Похожие презентации:
Objectives of Physics. Introduction and Chapter 1
1. Introduction and Chapter 1
INTRODUCTION AND CHAPTER 12. Physics
PHYSICSFundamental Science
Concerned with the fundamental principles of the Universe
Foundation of other physical sciences
Has simplicity of fundamental concepts
Divided into five major areas
Classical Mechanics
Relativity
Thermodynamics
Electromagnetism
Optics
Quantum Mechanics
3. Objectives of Physics
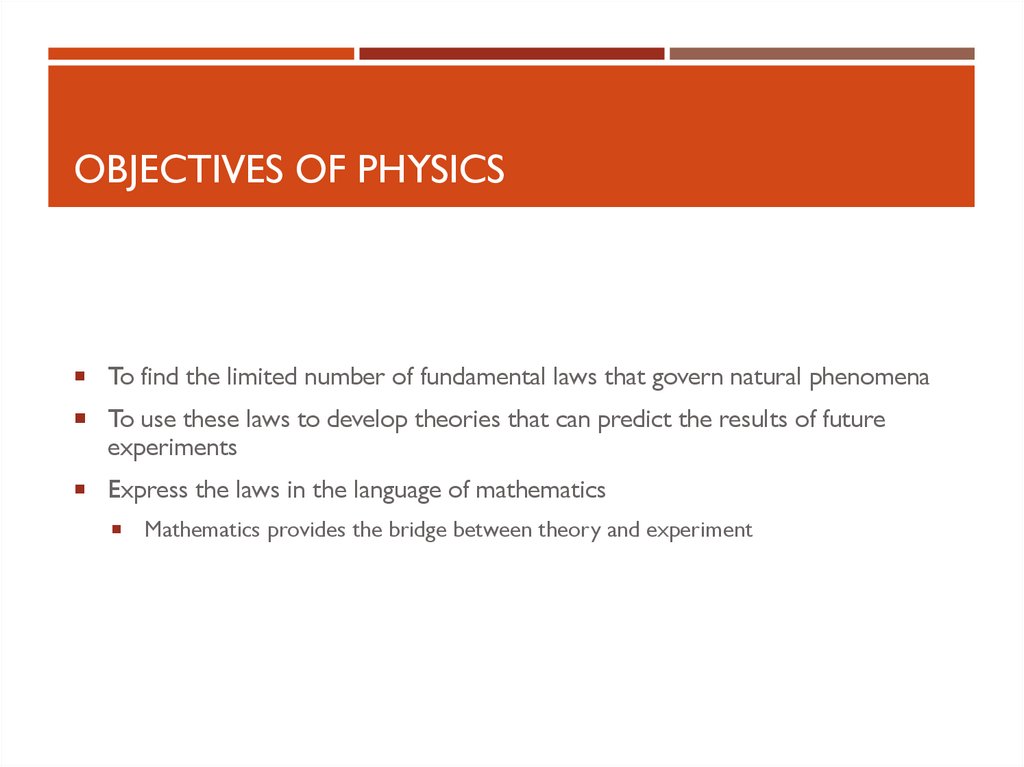
OBJECTIVES OF PHYSICSTo find the limited number of fundamental laws that govern natural phenomena
To use these laws to develop theories that can predict the results of future
experiments
Express the laws in the language of mathematics
Mathematics provides the bridge between theory and experiment
4. Measurements
MEASUREMENTSUsed to describe natural phenomena
Needs defined standards
Characteristics of standards for measurements
Readily accessible
Possess some property that can be measured reliably
Must yield the same results when used by anyone anywhere
Cannot change with time
5. Standards of Fundamental Quantities
STANDARDS OF FUNDAMENTAL QUANTITIESStandardized systems
Agreed upon by some authority, usually a governmental body
SI – Systéme International
Agreed to in 1960 by an international committee
Main system used in this text
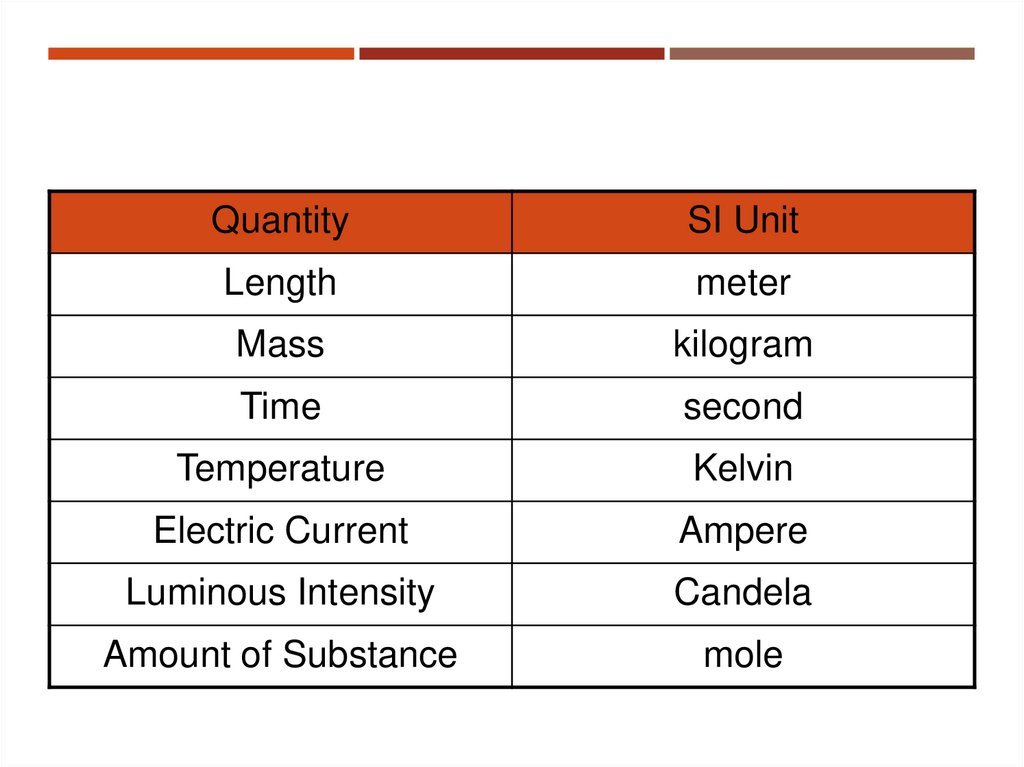
6. Fundamental Quantities and Their Units
FUNDAMENTAL QUANTITIES AND THEIRUNITS
Quantity
SI Unit
Length
meter
Mass
kilogram
Time
second
Temperature
Kelvin
Electric Current
Ampere
Luminous Intensity
Candela
Amount of Substance
mole
7. Quantities Used in Mechanics
QUANTITIES USED IN MECHANICSIn mechanics, three basic quantities are used
Length
Mass
Time
Will also use derived quantities
These are other quantities that can be expressed in terms of the basic quantities
Example: Area is the product of two lengths
Area is a derived quantity
Length is the fundamental quantity
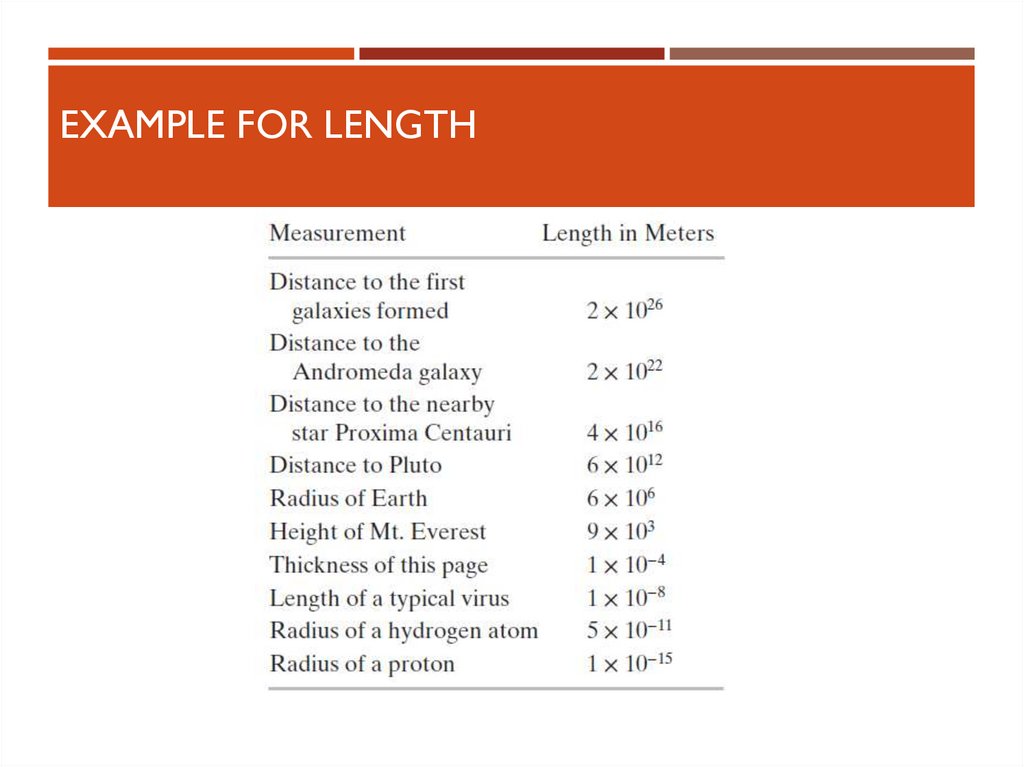
8. Length
LENGTHLength is the distance between two points in space
Units
SI – meter, m
Defined in terms of a meter – the distance traveled by light in a vacuum during a
given time
See Table for some examples of lengths
9. Example for Length
EXAMPLE FOR LENGTH10. Mass
MASSUnits
SI – kilogram, kg
Defined in terms of a kilogram, based on a specific cylinder kept at the International
Bureau of Standards
See Table for masses of various objects
11. Examples for Mass
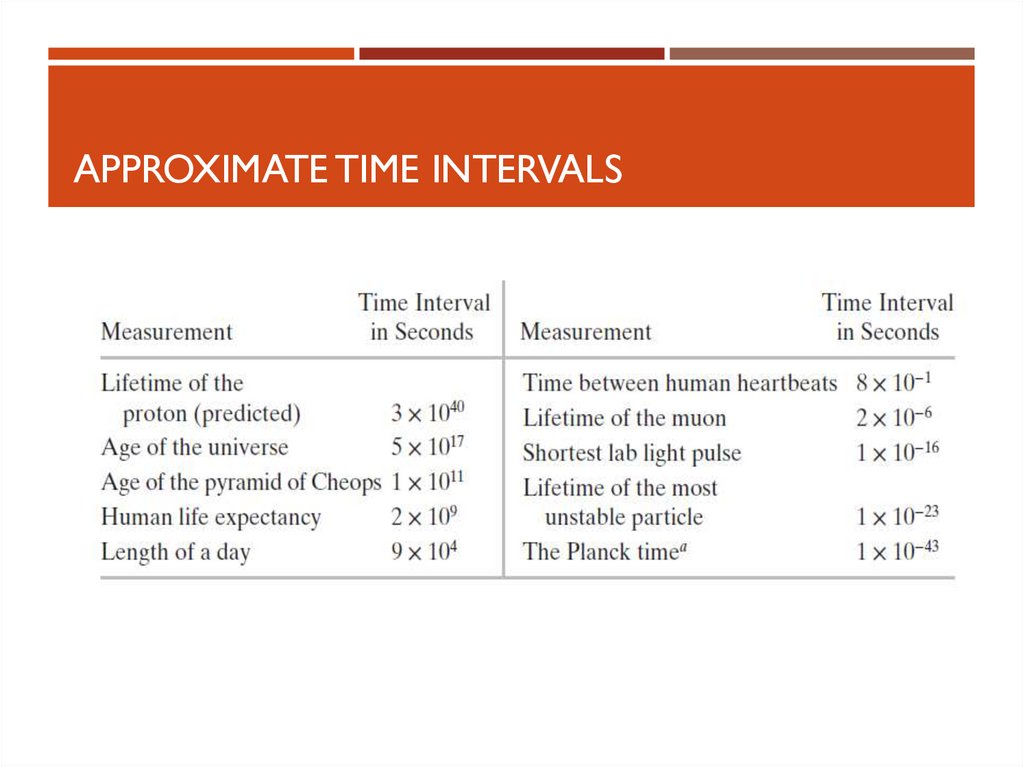
EXAMPLES FOR MASS12. Time
TIMEUnits
seconds, s
Defined in terms of the oscillation of radiation from a cesium atom
See Table for some approximate time intervals
13. Approximate Time Intervals
APPROXIMATE TIME INTERVALS14. Number Notation
NUMBER NOTATIONWhen writing out numbers with many digits, spacing in groups of three will be
used
No commas
Standard international notation
Examples:
25 100
5.123 456 789 12
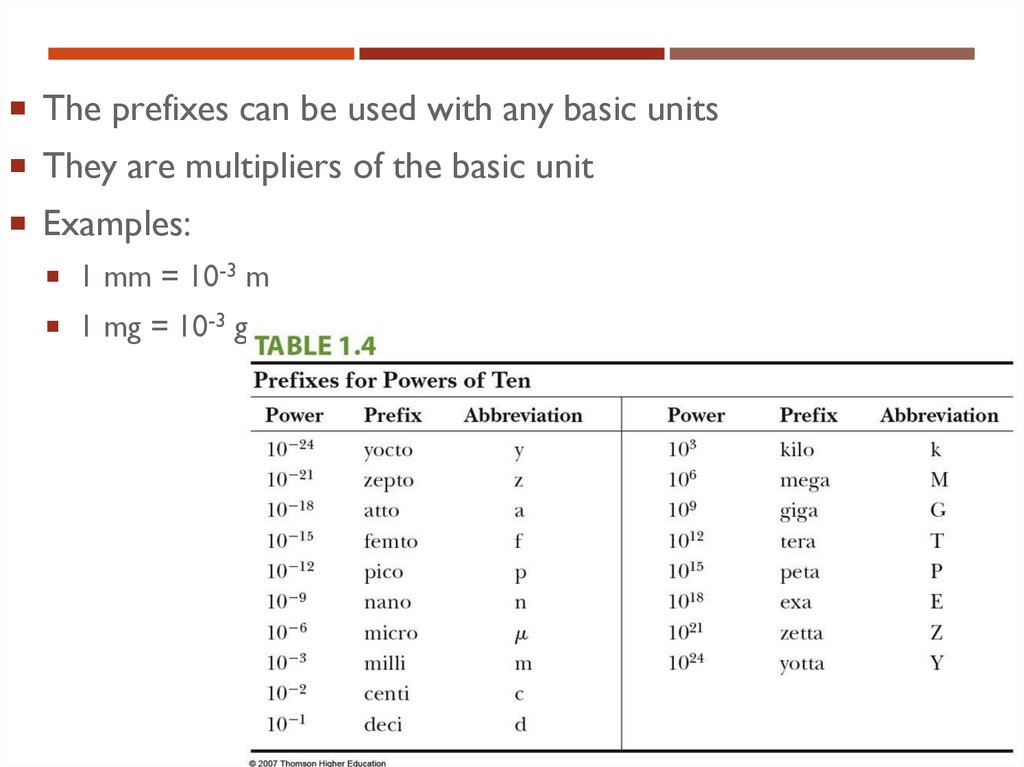
15. Prefixes
PREFIXESPrefixes correspond to powers of 10
Each prefix has a specific name
Each prefix has a specific abbreviation
16. Prefixes, cont.
The prefixes can be used with any basic unitsPREFIXES, CONT.
They are multipliers of the basic unit
Examples:
1 mm = 10-3 m
1 mg = 10-3 g
17. Model Building
MODEL BUILDINGA model is a system of physical components
Useful when you cannot interact directly with the phenomenon
Identifies the physical components
Makes predictions about the behavior of the system
The predictions will be based on interactions among the components and/or
Based on the interactions between the components and the environment
18. Modeling Technique
MODELING TECHNIQUEImportant technique is to build a model for a problem
Identify a system of physical components for the problem
Make predictions of the behavior of the system based on the interactions among the
components and/or the components and the environment
Important problem-solving technique to develop
19. Basic Quantities and Their Dimension
BASIC QUANTITIES AND THEIR DIMENSIONDimension has a specific meaning – it denotes the physical nature of a quantity
Dimensions are denoted with square brackets
Length [L]
Mass [M]
Time [T]
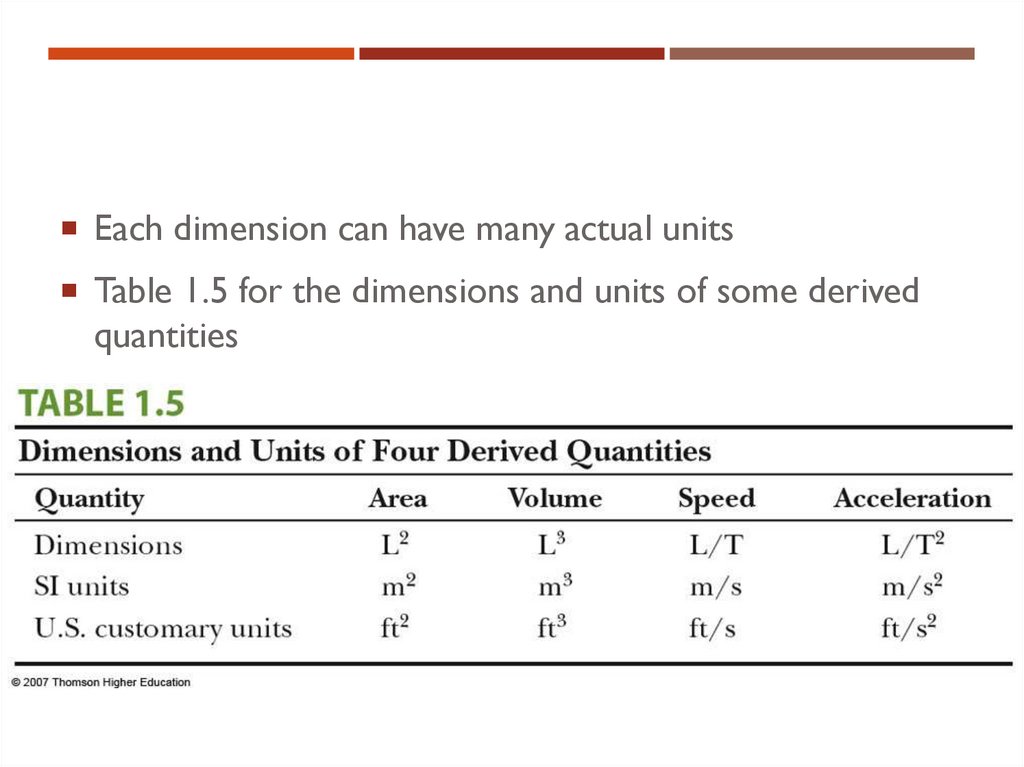
20. Dimensions and Units
DIMENSIONS AND UNITSEach dimension can have many actual units
Table 1.5 for the dimensions and units of some derived
quantities
21. Dimensional Analysis
DIMENSIONAL ANALYSISTechnique to check the correctness of an equation or to
assist in deriving an equation
Dimensions (length, mass, time, combinations) can be
treated as algebraic quantities
add, subtract, multiply, divide
Both sides of equation must have the same dimensions
Any relationship can be correct only if the dimensions on
both sides of the equation are the same
Cannot give numerical factors: this is its limitation
22. Dimensional Analysis, example
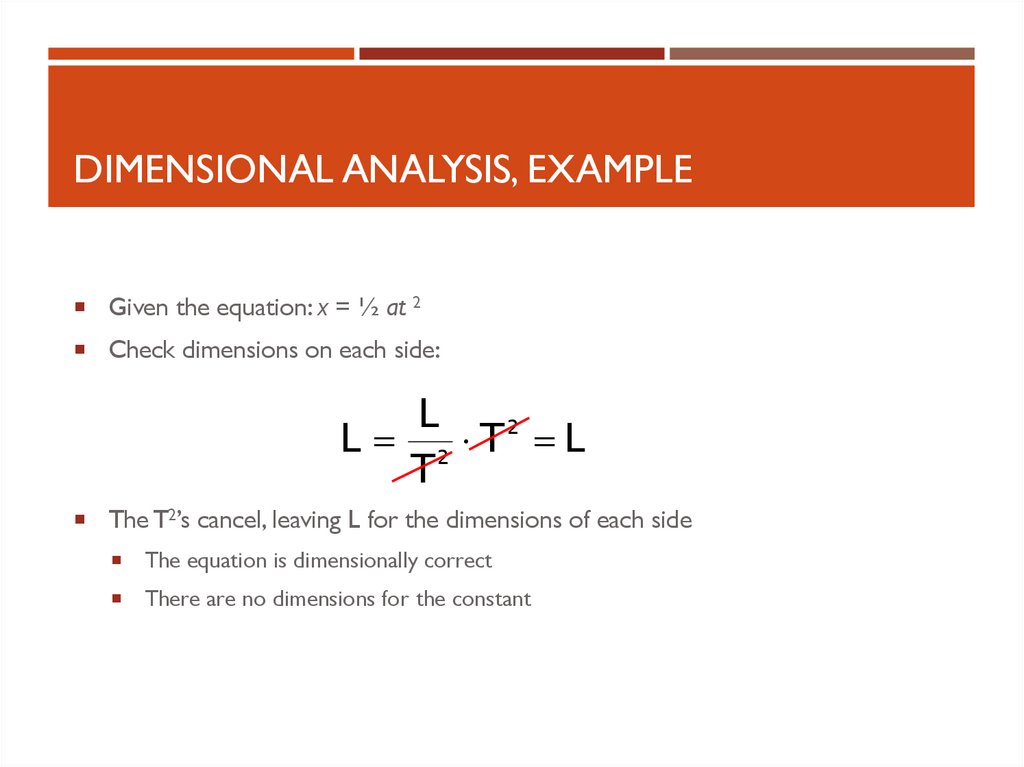
DIMENSIONAL ANALYSIS, EXAMPLEGiven the equation: x = ½ at 2
Check dimensions on each side:
L
L 2 T2 L
T
The T2’s cancel, leaving L for the dimensions of each side
The equation is dimensionally correct
There are no dimensions for the constant
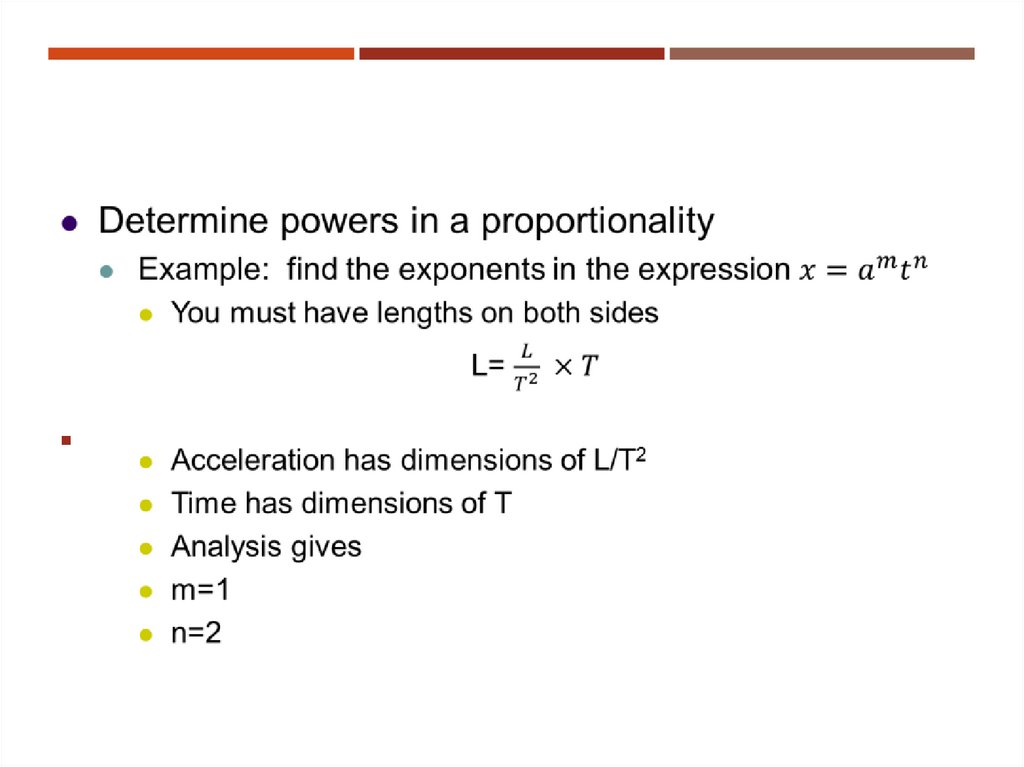
23. Dimensional Analysis to Determine a Power Law
DIMENSIONAL ANALYSIS TO DETERMINE APOWER LAW
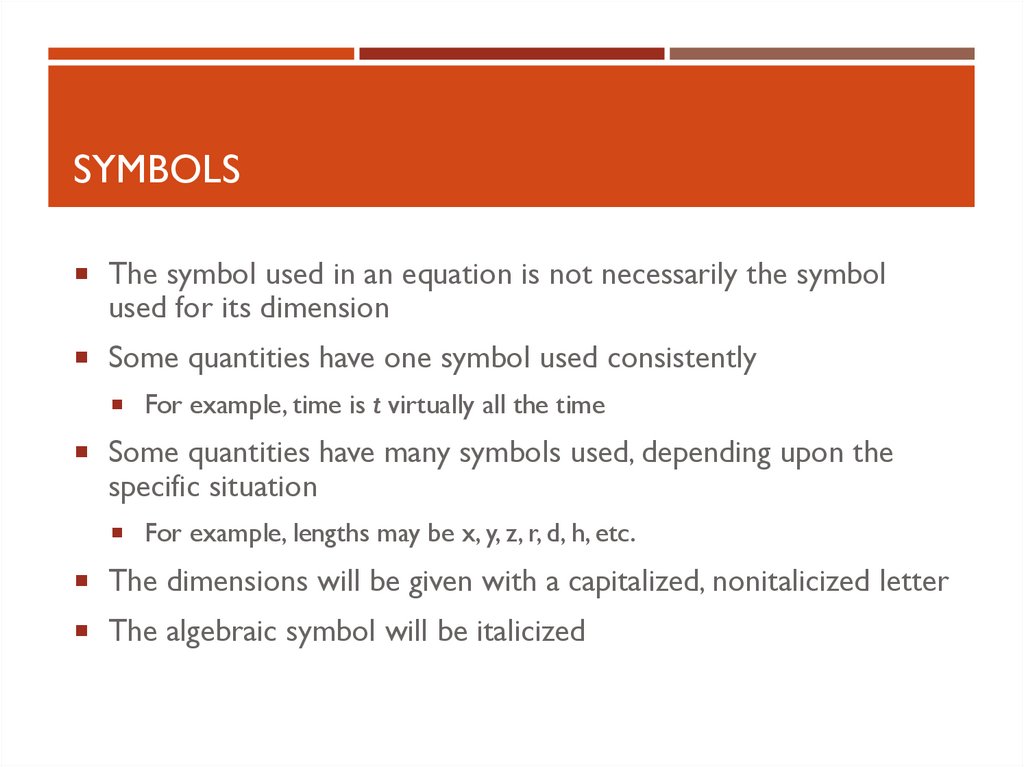
24. Symbols
SYMBOLSThe symbol used in an equation is not necessarily the symbol
used for its dimension
Some quantities have one symbol used consistently
For example, time is t virtually all the time
Some quantities have many symbols used, depending upon the
specific situation
For example, lengths may be x, y, z, r, d, h, etc.
The dimensions will be given with a capitalized, nonitalicized letter
The algebraic symbol will be italicized
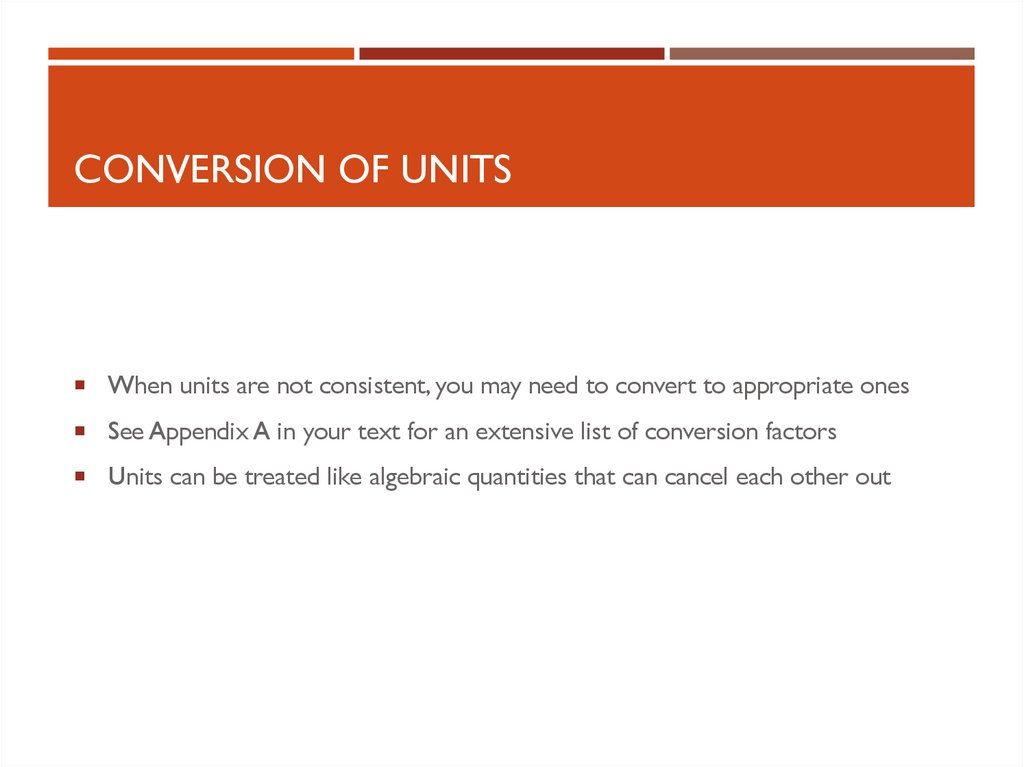
25. Conversion of Units
CONVERSION OF UNITSWhen units are not consistent, you may need to convert to appropriate ones
See Appendix A in your text for an extensive list of conversion factors
Units can be treated like algebraic quantities that can cancel each other out
26. Conversion
CONVERSIONAlways include units for every quantity, you can carry the
units through the entire calculation
Multiply original value by a ratio equal to one
Example
15.0 in ? cm
2.54 cm
15.0 in
38.1cm
1in
Note the value inside the parentheses is equal to 1 since 1 in. is
defined as 2.54 cm
27. Uncertainty in Measurements
UNCERTAINTY IN MEASUREMENTSThere is uncertainty in every measurement – this uncertainty carries over
through the calculations
May be due to the apparatus, the experimenter, and/or the number of measurements
made
Need a technique to account for this uncertainty
We will use rules for significant figures to approximate the uncertainty in results
of calculations
28. Significant Figures
SIGNIFICANT FIGURESA significant figure is one that is reliably known
Zeros may or may not be significant
Those used to position the decimal point are not significant
To remove ambiguity, use scientific notation
In a measurement, the significant figures include the first
estimated digit
29. Significant Figures, examples
SIGNIFICANT FIGURES, EXAMPLESAll nonzero digits are significant.
2.536 g has 4 significant figures
2.5 g has 2 s.f.
Zeros between nonzero digits are significant.
1005 kg has 4 s.f.
5.04 mLhas 3 s.f.
Zeros to the left of the first nonzero digits are not significant.
0.005 kg has only 1 s.f.
0.045 kg has 2 s.f.
30. Significant Figures, examples
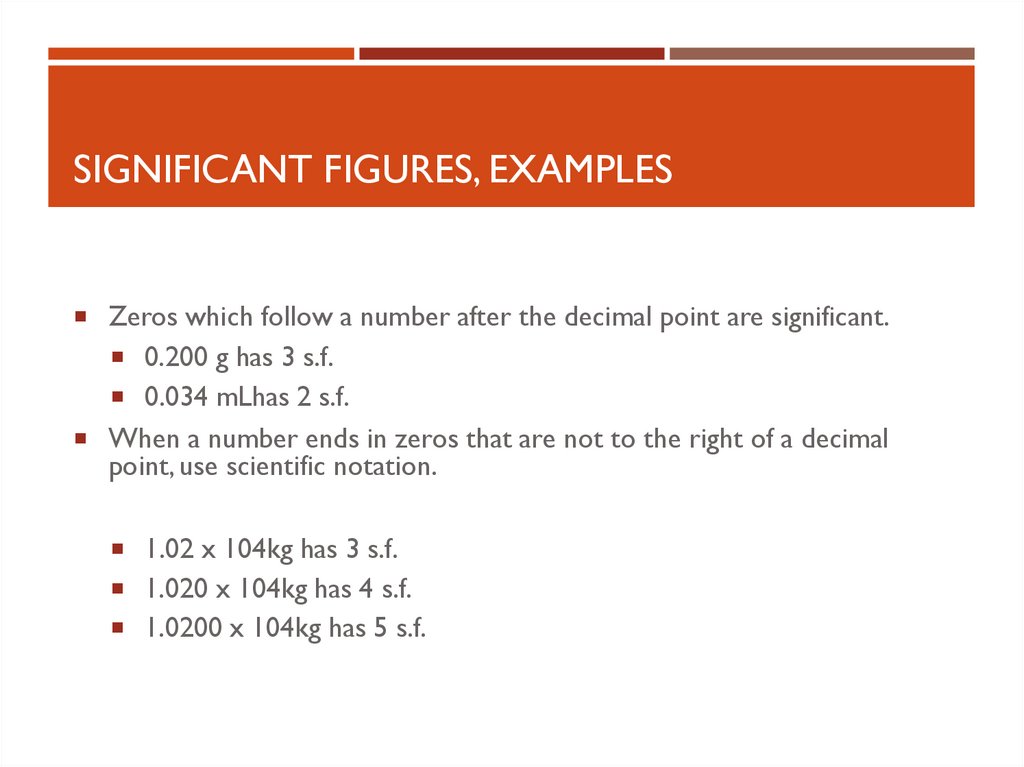
SIGNIFICANT FIGURES, EXAMPLESZeros which follow a number after the decimal point are significant.
0.200 g has 3 s.f.
0.034 mLhas 2 s.f.
When a number ends in zeros that are not to the right of a decimal
point, use scientific notation.
1.02 x 104kg has 3 s.f.
1.020 x 104kg has 4 s.f.
1.0200 x 104kg has 5 s.f.
31. Significant Figures, examples
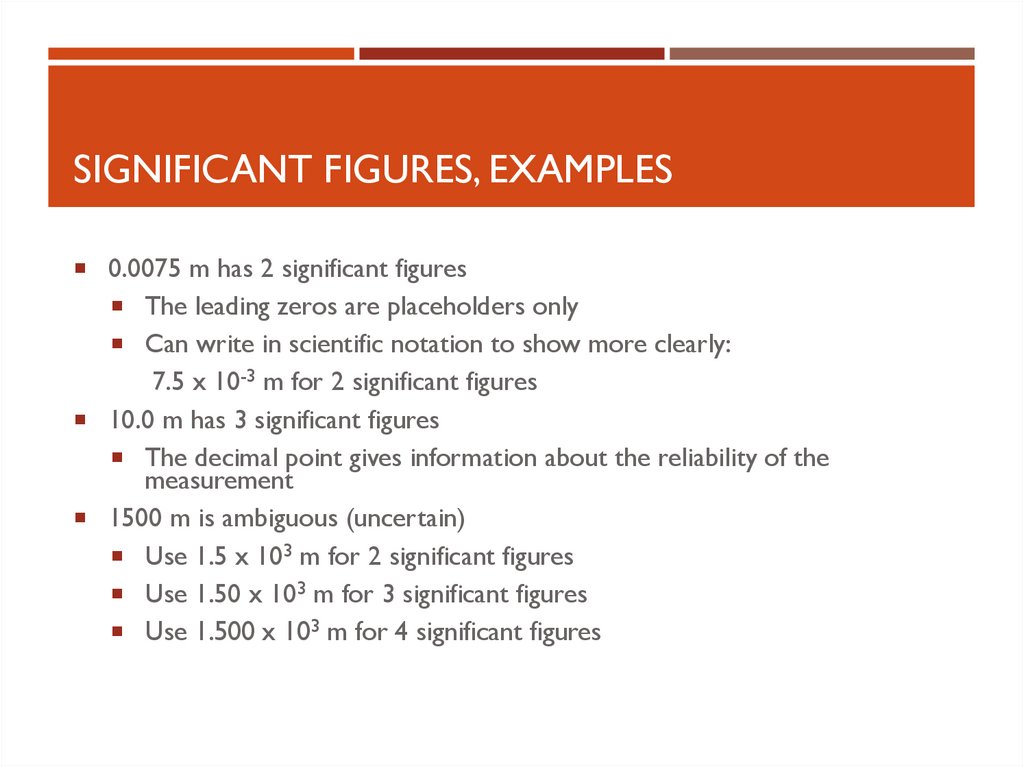
SIGNIFICANT FIGURES, EXAMPLES0.0075 m has 2 significant figures
The leading zeros are placeholders only
Can write in scientific notation to show more clearly:
7.5 x 10-3 m for 2 significant figures
10.0 m has 3 significant figures
The decimal point gives information about the reliability of the
measurement
1500 m is ambiguous (uncertain)
Use 1.5 x 103 m for 2 significant figures
Use 1.50 x 103 m for 3 significant figures
Use 1.500 x 103 m for 4 significant figures
32. Operations with Significant Figures – Multiplying or Dividing
OPERATIONS WITH SIGNIFICANT FIGURES– MULTIPLYING OR DIVIDING
When multiplying or dividing, the number of significant figures the final answer is
the same as the number of significant figures in the quantity having the lowest
number of significant figures.
Example: 25.57 m x 2.45 m = 62.6 m2
The 2.45 m limits your result to 3 significant figures
33. Operations with Significant Figures – Adding or Subtracting
OPERATIONS WITH SIGNIFICANT FIGURES– ADDING OR SUBTRACTING
When adding or subtracting, the number of decimal places in the result should
equal the smallest number of decimal places in any term in the sum.
Example: 135 cm + 3.25 cm = 138 cm
The 135 cm limits your answer to the units decimal value
34. Operations With Significant Figures – Summary
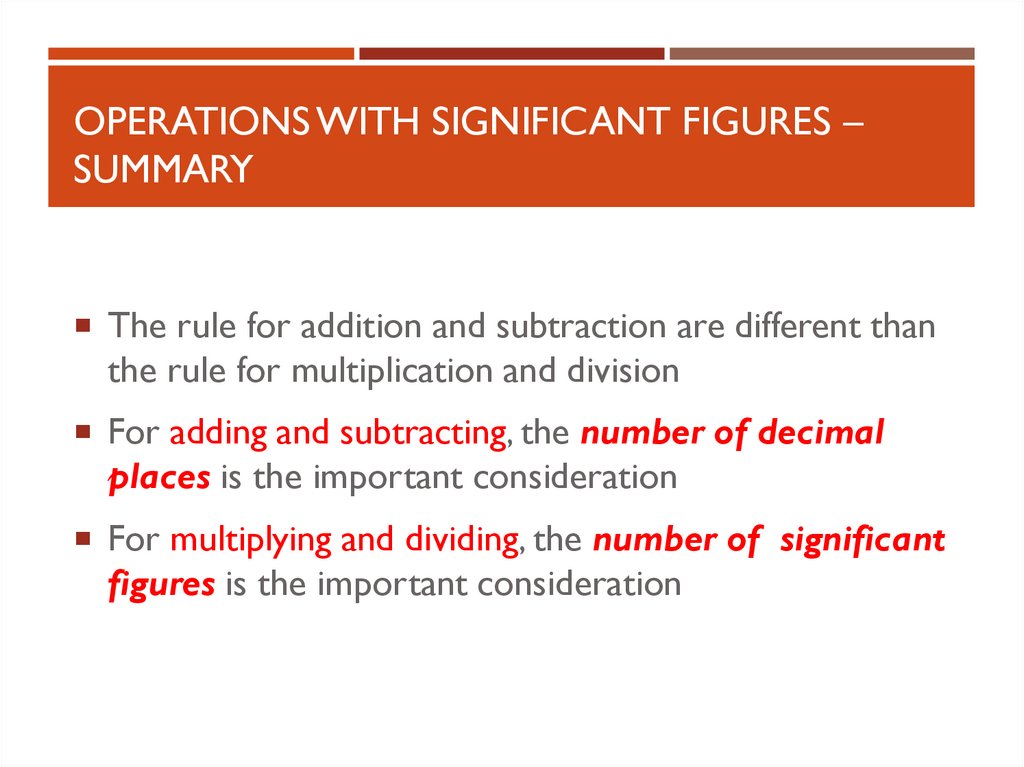
OPERATIONS WITH SIGNIFICANT FIGURES –SUMMARY
The rule for addition and subtraction are different than
the rule for multiplication and division
For adding and subtracting, the number of decimal
places is the important consideration
For multiplying and dividing, the number of significant
figures is the important consideration
35. Rounding
ROUNDINGLast retained digit is increased by 1 if the last digit
dropped is greater than 5
Last retained digit remains as it is if the last digit
dropped is less than 5
If the last digit dropped is equal to 5, the retained digit
should be rounded to the nearest even number
Saving rounding until the final result will help eliminate
accumulation of errors




















 Физика
Физика








