Похожие презентации:
Hepatitis viruses
1.
Privolzhsky Research Medical UniversityEpidemiology, Microbiology and EBM department
Hepatitis Viruses
Prof. Zaslavskaia M.I.
2.
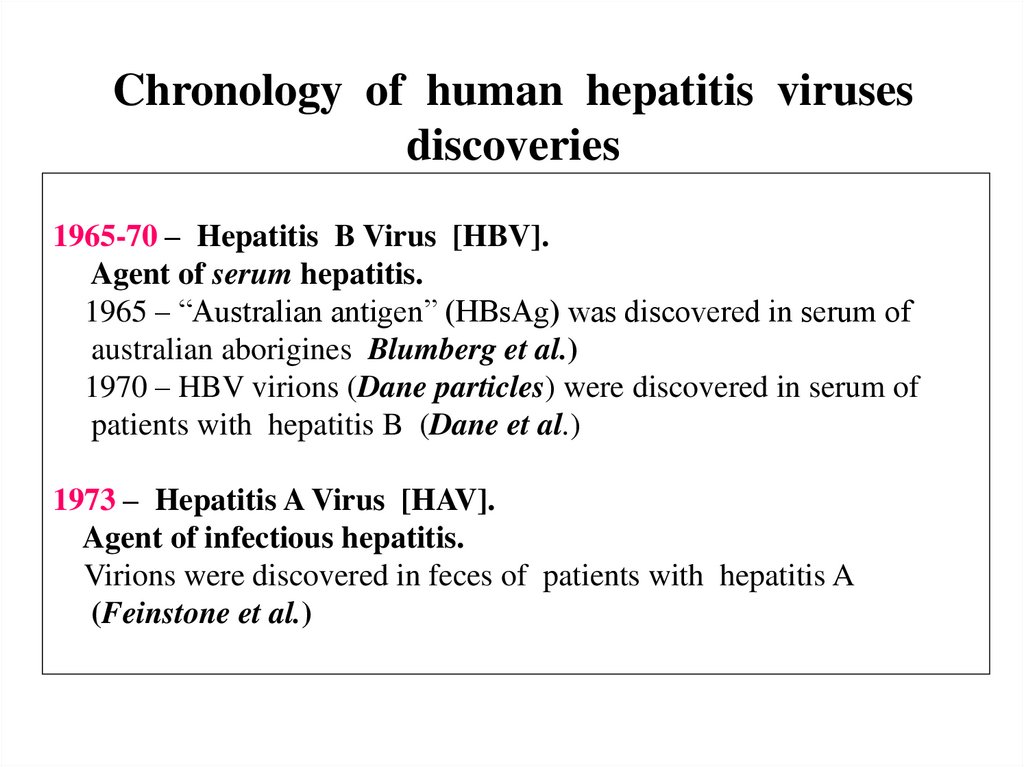
Chronology of human hepatitis virusesdiscoveries
1965-70 – Hepatitis B Virus [HBV].
Agent of serum hepatitis.
1965 – “Australian antigen” (HBsAg) was discovered in serum of
australian aborigines Blumberg et al.)
1970 – HBV virions (Dane particles) were discovered in serum of
patients with hepatitis B (Dane et al.)
1973 – Hepatitis A Virus [HAV].
Agent of infectious hepatitis.
Virions were discovered in feces of patients with hepatitis A
(Feinstone et al.)
3.
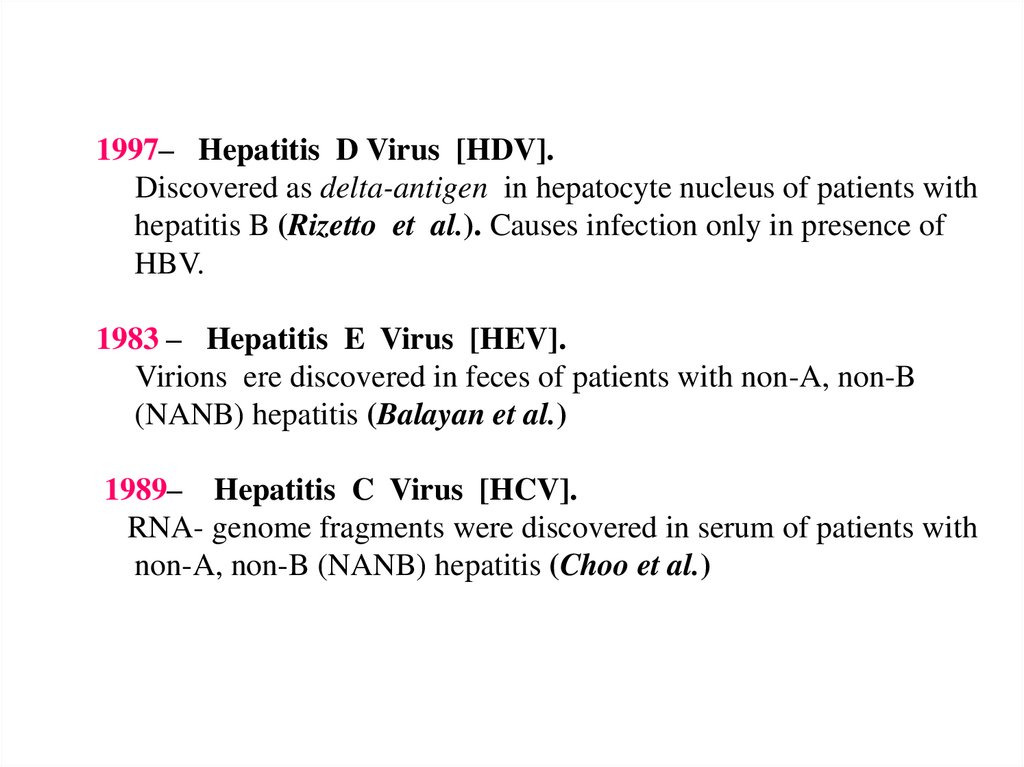
1997– Hepatitis D Virus [HDV].Discovered as delta-antigen in hepatocyte nucleus of patients with
hepatitis B (Rizetto et al.). Causes infection only in presence of
HBV.
1983 – Hepatitis E Virus [HEV].
Virions ere discovered in feces of patients with non-A, non-B
(NANB) hepatitis (Balayan et al.)
1989– Hepatitis C Virus [HCV].
RNA- genome fragments were discovered in serum of patients with
non-A, non-B (NANB) hepatitis (Choo et al.)
4.
5.
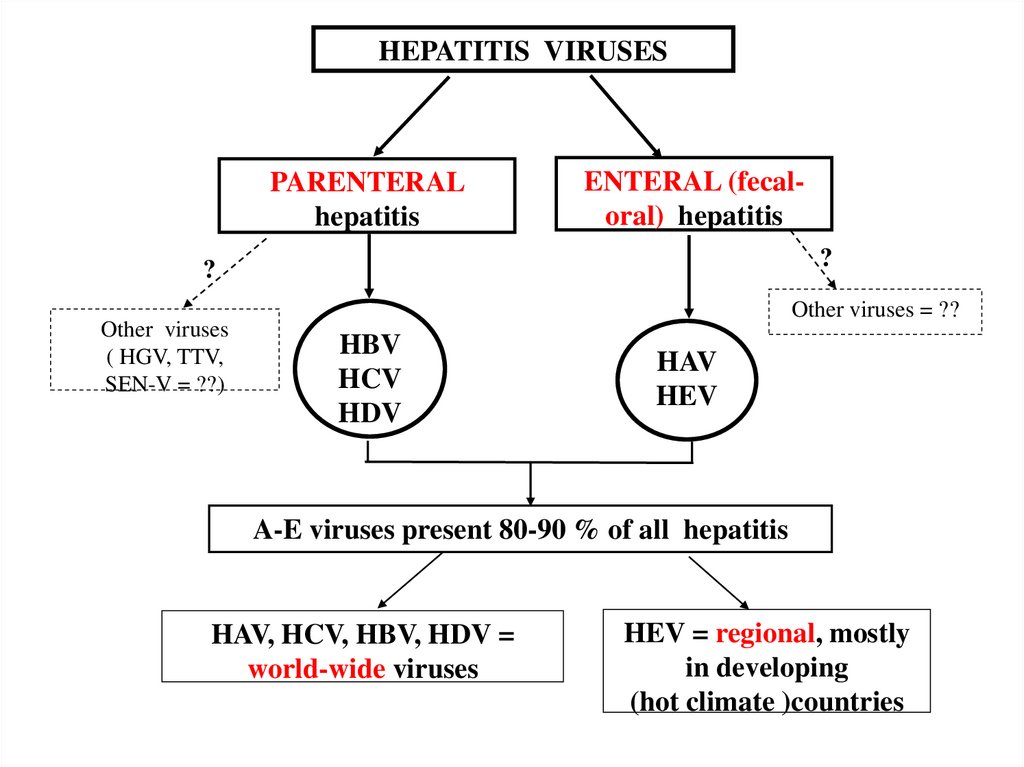
HEPATITIS VIRUSESPARENTERAL
hepatitis
ENTERAL (fecaloral) hepatitis
?
?
Other viruses
( HGV, TTV,
SEN-V = ??)
Other viruses = ??
HBV
HCV
HDV
HAV
HEV
A-E viruses present 80-90 % of all hepatitis
HAV, HCV, HBV, HDV =
world-wide viruses
HEV = regional, mostly
in developing
(hot climate )countries
6.
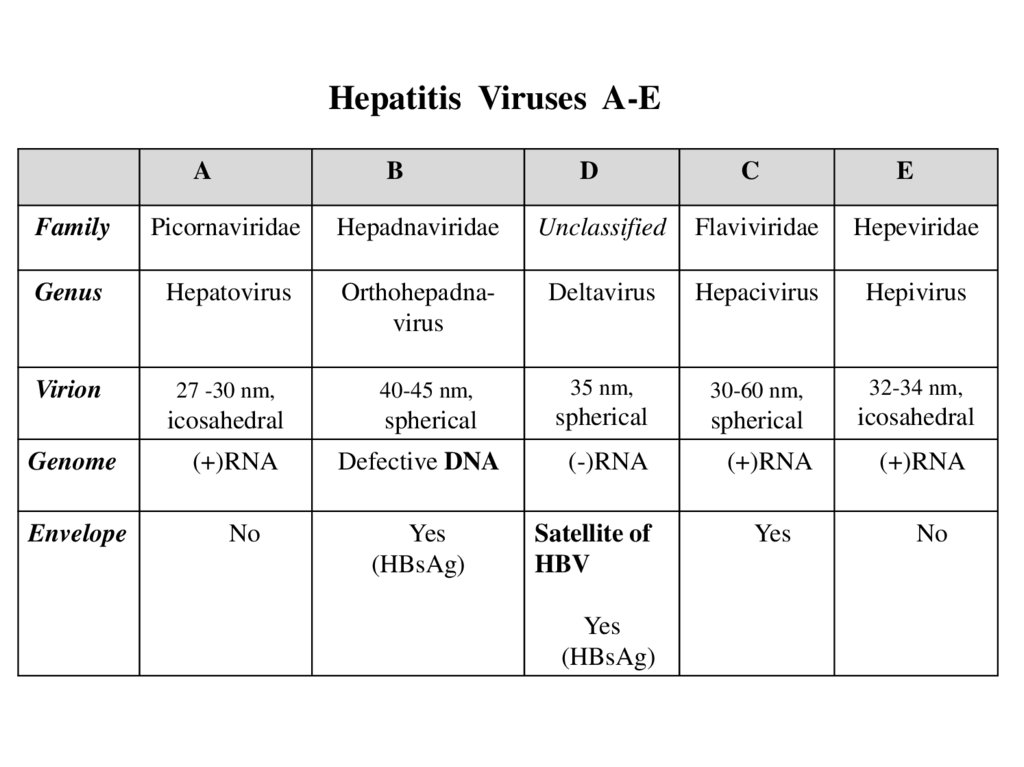
Hepatitis Viruses A-EA
B
D
C
E
Family
Picornaviridae
Hepadnaviridae
Unclassified
Flaviviridae
Hepeviridae
Genus
Hepatovirus
Orthohepаdnavirus
Deltavirus
Hepacivirus
Hepivirus
Virion
27 -30 nm,
40-45 nm,
35 nm,
30-60 nm,
32-34 nm,
icosahedral
spherical
spherical
spherical
icosahedral
Genome
(+)RNA
Defective DNA
(-)RNA
(+)RNA
(+)RNA
Envelope
No
Yes
(HBsAg)
Satellite of
HBV
Yes
No
Yes
(HBsAg)
7.
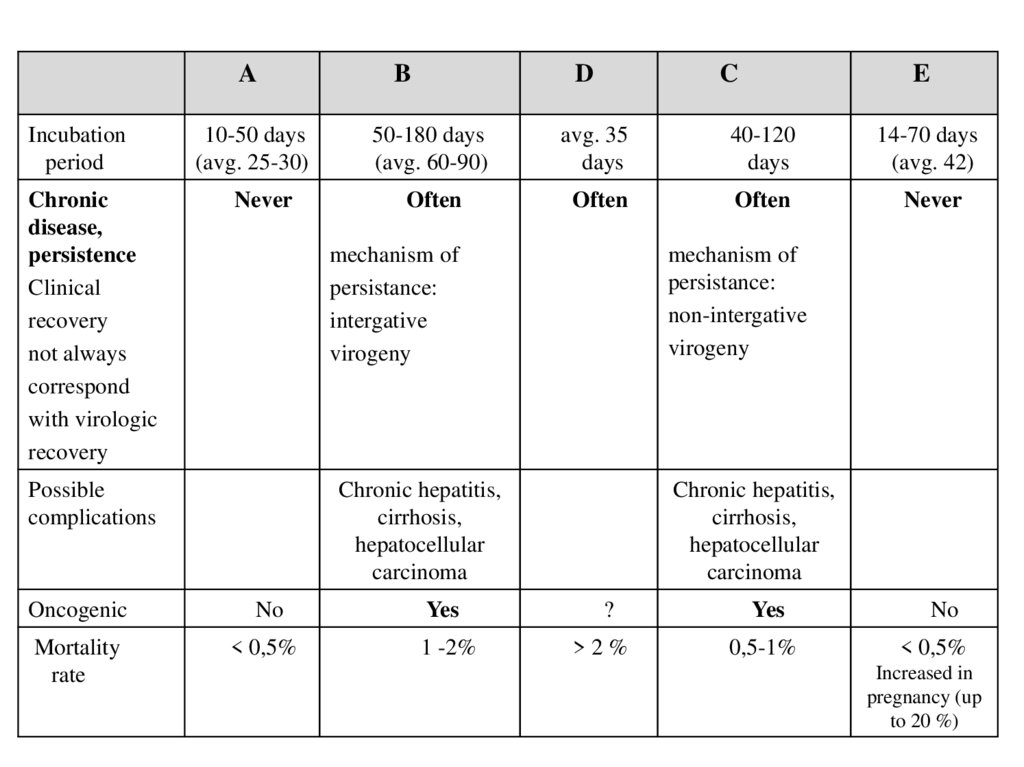
AB
D
C
E
Incubation
period
10-50 days
(avg. 25-30)
50-180 days
(avg. 60-90)
avg. 35
days
40-120
days
14-70 days
(avg. 42)
Chronic
disease,
persistence
Clinical
recovery
not always
correspond
with virologic
recovery
Never
Often
Often
Often
Never
Possible
complications
mechanism of
persistance:
intergative
virogeny
mechanism of
persistance:
non-intergative
virogeny
Chronic hepatitis,
cirrhosis,
hepatocellular
carcinoma
Chronic hepatitis,
cirrhosis,
hepatocellular
carcinoma
Oncogenic
No
Yes
?
Yes
No
Mortality
rate
< 0,5%
1 -2%
>2%
0,5-1%
< 0,5%
Increased in
pregnancy (up
to 20 %)
8.
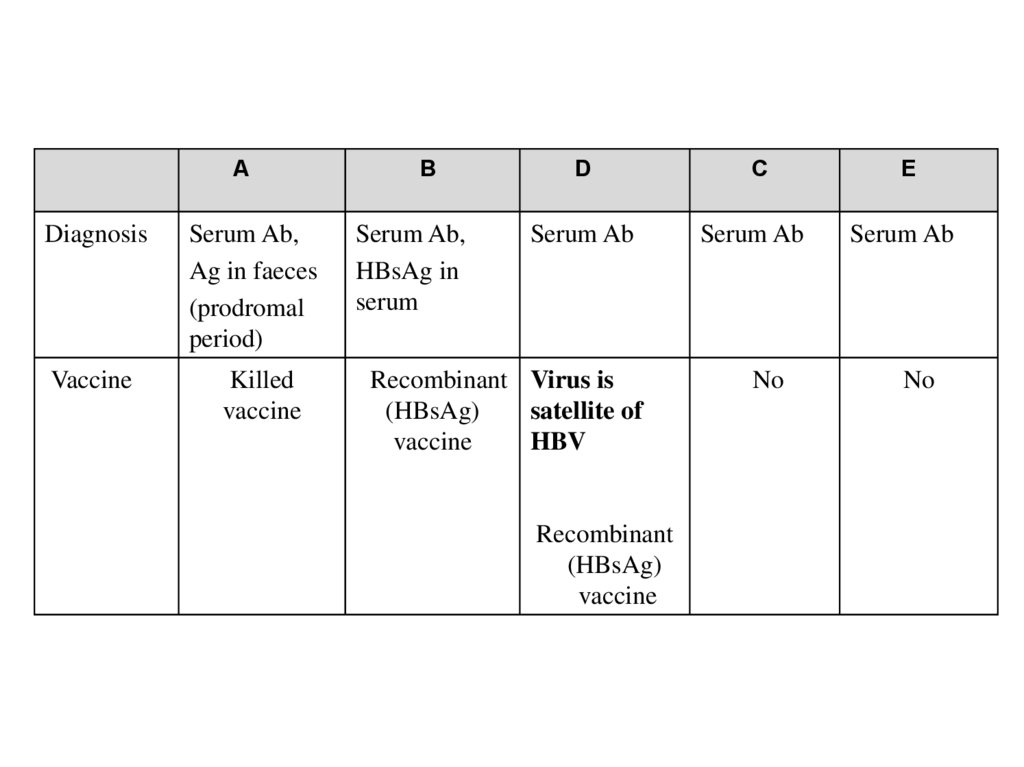
ADiagnosis
Serum Ab,
Ag in faeces
(prodromal
period)
Vaccine
Killed
vaccine
B
Serum Ab,
HBsAg in
serum
D
C
E
Serum Ab
Serum Ab
Serum Ab
No
No
Recombinant Virus is
(HBsAg)
satellite of
vaccine
HBV
Recombinant
(HBsAg)
vaccine
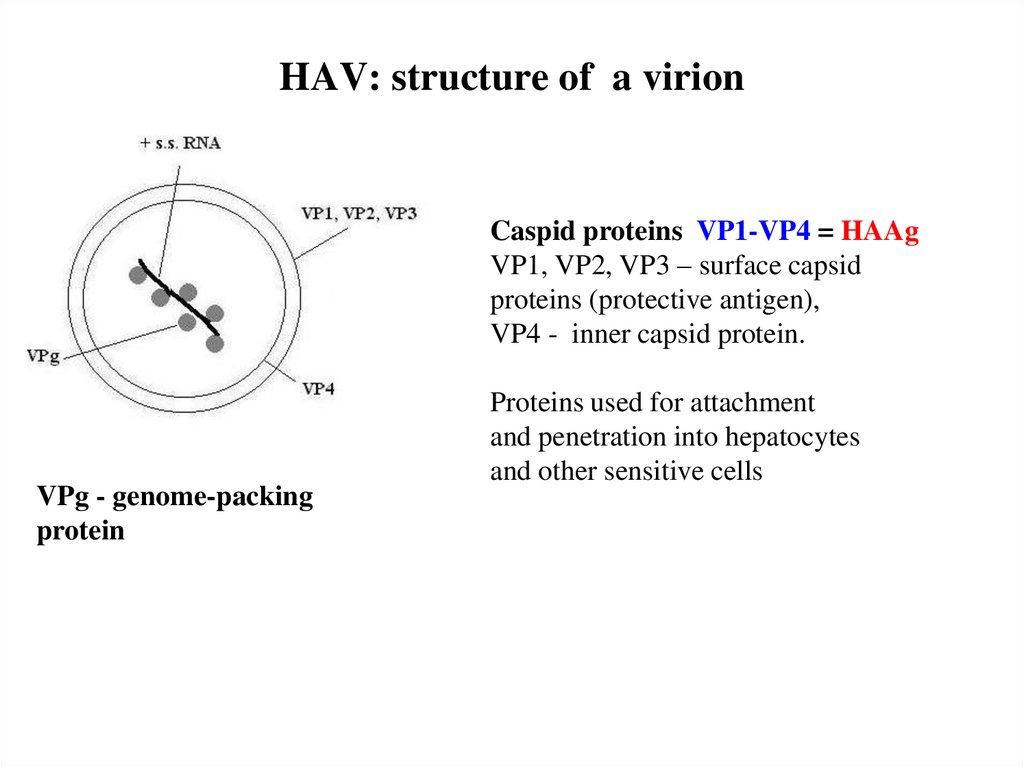
9. HAV: structure of a virion
Caspid proteins VP1-VP4 = HAAgVP1, VP2, VP3 – surface capsid
proteins (protective antigen),
VP4 - inner capsid protein.
VPg - genome-packing
protein
Proteins used for attachment
and penetration into hepatocytes
and other sensitive cells
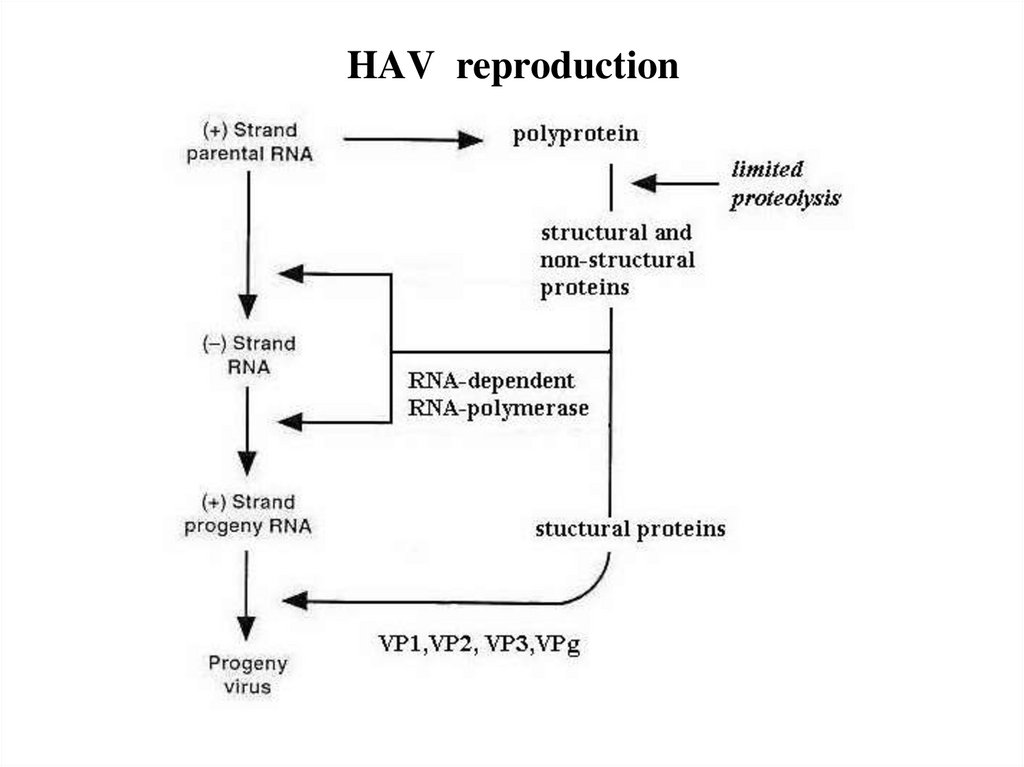
10. HAV reproduction
11.
12.
13.
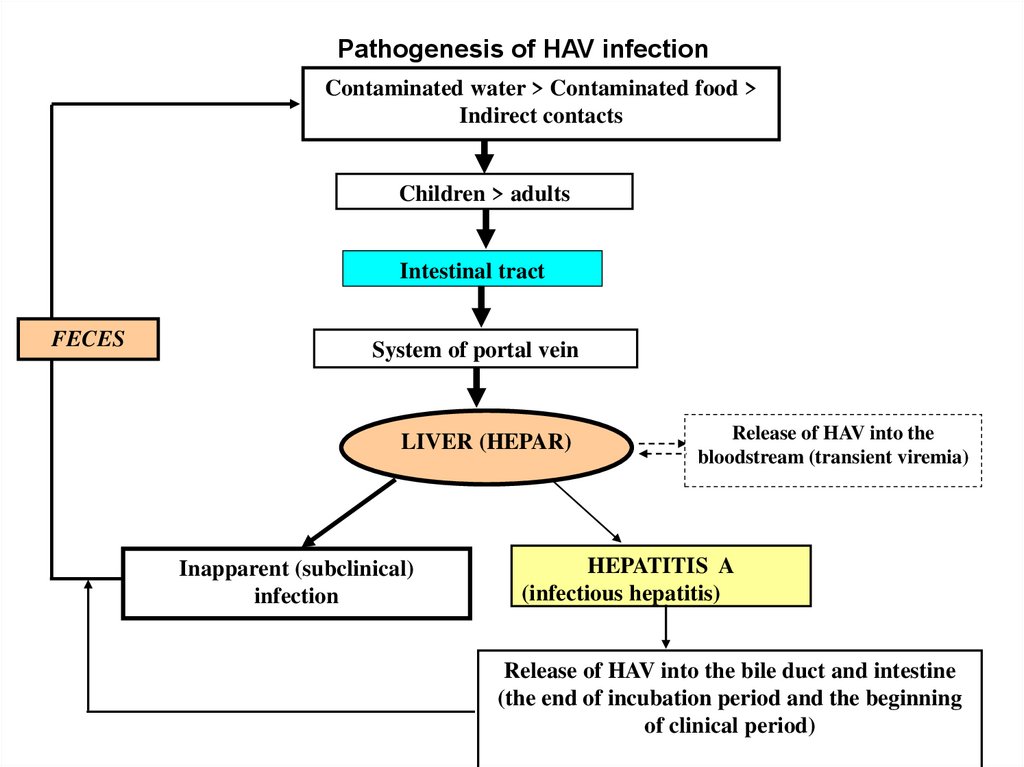
Pathogenesis of HAV infectionContaminated water > Contaminated food >
Indirect contacts
Children > adults
Intestinal tract
FECES
System of portal vein
LIVER (HEPAR)
Inapparent (subclinical)
infection
Release of HAV into the
bloodstream (transient viremia)
HEPATITIS A
(infectious hepatitis)
Release of HAV into the bile duct and intestine
(the end of incubation period and the beginning
of clinical period)
14.
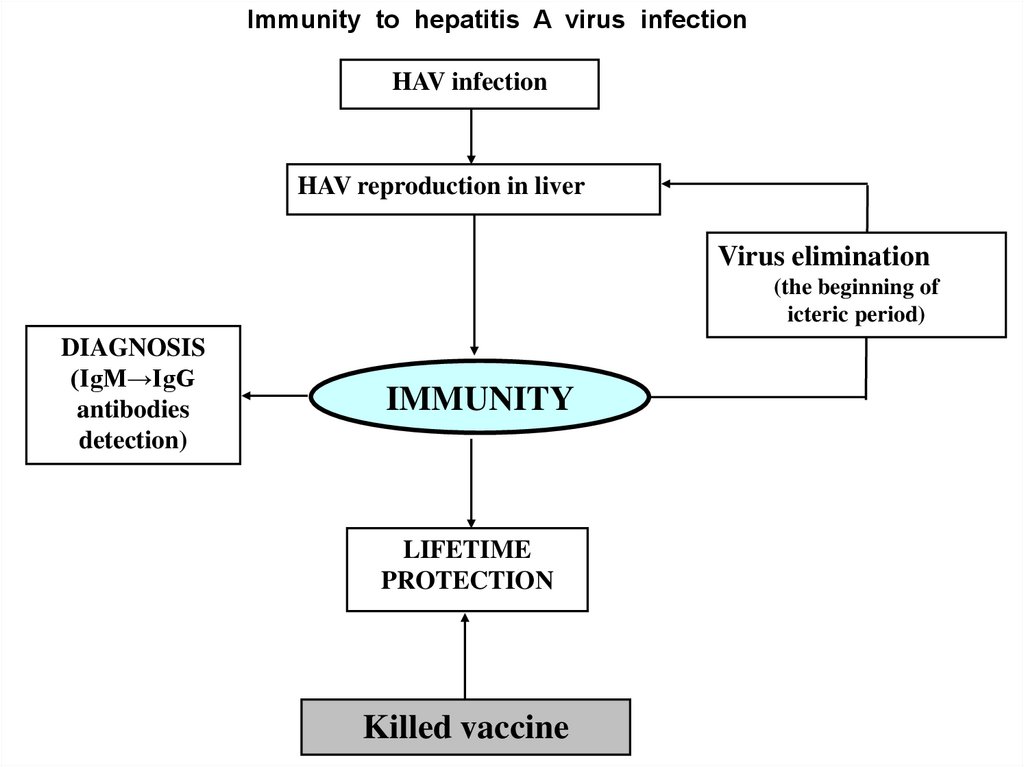
Immunity to hepatitis A virus infectionHAV infection
HAV reproduction in liver
Virus elimination
(the beginning of
icteric period)
DIAGNOSIS
(IgM→IgG
antibodies
detection)
IMMUNITY
LIFETIME
PROTECTION
Killed vaccine
15.
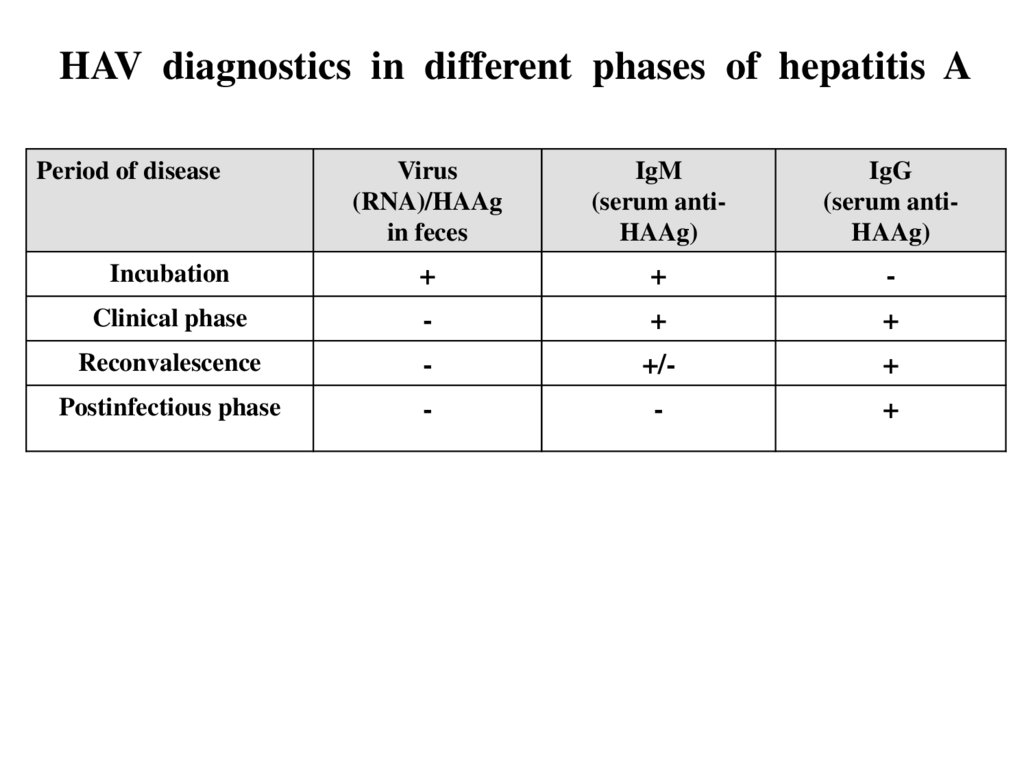
HAV diagnostics in different phases of hepatitis APeriod of disease
Virus
(RNA)/HAAg
in feces
IgM
(serum antiHAAg)
IgG
(serum antiHAAg)
Incubation
+
+
-
Clinical phase
-
+
+
Reconvalescence
-
+/-
+
Postinfectious phase
-
-
+
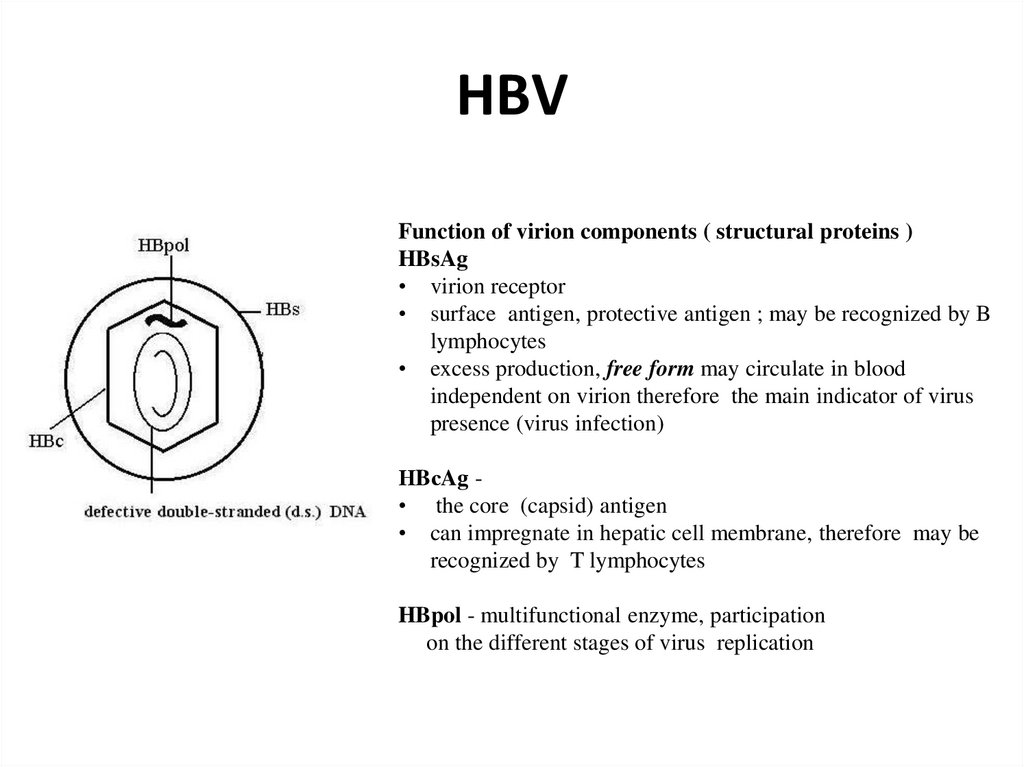
16. Hepatitis В virus (HBV)
17. HBV
Function of virion components ( structural proteins )HBsAg
• virion receptor
• surface antigen, protective antigen ; may be recognized by B
lymphocytes
• excess production, free form may circulate in blood
independent on virion therefore the main indicator of virus
presence (virus infection)
НBсAg • the core (capsid) antigen
• can impregnate in hepatic cell membrane, therefore may be
recognized by T lymphocytes
HBpol - multifunctional enzyme, participation
on the different stages of virus replication
18. Characteristics of nonstructural proteins (antigens) of HBV
HBеAg• Result of proteolysis/НBс Ag
• Can release in the blood , concentration in the blood
correlates with virus replication activity
HBxAg
• Possibility of autonomic synthesis
• May migrate into hepatocyte membrane, may be recognized
as T-epitopes by cytotoxic T lympocytes (cTL).
• can release in the blood
• can induce oncogenous transformation in hepatocytes
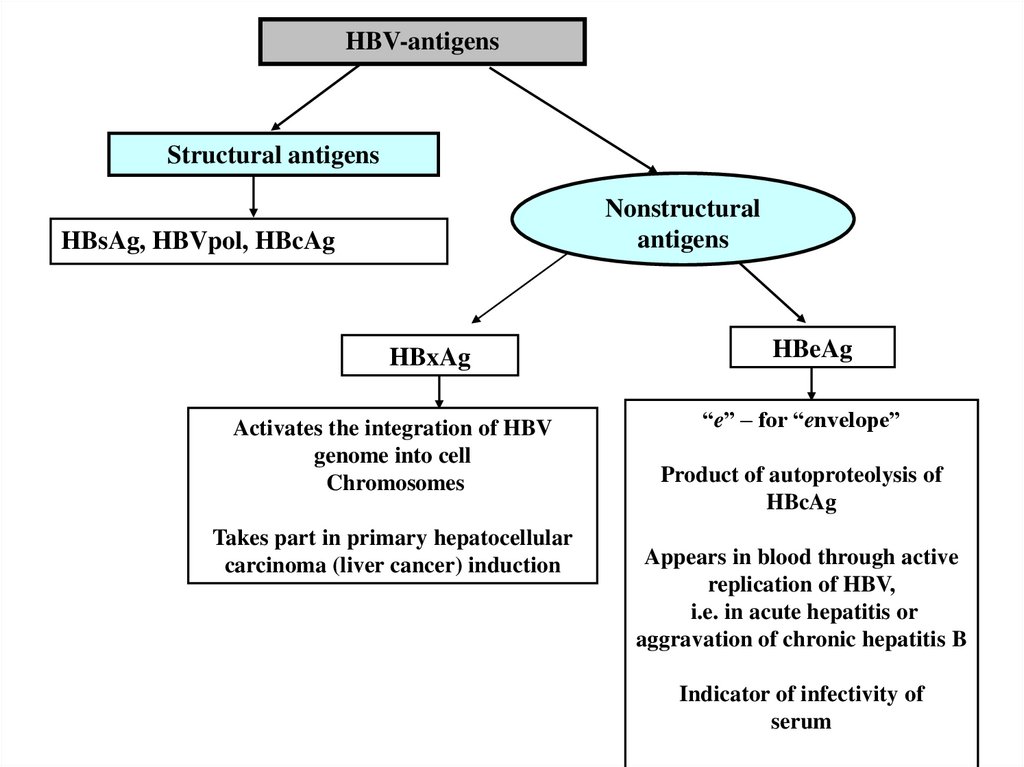
19.
HBV-antigensStructural antigens
Nonstructural
antigens
HBsAg, HBVpol, HBcAg
HBxAg
Activates the integration of HBV
genome into cell
Chromosomes
Takes part in primary hepatocellular
carcinoma (liver cancer) induction
HBeAg
“e” – for “envelope”
Product of autoproteolysis of
HBcAg
Appears in blood through active
replication of HBV,
i.e. in acute hepatitis or
aggravation of chronic hepatitis B
Indicator of infectivity of
serum
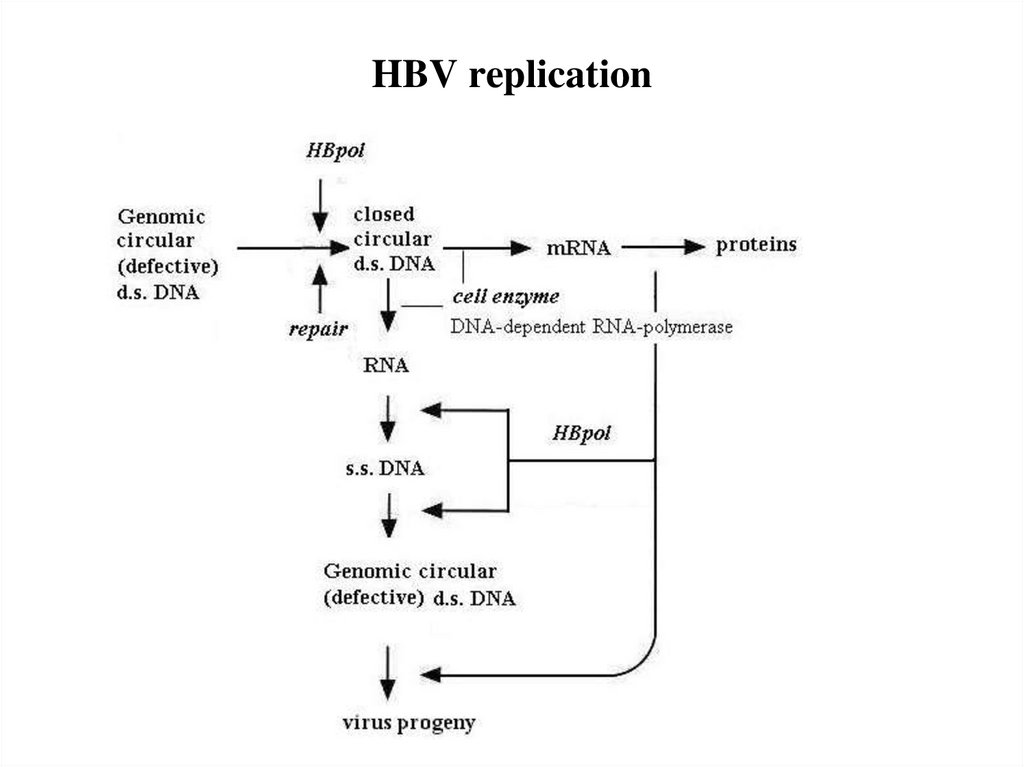
20. HBV replication
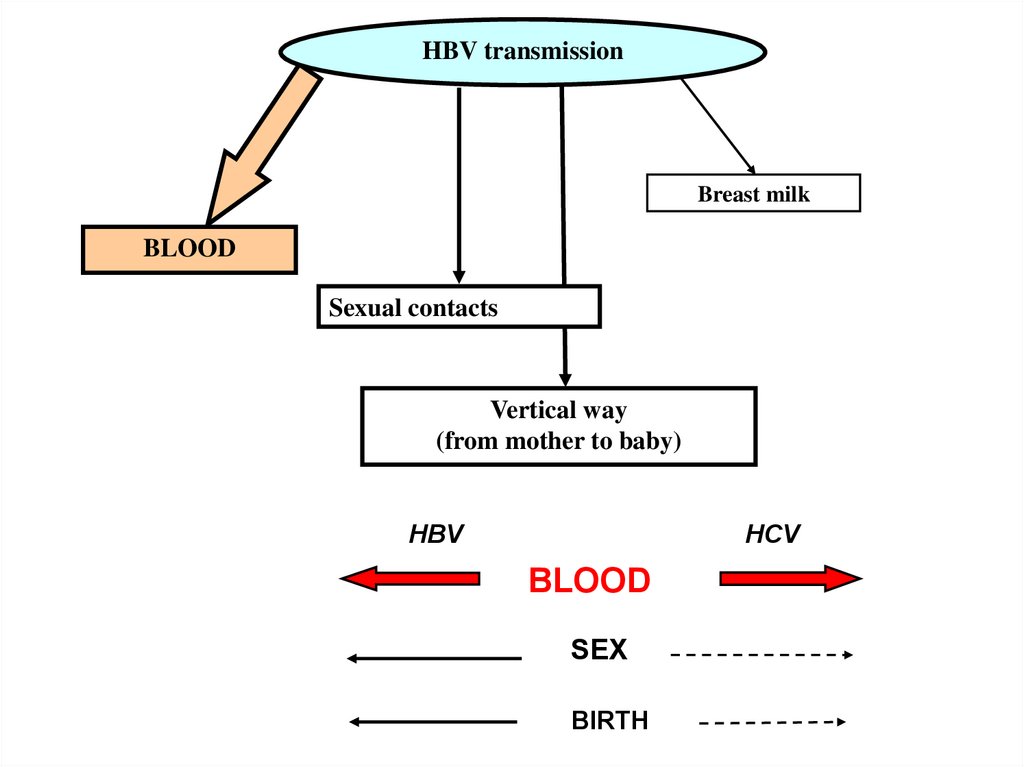
21.
HBV transmissionBreast milk
BLOOD
Sexual contacts
Vertical way
(from mother to baby)
HBV
HCV
BLOOD
SEX
BIRTH
22.
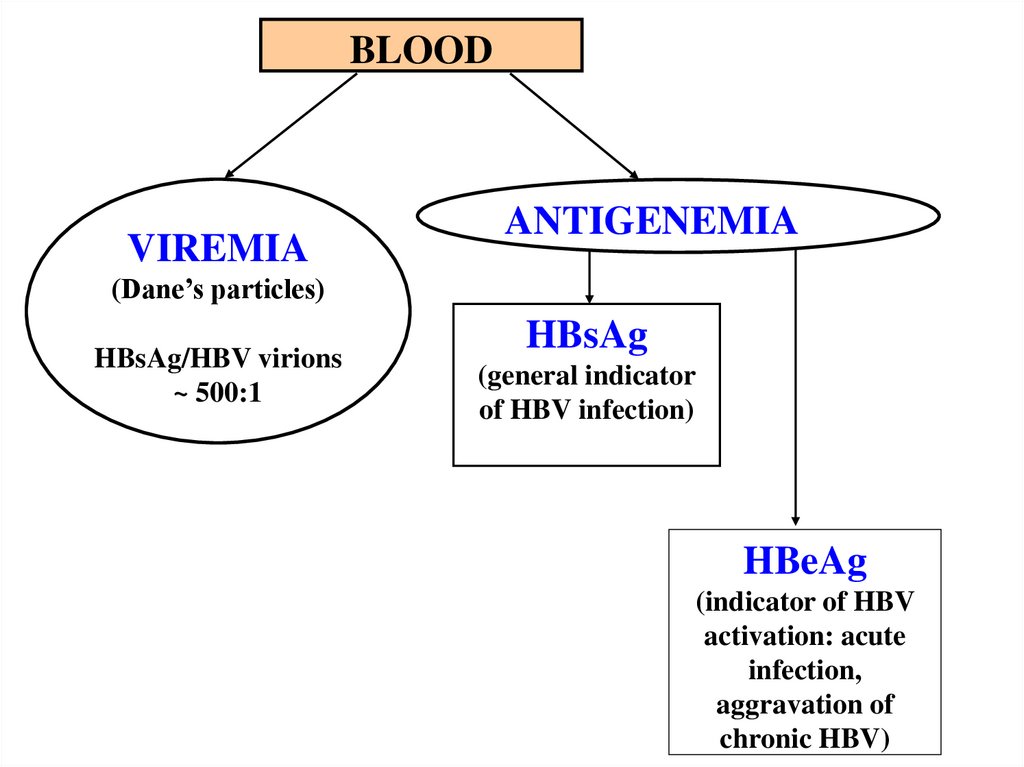
BLOODVIREMIA
ANTIGENEMIA
(Dane’s particles)
HBsAg/HBV virions
~ 500:1
HBsAg
(general indicator
of HBV infection)
HBeAg
(indicator of HBV
activation: acute
infection,
aggravation of
chronic HBV)
23.
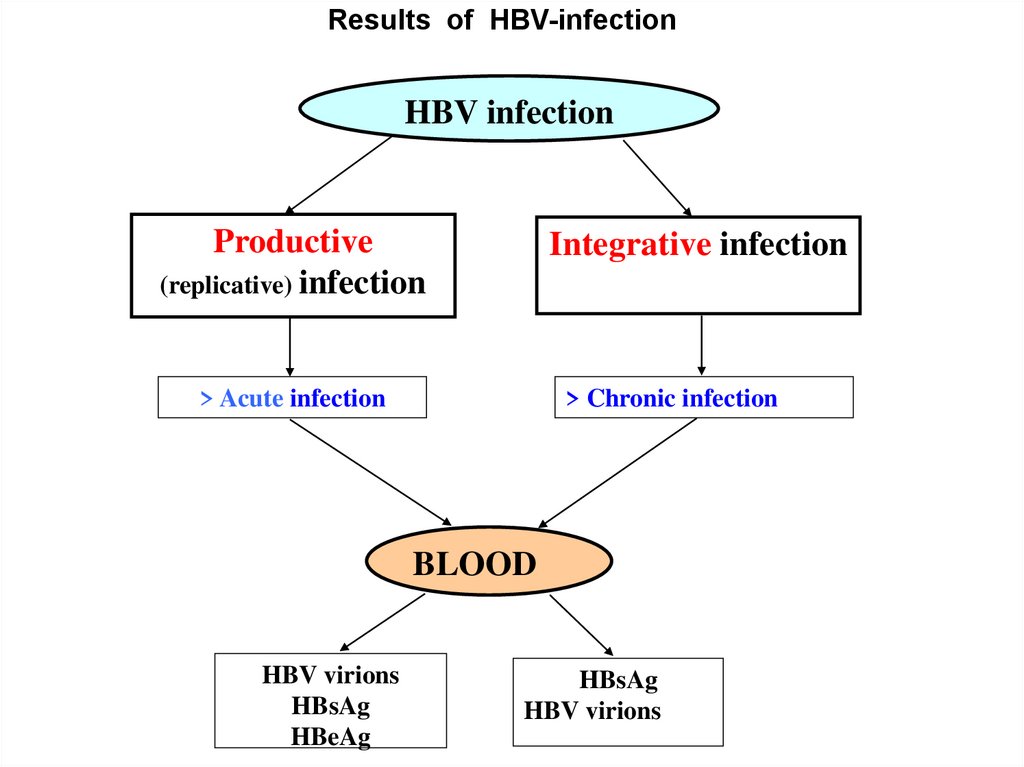
Results of HBV-infectionHBV infection
Productive
(replicative) infection
Integrative infection
> Acute infection
> Chronic infection
BLOOD
HBV virions
HBsAg
HBeAg
HBsAg
HBV virions
24.
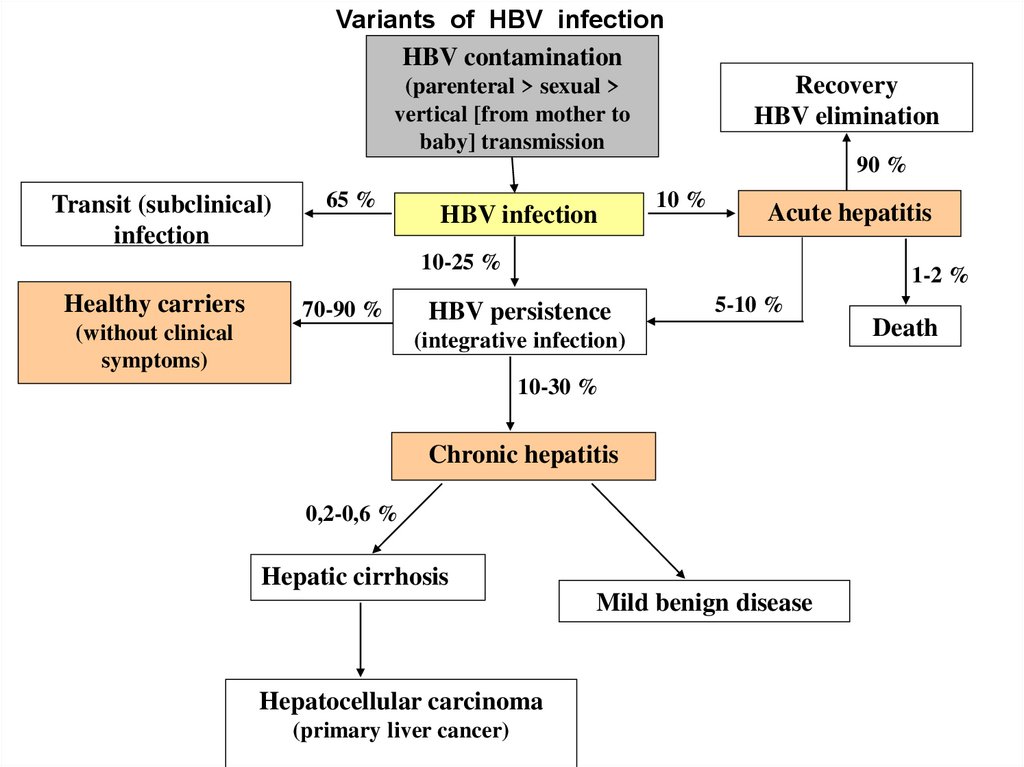
Variants of HBV infectionHBV contamination
Recovery
HBV elimination
(parenteral > sexual >
vertical [from mother to
baby] transmission
90 %
Transit (subclinical)
infection
65 %
HBV infection
10 %
Acute hepatitis
10-25 %
Healthy carriers
70-90 %
(without clinical
symptoms)
1-2 %
HBV persistence
5-10 %
(integrative infection)
10-30 %
Chronic hepatitis
0,2-0,6 %
Hepatic cirrhosis
Mild benign disease
Hepatocellular carcinoma
(primary liver cancer)
Death
25.
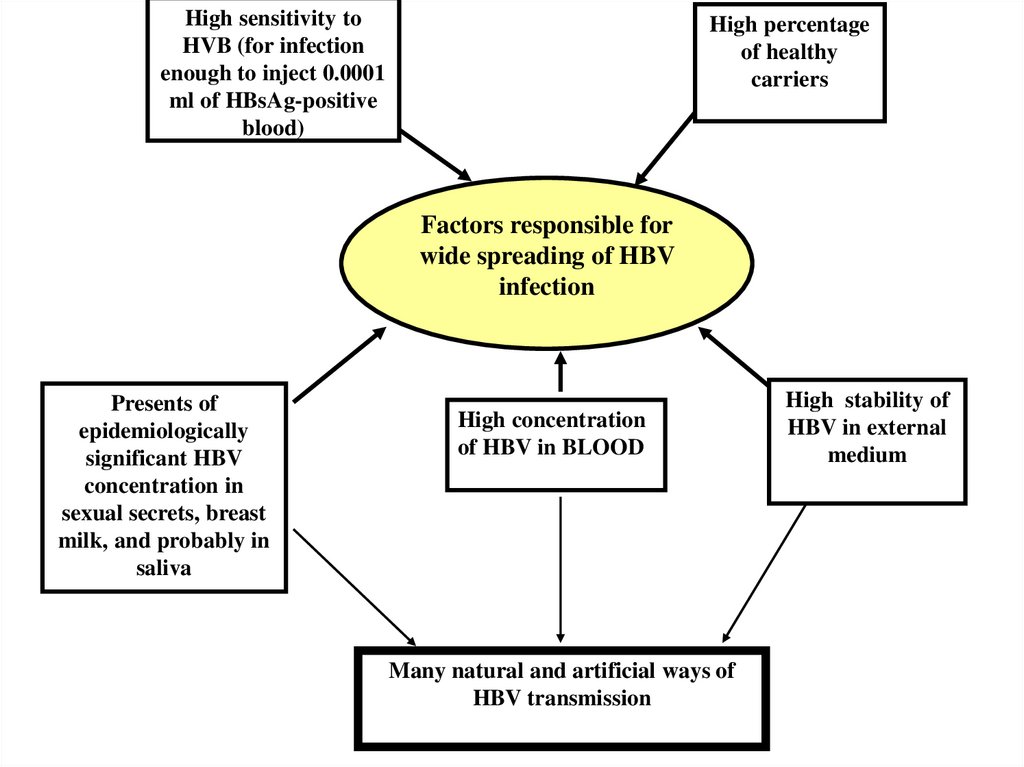
High sensitivity toHVB (for infection
enough to inject 0.0001
ml of HBsAg-positive
blood)
High percentage
of healthy
carriers
Factors responsible for
wide spreading of HBV
infection
Presents of
epidemiologically
significant HBV
concentration in
sexual secrets, breast
milk, and probably in
saliva
High concentration
of HBV in BLOOD
Many natural and artificial ways of
HBV transmission
High stability of
HBV in external
medium
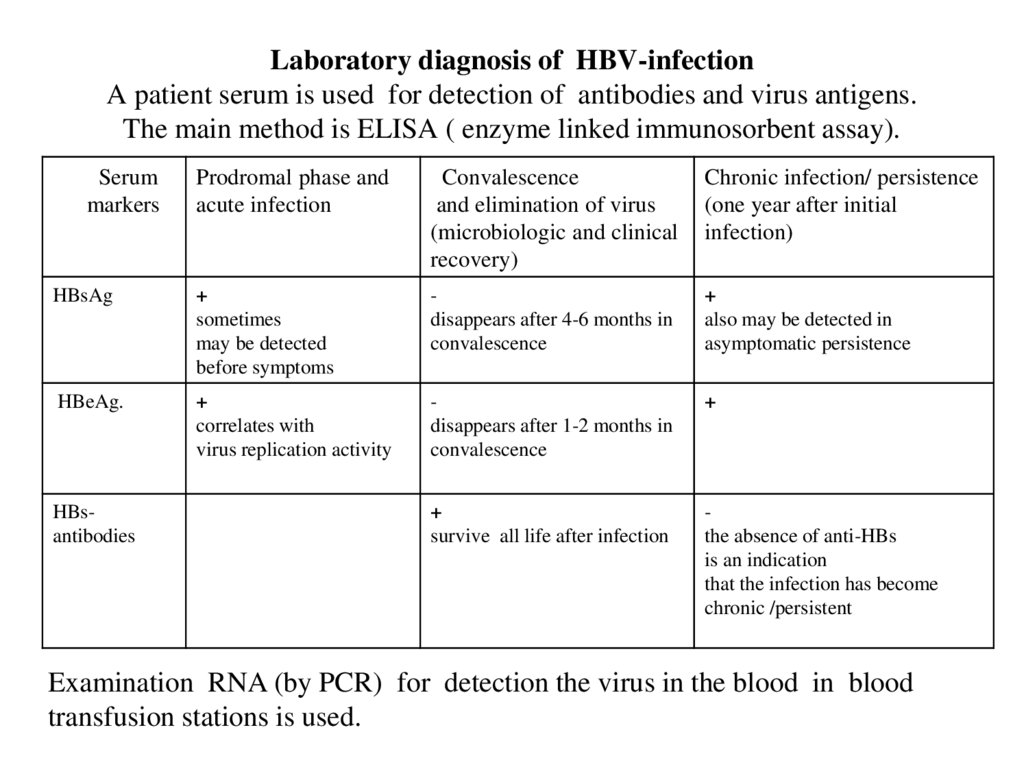
26. Laboratory diagnosis of HBV-infection A patient serum is used for detection of antibodies and virus antigens. The main method
is ELISA ( enzyme linked immunosorbent assay).Serum
markers
Prodromal phase and
acute infection
Convalescence
and elimination of virus
(microbiologic and clinical
recovery)
Chronic infection/ persistence
(one year after initial
infection)
HBsAg
+
sometimes
may be detected
before symptoms
disappears after 4-6 months in
convalescence
+
also may be detected in
asymptomatic persistence
HBeAg.
+
correlates with
virus replication activity
disappears after 1-2 months in
convalescence
+
+
survive all life after infection
the absence of anti-HBs
is an indication
that the infection has become
chronic /persistent
HBsantibodies
Examination RNA (by PCR) for detection the virus in the blood in blood
transfusion stations is used.
27. Hepatitis C virus (HCV)
Pathogenetic and epidemiologic similarity with HCV.Asymptomatic chronic infection is most often. Complete elimination
of viruses after acute phase is detected in 10-15% patients only.
Mechanism of persistance: non-intergative virogeny.
High antigenic variability. Vaccination is not available.
Slow antibody synthesis ( slow seroconversion) is due to virus
mutation. Therefore ”seronegative window” (undetectable period for
antibodies) is 4-6 months.
Examination RNA (by PCR) for detection the virus in the blood in
blood transfusion stations is used.



 Медицина
Медицина