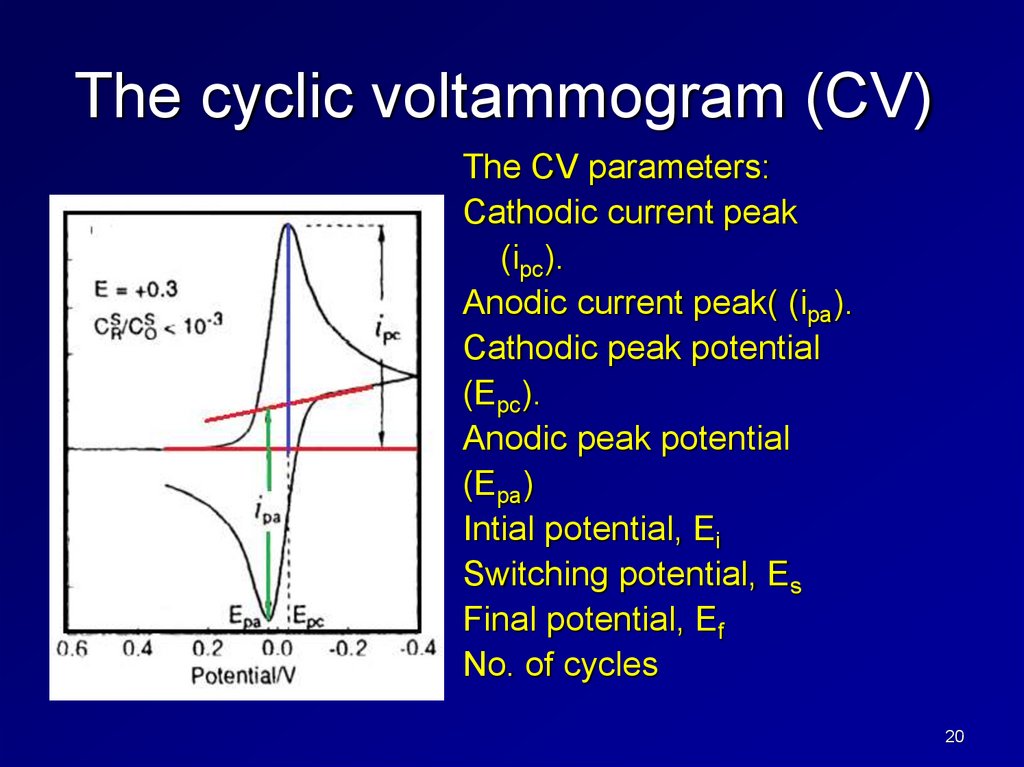
The cyclic voltammogram (CV)The CV parameters:
Cathodic current peak
(ipc).
Anodic current peak( (ipa).
Cathodic peak potential
(Epc).
Anodic peak potential
(Epa)
Intial potential, Ei
Switching potential, Es
Final potential, Ef
No. of cycles
20
21.
Cyclic voltammetryCyclic voltammetry is often the first experiment
performed in an electrochemical study of an
inorganic or organic compound, a biological
material, or an electrode surface.
The effectiveness of CV results from its
capability for rapid observation of redox behavior
over the available potential window.
The voltammogram in a sense is analogous to a
conventional spectrum in spectroscopy.
21
22.
Cyclic voltammetric experimentSame conditions established for LSV.
-Quiescent (stagnant) solution.
- A supporting electrolyte is added.
- A stationary electrode.
- Many materials can be used as electrodes
depending on the purpose of the experiment.
- Metals and noble metals, hanging drop
mercury electrode (HDME) semiconductors,
conducting polymers, carbon electrodes,
modified metallic electrodes, …….etc.
22
23.
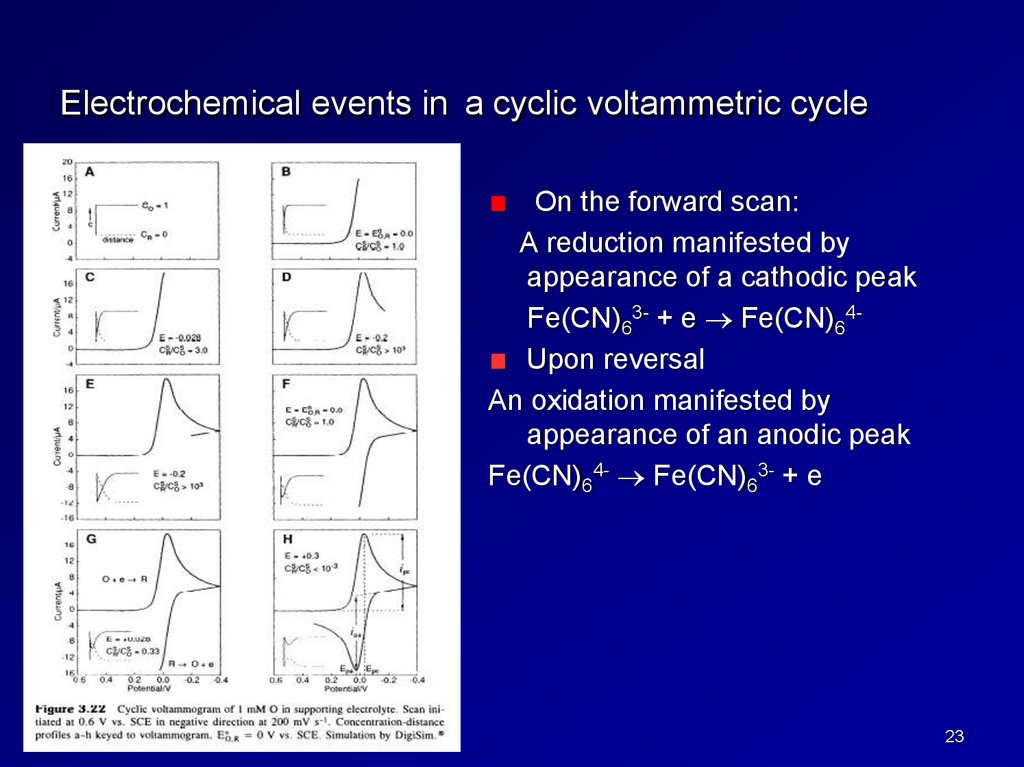
Electrochemical events in a cyclic voltammetric cycleOn the forward scan:
A reduction manifested by
appearance of a cathodic peak
Fe(CN)63- + e Fe(CN)64Upon reversal
An oxidation manifested by
appearance of an anodic peak
Fe(CN)64- Fe(CN)63- + e
23
24.
Voltammetric featuresReversible systems:
By solving Fick’s laws, we get the Randles-Sevcik
equation
ip = (2.69x105)n3/2ADo1/2Co* 1/2
The values of ipc and ipa should be close for a
reversible stable system.
(ipa/ipc) 1
This value, however, will be less if the
electrochemical reaction is coupled to a following
chemistry.
24
25.
Voltammetric featuresFor a reversible couple
E ’ = (Epc+Epa)/2
Ep = Epa – Epc = 0.0592/n
at 25 C
Define “current function”
Current function =
= ip/( 1/2C *)
= 2.69x105n3/2Do1/2A
The current function is independent of scan
rate for a reversible stable system.
o
25
26.
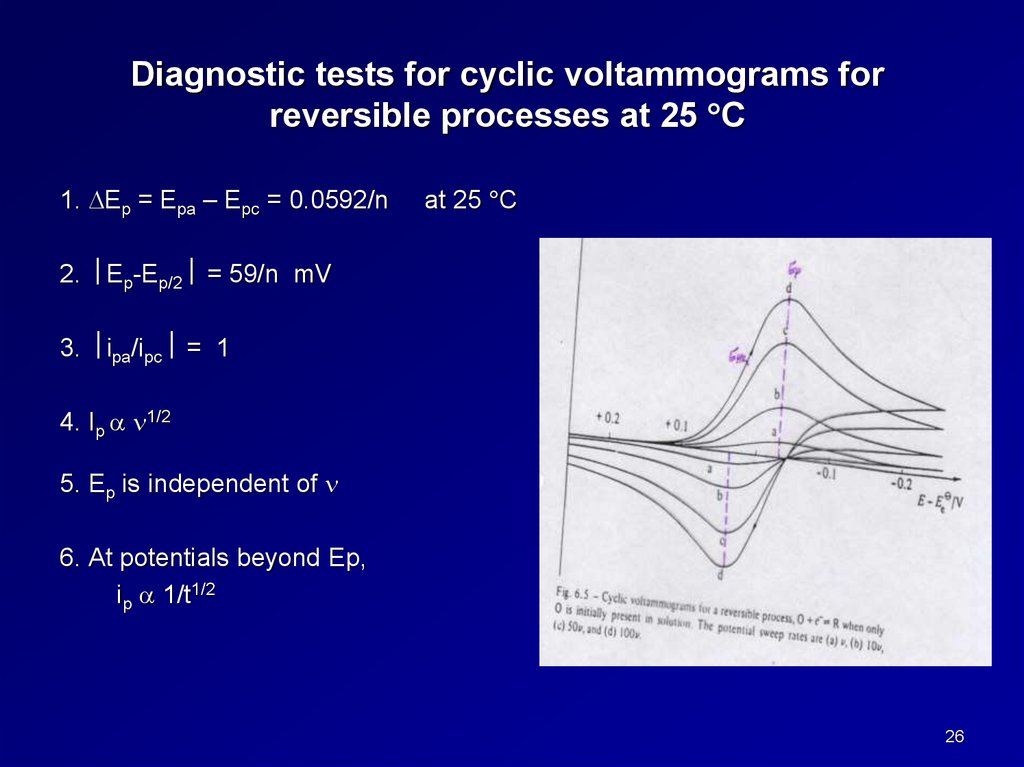
Diagnostic tests for cyclic voltammograms forreversible processes at 25 C
1. Ep = Epa – Epc = 0.0592/n
at 25 C
2. Ep-Ep/2 = 59/n mV
3. ipa/ipc = 1
4. Ip 1/2
5. Ep is independent of
6. At potentials beyond Ep,
ip 1/t1/2
26
27.
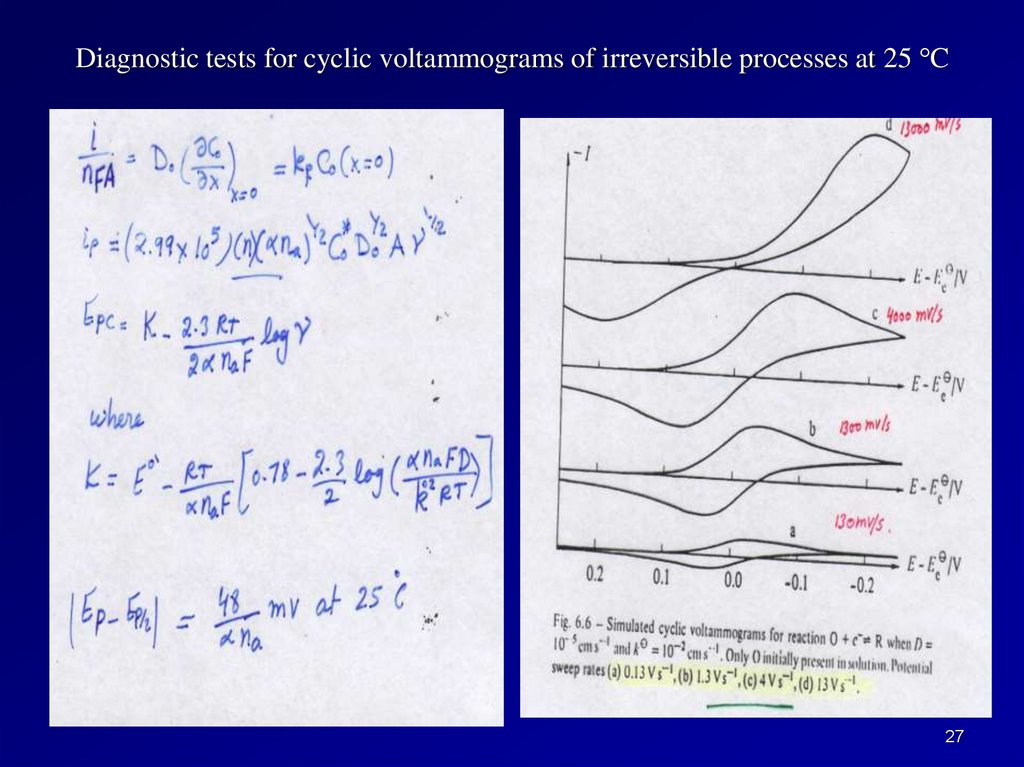
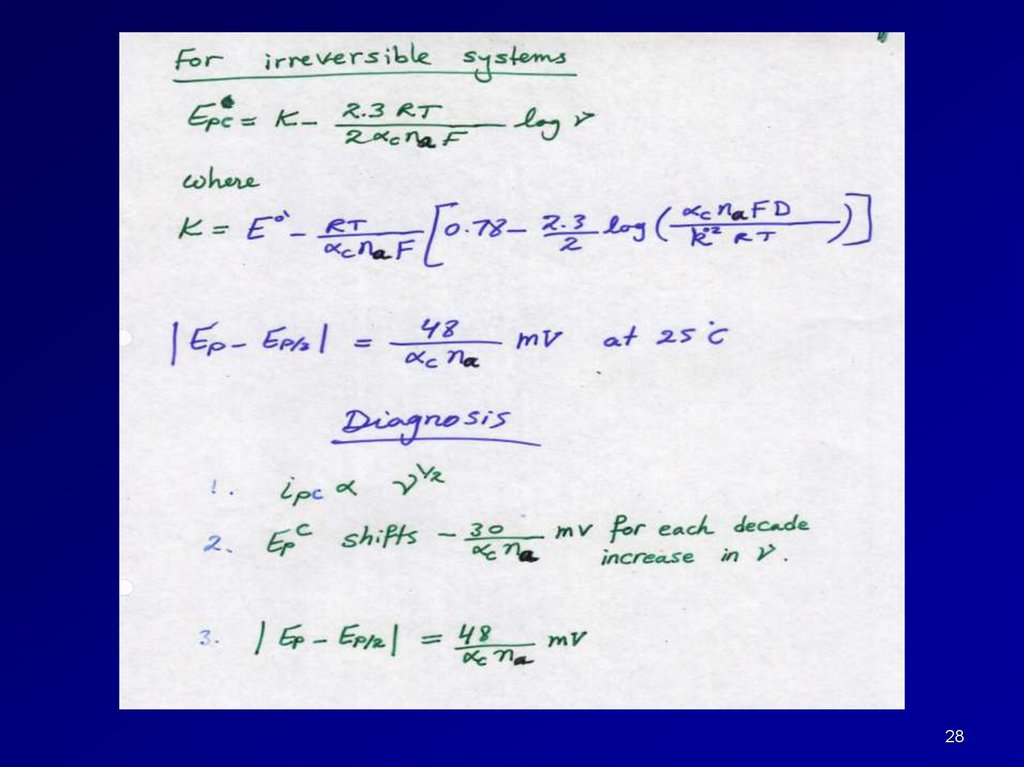
Diagnostic tests for cyclic voltammograms of irreversible processes at 25 C27
28.
2829.
2930.
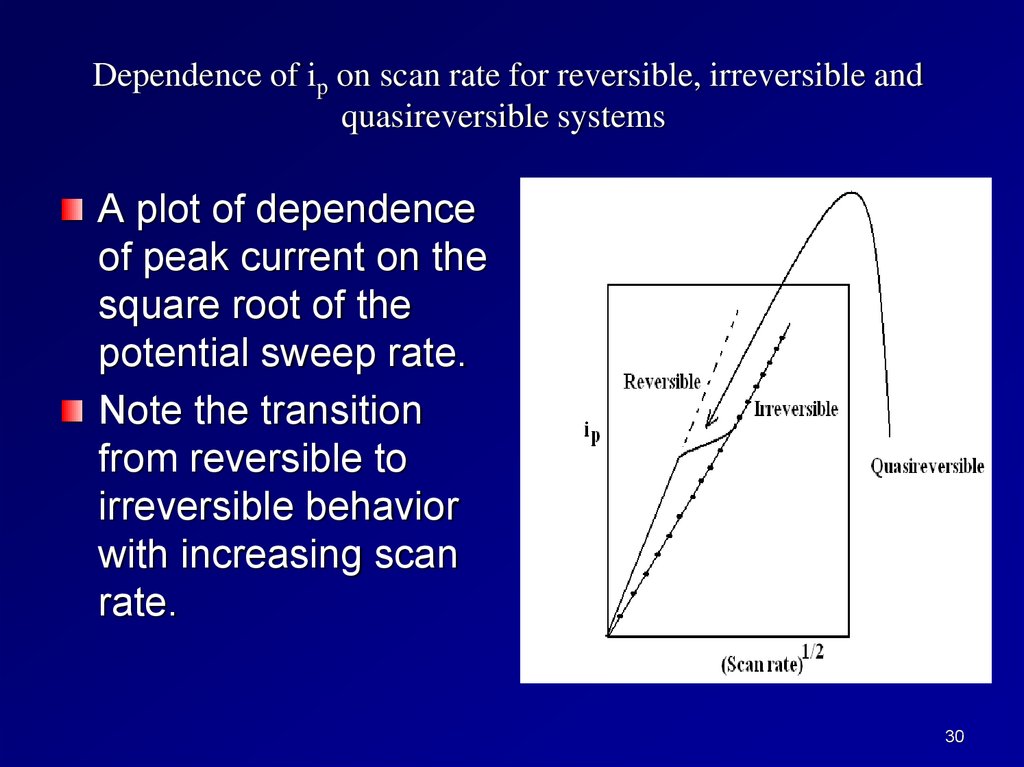
Dependence of ip on scan rate for reversible, irreversible andquasireversible systems
A plot of dependence
of peak current on the
square root of the
potential sweep rate.
Note the transition
from reversible to
irreversible behavior
with increasing scan
rate.
30
31.
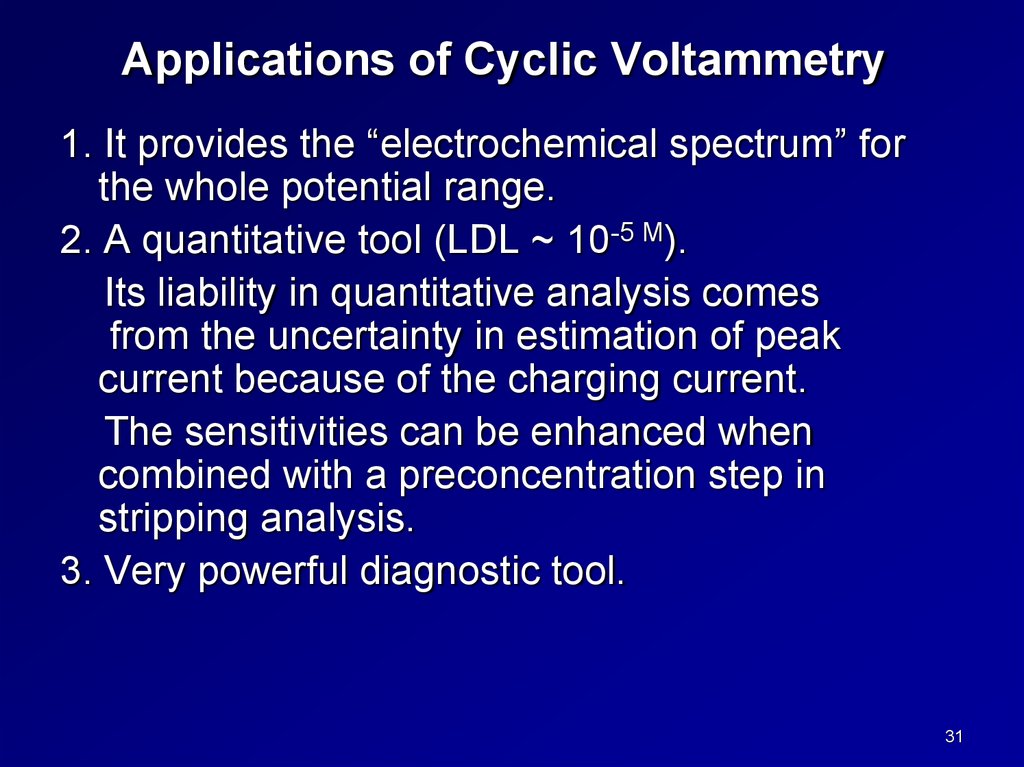
Applications of Cyclic Voltammetry1. It provides the “electrochemical spectrum” for
the whole potential range.
2. A quantitative tool (LDL ~ 10-5 M).
Its liability in quantitative analysis comes
from the uncertainty in estimation of peak
current because of the charging current.
The sensitivities can be enhanced when
combined with a preconcentration step in
stripping analysis.
3. Very powerful diagnostic tool.
31
32.
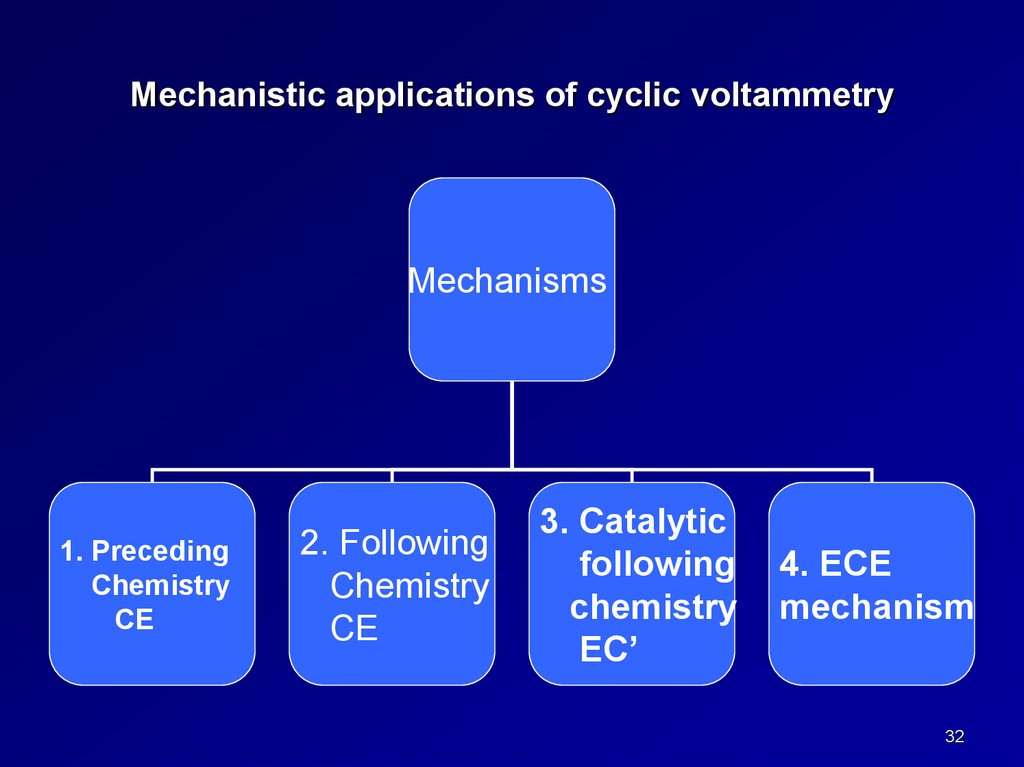
Mechanistic applications of cyclic voltammetryMechanisms
1. Preceding
Chemistry
CE
2. Following
Chemistry
CE
3. Catalytic
following
chemistry
EC’
4. ECE
mechanism
32
33.
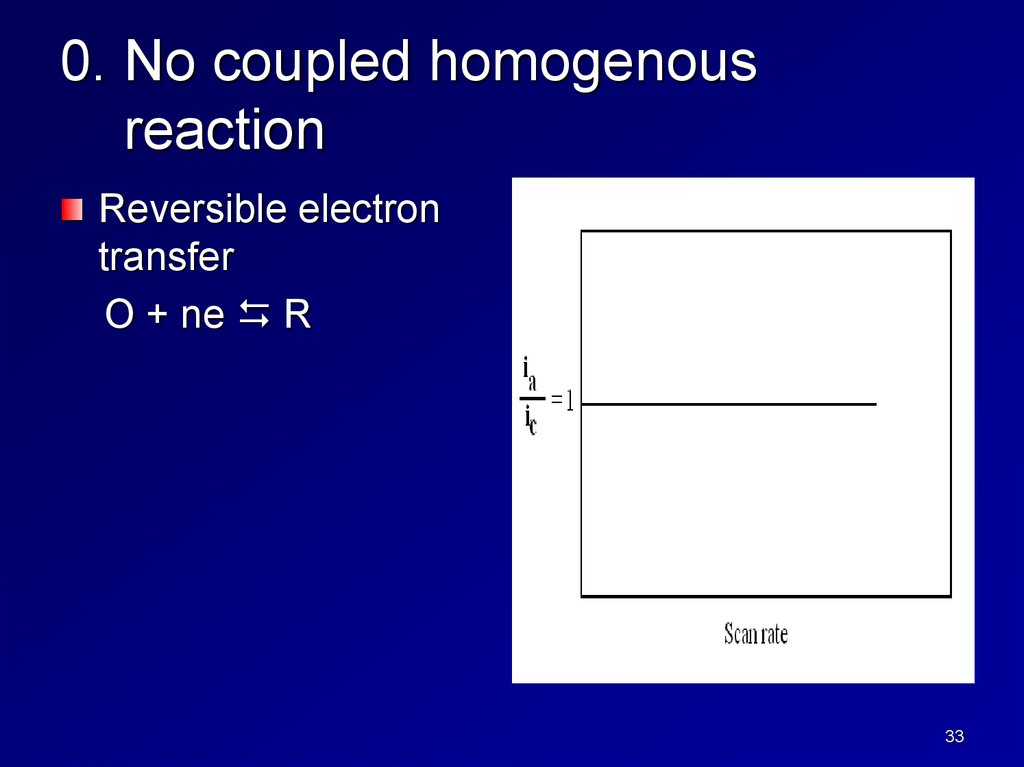
0. No coupled homogenousreaction
Reversible electron
transfer
O + ne R
33
34.
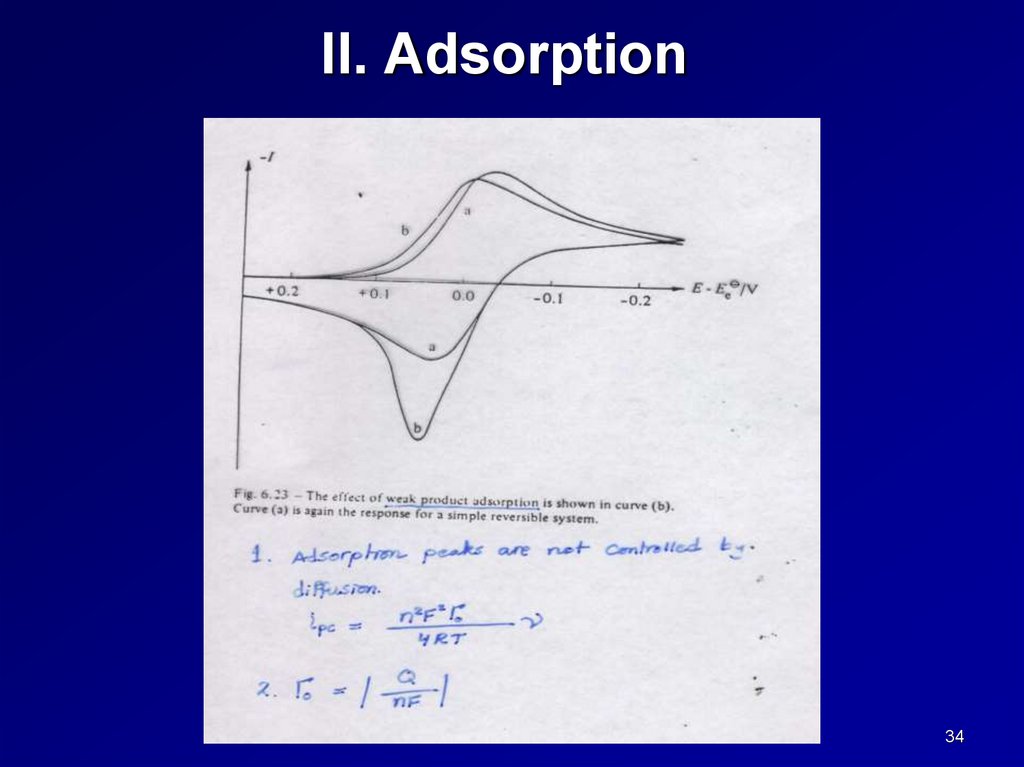
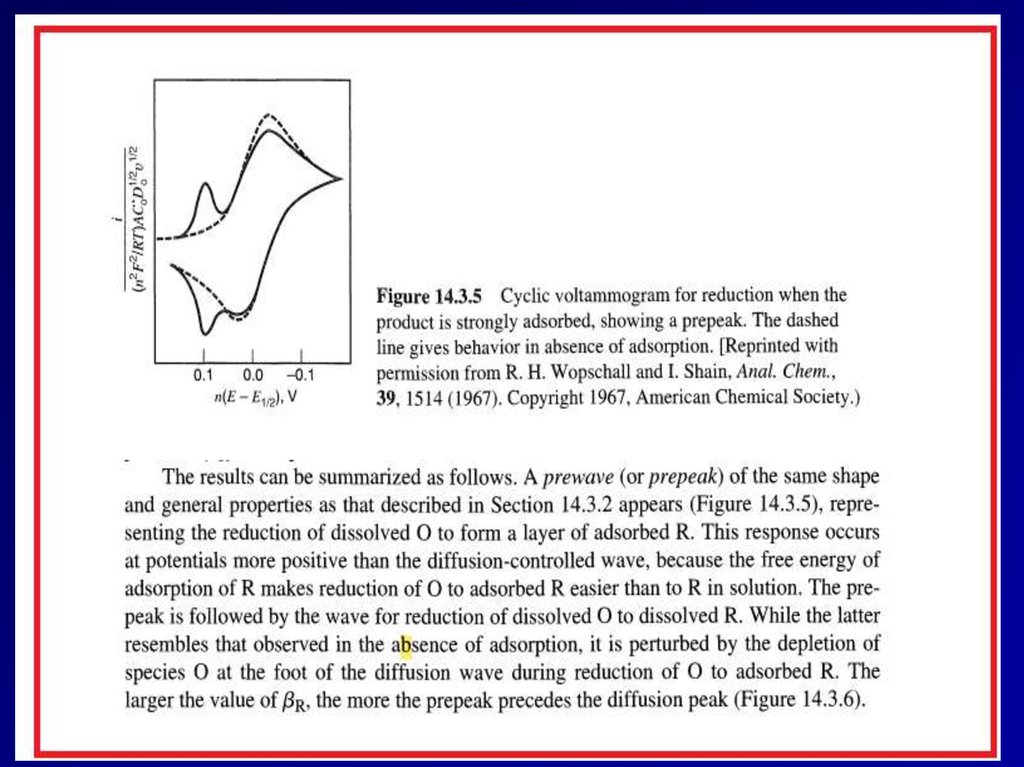
II. Adsorption34
35.
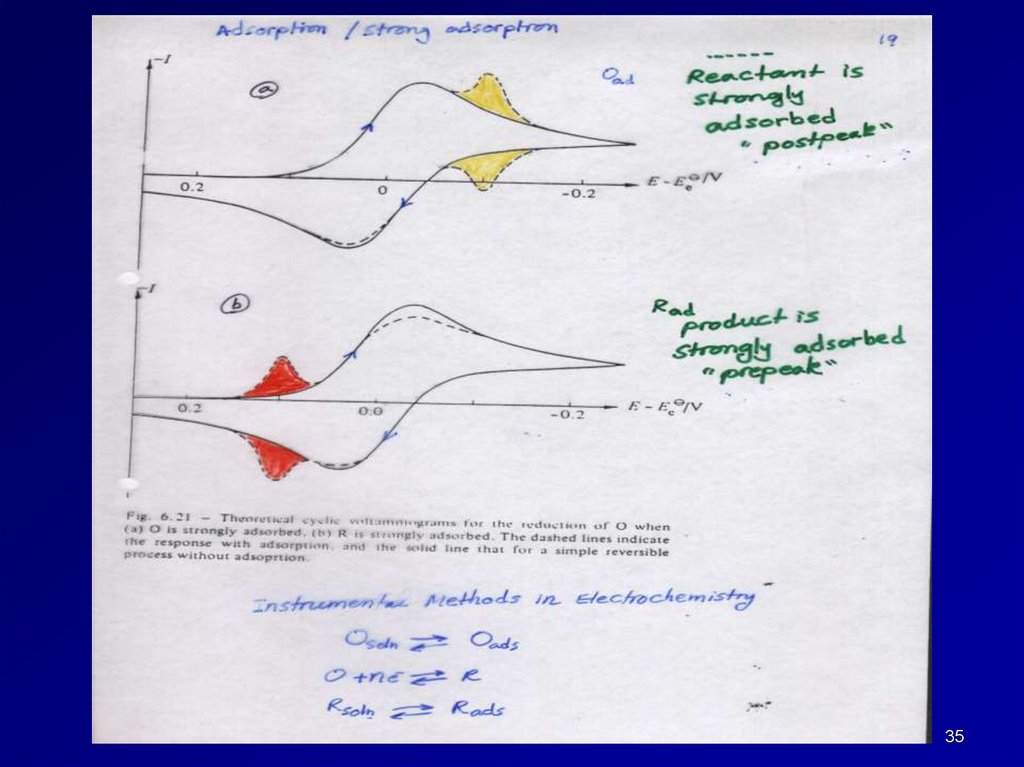
3536.
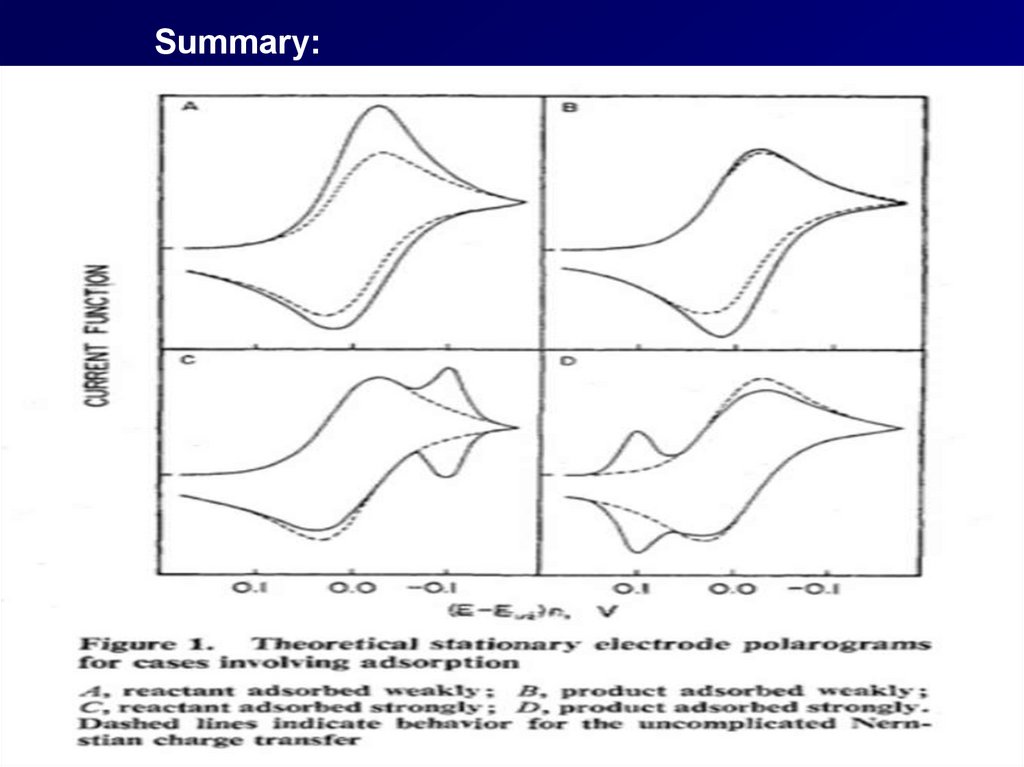
Summary:36
37.
3738.
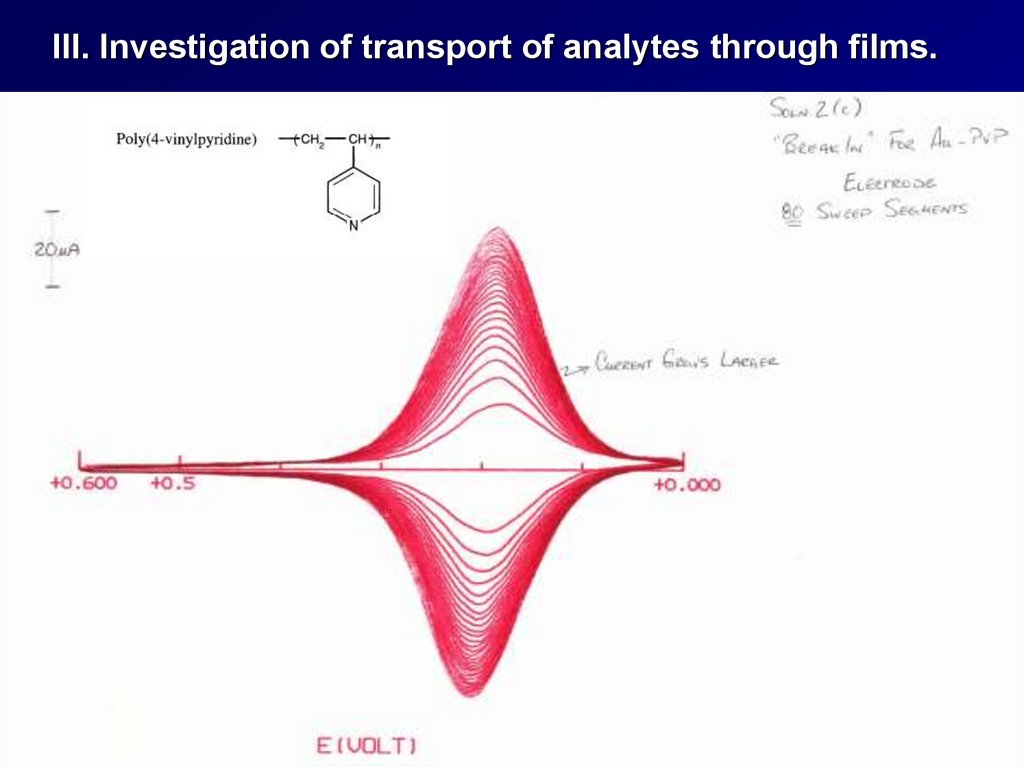
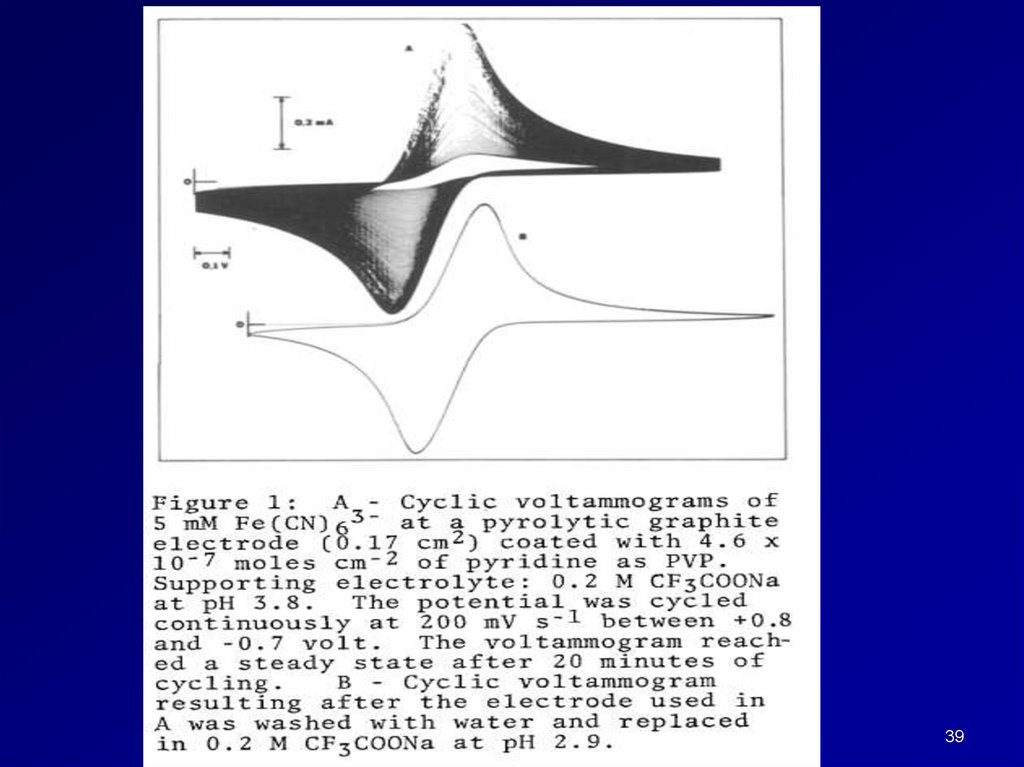
III. Investigation of transport of analytes through films.38
39.
3940.
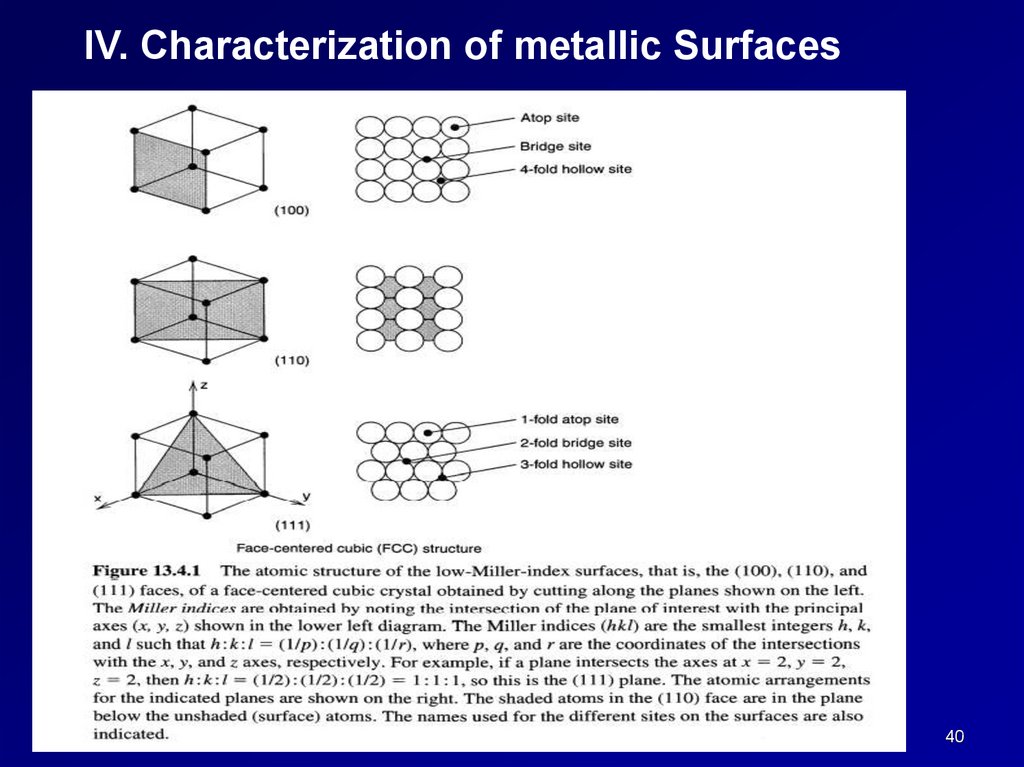
IV. Characterization of metallic Surfaces40
41.
4142.
4243.
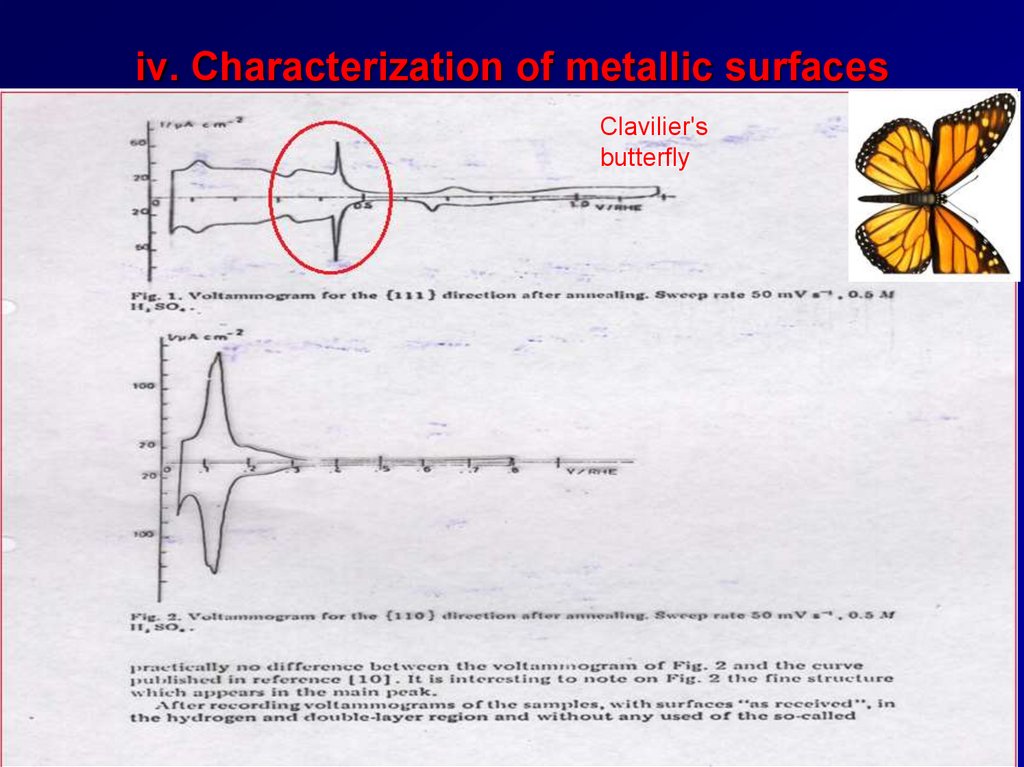
iv. Characterization of metallic surfacesClavilier's
butterfly
43
44.
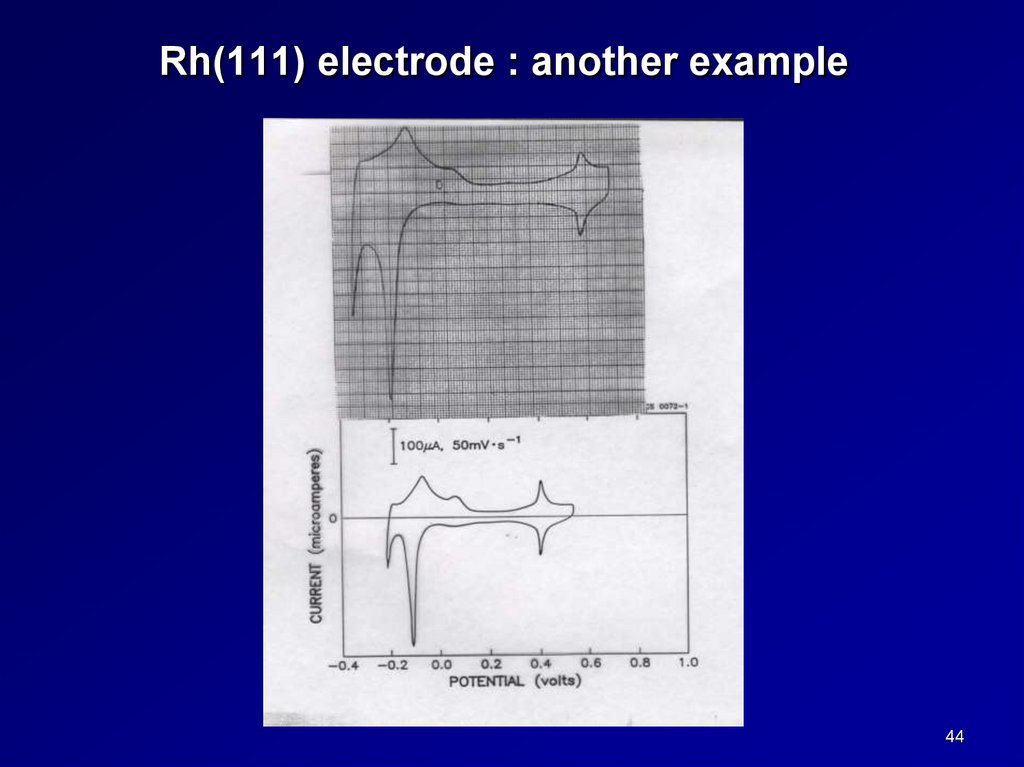
Rh(111) electrode : another example44
45.
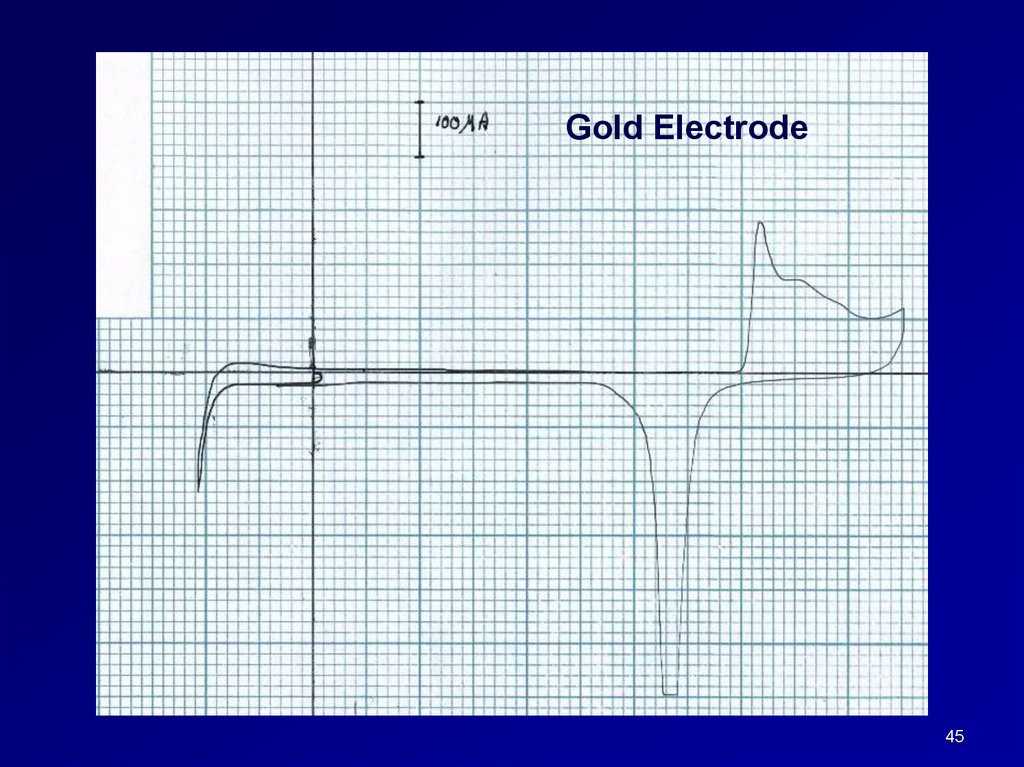
Gold Electrode45
46.
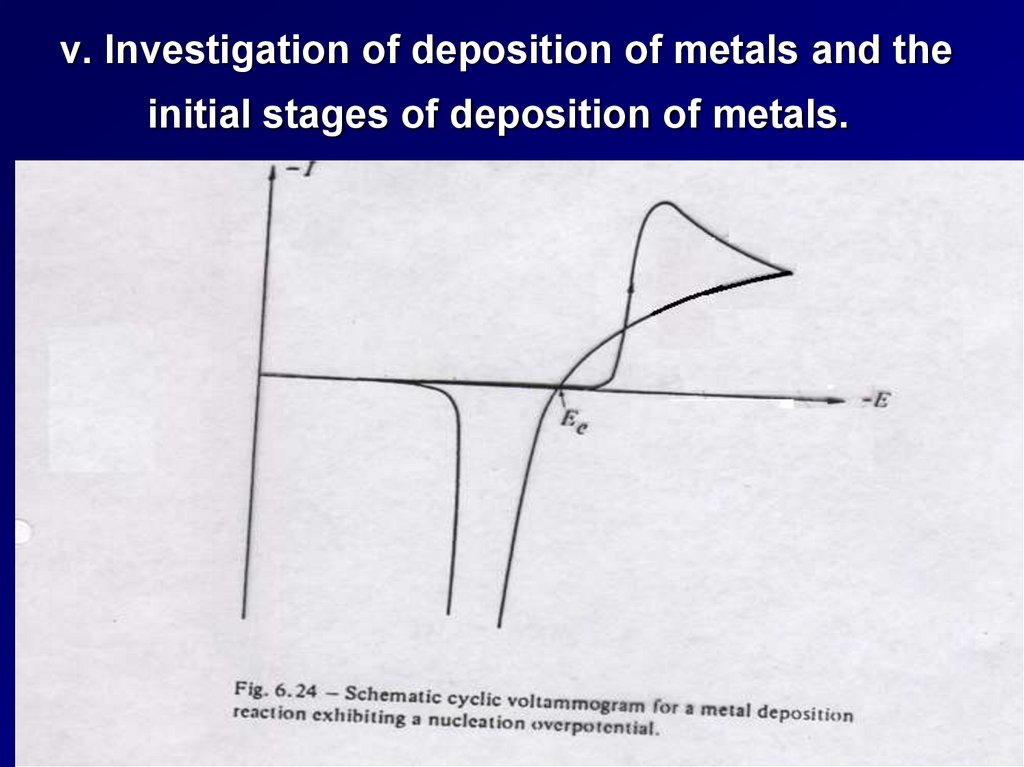
v. Investigation of deposition of metals and theinitial stages of deposition of metals.
46
47.
Some other applicationsvi. Evaluation of E ’ and the other
thermodynamic functions.
vii. Passivation of metallic surfaces and
corrosion inhibition.
viii. Modification of surfaces for analytical or
electrocatalytic purposes
ix. Semiconductors.
……………etc.
47
48.
InstrumentationPotentiostat
Electrochemical cell
48
49.
4950.
5051.
5152.
5253.
5354.
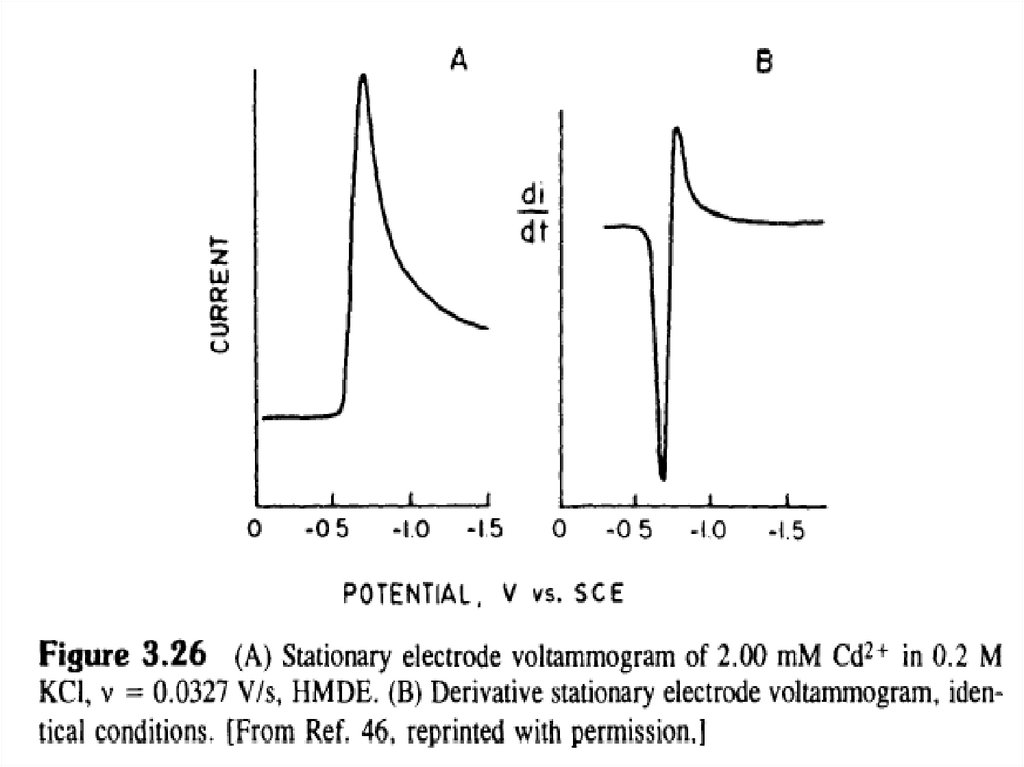
Derivative linear sweepvoltammogram and derivative CV.
The same excitation signal in LSV and
CV
The current is electronically
differentiated with respect to time and
the resulting derivative (di/dt) is
recorded as a function of the applied
potential.
54
55.
5556.
Derivative LSV(cont’d)The first derivative peak height is directly proportional to
the concentration of the electroactive species. Several
advantages accrue from monitoring the derivative
response, including increased sensitivity, resolution and
ease of obtaining rate parameters of coupled chemical
reactions. An enhanced signal-to-background ratio
derives from the relatively small variation in double-layer
charging current with potential, making its contribution to
the derivative small. This advantage becomes important
at high scan rates where charging current grows in
relation to the faradaic response.
56
57.
Some practical aspects of cyclicvoltammetry
Utmost cleanliness
must be established
-Chromerge (Chromic
acid solution).
Chemicals.
Water
Design of the cell
57












 Физика
Физика








