Похожие презентации:
Pulmonary disorders
1.
Pulmonary disorders2.
ASTHMA3.
4.
A. Definition:Asthma is a chronic inflammatory disorder of the airways
causing recurrent episodes of wheezing, breathlessness, cough,
and chest tightness, particularly at night or early in the
morning.
During episodes, there is variable airway obstruction, often
reversible spontaneously or with treatment.
There is also increased bronchial hyper-responsiveness to a
variety of stimuli.
5.
• Asthma is commonly divided into two types:1) allergic (extrinsic) asthma and
2) non-allergic (intrinsic)
• Allergic (extrinsic) asthma (asthma symptoms triggered
by an allergic reaction).
• Allergic asthma is the most common form of asthma.
• Allergic asthma is triggered by inhaling allergens such as
dust mites, pet dander, pollens, mold, etc.
6.
• Non-Allergic (intrinsic) asthma (asthma symptoms triggered byfactors not related to allergies):
• Like allergic asthma, non-allergic asthma is characterized by
airway obstruction and inflammation that is at least partially
reversible with medication, however symptoms in this type of
asthma are NOT associated with an allergic reaction.
• Non-allergic asthma is triggered by other factors such as
anxiety, stress, exercise, cold air, dry air, hyperventilation,
smoke, viruses or other irritants.
7.
B. Diagnosis• Episodic symptoms of airflow obstruction are
present.
• Airway obstruction is reversible (FEV1 improves
by 12% or more after short-acting β2-agonists
[SABAs]).
• Alternative diagnoses are excluded.
8.
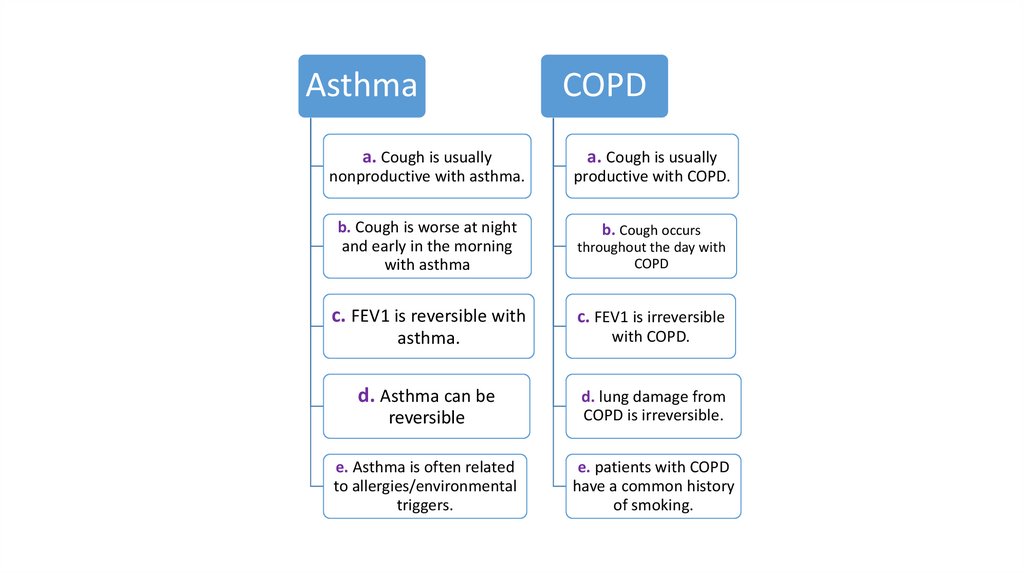
AsthmaCOPD
a. Cough is usually
a. Cough is usually
nonproductive with asthma.
productive with COPD.
b. Cough is worse at night
and early in the morning
with asthma
b. Cough occurs
throughout the day with
COPD
c. FEV1 is reversible with
c. FEV1 is irreversible
asthma.
with COPD.
d. Asthma can be
reversible
d. lung damage from
COPD is irreversible.
e. Asthma is often related
to allergies/environmental
triggers.
e. patients with COPD
have a common history
of smoking.
9.
•Q. what is spirometry?•A. Meaning the measuring of breath and is
the most common of the pulmonary
function test, measuring lung function,
specially the amount (volume) and/or speed
(flow) of air that can be inhaled and
exhaled.
10.
11.
Table 1, spirometer readingsComponent
FEV1 3-4liter
What It Measures
Volume of air exhaled forcefully in
the first second of maximal
expiration
FVC 4-5liter
The maximum volume of air that
Reported in liters and % predicted
can be exhaled after full inspiration
Normal adults can empty 80% of air
in < 6 seconds
Differentiates between obstructive 1) Normal: Within 5% of predicted
and restrictive disease
range, which varies with age;
usually 75%–80% in adults
2) Decreased in obstructive
disease (mainly COPD) (< 70%)
3) Normal/high in restrictive
disease (pulmonary fibrosis)
FEV1/FVC ratio
Normal Values
1) Normal is 75-80% of FVC
2) In asthma, reversibility is shown
by an increase of FEV1by ≥ 12%
after SABA
12.
• Q. what is peak flow meter?• A. is a small handheld device used to monitor a
person’s ability to breath out air and the person’s
maximum speed of expiration is called peak
expiratory flow or peak expiratory flow rate (PEF or
PEFR, respectively) normal value of PEFR is for
male 550-650 liter/minutes and for female 420460L/min).
13.
14.
• Q. what is spacer or holding chamber?• A. a device that is placed on the mouthpiece of the metered dose
inhaler (MDI) that facilitate the inhalation technique for children and
elderly patients. We may use face mask for young children.
15.
• Q. What are types of inhalers?• A.
1) MDI
• Examples: ventolin, clenil spray
16.
2) Dry powder inhaler (DPI)• Examples: foradil inhaler capsule, sevent
17.
3) Nebulizer18.
• Q. what is the meaning of personal best or predictedvalue?
• A.
1) Personal best is the maximum reading (best reading) (of
PEFR, by peak flow meter or of FEV1 by spirometer) during
two weeks with well controlled disease
2) Predicted value is the normal mean average value of
healthy based on age and height (there is a peak flow
predicted value calculator)
19.
C. Asthma classification and recommended steps for treatmentComponents
Age group
Intermittent
Frequency of
All ages
≤ 2 days/week
Mild Persistent Moderate
Persistent
> 2 days/week, Daily
≤ 2 days/week
but not daily
> 2 days/week, Daily
symptoms
Short-acting
β2- agonist;
use for
symptom
control
All ages
but not daily
Severe
Persistent
Throughout the
day
Several times a
day
20.
ComponentsAge group
Nighttime ≥ 12
awakening
5–11
0-4
Intermittent
≤ 2 times/
Month
Mild Persistent Moderate
Severe
Persistent
Persistent
3 or 4 times/
More than once Often 7
weekly,
times/week
month
≤ 2 times/
3 or 4 times/
month
month
but not nightly
More than once Often 7
weekly,
times/week
1 or 2
times/month
but not nightly
3 or 4
times/month
0
More than once
weekly
21.
ComponentsAge group
Intermittent
Mild Persistent
Moderate
Persistent
Severe Persistent
FEV1/FVC
≥ 12
Normal
Normal
Reduced 5%
Reduced > 5%
5–11
0-4
> 80%
> 80%
75%–80%
< 75%
≥ 12
0 or 1/yr
5–11
0-4
≥ 12
0 or 1/yr
≥ 2 in 6 mo or =4 wheezing episodes per year
> 80%
> 80%
> 60% to < 80%
< 60%
(normal)
> 80%
(normal)
> 80%
> 80%
> 60% to < 80%
(normal)
(normal)
(normal)
Exacerbations
requiring oral
steroids
FEV1
(% of normal)
5–11
0-4
N/A
N/A
≥ 2/yr
22.
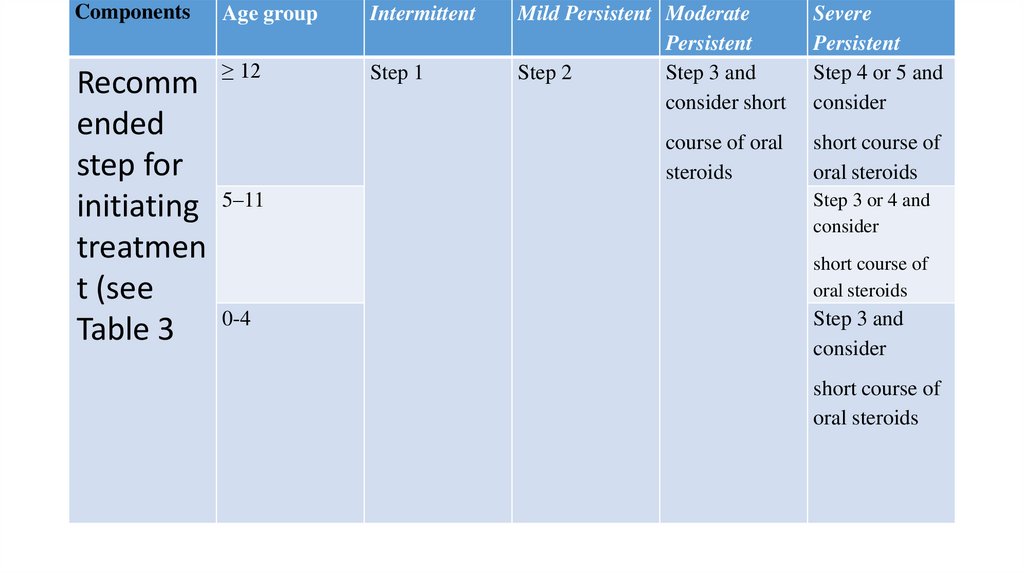
ComponentsAge group
Intermittent
≥ 12
Step 1
Recomm
ended
step for
initiating 5–11
treatmen
t (see
Table 3 0-4
Mild Persistent Moderate
Persistent
Step 2
Step 3 and
consider short
Severe
Persistent
Step 4 or 5 and
consider
course of oral
steroids
short course of
oral steroids
Step 3 or 4 and
consider
short course of
oral steroids
Step 3 and
consider
short course of
oral steroids
23.
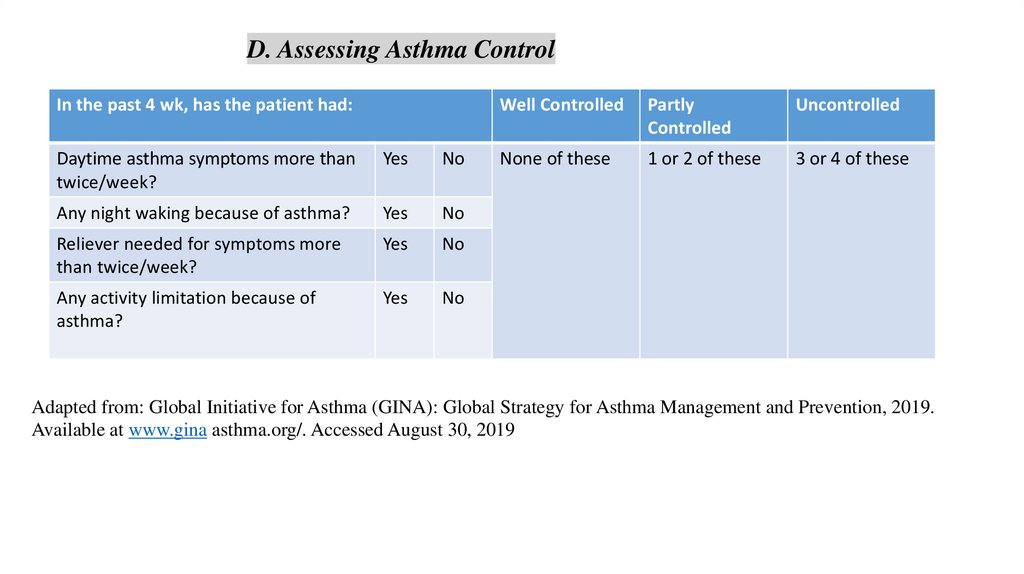
D. Assessing Asthma ControlIn the past 4 wk, has the patient had:
Daytime asthma symptoms more than
twice/week?
Yes
No
Any night waking because of asthma?
Yes
No
Reliever needed for symptoms more
than twice/week?
Yes
No
Any activity limitation because of
asthma?
Yes
No
Well Controlled
Partly
Controlled
Uncontrolled
None of these
1 or 2 of these
3 or 4 of these
Adapted from: Global Initiative for Asthma (GINA): Global Strategy for Asthma Management and Prevention, 2019.
Available at www.gina asthma.org/. Accessed August 30, 2019
24.
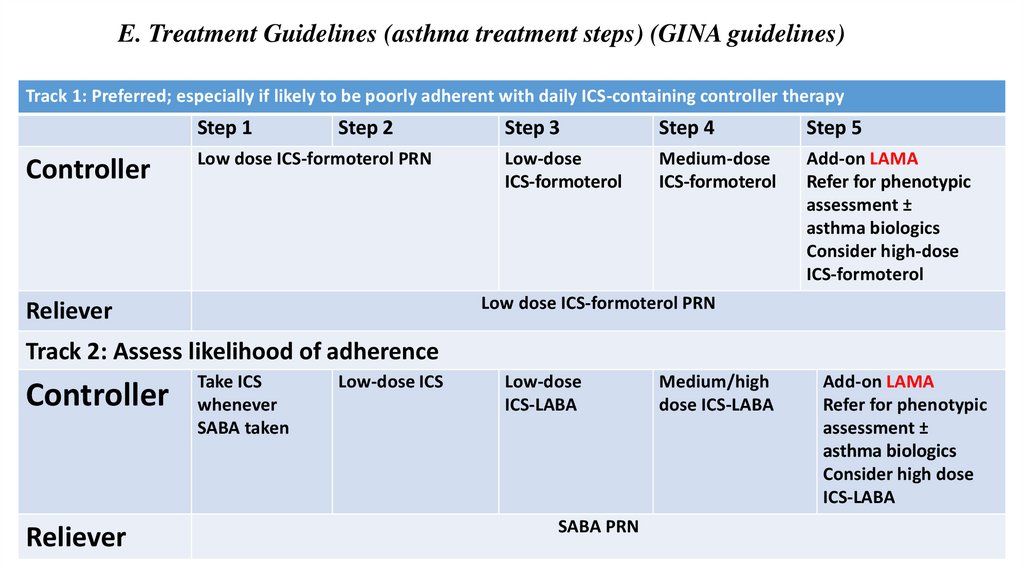
E. Treatment Guidelines (asthma treatment steps) (GINA guidelines)Track 1: Preferred; especially if likely to be poorly adherent with daily ICS-containing controller therapy
Step 1
Controller
Step 2
Low dose ICS-formoterol PRN
Step 3
Step 4
Step 5
Low-dose
ICS-formoterol
Medium-dose
ICS-formoterol
Add-on LAMA
Refer for phenotypic
assessment ±
asthma biologics
Consider high-dose
ICS-formoterol
Low dose ICS-formoterol PRN
Reliever
Track 2: Assess likelihood of adherence
Controller
Reliever
Take ICS
whenever
SABA taken
Low-dose ICS
Low-dose
ICS-LABA
SABA PRN
Medium/high
dose ICS-LABA
Add-on LAMA
Refer for phenotypic
assessment ±
asthma biologics
Consider high dose
ICS-LABA
25.
Alternative controllers for either trackStep 1
Step 2
Step 3
Step 4
Low dose ICS
whenever SABA
taken, or daily
LTRA or HDM
SLIT
Medium-dose
ICS, or add
LTRA, or add
HDM SLIT
Add LAMA or
LTRA or HDM
SLIT, or switch
to high dose ICS
Step 5
Add
azithromycin
(adults) or
LTRA; add low
dose OCS
GINA = Global Initiative for Asthma; HDM = house dust mite; ICS = inhaled corticosteroid; LABA = long-acting
β2-agonist; LTRA = leukotriene receptor antagonist;; OCS = oral corticosteroid; PRN = as needed; RTI =
respiratory tract infection; SABA = short-acting β2-agonist; SLIT = sublingual immunotherapy.
a Updates based on the 2020 guidelines.
b Cromolyn, LTRAs, and theophylline were not considered in 2020 update and/or have limited availability in the
United States, and/or have increased risk of adverse drug reactions and need for monitoring making use less
desirable.
26.
• Q. is clenil spray (ICS+SABA) an appropriate combination?• Q. what is HFA?
27.
F. Pharmacologic AgentsActive Ingredients
Adverse effect
Comments
Corticosteroid inhalers
Corticosteroid inhalers
Corticosteroid inhalers
Beclomethasone MDI
Fluticasone MDI
Fluticasone DPI
Mometasone DPI
Budesonide DPI and 1-mg/2mL nebs (pulmicort)
Ciclesonide MDI
• Oral candidiasis
• Hoarseness of voice
• May slow bone growth in
children but similar adult
height
• ICSs are first line for
persistent asthma
• RINSE MOUTH with water
after inhalations (to avoid
oral candidiasis)
• Use corticosteroid inhaler
as SCHEDULED, not as
needed
• Onset of improvement is 5–
7 days. Additional benefit
may occur over several
weeks
• Consider calcium and
vitamin D supplements in
adults, particularly in
perimenopausal women
28.
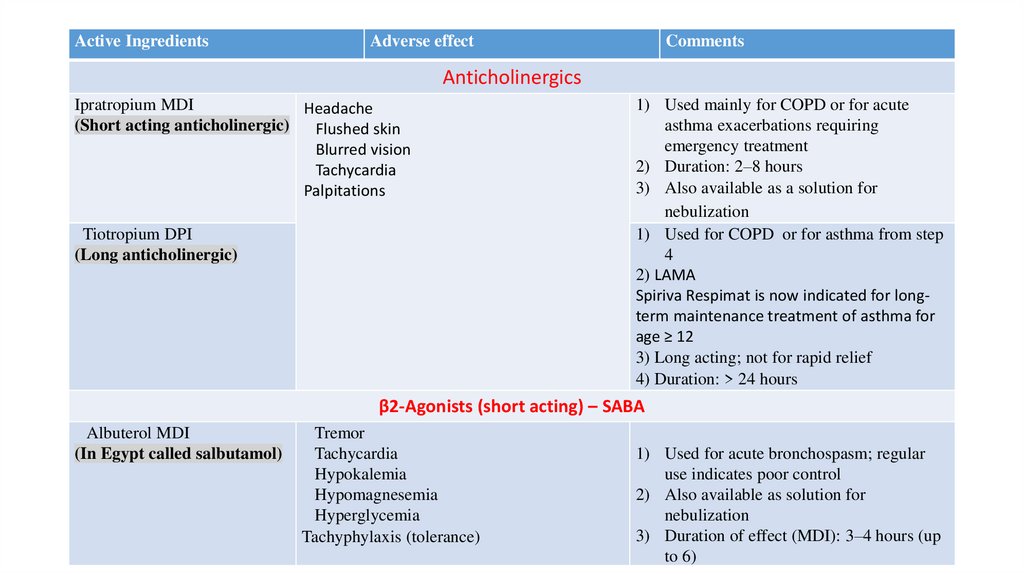
Active IngredientsAdverse effect
Comments
Anticholinergics
Ipratropium MDI
Headache
(Short acting anticholinergic)
Flushed skin
Blurred vision
Tachycardia
Palpitations
Tiotropium DPI
(Long anticholinergic)
1) Used mainly for COPD or for acute
asthma exacerbations requiring
emergency treatment
2) Duration: 2–8 hours
3) Also available as a solution for
nebulization
1) Used for COPD or for asthma from step
4
2) LAMA
Spiriva Respimat is now indicated for longterm maintenance treatment of asthma for
age ≥ 12
3) Long acting; not for rapid relief
4) Duration: > 24 hours
β2-Agonists (short acting) – SABA
Albuterol MDI
(In Egypt called salbutamol)
Tremor
Tachycardia
Hypokalemia
Hypomagnesemia
Hyperglycemia
Tachyphylaxis (tolerance)
1) Used for acute bronchospasm; regular
use indicates poor control
2) Also available as solution for
nebulization
3) Duration of effect (MDI): 3–4 hours (up
to 6)
29.
Active IngredientsAdverse effect
comments
β2-Agonists (long acting) – LABA
Salmeterol DPI
Formoterol DPI
Tremor
Tachycardia
Electrolyte effects rare
Formoterol 20-mcg/2- mL
nebs
1) Not for acute symptoms
2) Should NOT be used as
monotherapy for asthma
3) Duration: 8–12 hours
Methylxanthine
Theophylline
At high levels:
Nausea
Vomiting
CNS stimulation
Headache
Tachycardia
Seizures
Hematemesis
Hyperglycemia
Hypokalemia
At therapeutic levels:
Insomnia
GI upset
increased hyperactivity in some children
Difficult urination in BPH
1) Achieve concentrations of 5–15
mcg/mL
2) Beneficial for night symptoms
3) Not for acute relief
4) Duration: variable; up to 24 hours
30.
Active IngredientsAdverse effect
Comments
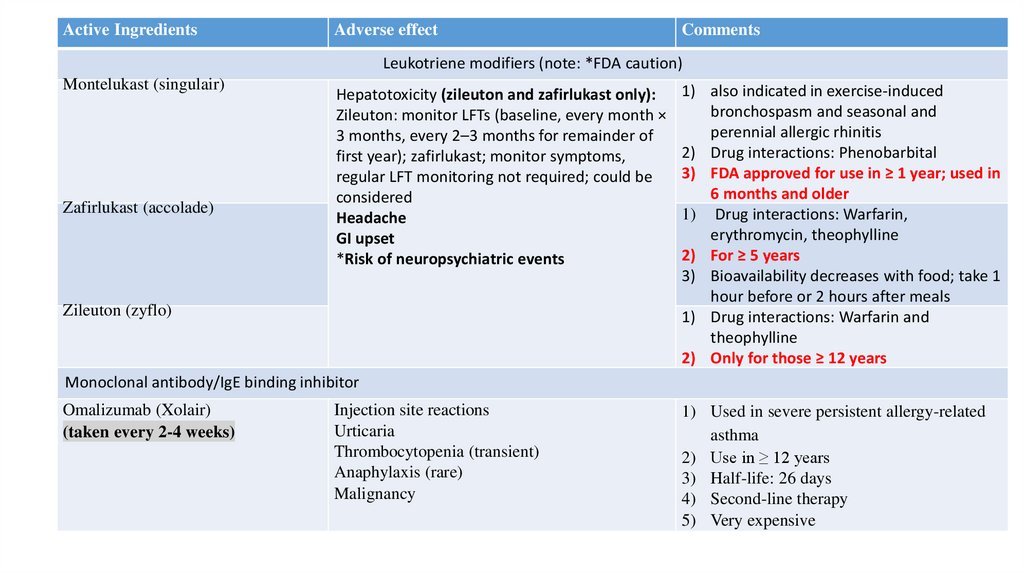
Leukotriene modifiers (note: *FDA caution)
Montelukast (singulair)
Zafirlukast (accolade)
Hepatotoxicity (zileuton and zafirlukast only):
Zileuton: monitor LFTs (baseline, every month ×
3 months, every 2–3 months for remainder of
first year); zafirlukast; monitor symptoms,
regular LFT monitoring not required; could be
considered
Headache
GI upset
*Risk of neuropsychiatric events
Zileuton (zyflo)
1) also indicated in exercise-induced
bronchospasm and seasonal and
perennial allergic rhinitis
2) Drug interactions: Phenobarbital
3) FDA approved for use in ≥ 1 year; used in
6 months and older
1) Drug interactions: Warfarin,
erythromycin, theophylline
2) For ≥ 5 years
3) Bioavailability decreases with food; take 1
hour before or 2 hours after meals
1) Drug interactions: Warfarin and
theophylline
2) Only for those ≥ 12 years
Monoclonal antibody/IgE binding inhibitor
Omalizumab (Xolair)
(taken every 2-4 weeks)
Injection site reactions
Urticaria
Thrombocytopenia (transient)
Anaphylaxis (rare)
Malignancy
1) Used in severe persistent allergy-related
asthma
2) Use in ≥ 12 years
3) Half-life: 26 days
4) Second-line therapy
5) Very expensive
31.
IL5 antagonistMepolizumab
Injection-site reactions:
100 mg SC every 4 wk in the upper arm, Headache
thigh, or abdomen
Fatigue
Herpes zoster infection
Hypersensitivity reaction (rare)
FDA-approved November 2015
Add-on maintenance therapy in
severe asthma in ages ≥ 12 with an
eosinophilic phenotype
Reduces rate of asthma exacerbations
by > 50%; reduces corticosteroid dose
by 50%
Reslizumab
3 mg/kg IV infusion over 20–50 min
every 4 wk
Increased creatine kinase
concentration
Myalgia
Throat pain
Anaphylaxis (rare)
FDA approved March 2016
Add-on maintenance therapy in
severe asthma in those ≥ 18 with an
eosinophilic phenotype
Reduces rate of asthma exacerbations
by about 50% in these patients
Benralizumab (Fasenra)
30 mg SC every 4 wk for the first 3
doses, followed by once every 8 wk
in upper arm, thigh, or abdomen
Headache
Pharyngitis
Fever
Hypersensitivity reaction
FDA-approved November 2017
Add-on maintenance treatment of
patients with severe asthma aged
12 years and older, and with an
eosinophilic phenotype
32.
In clinical trials, the eosinophilic phenotype was defined as a peripheral bloodabsolute eosinophil count of ≥ 400/mm3 if patient not taking daily systemic
corticosteroids
33.
Notes on Long-Acting β2-Agonists (LABAs):1) Use of a LABA alone without a long-term asthma control drug such as an inhaled
corticosteroid (ICS) is contraindicated.
2) LABAs should not be used in patients whose asthma is adequately controlled on
low- or medium-dose ICSs.
3) LABAs should be used only as additional therapy for patients who are currently
taking, but not adequately controlled on, a long-term asthma control agent (e.g.,
an ICS).
4) Once asthma control is achieved and maintained, patients should be assessed at
regular intervals and stepped-down (e.g., discontinue the LABA), if possible, and
the patient should continue to be treated with a long-term asthma control agent
(e.g., an ICS).
5) Pediatric and adolescent patients who require a LABA and an ICS should use a
combination product to ensure adherence to both medications.
34.
G. Monitoring• 1. Peak flow monitoring
• a. Symptom-based and peak flow–based monitoring have similar benefits; either is
appropriate for most patients. Symptom-based monitoring is more convenient.
• b. Consider daily home peak flow monitoring for moderate-severe persistent asthma
if patient has history of severe exacerbations or has poor perception of worsening of
asthma symptoms.
• c. Personal best peak expiratory flow rate (PEFR), not predicted PEFR, should be
determined if using peak flow–based asthma action plan.
• i. Personal best PEFR is the highest number attained after daily monitoring for 2
weeks twice daily when asthma is under good control.
• ii. Predicted PEFR is based on population normal using sex, height, and age.
35.
• 2. Spirometry (if 5 years or older)• a. At initial assessment
• b. After treatment is started and symptoms are stabilized
• c. If prolonged or progressive loss of asthma control
• d. At least every 1–2 years or more often, depending on response to therapy
36.
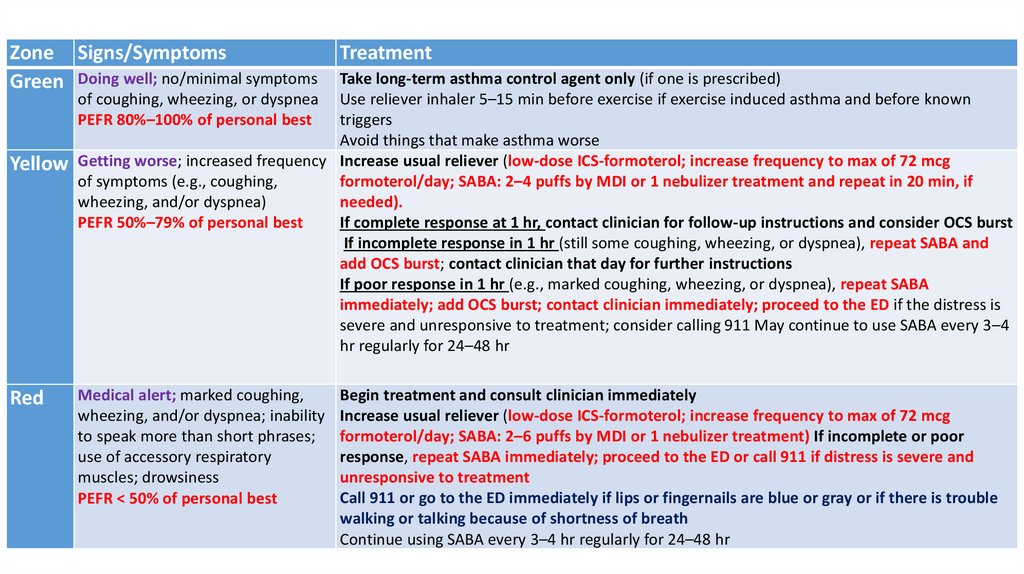
H. Asthma Action Plan: home treatment of an asthmaexacerbation
• Usually symptom based or peak flow–based (table7)
37.
Zone Signs/SymptomsGreen Doing well; no/minimal symptoms
Treatment
Take long-term asthma control agent only (if one is prescribed)
of coughing, wheezing, or dyspnea Use reliever inhaler 5–15 min before exercise if exercise induced asthma and before known
triggers
PEFR 80%–100% of personal best
Avoid things that make asthma worse
Yellow Getting worse; increased frequency Increase usual reliever (low-dose ICS-formoterol; increase frequency to max of 72 mcg
of symptoms (e.g., coughing,
formoterol/day; SABA: 2–4 puffs by MDI or 1 nebulizer treatment and repeat in 20 min, if
wheezing, and/or dyspnea)
needed).
If complete response at 1 hr, contact clinician for follow-up instructions and consider OCS burst
PEFR 50%–79% of personal best
If incomplete response in 1 hr (still some coughing, wheezing, or dyspnea), repeat SABA and
add OCS burst; contact clinician that day for further instructions
If poor response in 1 hr (e.g., marked coughing, wheezing, or dyspnea), repeat SABA
immediately; add OCS burst; contact clinician immediately; proceed to the ED if the distress is
severe and unresponsive to treatment; consider calling 911 May continue to use SABA every 3–4
hr regularly for 24–48 hr
Red
Medical alert; marked coughing,
wheezing, and/or dyspnea; inability
to speak more than short phrases;
use of accessory respiratory
muscles; drowsiness
PEFR < 50% of personal best
Begin treatment and consult clinician immediately
Increase usual reliever (low-dose ICS-formoterol; increase frequency to max of 72 mcg
formoterol/day; SABA: 2–6 puffs by MDI or 1 nebulizer treatment) If incomplete or poor
response, repeat SABA immediately; proceed to the ED or call 911 if distress is severe and
unresponsive to treatment
Call 911 or go to the ED immediately if lips or fingernails are blue or gray or if there is trouble
walking or talking because of shortness of breath
Continue using SABA every 3–4 hr regularly for 24–48 hr
38.
I. Managing Exacerbations: in Emergency Department (ED) or Hospital• 1. Mild (heart rate < 100 beats/min, FEV1≥ 70% of predicted or personal best, Dyspnea only with activity) to
moderate (heart rate 100-120, dyspnea interferes with or limits usual activity, FEV1 of 40%-69%)
• a. Oxygen to achieve Sao2 of 90% or more
• b. An inhaled SABA (MDI with valved holding chamber or nebulizer) up to three doses in the first hour
• i. Adult dose: Albuterol MDI 4–10 puffs every 20 minutes for up to 4 hours; then every 1–4 hours as
needed or by nebulizer 2.5–5 mg every 20 minutes for three doses; then 2.5–10 mg every 1–4 hours as
needed
• ii. Pediatric dose (12 years or younger): Albuterol MDI 4–8 puffs every 20 minutes for three doses; then
every 1–4 hours as needed; use holding chamber (add mask if younger than 4 years) or by nebulizer 0.15
mg/kg (minimal dose 2.5 mg) every 20 minutes for three doses; then 0.15–0.3 mg/kg up to 10 mg every 1–
4 hours as needed
• c. Oral corticosteroid (OCS) if no response immediately or if patient recently took an OCS
39.
• 2. Severe exacerbation (heart rate > 120 beats/min, FEV1 less than 40%, dyspnea atrest; interferes with conversation)
• a. Oxygen to achieve Sao2 of 90% or more
• b. High-dose inhaled SABA plus ipratropium by MDI plus valved holding chamber or
nebulizer every 20 minutes or continuously for 1 hour
• c. Oral corticosteroids
• i. Adult dose: Prednisone 40–80 mg/day in one or two divided doses until peak
expiratory flow reaches 70% of predicted
• ii. Pediatric dose (12 years or younger): 1–2 mg/kg in two divided doses
(maximum 60 mg/day) until peak expiratory flow is 70% of predicted
• d. Consider adjunctive therapies (intravenous magnesium or heliox) if still
unresponsive
40.
• 3. Impending or actual respiratory arrest• a. Intubation and mechanical ventilation with oxygen 100%
• b. Nebulized SABA plus ipratropium
• c. Intravenous corticosteroids
• d. Consider adjunctive therapies (intravenous magnesium or heliox) if
patient is still unresponsive to therapy.
• e. Admit to intensive care.
41.
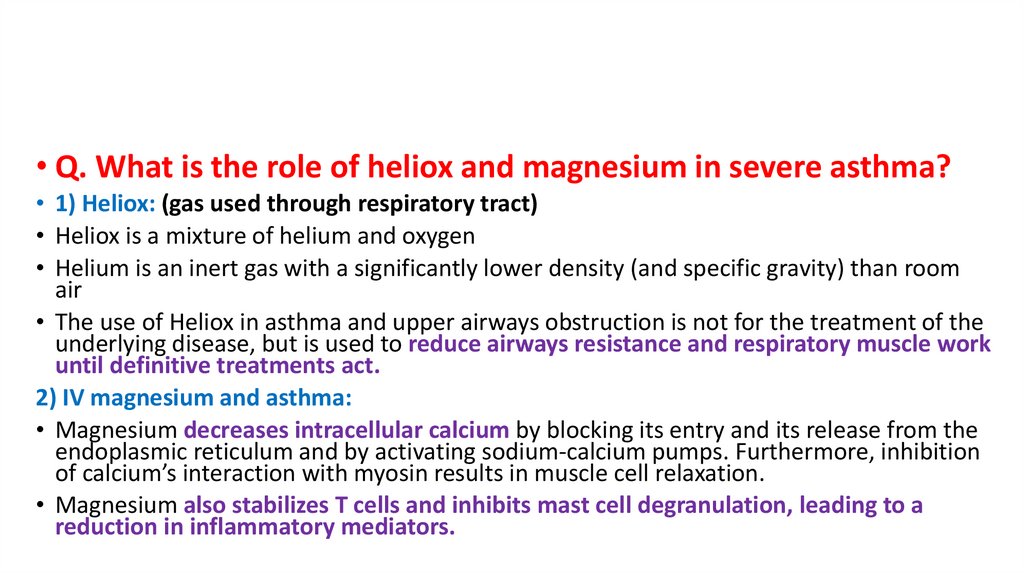
• Q. What is the role of heliox and magnesium in severe asthma?• 1) Heliox: (gas used through respiratory tract)
• Heliox is a mixture of helium and oxygen
• Helium is an inert gas with a significantly lower density (and specific gravity) than room
air
• The use of Heliox in asthma and upper airways obstruction is not for the treatment of the
underlying disease, but is used to reduce airways resistance and respiratory muscle work
until definitive treatments act.
2) IV magnesium and asthma:
• Magnesium decreases intracellular calcium by blocking its entry and its release from the
endoplasmic reticulum and by activating sodium-calcium pumps. Furthermore, inhibition
of calcium’s interaction with myosin results in muscle cell relaxation.
• Magnesium also stabilizes T cells and inhibits mast cell degranulation, leading to a
reduction in inflammatory mediators.
42.
J. Vaccines – Adults with asthma (19–64 years of age)should receive:
1) The 23-valent pneumococcal polysaccharide vaccine
(PPSV; Pneumovax) once
2) Influenza vaccine every fall/winter
43.
K. Asthma in Pregnancy1. Asthma may worsen, improve, or stay the same during pregnancy.
2. Asthma may increase the risk of perinatal mortality, hyperemesis, preeclampsia, neonatal
mortality, prematurity, and low-birth-weight infants, especially if uncontrolled.
3. Medications:
a. Preferred controller: Budesonide ICS (only category B ICS); however, if well controlled on other
ICS before pregnancy, it may be continued
b. Preferred rescue: Albuterol (category C)
c. LABAs are category C; less clinical experience. Use during pregnancy is reasonable if necessary
for asthma control. Salmeterol is preferred LABA.
d. LTM: montelukast (category B), good
e. Prednisone is category C; potential adverse effects in pregnancy are cleft palate, preeclampsia,
gestational diabetes, low birth weight, prematurity. Prednisone should be used, if necessary,
for acute exacerbations in pregnancy.
44.
L. Exercise-induced bronchospasma. Presents with cough, shortness of breath, chest pain or tightness,
wheezing, or endurance problems during exercise
b. Diagnosis is made by an exercise challenge in which a 15% decrease in
FEV1 or peak expiratory flow occurs before and after exercise.
c. Prevention and Treatment of Symptoms
1. Long-term control therapy
2. Pretreatment with a SABA before exercise
3. Leukotriene modifiers (LTMs) can attenuate symptoms in 50% of patients.




































 Медицина
Медицина