Похожие презентации:
Acyl chlorides 5
1.
Acyl (or acid) chloridesand
acid anhydrides
2.
Learning Objectives• recognise acid chlorides and acid anhydrides
• know and be able to use the mechanism of
acylation reactions
• understand their reactions and importance in
organic synthesis
3.
Expected Outcomes• Explain the high reactivity of acid chlorides.
• Name and draw structures of acid chlorides and
acid anhydrides.
• Draw and outline reaction mechanisms for various
acylation reactions.
• Explain the importance of acylation in organic
synthesis.
4.
KeywordsAcid (acyl) chlorides
Acid anhydrides
Nucleophilic substitution
Nucleophilic addition-elimination
Acylation
Alkylation
Carboxylic acid derivatives
Acyl group
Nucleophilic attack
Nucleophile
Leaving group
Deprotonation
5.
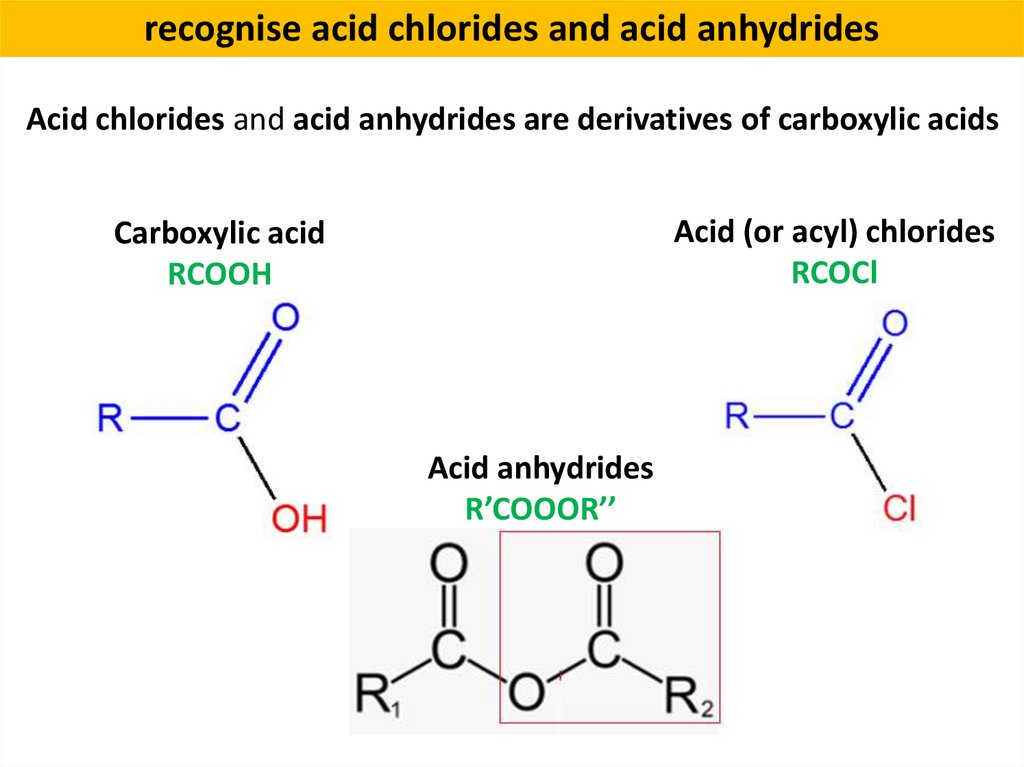
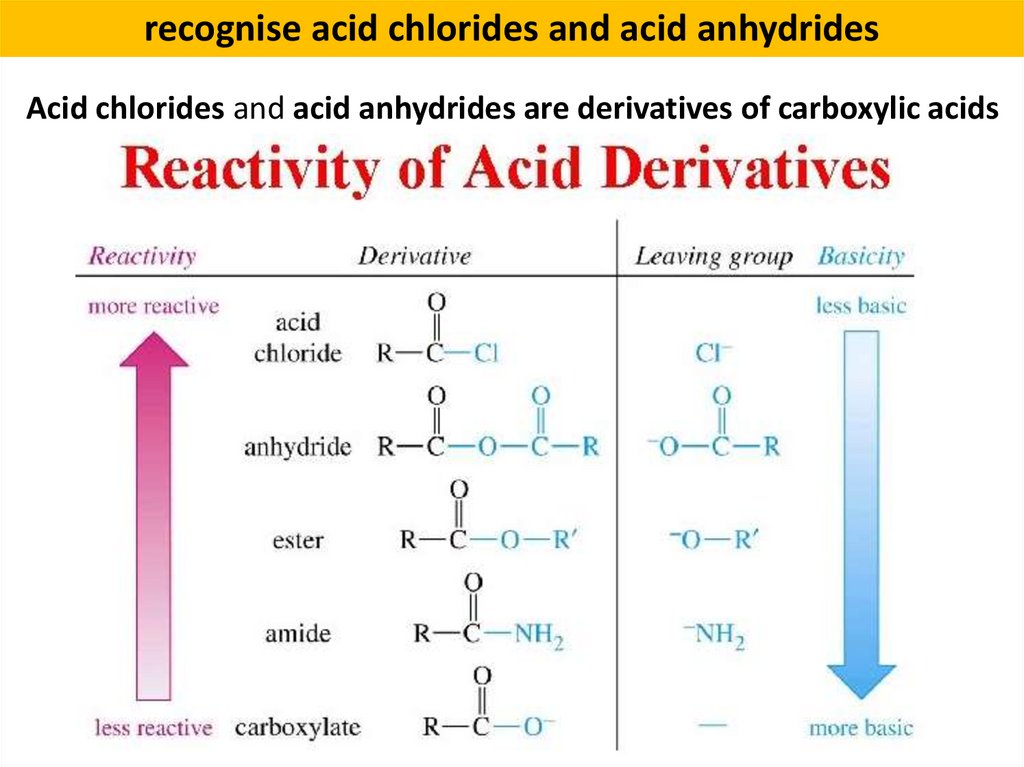
recognise acid chlorides and acid anhydridesAcid chlorides and acid anhydrides are derivatives of carboxylic acids
Acid (or acyl) chlorides
RCOCl
Carboxylic acid
RCOOH
Acid anhydrides
R’COOOR’’
6.
recognise acid chlorides and acid anhydridesAcid chlorides and acid anhydrides are derivatives of carboxylic acids
7.
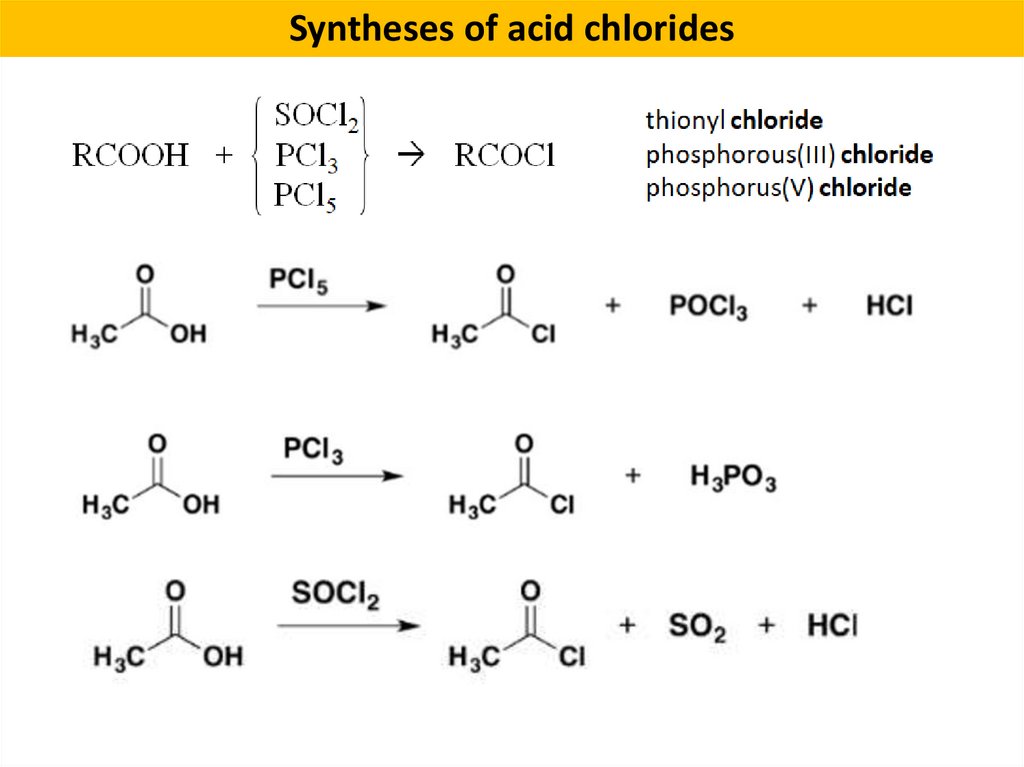
Syntheses of acid chlorides8.
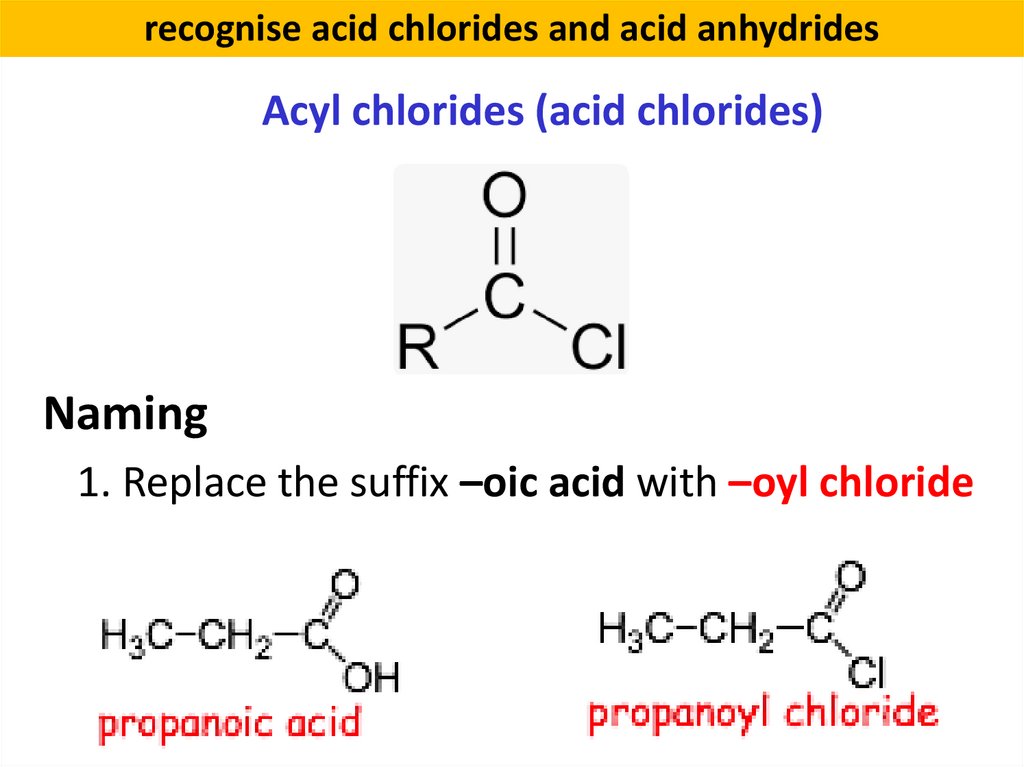
recognise acid chlorides and acid anhydridesAcyl chlorides (acid chlorides)
Naming
1. Replace the suffix –oic acid with –oyl chloride
9.
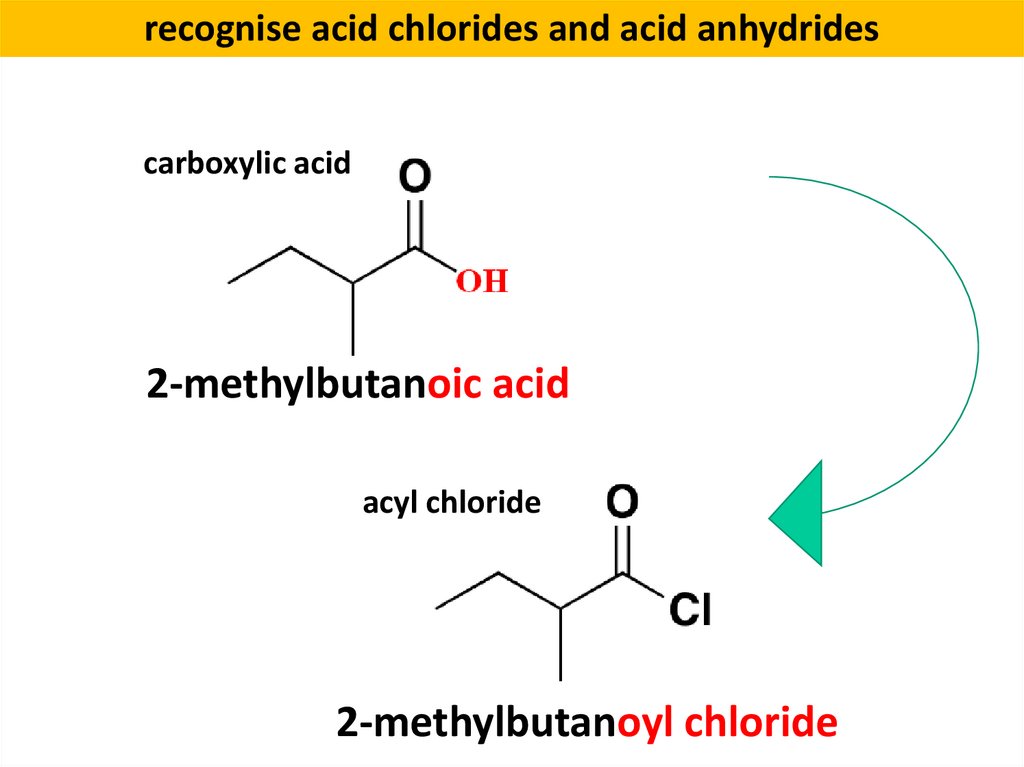
recognise acid chlorides and acid anhydridescarboxylic acid
2-methylbutanoic acid
acyl chloride
2-methylbutanoyl chloride
10.
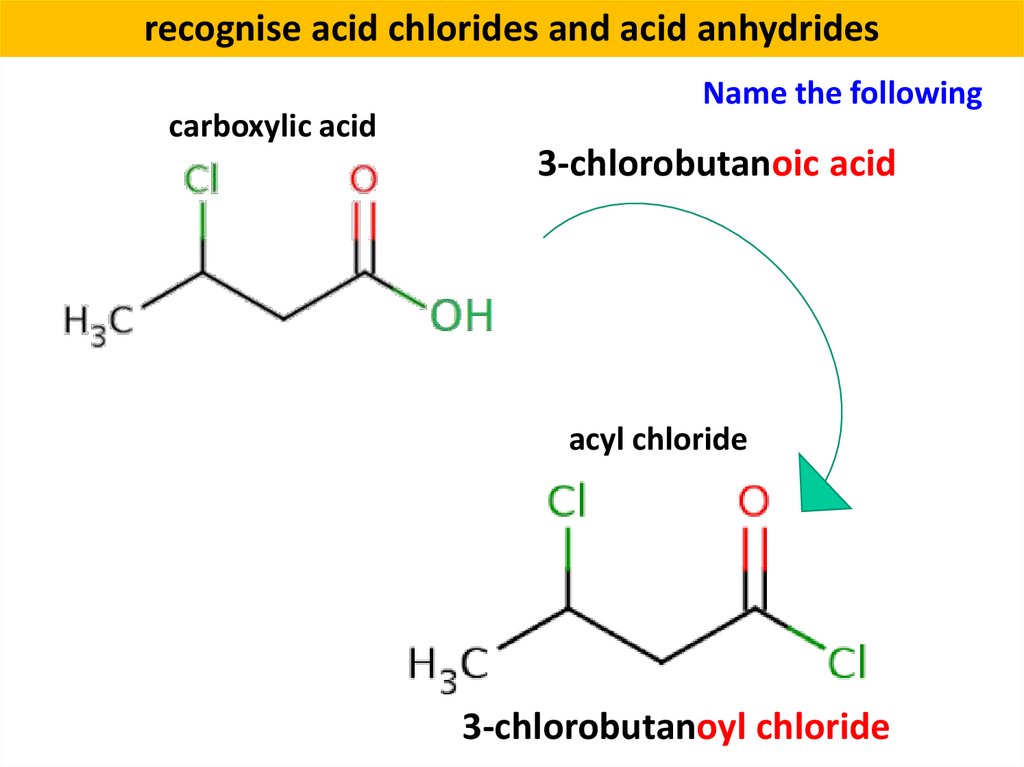
recognise acid chlorides and acid anhydridescarboxylic acid
Name the following
3-chlorobutanoic acid
acyl chloride
3-chlorobutanoyl chloride
11.
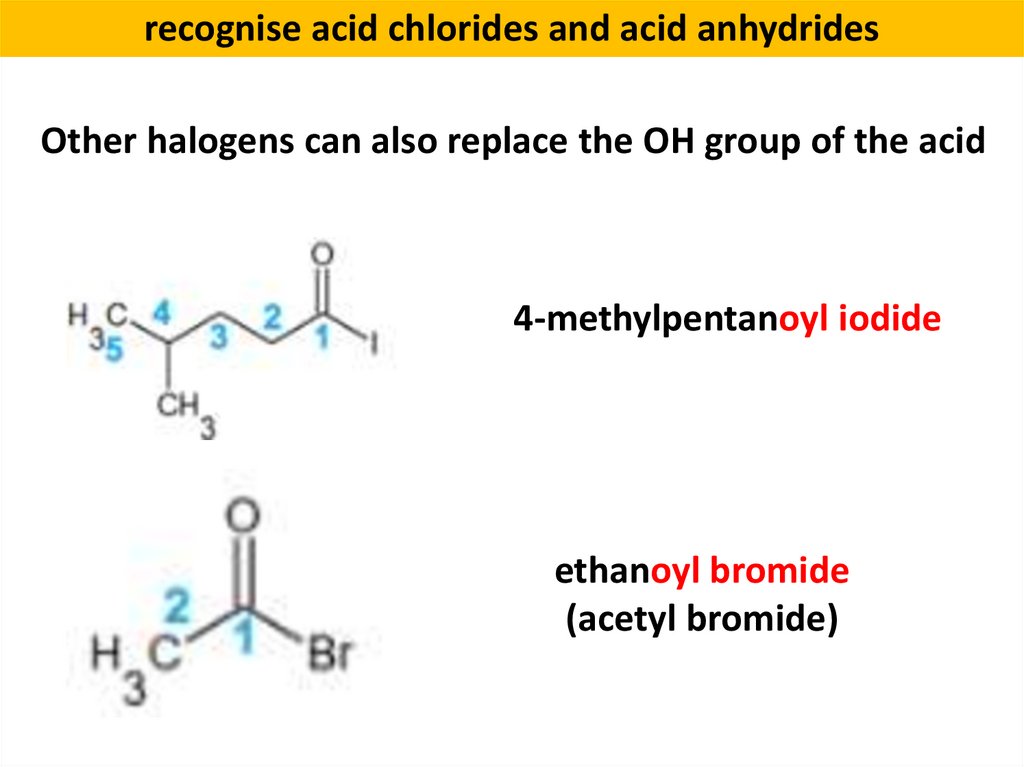
recognise acid chlorides and acid anhydridesOther halogens can also replace the OH group of the acid
4-methylpentanoyl iodide
ethanoyl bromide
(acetyl bromide)
12.
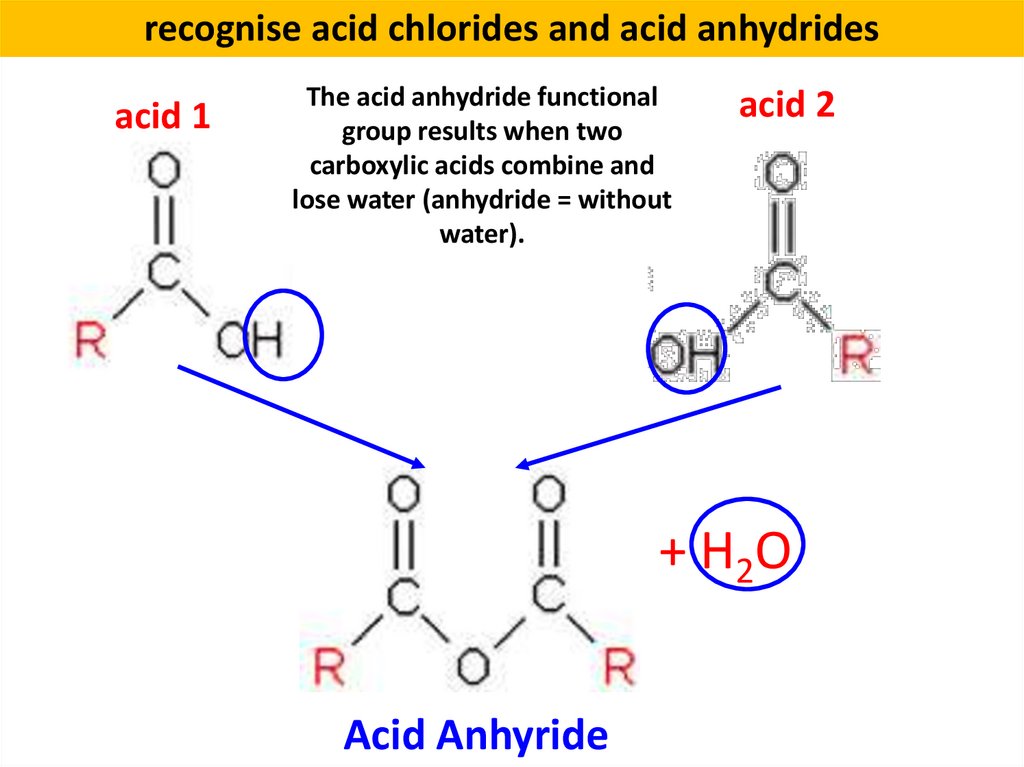
recognise acid chlorides and acid anhydridesacid 1
The acid anhydride functional
group results when two
carboxylic acids combine and
lose water (anhydride = without
water).
acid 2
+ H2O
Acid Anhyride
13.
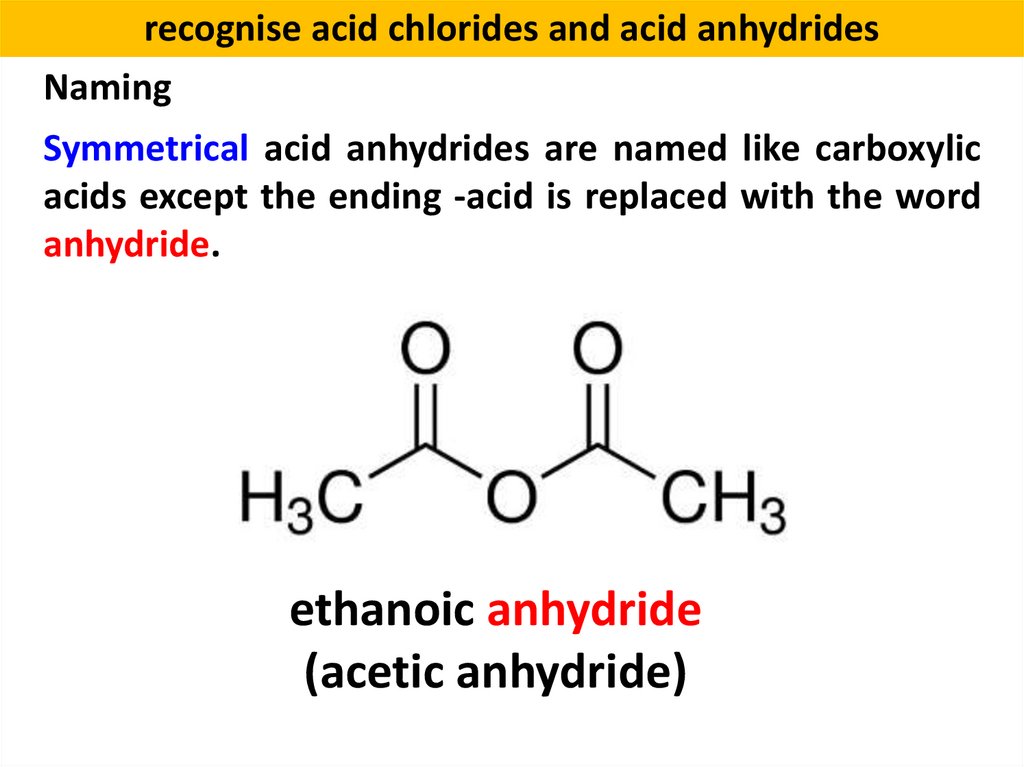
recognise acid chlorides and acid anhydridesNaming
Symmetrical acid anhydrides are named like carboxylic
acids except the ending -acid is replaced with the word
anhydride.
ethanoic anhydride
(acetic anhydride)
14.
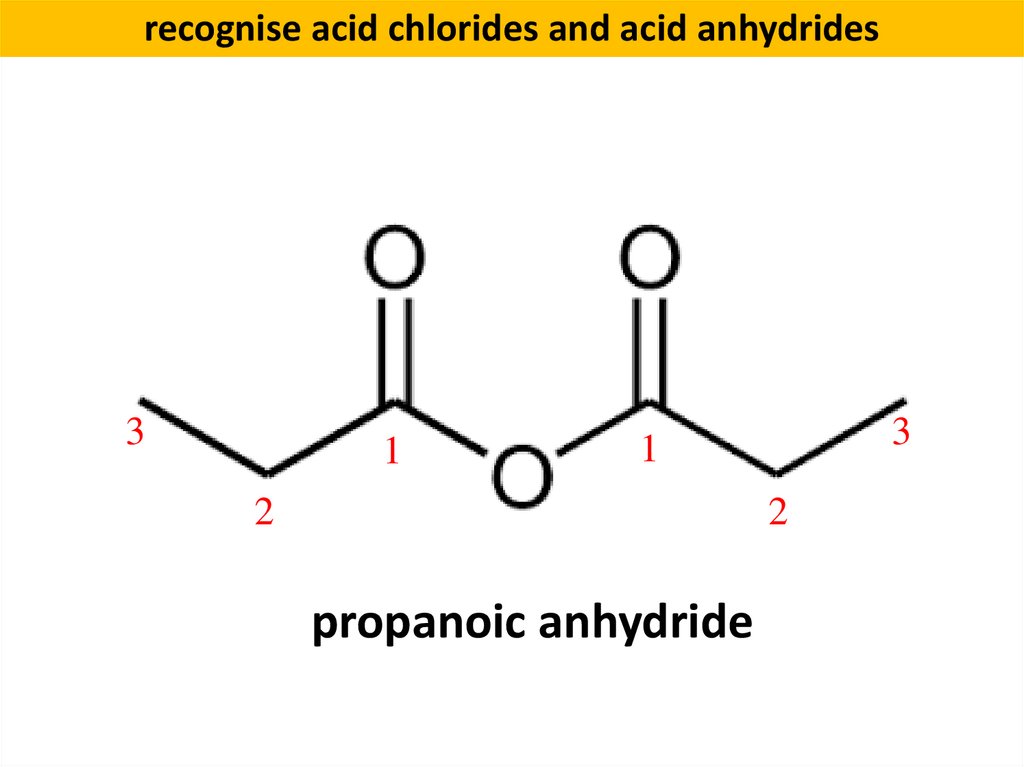
recognise acid chlorides and acid anhydrides3
1
3
1
2
2
propanoic anhydride
15.
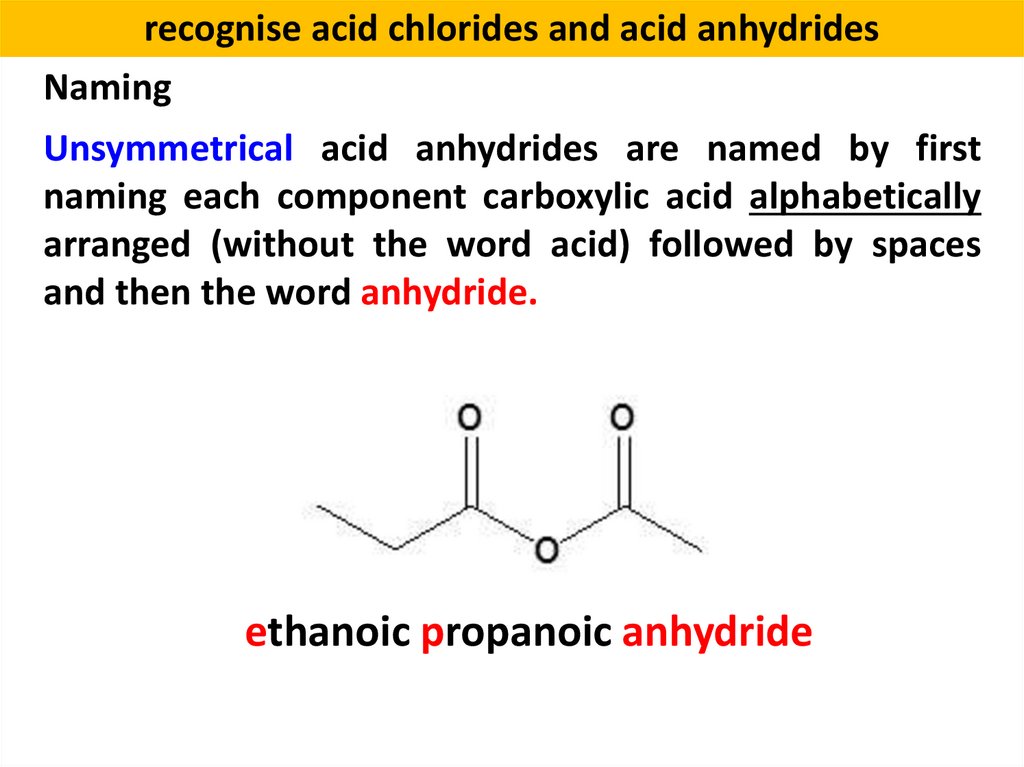
recognise acid chlorides and acid anhydridesNaming
Unsymmetrical acid anhydrides are named by first
naming each component carboxylic acid alphabetically
arranged (without the word acid) followed by spaces
and then the word anhydride.
ethanoic propanoic anhydride
16.
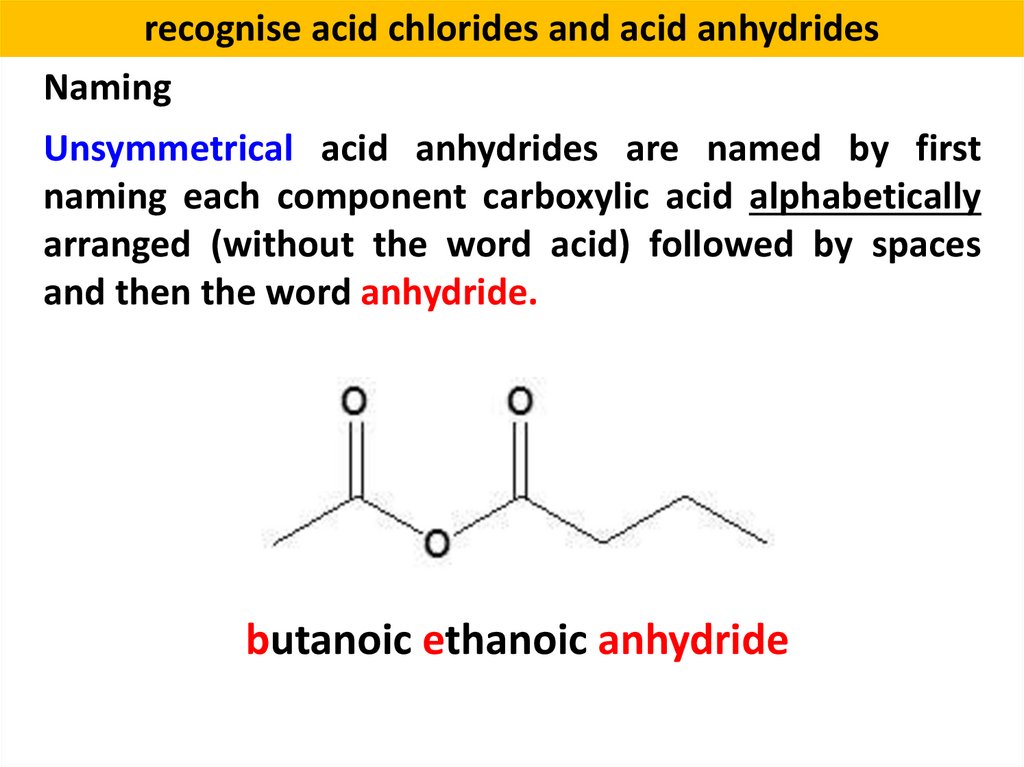
recognise acid chlorides and acid anhydridesNaming
Unsymmetrical acid anhydrides are named by first
naming each component carboxylic acid alphabetically
arranged (without the word acid) followed by spaces
and then the word anhydride.
butanoic ethanoic anhydride
17.
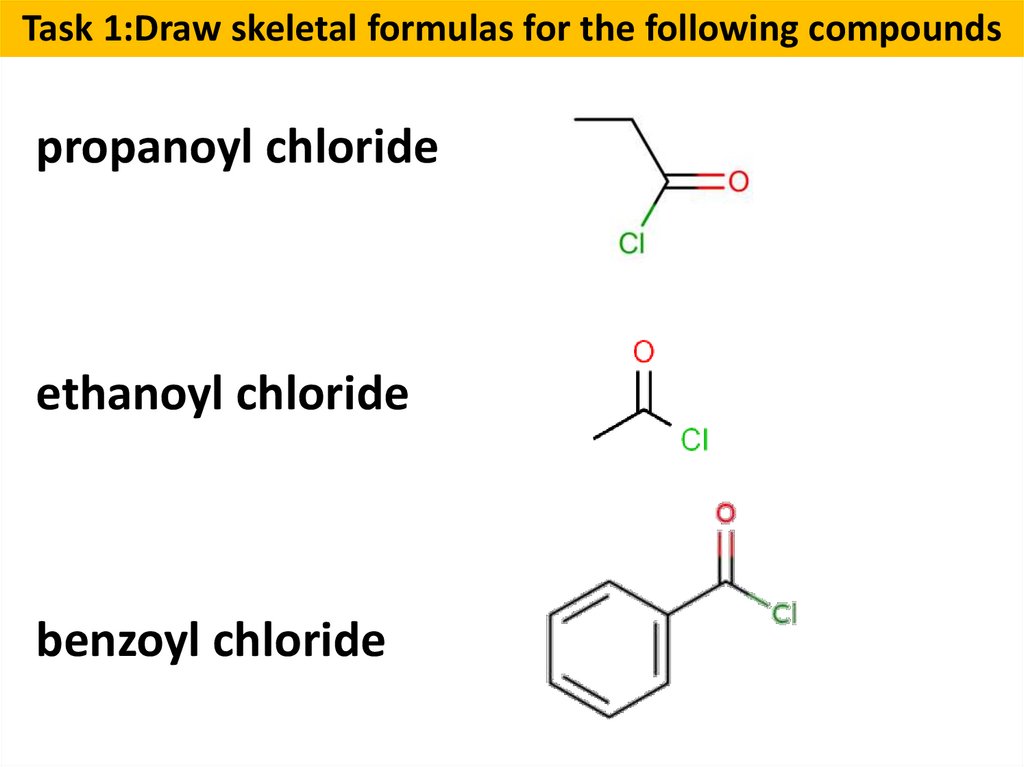
Task 1:Draw skeletal formulas for the following compoundspropanoyl chloride
ethanoyl chloride
benzoyl chloride
18.
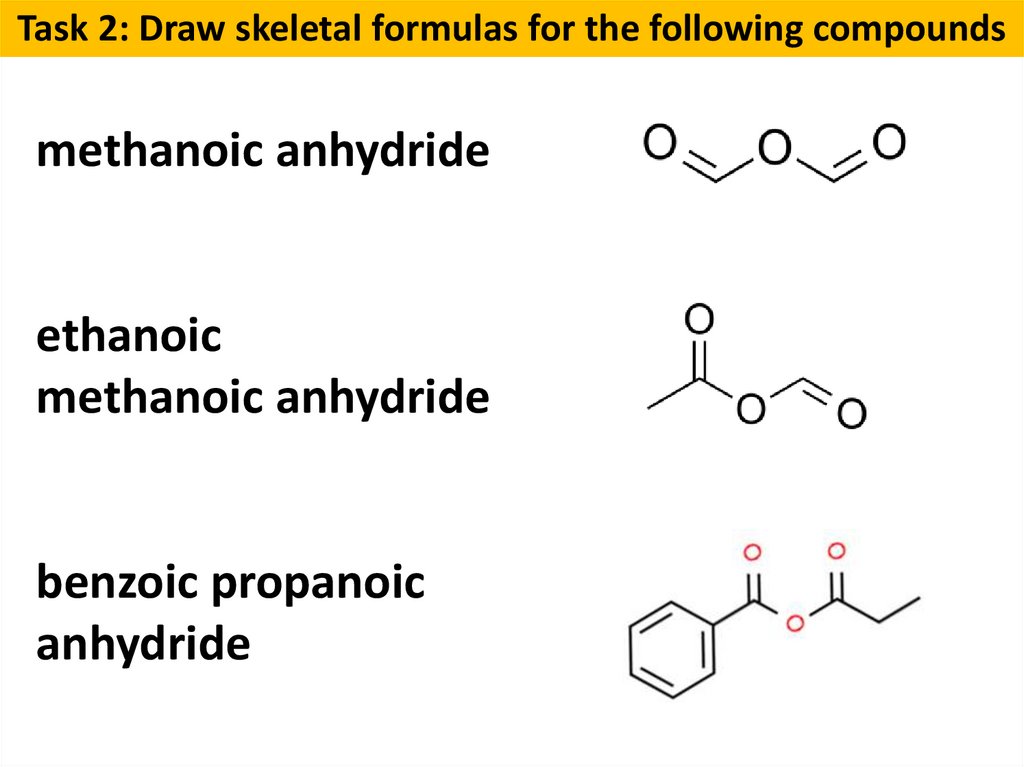
Task 2: Draw skeletal formulas for the following compoundsmethanoic anhydride
ethanoic
methanoic anhydride
benzoic propanoic
anhydride
19.
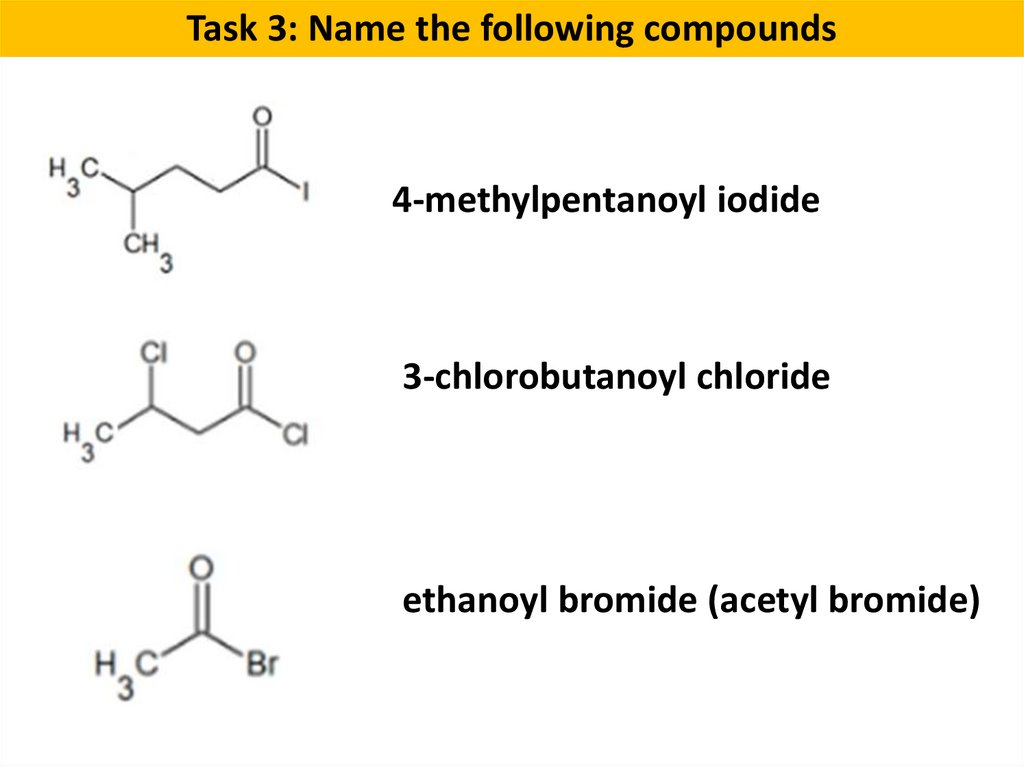
Task 3: Name the following compounds4-methylpentanoyl iodide
3-chlorobutanoyl chloride
ethanoyl bromide (acetyl bromide)
20.
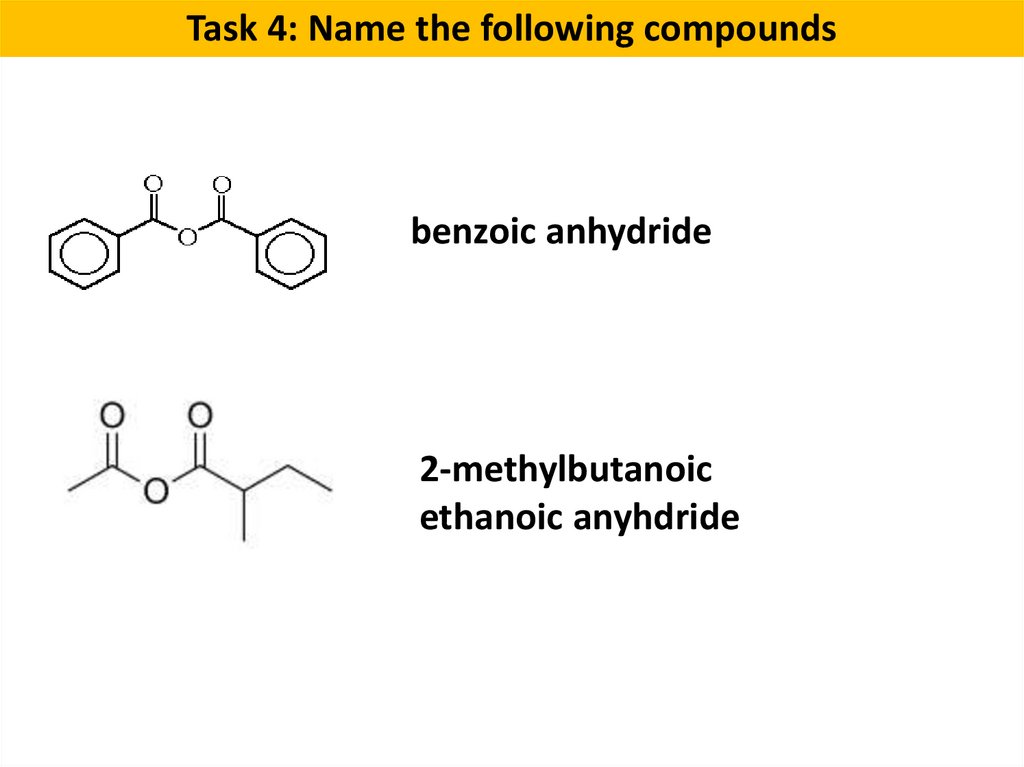
Task 4: Name the following compoundsbenzoic anhydride
2-methylbutanoic
ethanoic anyhdride
21.
recognise acid chlorides and acid anhydridesI can recognise and name acid chlorides and acid
anhydrides
22.
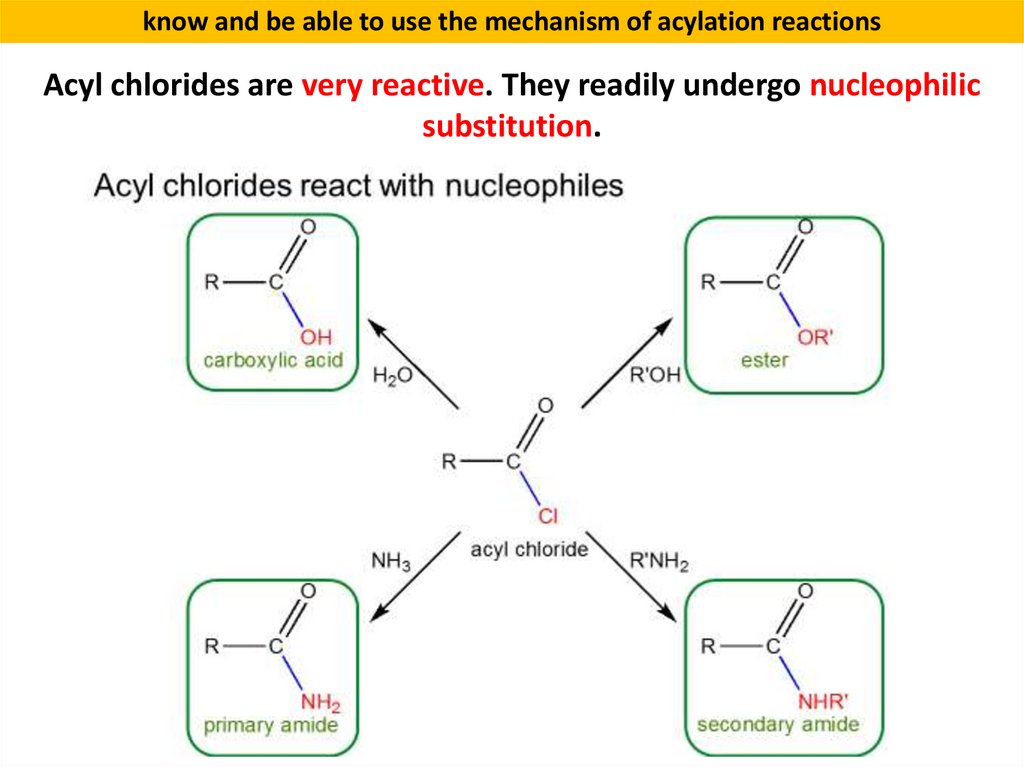
know and be able to use the mechanism of acylation reactionsAcyl chlorides are very reactive. They readily undergo nucleophilic
substitution.
23.
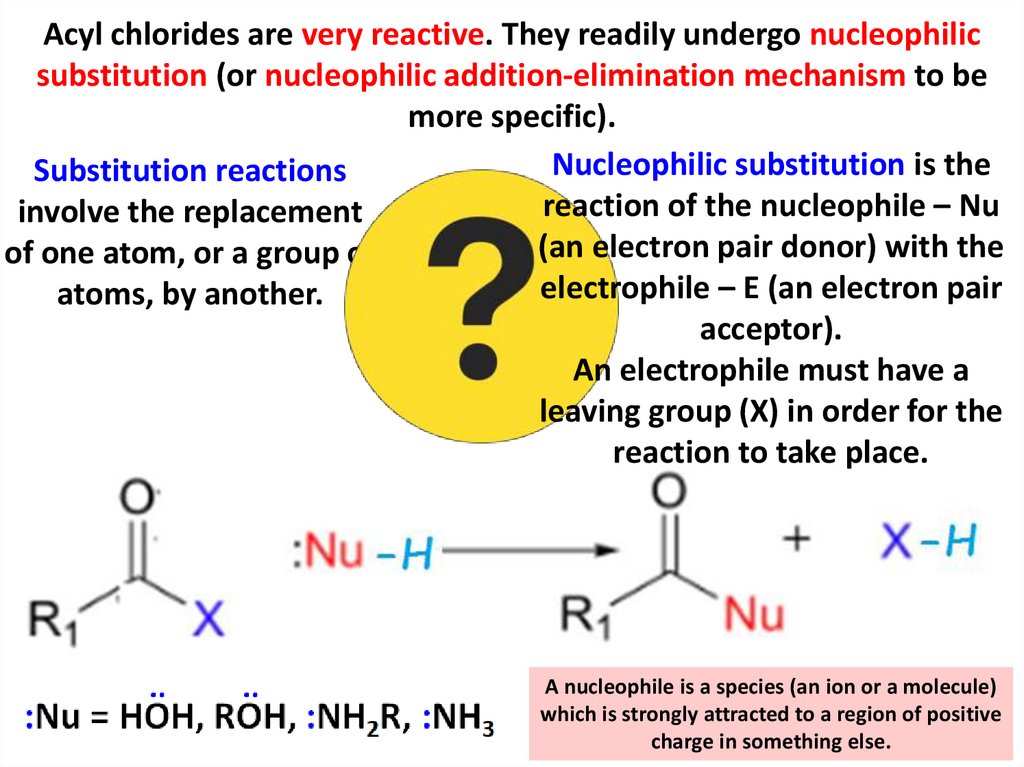
Acyl chlorides are very reactive. They readily undergo nucleophilicsubstitution (or nucleophilic addition-elimination mechanism to be
more specific).
Nucleophilic substitution is the
Substitution reactions
reaction of the nucleophile – Nu
involve the replacement
(an electron pair donor) with the
of one atom, or a group of
electrophile – E (an electron pair
atoms, by another.
acceptor).
An electrophile must have a
leaving group (X) in order for the
reaction to take place.
A nucleophile is a species (an ion or a molecule)
which is strongly attracted to a region of positive
charge in something else.
24.
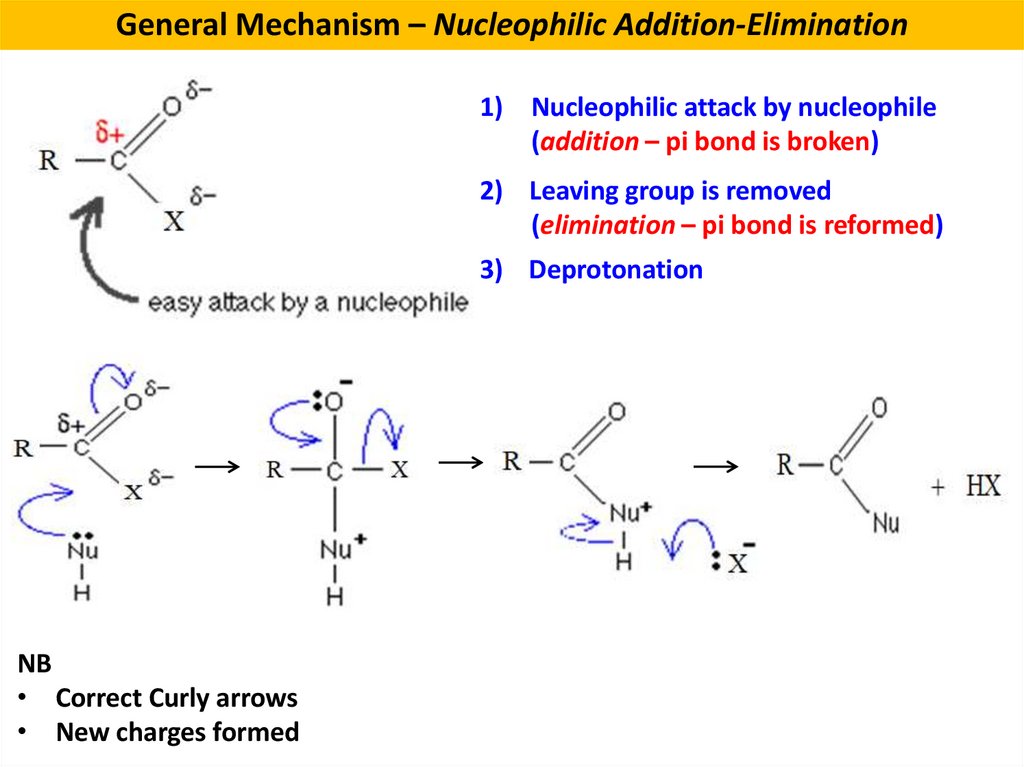
General Mechanism – Nucleophilic Addition-Elimination1) Nucleophilic attack by nucleophile
(addition – pi bond is broken)
2) Leaving group is removed
(elimination – pi bond is reformed)
3) Deprotonation
NB
• Correct Curly arrows
• New charges formed
25.
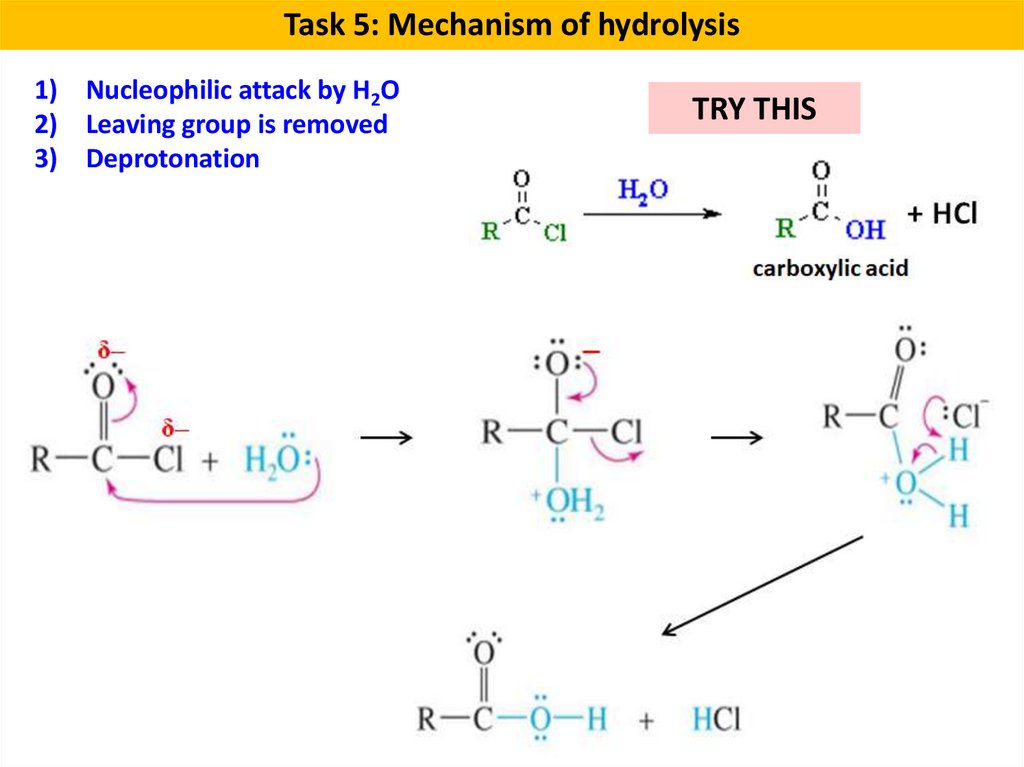
Task 5: Mechanism of hydrolysis1) Nucleophilic attack by H2O
2) Leaving group is removed
3) Deprotonation
TRY THIS
26.
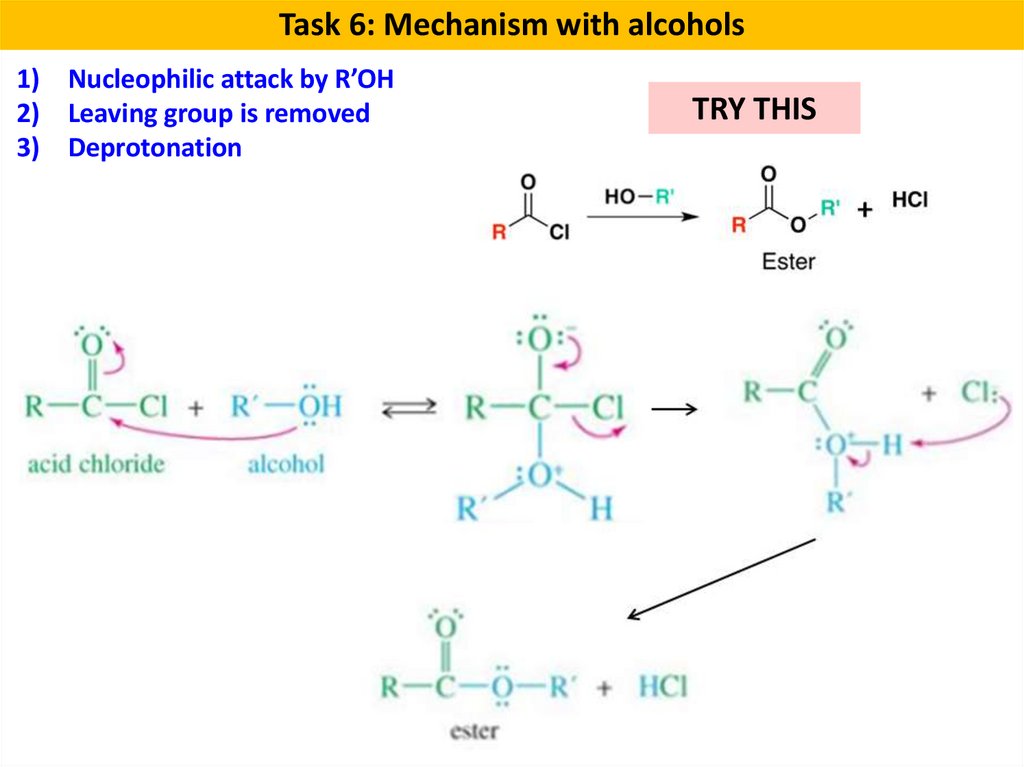
Task 6: Mechanism with alcohols1) Nucleophilic attack by R’OH
2) Leaving group is removed
3) Deprotonation
TRY THIS
27.
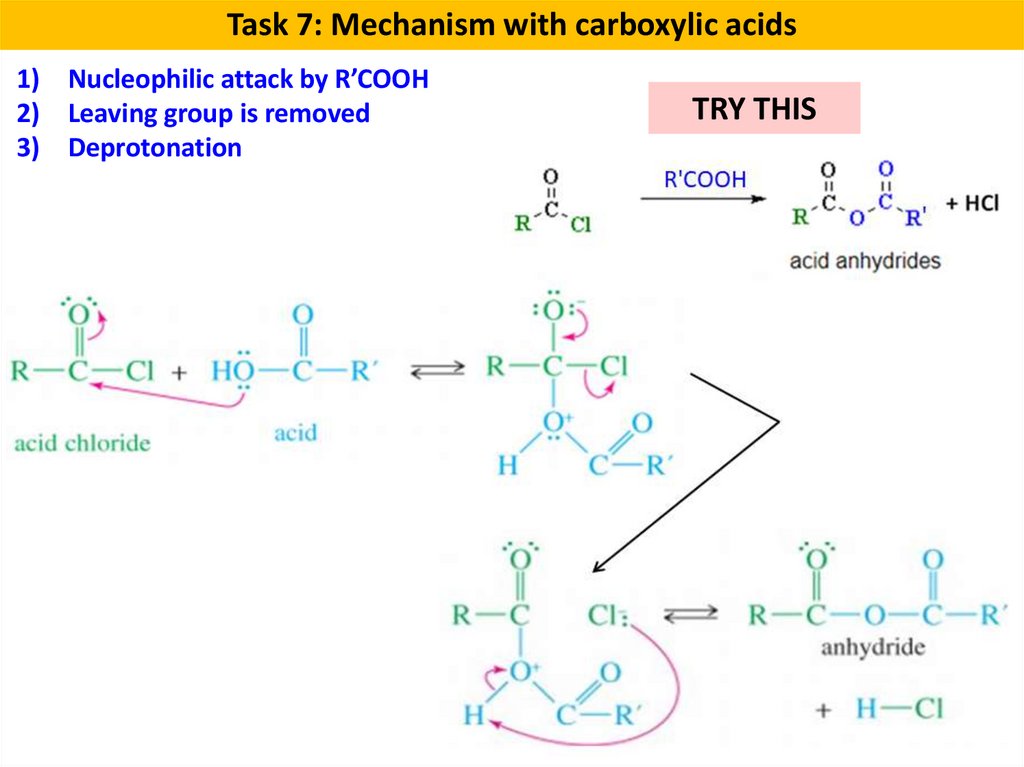
Task 7: Mechanism with carboxylic acids1) Nucleophilic attack by R’COOH
2) Leaving group is removed
3) Deprotonation
TRY THIS
28.
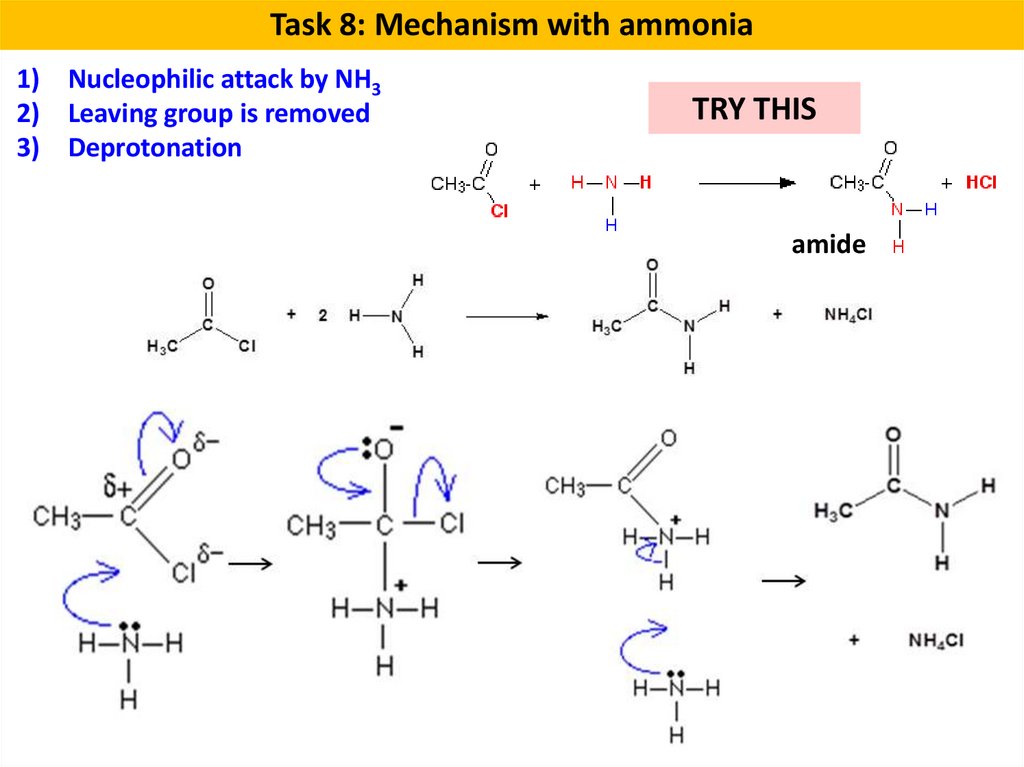
Task 8: Mechanism with ammonia1) Nucleophilic attack by NH3
2) Leaving group is removed
3) Deprotonation
TRY THIS
amide
29.
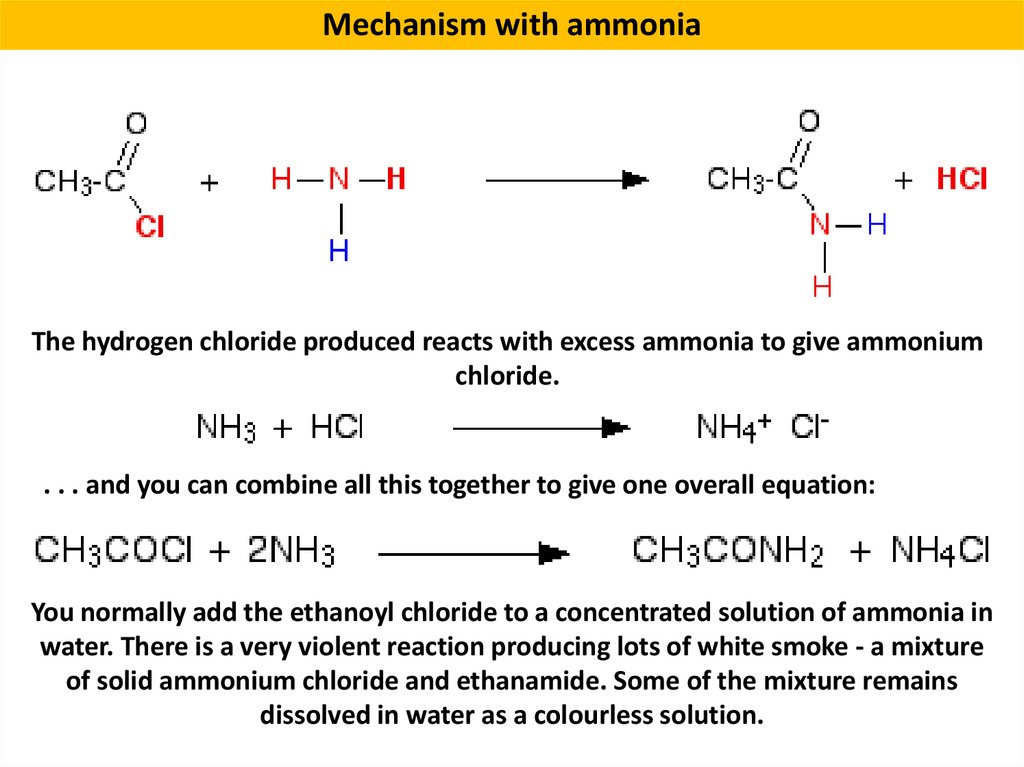
Mechanism with ammoniaThe hydrogen chloride produced reacts with excess ammonia to give ammonium
chloride.
. . . and you can combine all this together to give one overall equation:
You normally add the ethanoyl chloride to a concentrated solution of ammonia in
water. There is a very violent reaction producing lots of white smoke - a mixture
of solid ammonium chloride and ethanamide. Some of the mixture remains
dissolved in water as a colourless solution.
30.
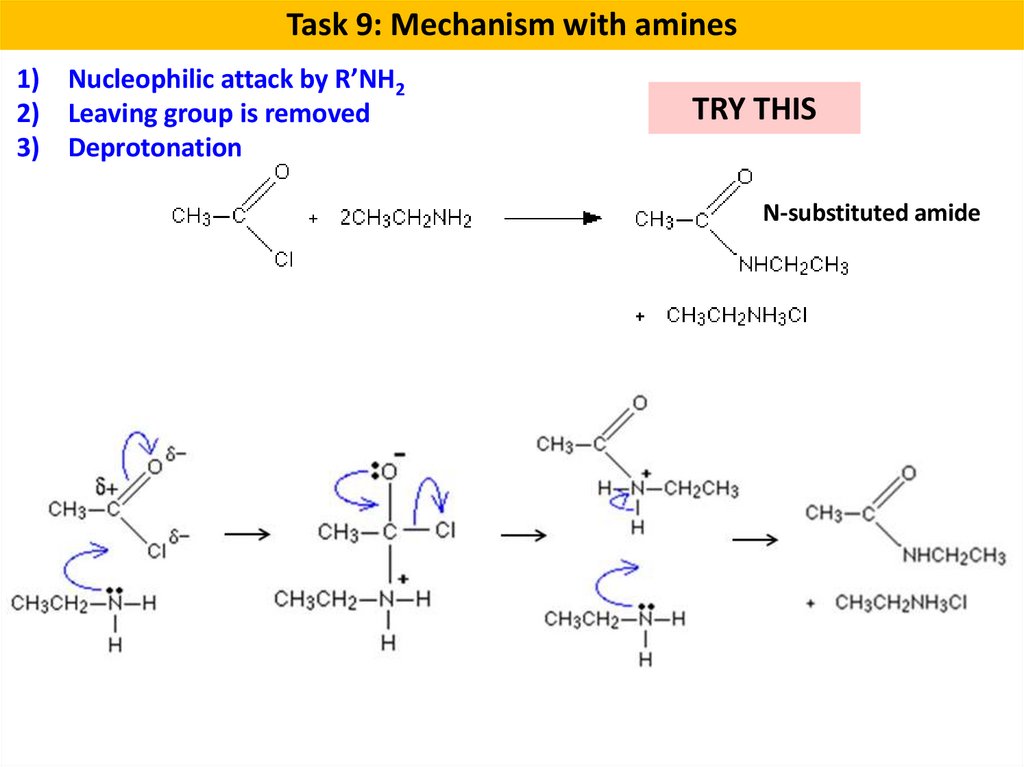
Task 9: Mechanism with amines1) Nucleophilic attack by R’NH2
2) Leaving group is removed
3) Deprotonation
TRY THIS
N-substituted amide
31.
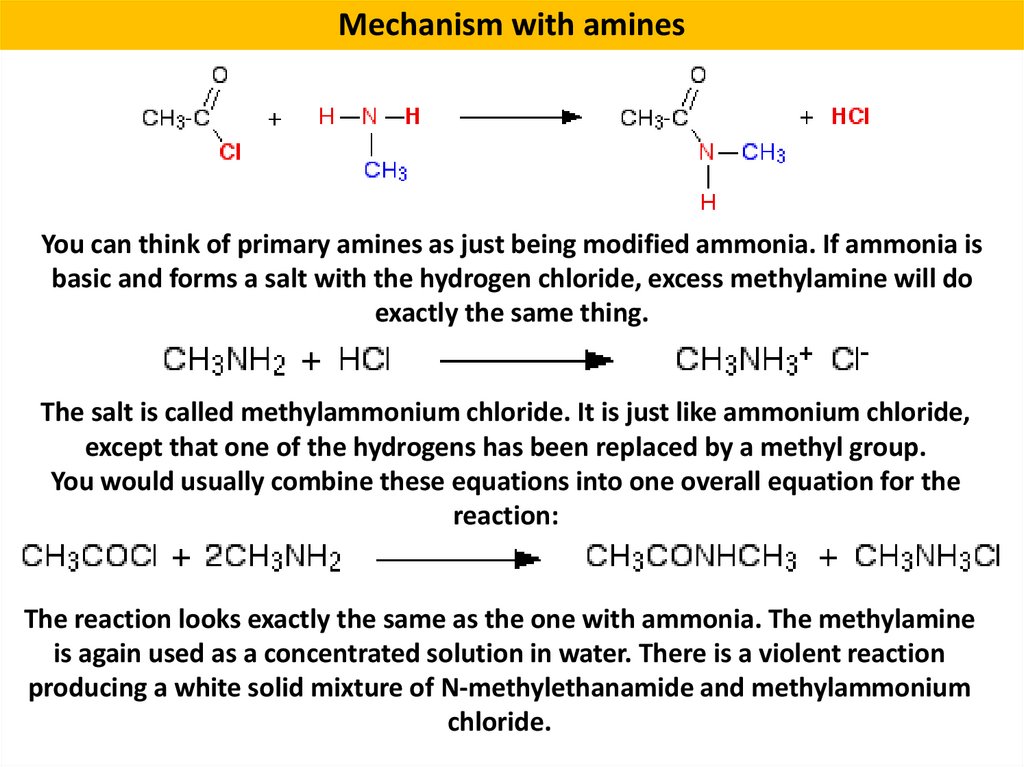
Mechanism with aminesYou can think of primary amines as just being modified ammonia. If ammonia is
basic and forms a salt with the hydrogen chloride, excess methylamine will do
exactly the same thing.
The salt is called methylammonium chloride. It is just like ammonium chloride,
except that one of the hydrogens has been replaced by a methyl group.
You would usually combine these equations into one overall equation for the
reaction:
The reaction looks exactly the same as the one with ammonia. The methylamine
is again used as a concentrated solution in water. There is a violent reaction
producing a white solid mixture of N-methylethanamide and methylammonium
chloride.
32.
know and be able to use the mechanism of acylation reactionsI know and I am able to use the mechanism of
acylation reactions
33.
Reflection• What has been learned
• What remained unclear
• What is necessary to work on