Похожие презентации:
Copy of Member Profile Slides
1.
Ace Your InterviewA Guide to Clinical Trial Auditor Questions
(Beginner, Intermediate, & Advanced)
clinilaunchresearch.in
+91
2.
Who is a Clinical Trial Auditor?An Independent Guardian of Quality
A Clinical Trial Auditor ensures trials
follow protocols and regulations,
safeguarding data and participant
safety.
clinilaunchresearch.in
+91
3.
Clinical Trial AuditorWho we are?
What we do?
clinilaunchresearch.in
Independently verifies trial
compliance and data integrity.
checks trials for compliance,
accuracy, and participant
safety.
+91
4.
QuestionnariesBeginner Questions
Intermediate Questions
Advanced Scenarios
clinilaunchresearch.in
+91
5.
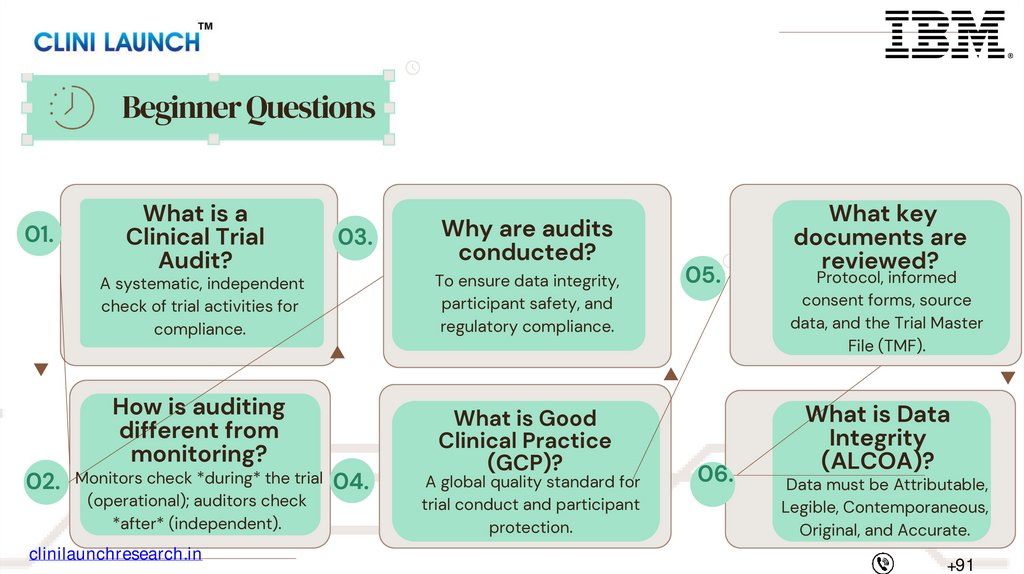
Beginner Questions01.
What is a
Clinical Trial
Audit?
03.
Why are audits
conducted?
A systematic, independent
check of trial activities for
compliance.
To ensure data integrity,
participant safety, and
regulatory compliance.
How is auditing
different from
monitoring?
What is Good
Clinical Practice
(GCP)?
02. Monitors check *during* the trial 04.
(operational); auditors check
*after* (independent).
clinilaunchresearch.in
A global quality standard for
trial conduct and participant
protection.
05.
06.
What key
documents are
reviewed?
Protocol, informed
consent forms, source
data, and the Trial Master
File (TMF).
What is Data
Integrity
(ALCOA)?
Data must be Attributable,
Legible, Contemporaneous,
Original, and Accurate.
+91
6.
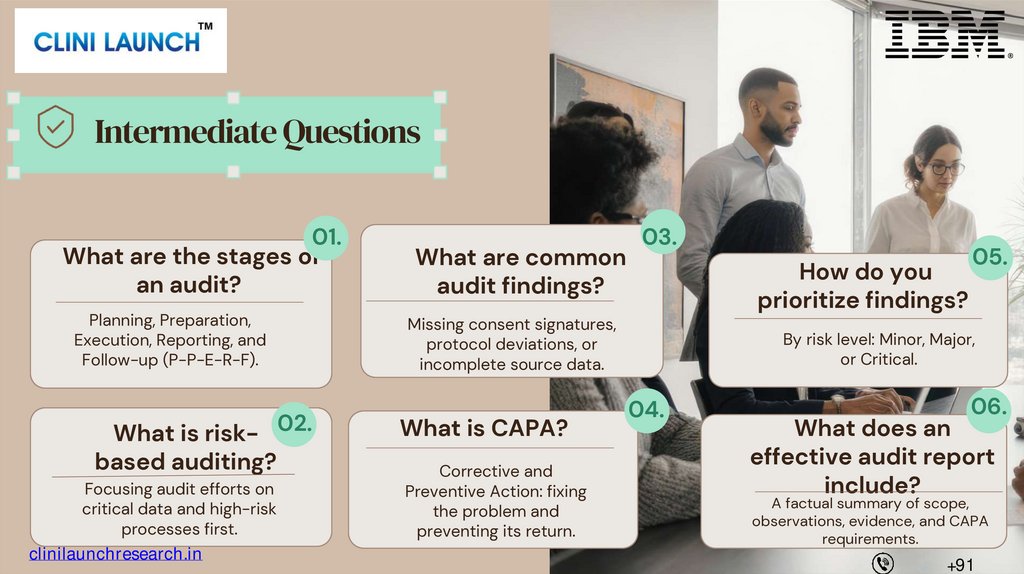
Intermediate Questions01.
What are the stages of
an audit?
Planning, Preparation,
Execution, Reporting, and
Follow-up (P-P-E-R-F).
02.
What is riskbased auditing?
Focusing audit efforts on
critical data and high-risk
processes first.
clinilaunchresearch.in
What are common
audit findings?
03.
How do you
prioritize findings?
Missing consent signatures,
protocol deviations, or
incomplete source data.
What is CAPA?
Corrective and
Preventive Action: fixing
the problem and
preventing its return.
05.
By risk level: Minor, Major,
or Critical.
04.
06.
What does an
effective audit report
include?
A factual summary of scope,
observations, evidence, and CAPA
requirements.
+91
7.
Advanced Scenarios01. Scenario: Missing Signature
• You notice a missing
investigator signature on an
informed-consent form.
Takeaway:
This is a critical compliance issue;
it must be noted for the formal QA
correction process.
clinilaunchresearch.in
+91
8.
01. Scenario: Date Discrepancy• You find a date discrepancy
between the source
document and the CRF.
Takeaway:
This is a data integrity error; it
must be clarified by the site, not
assumed or edited.
clinilaunchresearch.in
+91
9.
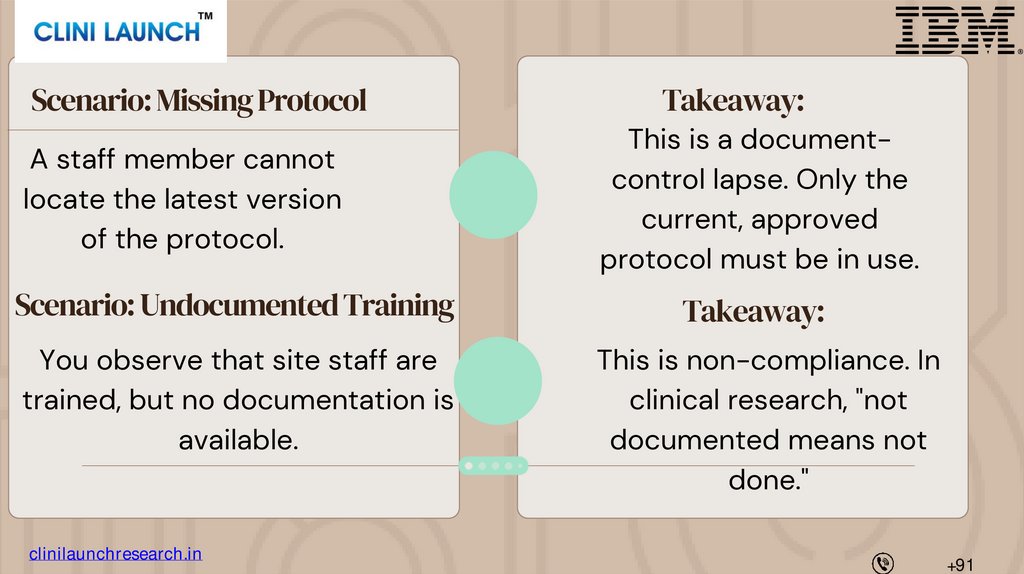
Scenario: Missing ProtocolA staff member cannot
locate the latest version
of the protocol.
Takeaway:
This is a documentcontrol lapse. Only the
current, approved
protocol must be in use.
Scenario: Undocumented Training
Takeaway:
You observe that site staff are
trained, but no documentation is
available.
This is non-compliance. In
clinical research, "not
documented means not
done."
clinilaunchresearch.in
+91
10.
Your Career in Clinical AuditKnow the rules, understand their
purpose, and commit to quality.
TO KNOW MORE ENROLL IN
CliniLaunch’s PG Diploma in
Clinical Research
clinilaunchresearch.in
+91