Похожие презентации:
Сложные эфиры
1.
2.
3.
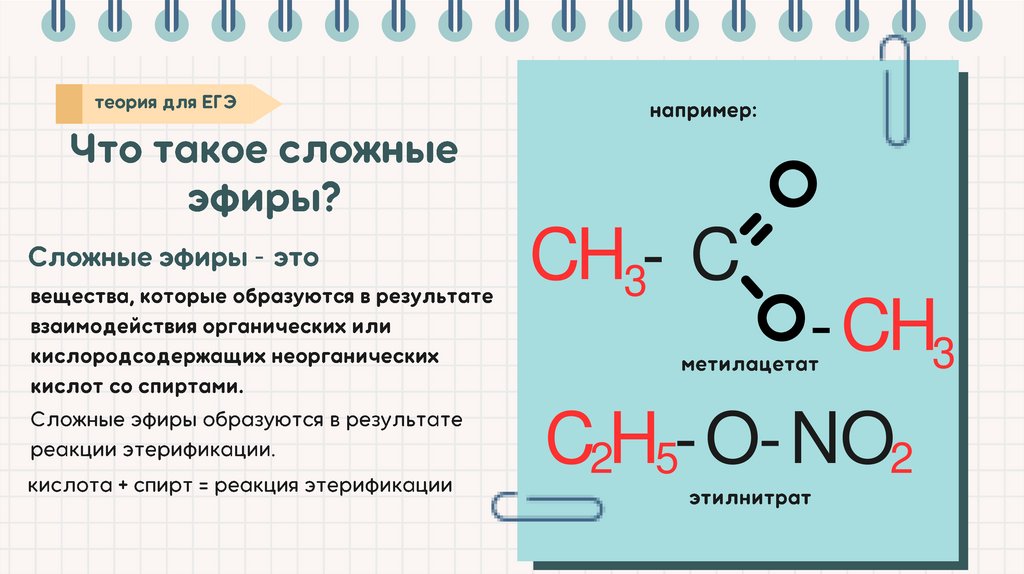
-CH3- C
-CH3
C2H5-O-NO2
4.
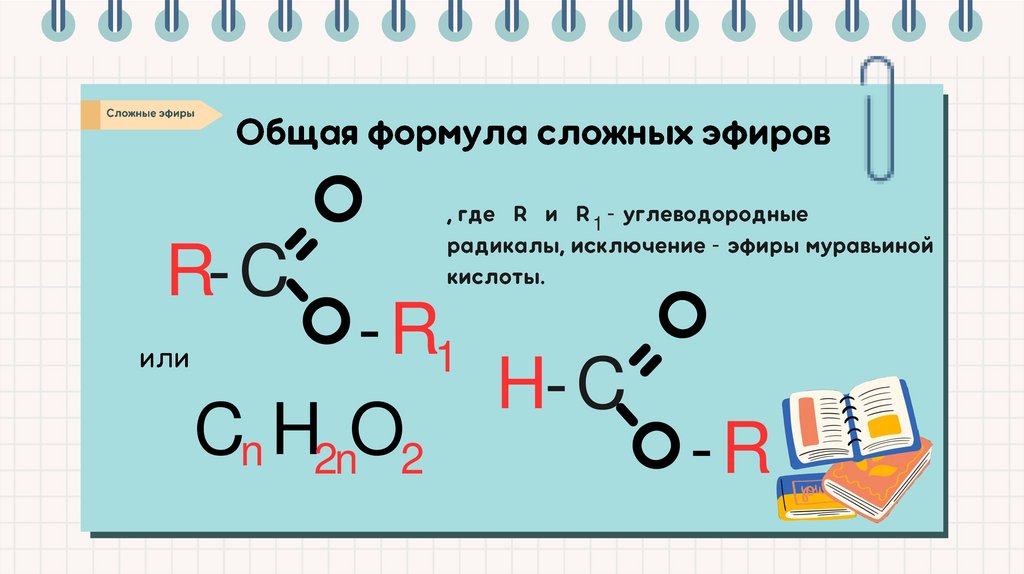
1 -R-C
-
-R 1
H-C
Cn H2nO2
-R
5.
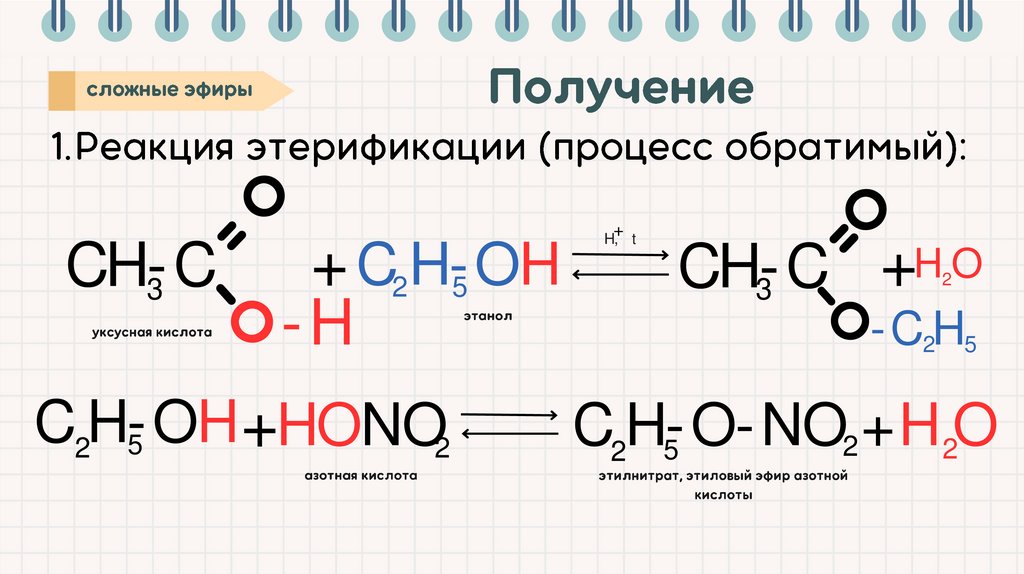
+CH-C
3
+ C2 H-OH
5
-H
C 2H-OH
+ HONO2
5
H, t
H
O
+
CH-C
3
2
-C 2H5
C2H-O-NO
+
H
O
2
2
5
6.
+H
2O
+ -CH
-CH
3
CH 3
CH-O-NO
2
2
2H
O
+
2
CH-O-NO
2
2
-
-
CH-OH
2
2HONO
2
+
CH-OH
2
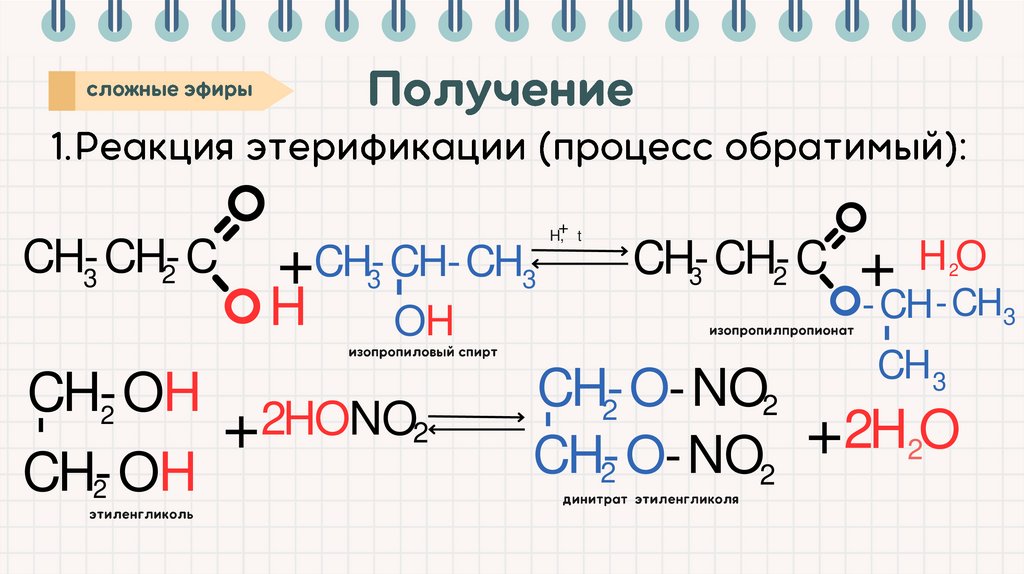
CH-CH-C
3
2
-
+ CH-CH-CH
3
3
H
OH
-
CH-CH-C
2
3
H, t
7.
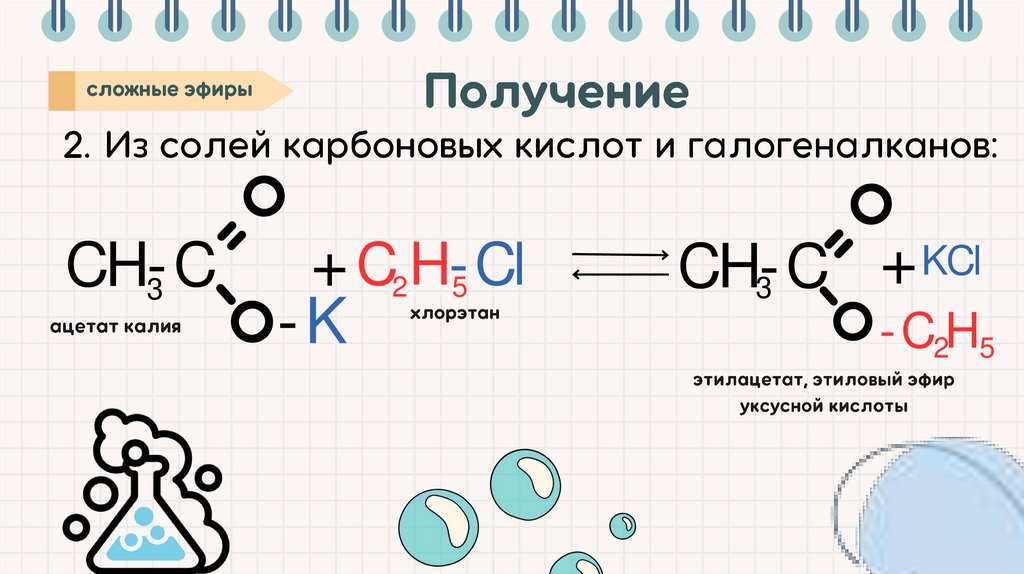
CH-C3
+ C2 H-Cl
5
-K
KCl
+
CH-C
3
-C2H5
8.
12
3
9.
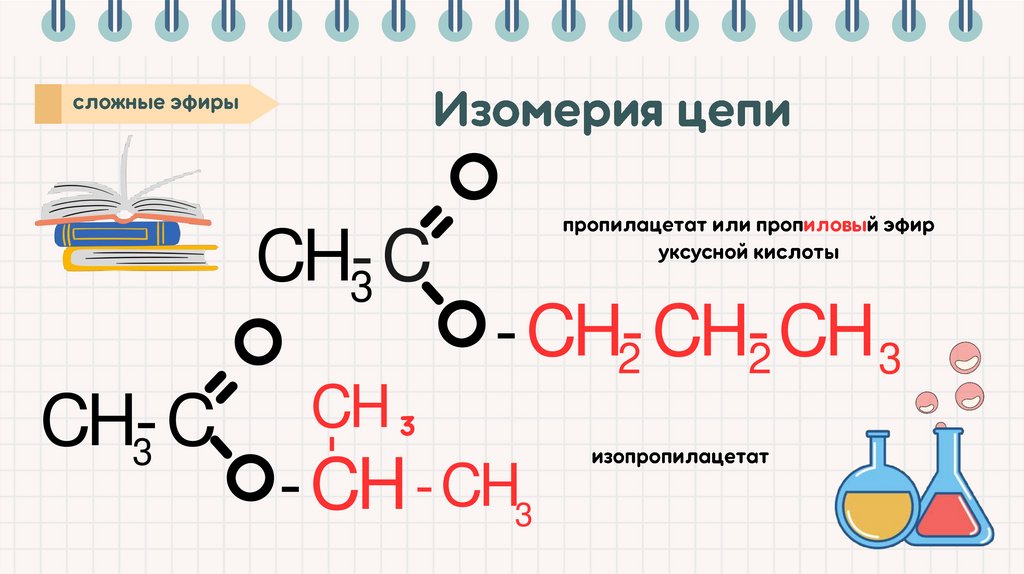
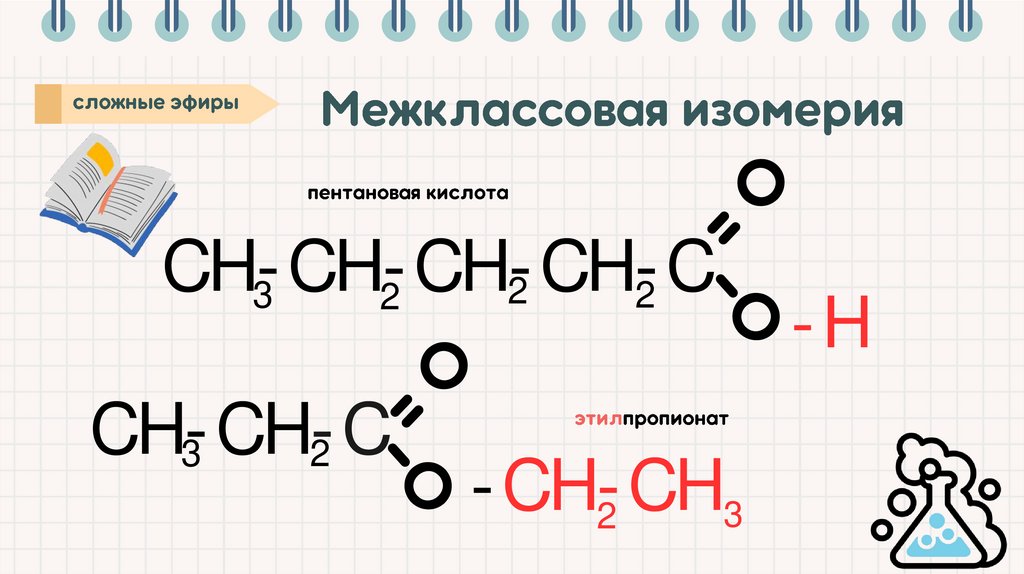
CH-C3
-CH-CH-CH
2
2
3
-
CH-C
3
CH
-CH -CH3
10.
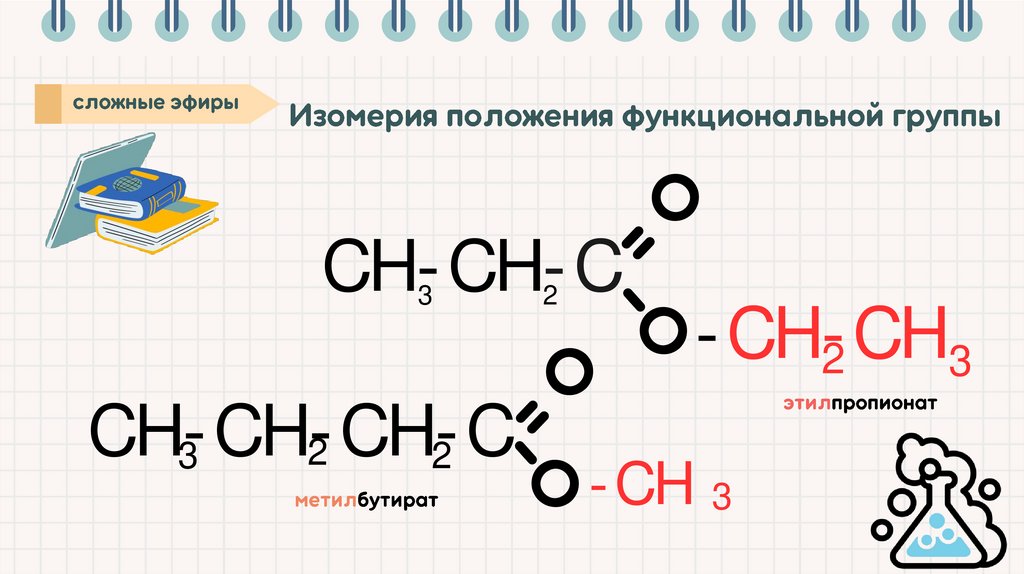
CH-CH-C3
2
-CH-CH
2
3
CH-CH-CH-C
2
3
2
-CH 3
11.
CH-CH-CH-CH-C2
3
2
2
CH-CH-C
3
2
-CH-CH
3
2
-H
12.
13.
14.
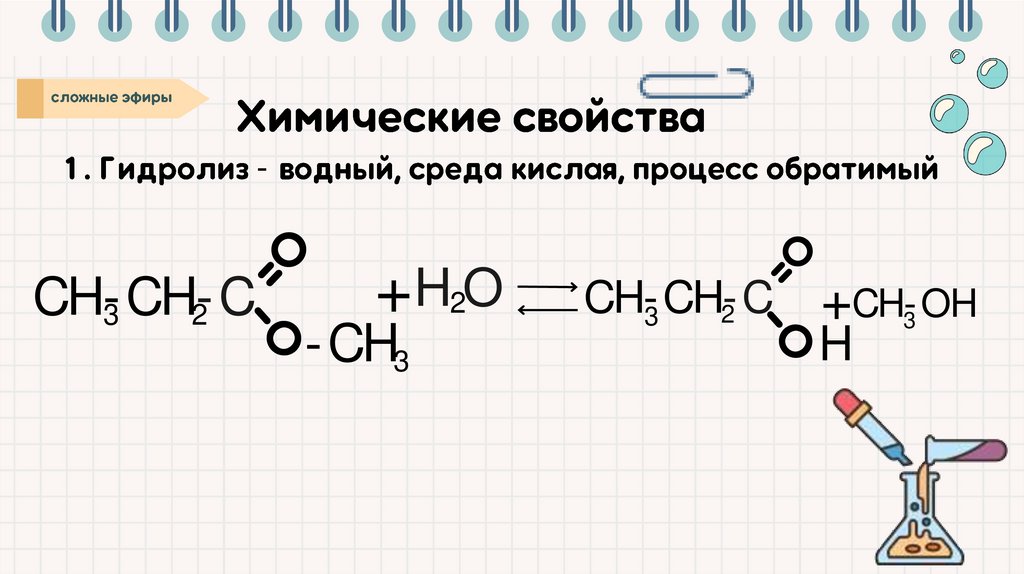
-CH-CH-C
3
2
+ H2O
-CH3
CH-CH-C
2
3
+ CH-OH
3
H
15.
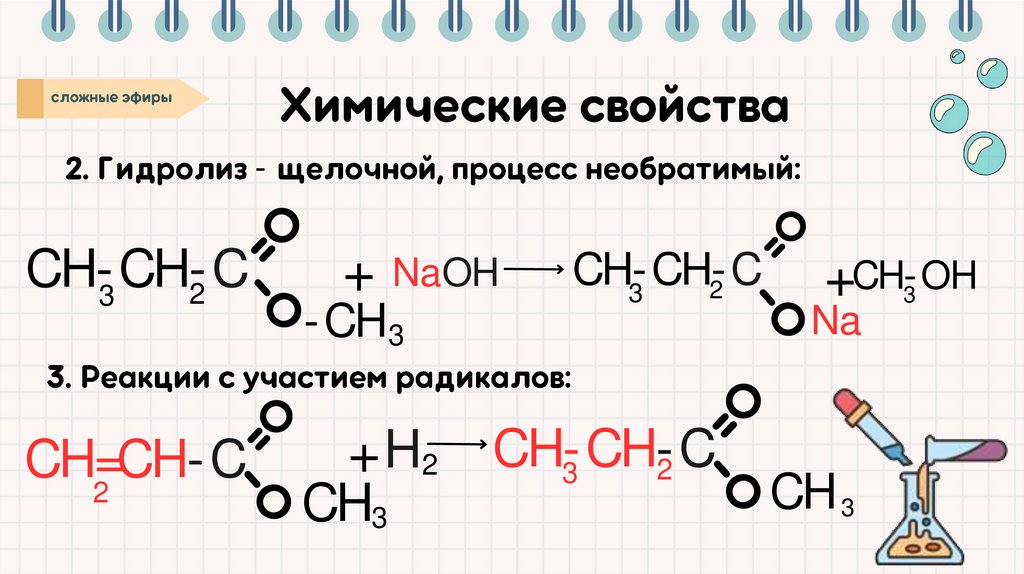
-CH-CH-C
2
3
CH=CH-C
2
+ NaOH
CH-CH-C
2
3
-CH 3
+ H2
CH3
+ CH-OH
3
Na
CH-CH-C
2
3
CH 3
16.
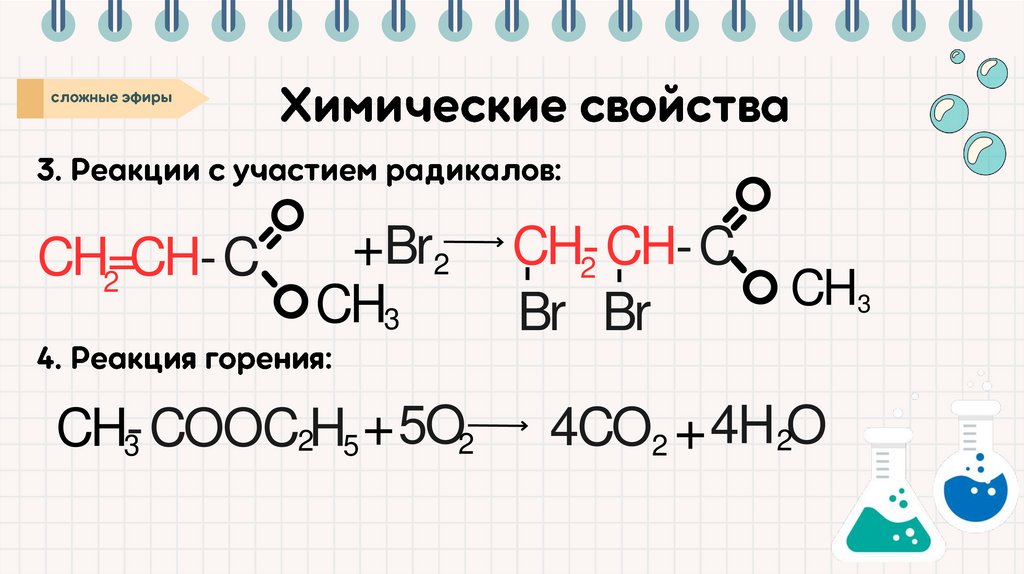
CH35O
+
CH-COOC
H
2
2
3
5
CH-CH-C
2
Br Br
-
+ Br2
-
CH=CH-C
2
CH3
4CO2 + 4H 2O





 Химия
Химия
