Похожие презентации:
presnt-e
1.
First Novartis Press Conference:Presentations given by
Daniel Vasella
(The Birth of Novartis)
and
Raymund Breu
(Financial Overview)
press1
2.
The Birth of Novartis:A Frontier of
Promises and Challenges
press2
3.
Novartis Aspirations - “Beat theBest”
To capture and hold worldwide leadership positions with a
strong, sustainable performance based on continuous
innovation
To create a fast, focused, flexible company with a passion for
competitiveness and implementation
press3
4.
Novartis: Global Leadershippress4
#1
in Life Sciences
#2
in Healthcare
#1
in Agribusiness
#2
in Medical Nutrition
5.
Advantages of Global LeadershipAchieve critical competitive mass
Reach scope and depth in our franchises
Hire, retain the best associates
Be our customers’ source of reference
Preferred partner for alliances and licensing cooperations
press5
6.
Integration:Rapid Pace, Decisive
Actions
press6
7.
ObjectivesMarch Projection
Current Estimate
Cost synergies*
CHF 1.8 Bio
CHF 2 Bio
Job reductions*
10 %
10 %
Realization
of synergies*
Within 3 years of approval
Within 3 years
Restructuring
charges
Approximately CHF 2 Bio
CHF 2 Bio
(net of one-time gains)
MBT (1996)
CSC (before AGM 1997)
MBT (sale closed Dec. 2, 1996)
CSC (March/April 1997)
Spin-offs
*excluding CSC
press7
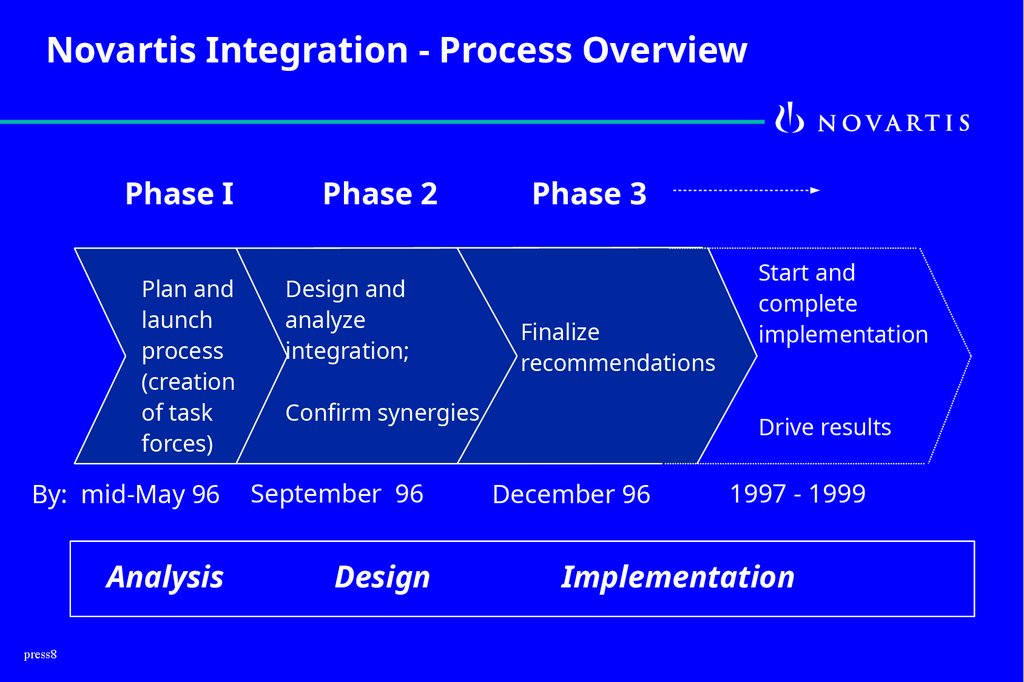
8.
Novartis Integration - Process OverviewPhase I
Phase 2
Plan and
launch
process
(creation
of task
forces)
Design and
analyze
integration;
By: mid-May 96
September 96
Analysis
Design
press8
Phase 3
Finalize
recommendations
Confirm synergies
Start and
complete
implementation
Drive results
December 96
1997 - 1999
Implementation
9.
IntegrationMilestones
On target, on time
Management team in place worldwide
All 600 sites selected
Worldwide organizational structures and headcount determined
Fact-based evaluation of business processes, transparency of activities
Synergy targets confirmed
press9
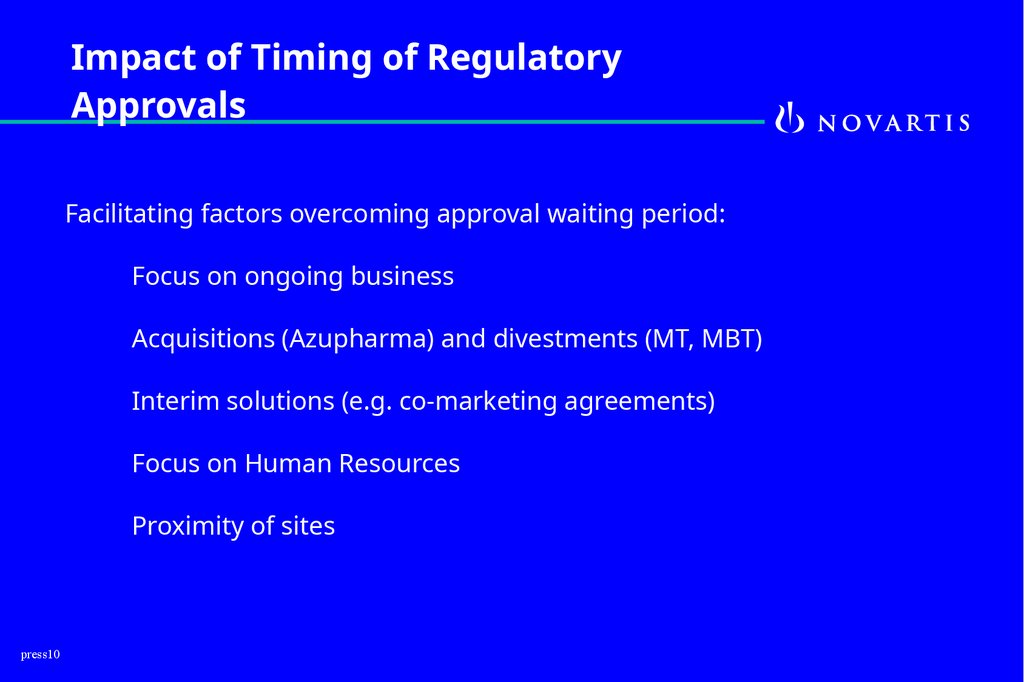
10.
Impact of Timing of RegulatoryApprovals
Facilitating factors overcoming approval waiting period:
Focus on ongoing business
Acquisitions (Azupharma) and divestments (MT, MBT)
Interim solutions (e.g. co-marketing agreements)
Focus on Human Resources
Proximity of sites
press10
11.
Conditions for RegulatoryApprovals
EU approval obtained July 17, 1996
Non-exclusive licenses of Methoprene; favorable towards Chiron licensing
out its HSV-tk patents
FTC clearance obtained December 17, 1996
Divestment of corn herbicides: dicamba in North America; dimethenamid
worldwide
Divestment of Sandoz’ North American Animal Health business
Non-exclusive licenses for HSV-tk technology, ex-vivo gene therapy
press11
6
patents, Factor VIII for hemophilia gene therapy, and interleukin-2,
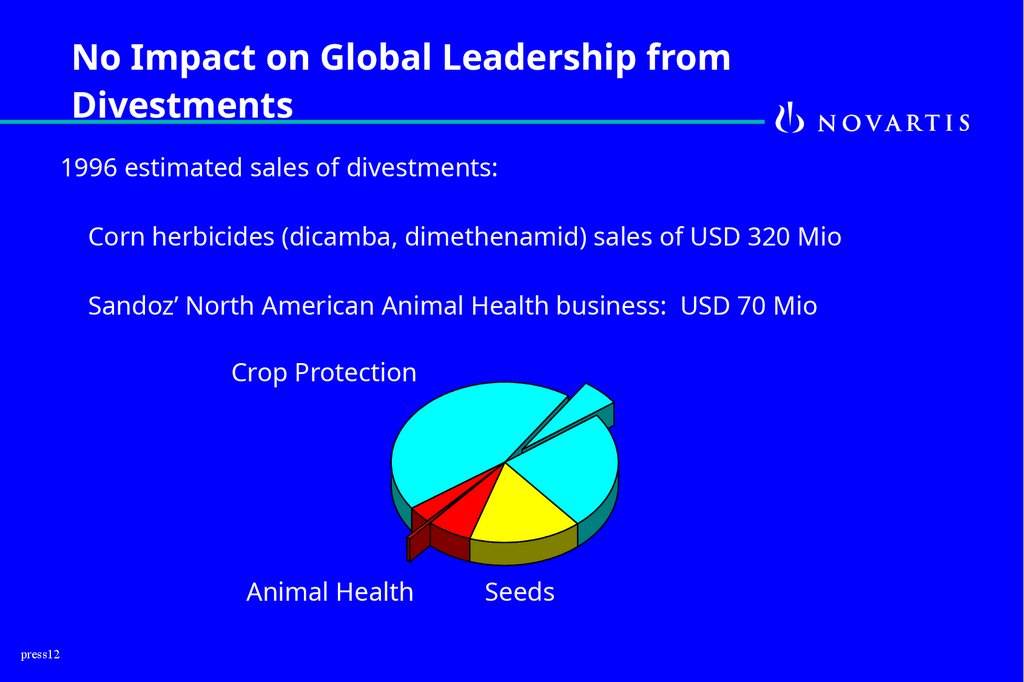
12.
No Impact on Global Leadership fromDivestments
1996 estimated sales of divestments:
Corn herbicides (dicamba, dimethenamid) sales of USD 320 Mio
Sandoz’ North American Animal Health business: USD 70 Mio
Crop Protection
Animal Health
press12
Seeds
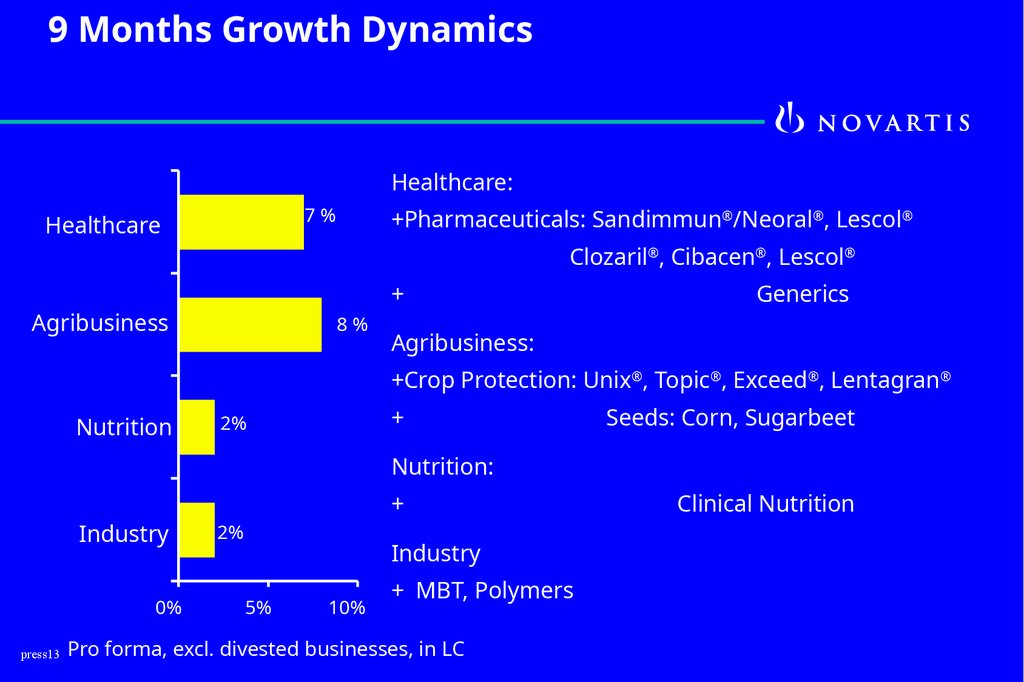
13.
9 Months Growth DynamicsHealthcare:
7%
Healthcare
+Pharmaceuticals: Sandimmun®/Neoral®, Lescol®
Clozaril®, Cibacen®, Lescol®
+
Agribusiness
8%
Generics
Agribusiness:
+Crop Protection: Unix®, Topic®, Exceed®, Lentagran®
Nutrition
+
2%
Seeds: Corn, Sugarbeet
Nutrition:
Industry
0%
+
2%
Industry
5%
10%
+ MBT, Polymers
press13 Pro forma, excl. divested businesses, in LC
Clinical Nutrition
14.
Novartis Core Strategies for FutureGrowth
Innovation, including cutting-edge technologies
Customer focus
Operational excellence with continuing productivity improvements
press14
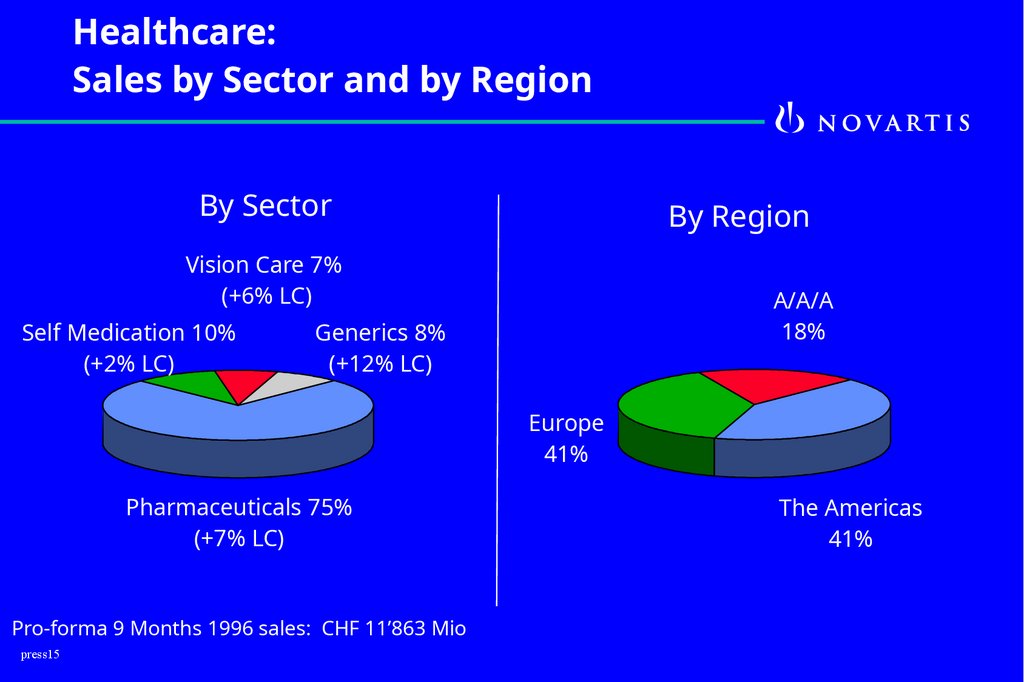
15.
Healthcare:Sales by Sector and by Region
By Sector
By Region
Vision Care 7%
(+6% LC)
Self Medication 10%
(+2% LC)
A/A/A
18%
Generics 8%
(+12% LC)
Europe
41%
Pharmaceuticals 75%
(+7% LC)
Pro-forma 9 Months 1996 sales: CHF 11’863 Mio
press15
The Americas
41%
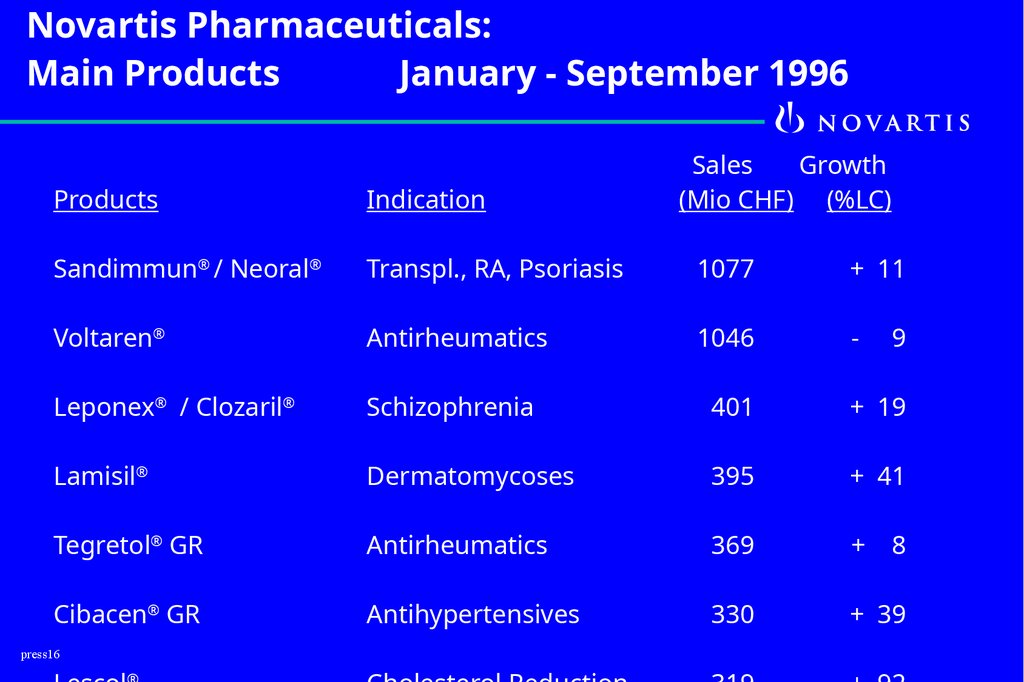
16.
Novartis Pharmaceuticals:Main Products
January - September 1996
Sales
Growth
(Mio CHF) (%LC)
Products
Indication
Sandimmun® / Neoral®
Transpl., RA, Psoriasis
1077
+ 11
Voltaren®
Antirheumatics
1046
-
Leponex® / Clozaril®
Schizophrenia
401
+ 19
Lamisil®
Dermatomycoses
395
+ 41
Tegretol® GR
Antirheumatics
369
+
Cibacen® GR
Antihypertensives
330
+ 39
press16
9
8
17.
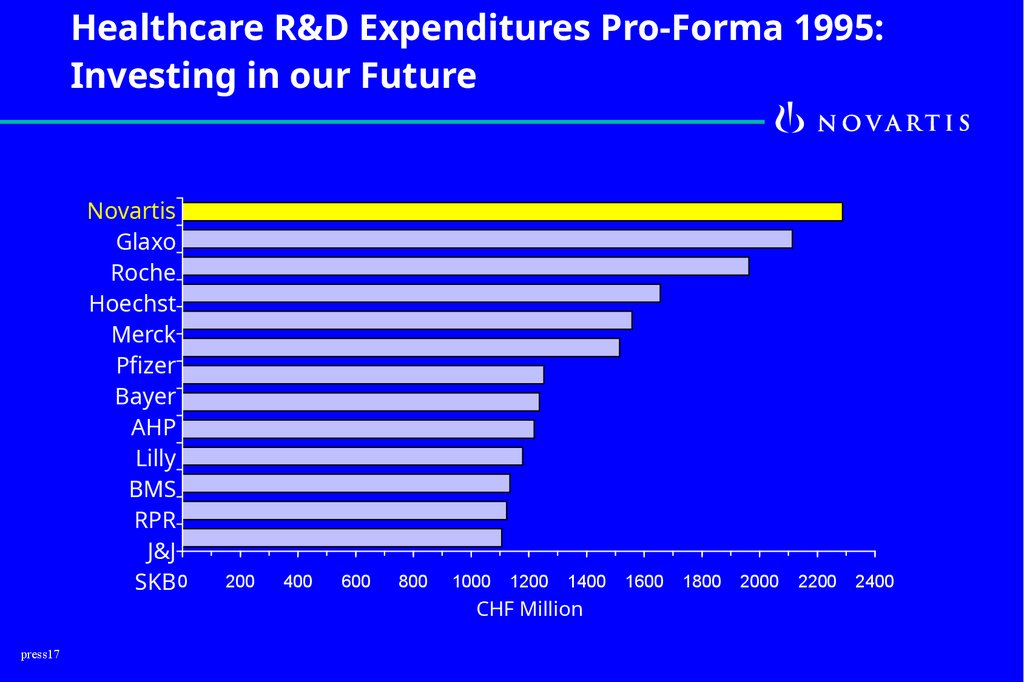
Healthcare R&D Expenditures Pro-Forma 1995:Investing in our Future
Novartis
Glaxo
Roche
Hoechst
Merck
Pfizer
Bayer
AHP
Lilly
BMS
RPR
J&J
SKB 0
press17
200
400
600
800
1000
1200
1400
CHF Million
1600
1800
2000
2200
2400
18.
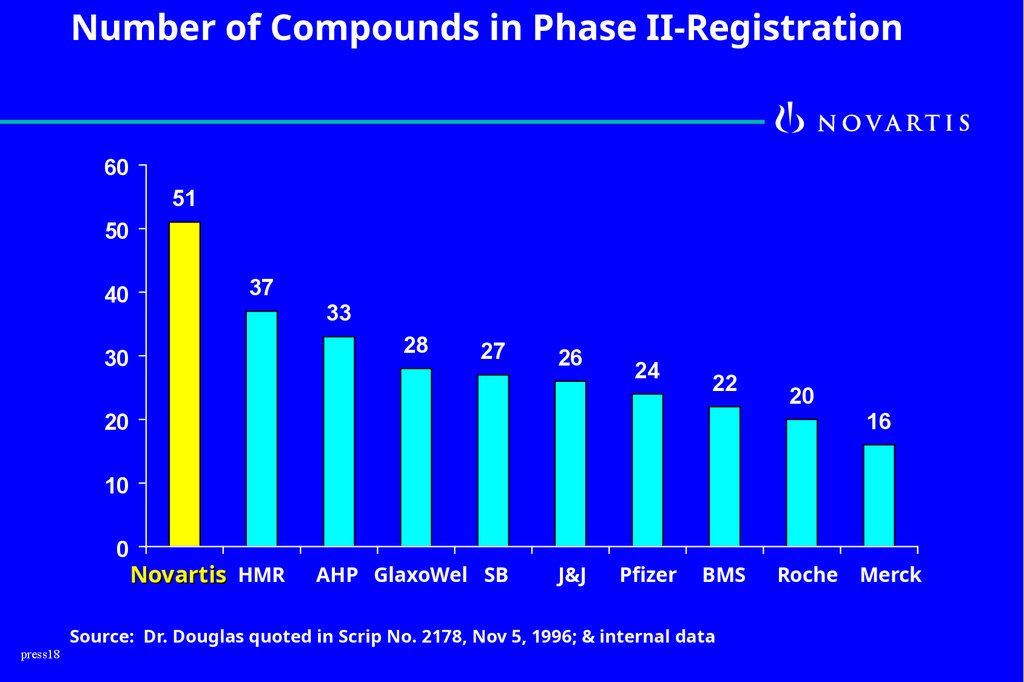
Number of Compounds in Phase II-Registration60
51
50
40
37
33
28
30
27
26
24
22
20
16
20
10
0
press18
Novartis HMR
AHP GlaxoWel SB
J&J
Pfizer
BMS
Source: Dr. Douglas quoted in Scrip No. 2178, Nov 5, 1996; & internal data
Roche
Merck
19.
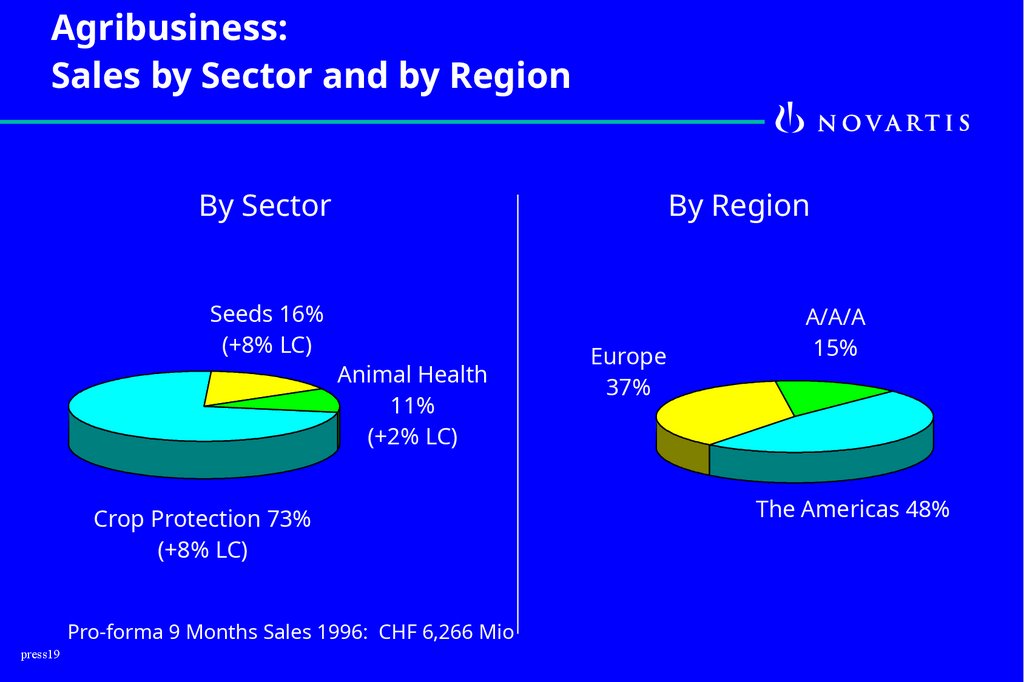
Agribusiness:Sales by Sector and by Region
By Sector
Seeds 16%
(+8% LC)
By Region
Animal Health
11%
(+2% LC)
Crop Protection 73%
(+8% LC)
Pro-forma 9 Months Sales 1996: CHF 6,266 Mio
press19
Europe
37%
A/A/A
15%
The Americas 48%
20.
Crop Protection OutlookMaintain leadership position with profitability above industry average
Derive competitive advantage from innovation
Focus on core business areas, profitable markets and high value adding
products
Achieve continuous productivity improvements
press20
21.
Animal Health OutlookContinued dynamic growth with Program® in the companion animal
business
Tiamutin® (against bacterial infections in pig & poultry) brings growth
opportunities with worldwide Novartis organization
Flexible and lean infrastructure
Increasing market share both in companion and farm animals
press21
22.
Seeds:Outlook
Focus on high value-added products and markets in corn, sugarbeet,
vegetables, flowers and oilseeds
Forceful global #2 position in corn
Strengthened competitive advantage through leadership in relevant applied
technologies
Above industry-average profitability and growth
press22
23.
Novartis Top Rankings in Nutrition# 1 brand in jarred baby food
# 1 health food marketer in Europe
# 2 worldwide in clinical nutrition
press23
24.
Nutrition - OutlookFocus on high growth, high value-added areas
Health-oriented products
Above industry-average volume growth
press24
25.
Building NovartisGlobal leadership in core areas with broad and deep market penetration
Top-ranked position in R&D investment creates innovation powerhouse
Commitment to stay in the forefront with accelerated growth
Dynamic earnings potential
Demerger unlocks shareholder value with enhanced focus on life sciences
Strong balance sheet
Fulfill the powerful logic of Novartis
press25
26.
The Promise of NovartisTo accomplish great things, we must not
only act but also believe
Anatole France
press26
27.
Financial Overviewpress27
28.
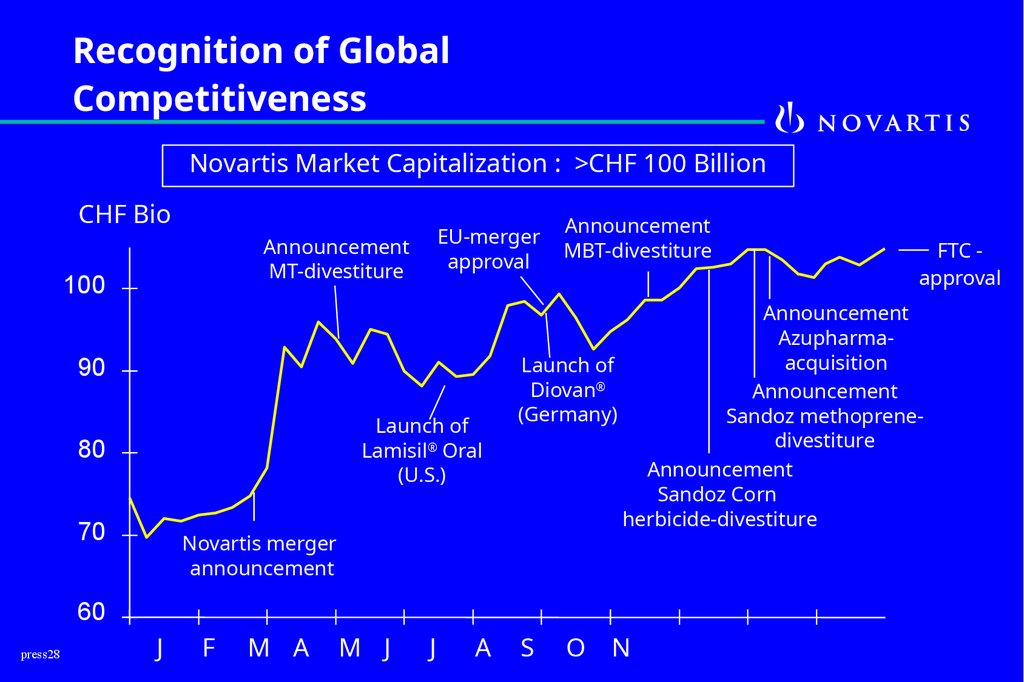
Recognition of GlobalCompetitiveness
Novartis Market Capitalization : >CHF 100 Billion
CHF Bio
EU-merger
approval
Announcement
MT-divestiture
100
90
Launch of
Lamisil® Oral
(U.S.)
80
70
Novartis merger
announcement
Announcement
MBT-divestiture
Announcement
Azupharmaacquisition
Launch of
Diovan®
Announcement
(Germany)
Sandoz methoprenedivestiture
Announcement
Sandoz Corn
herbicide-divestiture
60
press28
J
F
M A
M J
J
A
FTC approval
S
O
N
29.
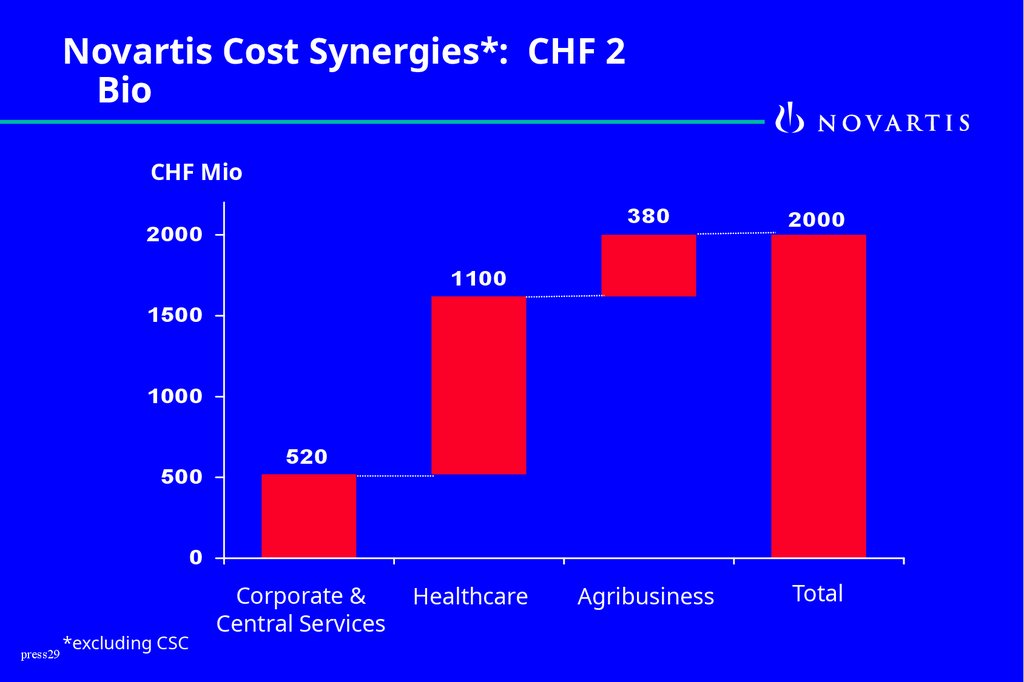
Novartis Cost Synergies*: CHF 2Bio
CHF Mio
2000
380
2000
Agribusiness
Total
1100
1500
1000
500
520
0
press29
*excluding CSC
Corporate &
Central Services
Healthcare
30.
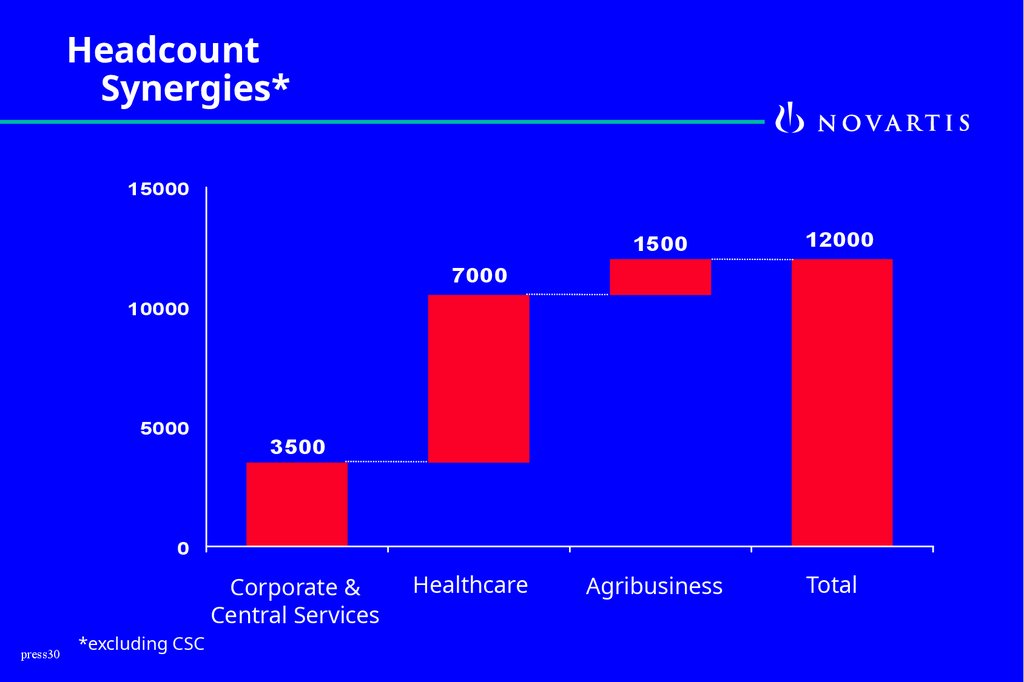
HeadcountSynergies*
15000
1500
12000
Agribusiness
Total
7000
10000
5000
3500
0
Corporate &
Central Services
press30
*excluding CSC
Healthcare
31.
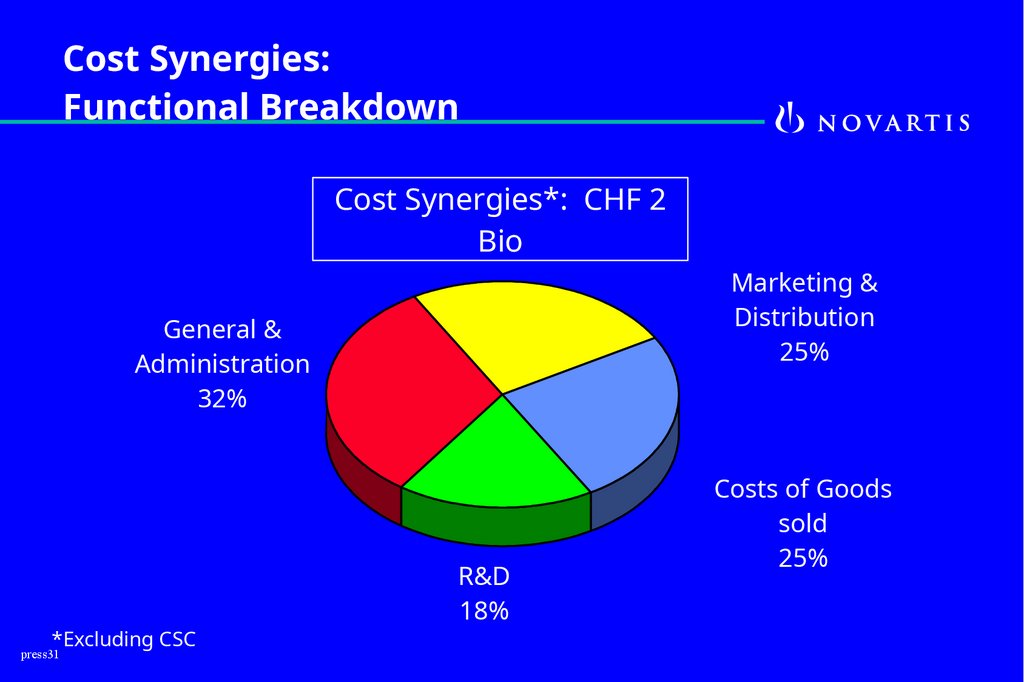
Cost Synergies:Functional Breakdown
Cost Synergies*: CHF 2
Bio
Marketing &
Distribution
25%
General &
Administration
32%
*Excluding CSC
press31
R&D
18%
Costs of Goods
sold
25%
32.
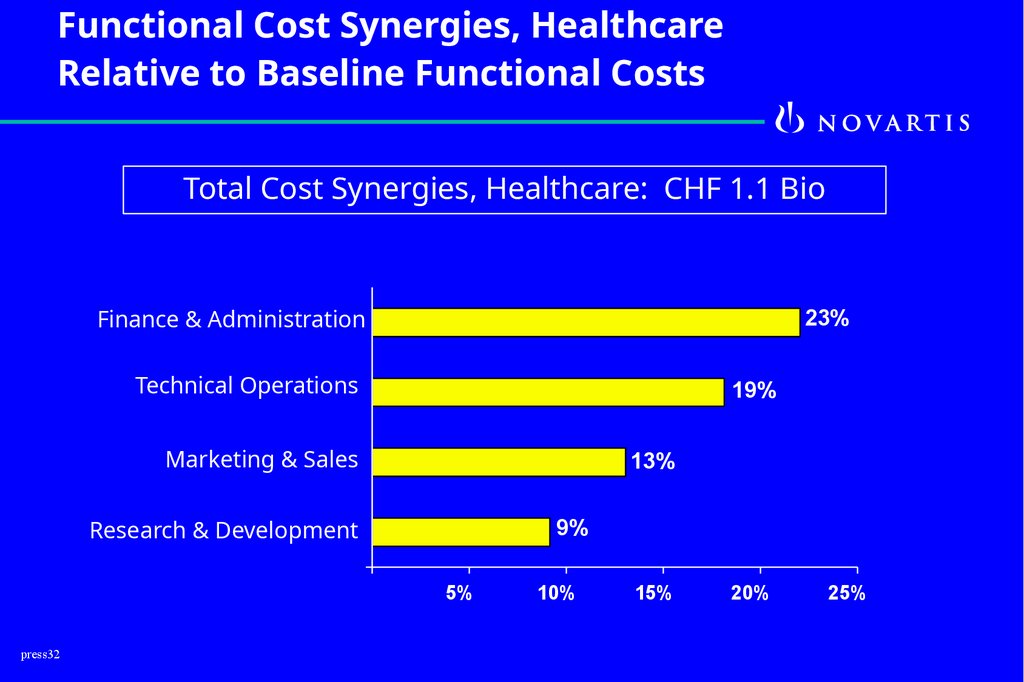
Functional Cost Synergies, HealthcareRelative to Baseline Functional Costs
Total Cost Synergies, Healthcare: CHF 1.1 Bio
Finance & Administration
23%
Technical Operations
19%
Marketing & Sales
13%
Research & Development
9%
5%
press32
10%
15%
20%
25%
33.
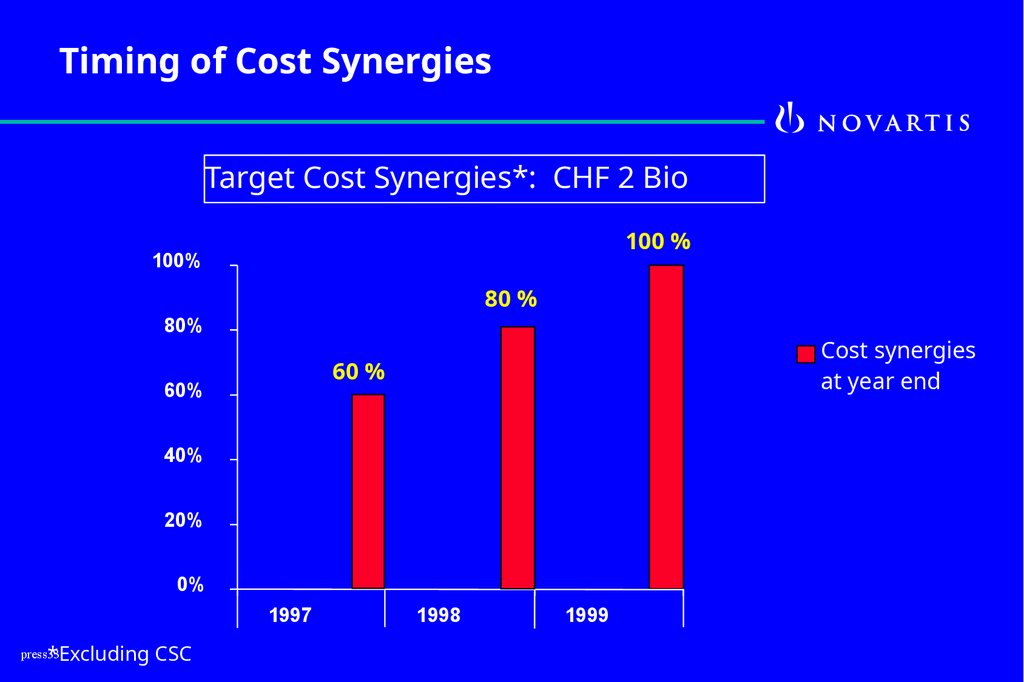
Timing of Cost SynergiesTarget Cost Synergies*: CHF 2 Bio
100 %
100%
80 %
80%
Cost synergies
at year end
60 %
60%
40%
20%
0%
1997
press33
*Excluding CSC
1998
1999
34.
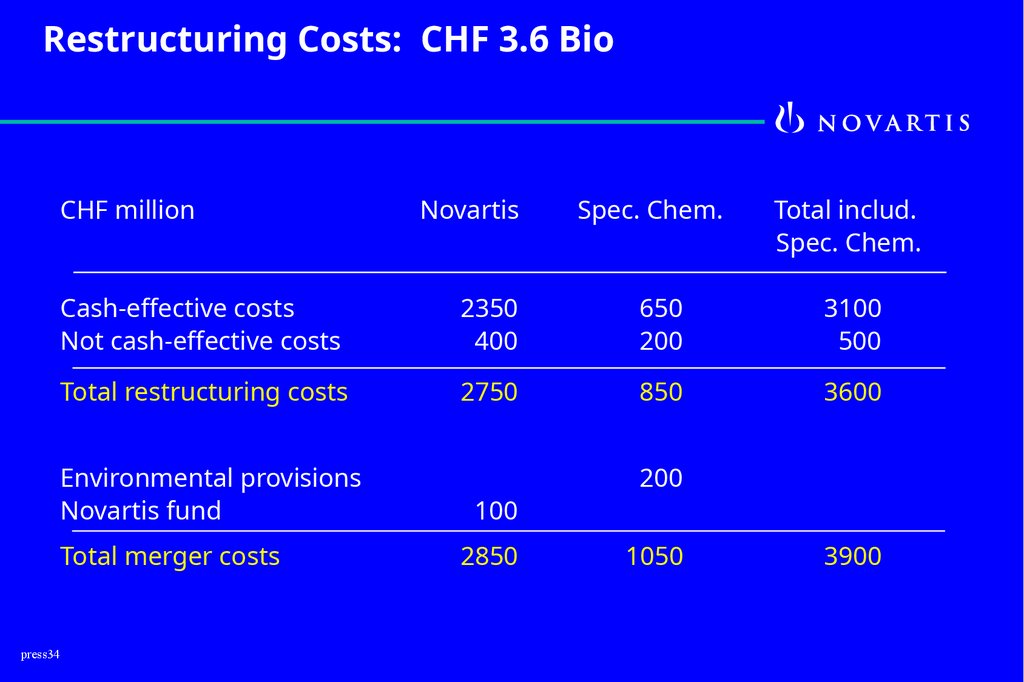
Restructuring Costs: CHF 3.6 BioCHF million
press34
Novartis
Spec. Chem.
Total includ.
Spec. Chem.
Cash-effective costs
Not cash-effective costs
2350
400
650
200
3100
500
Total restructuring costs
2750
850
3600
Environmental provisions
Novartis fund
100
Total merger costs
2850
200
1050
3900
35.
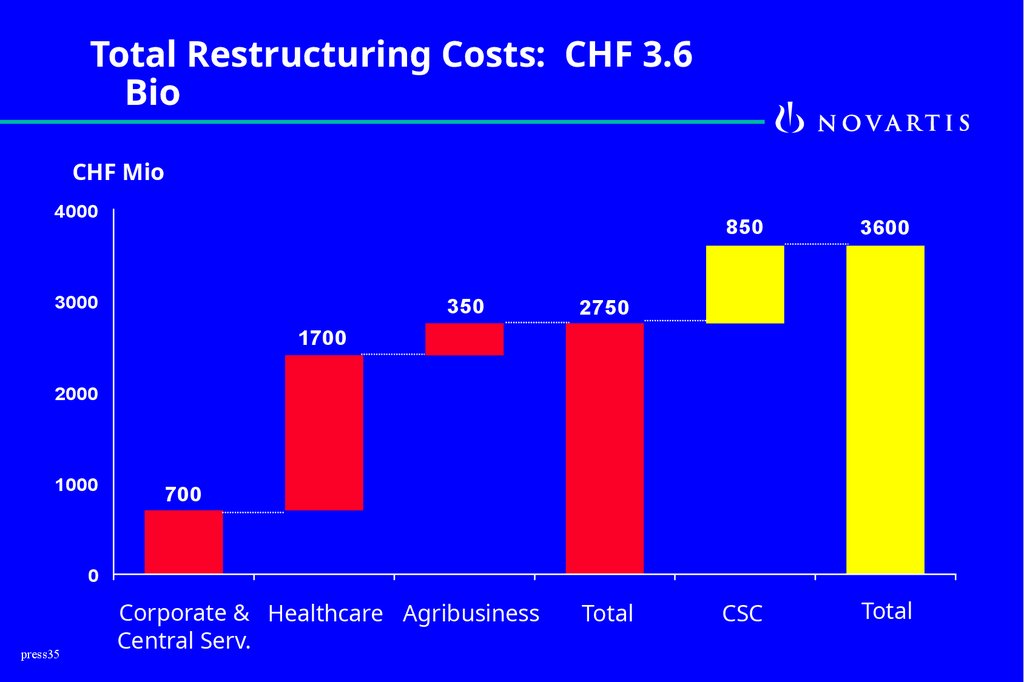
Total Restructuring Costs: CHF 3.6Bio
CHF Mio
4000
3000
350
850
3600
CSC
Total
2750
1700
2000
1000
700
0
press35
Corporate & Healthcare Agribusiness
Central Serv.
Total
36.
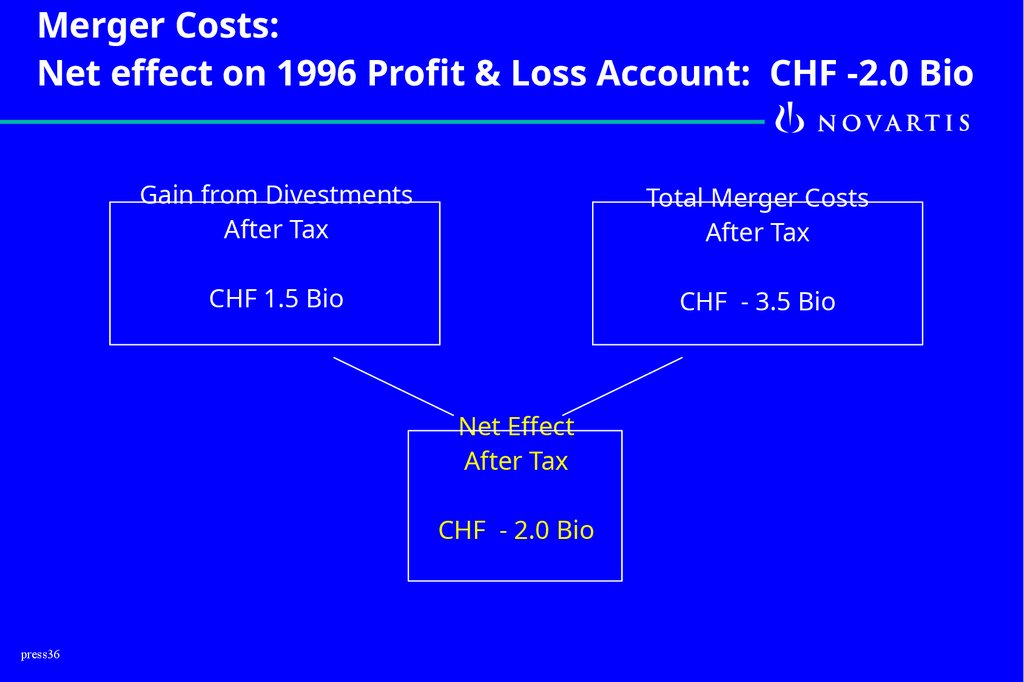
Merger Costs:Net effect on 1996 Profit & Loss Account: CHF -2.0 Bio
Gain from Divestments
After Tax
Total Merger Costs
After Tax
CHF 1.5 Bio
CHF - 3.5 Bio
Net Effect
After Tax
CHF - 2.0 Bio
press36
37.
Novartis LaunchTime Schedule
Dec. 17:
FTC Clearance
Dec. 20:
Entry into the commercial register
End trading of Ciba and Sandoz shares
Exchange:
1 Sandoz -> 1
1 Ciba
Novartis
-> 1 1/15 Novartis
Bearer shares into Bearer shares,
Registered shares into Registered shares
press37
Dec. 23:
Start trading Novartis shares / ADRs
Jan. 9:
Settlement of fractional shares ends
38.
Ciba Specialty ChemicalsExpected Timetable for SpinOff
Jan. 1, ‘97
CSC operationally autonomous, figures deconsolidated
Feb. ‘97
Issue Listing Prospectus
Feb. ‘97
Roadshow and bookbuilding
Feb./ Mar. ‘97
Rights issue subscription and trading period
Mar. ‘97
Begin trading CSC-shares
press38