Похожие презентации:
Acid anhydrides and aspirin 2
1.
Acid anhydrides and Aspirin2.
Learning Objectives• know and be able to use the mechanism of
acylation reactions
• understand the role of acylation in the manufacture
of aspirin
3.
Expected Outcomes• Draw and outline reaction mechanisms for various
acylation reactions
• Differentiate alkylation and acylation and identify
their uses
• Explain the role of acylation in the manufacture of
aspirin and write pertinent equation for this
4.
KeywordsAcid anhydrides
Nucleophilic substitution
Acylation
Alkylation
Acyl group
Nucleophilic attack
Nucleophile
Leaving group
Deprotonation
5.
Review Task – Silent Reading Activity (12 mins)• Read on pp. 386-387 of your coursebook.
• Answer Check-up question nos. 3 and 4.
• Be ready to discuss your answers in class.
6.
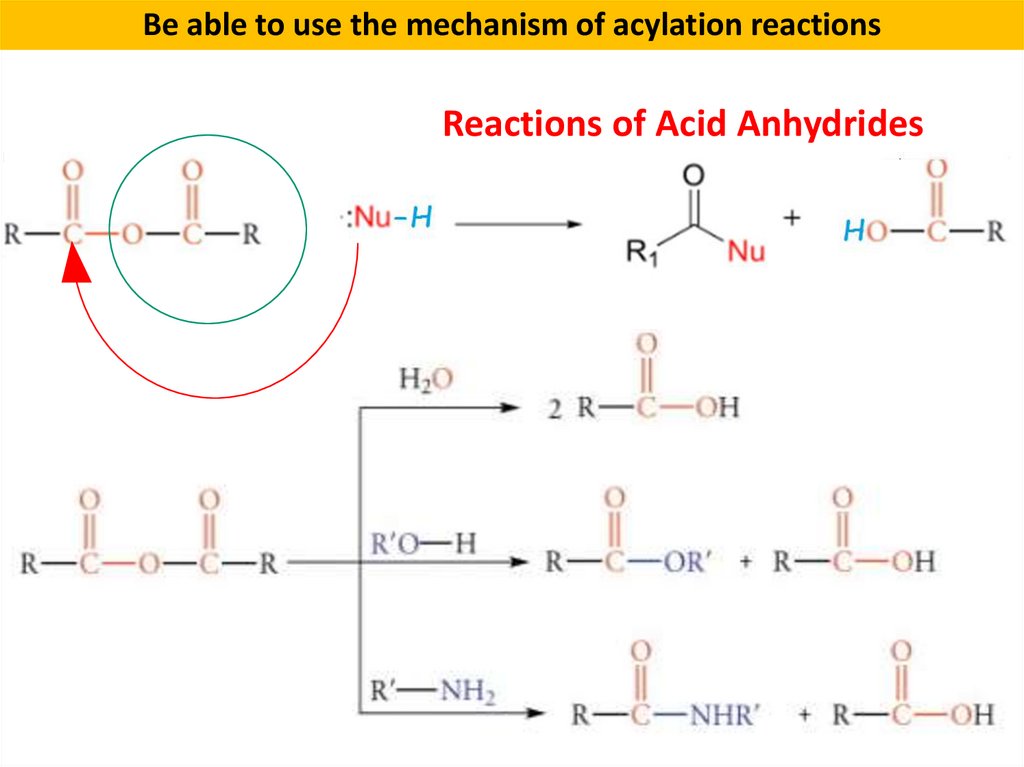
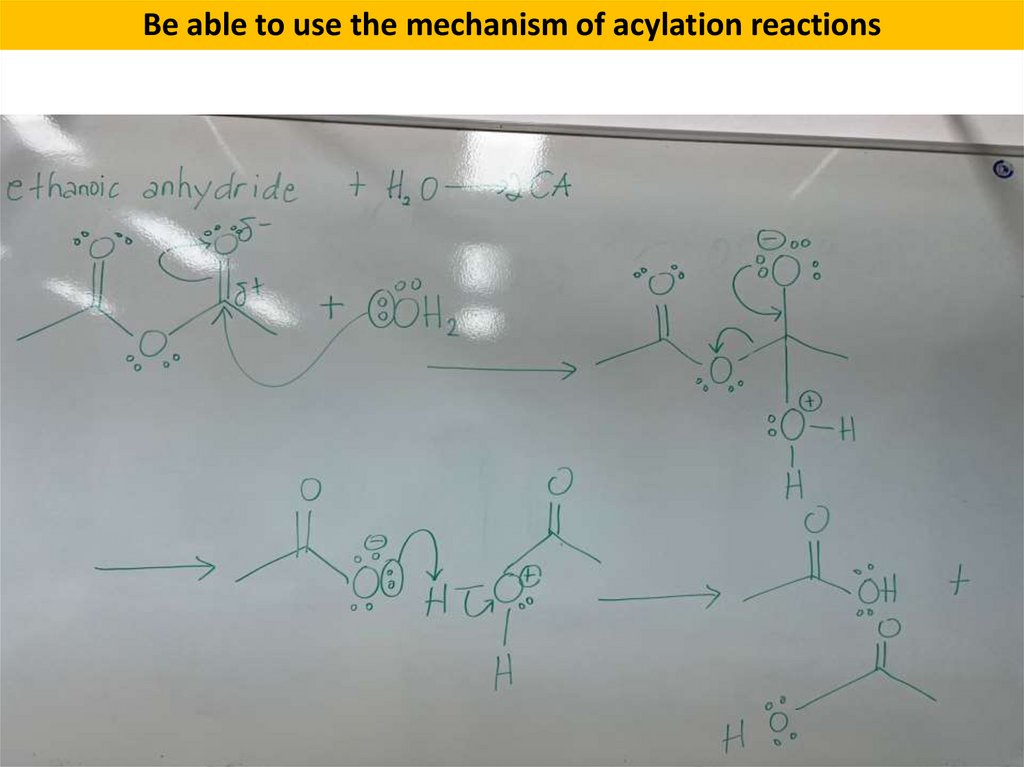
Be able to use the mechanism of acylation reactionsReactions of Acid Anhydrides
7.
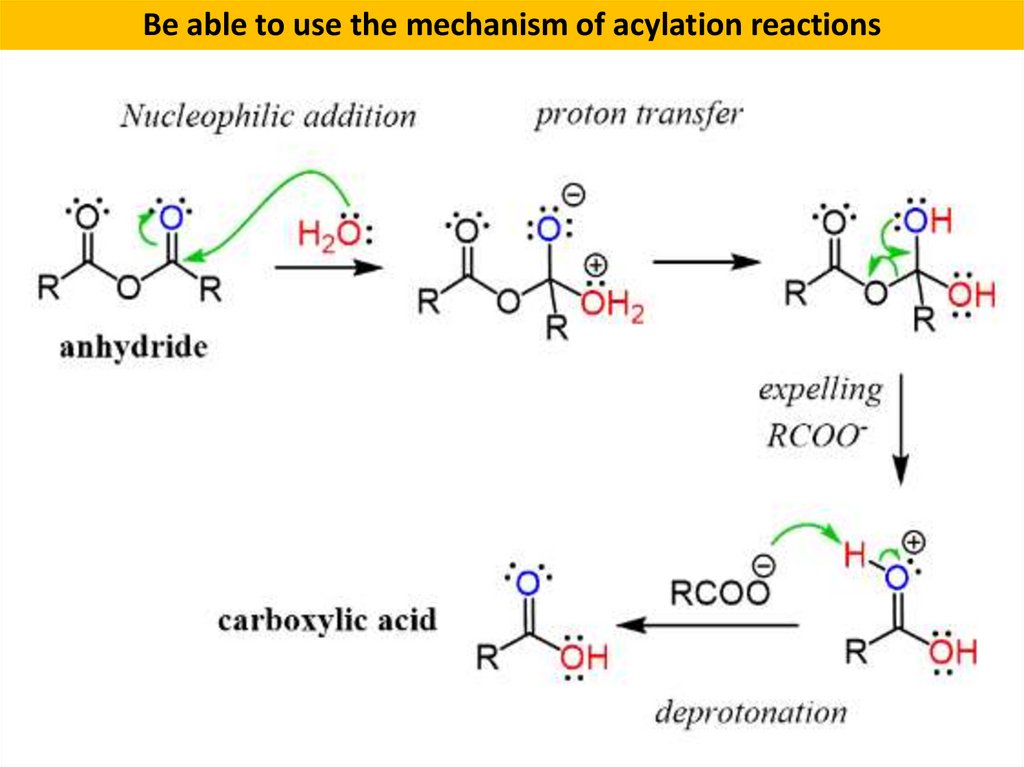
Be able to use the mechanism of acylation reactions8.
Be able to use the mechanism of acylation reactions9.
PAIR WORK ACTIVITYTask: Show the complete mechanism for the following reactions.
1. Ethanoic methanoic anhydride + water
2. Ethanoic methanoic anhydride + ethanol
10.
Be able to use the mechanism of acylation reactionsI can use the mechanism of acylation in various reactions of acid
anhydrides.
11.
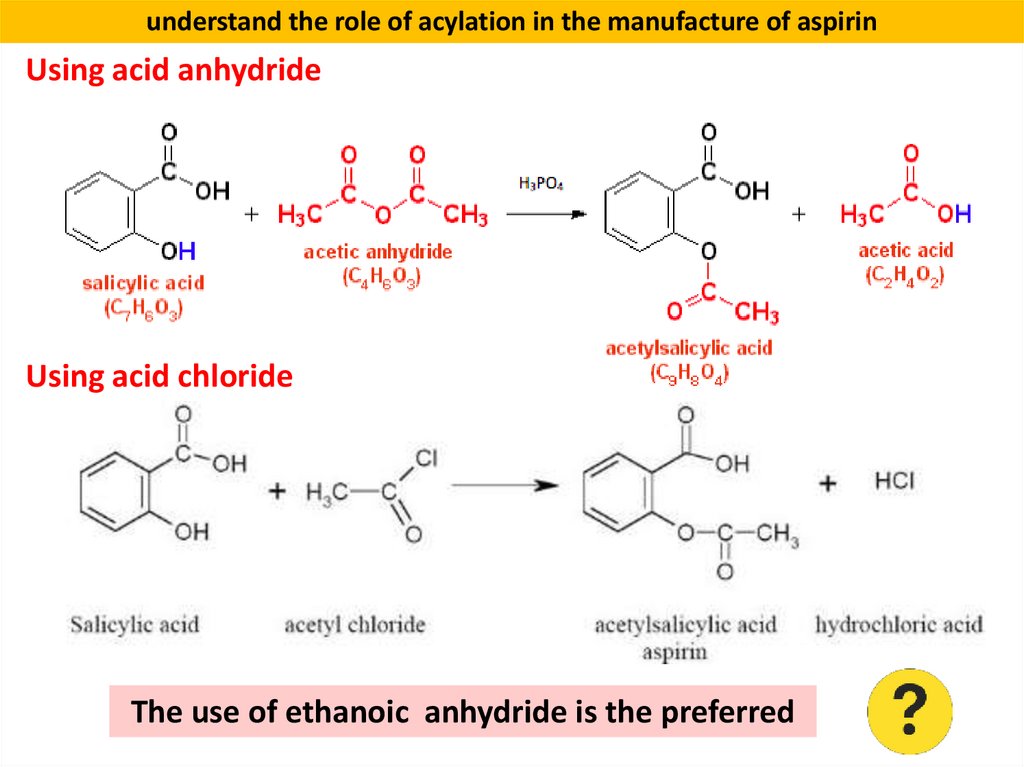
understand the role of acylation in the manufacture of aspirinSynthesis of Aspirin (acetylsalicylic acid) is a medication
used to reduce pain, fever, or inflammation.
12.
understand the role of acylation in the manufacture of aspirinUsing acid anhydride
Using acid chloride
The use of ethanoic anhydride is the preferred
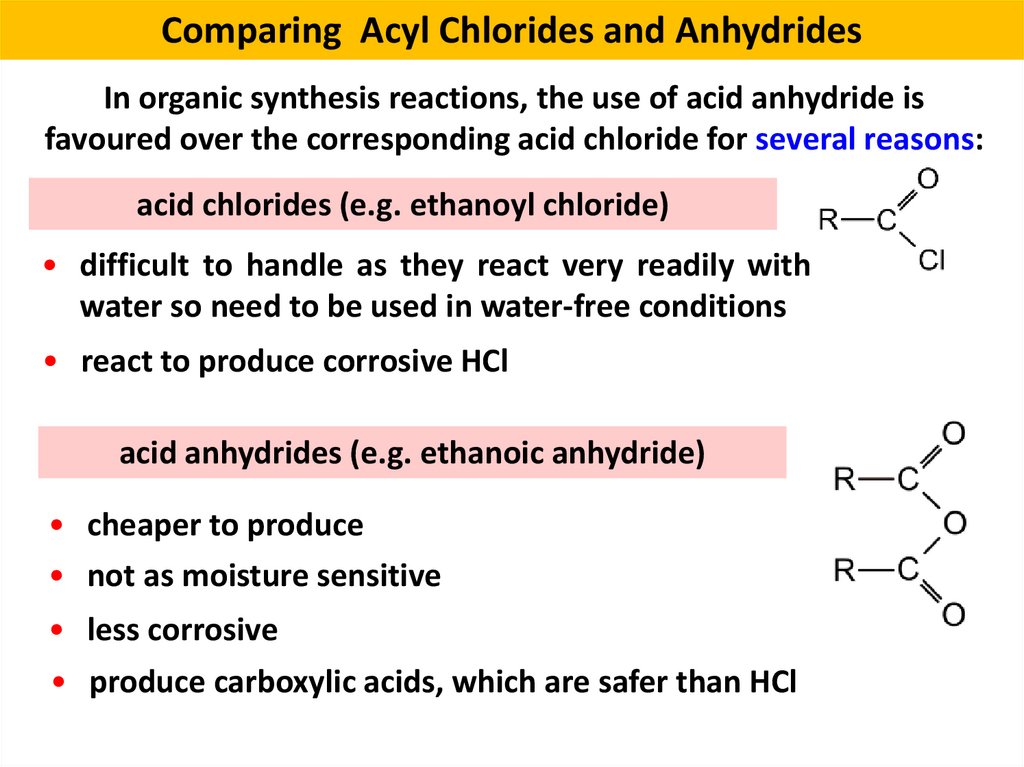
13. Comparing Acyl Chlorides and Anhydrides
In organic synthesis reactions, the use of acid anhydride isfavoured over the corresponding acid chloride for several reasons:
acid chlorides (e.g. ethanoyl chloride)
• difficult to handle as they react very readily with
water so need to be used in water-free conditions
• react to produce corrosive HCl
acid anhydrides (e.g. ethanoic anhydride)
• cheaper to produce
• not as moisture sensitive
• less corrosive
• produce carboxylic acids, which are safer than HCl
14.
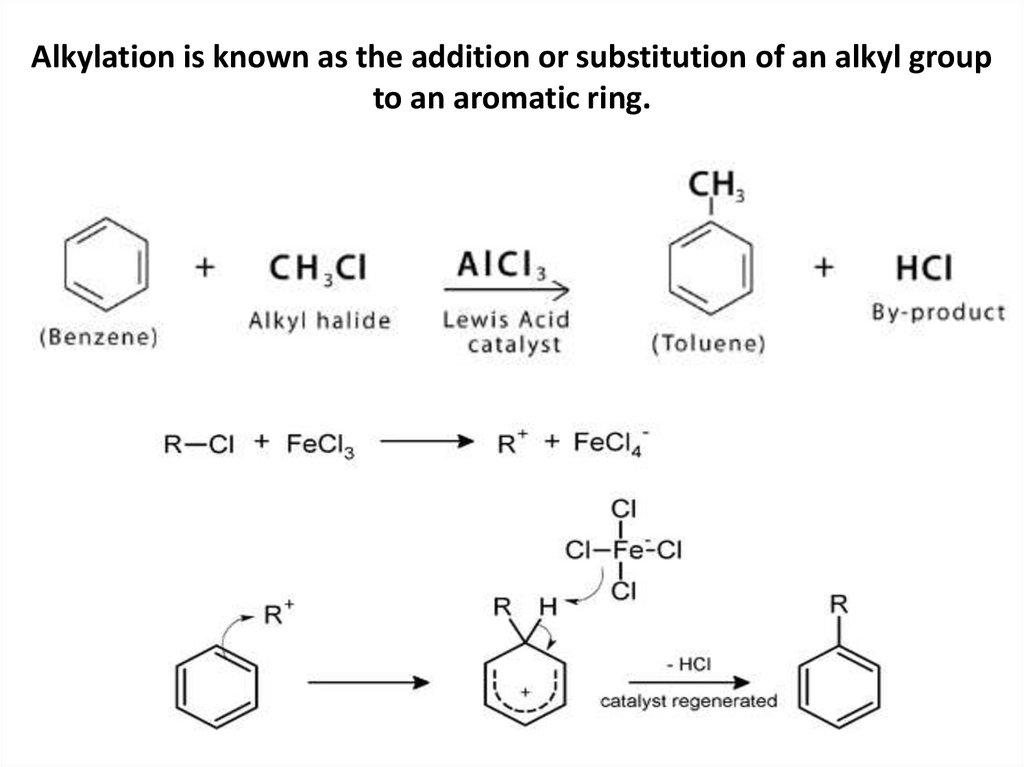
Alkylation is known as the addition or substitution of an alkyl groupto an aromatic ring.
15.
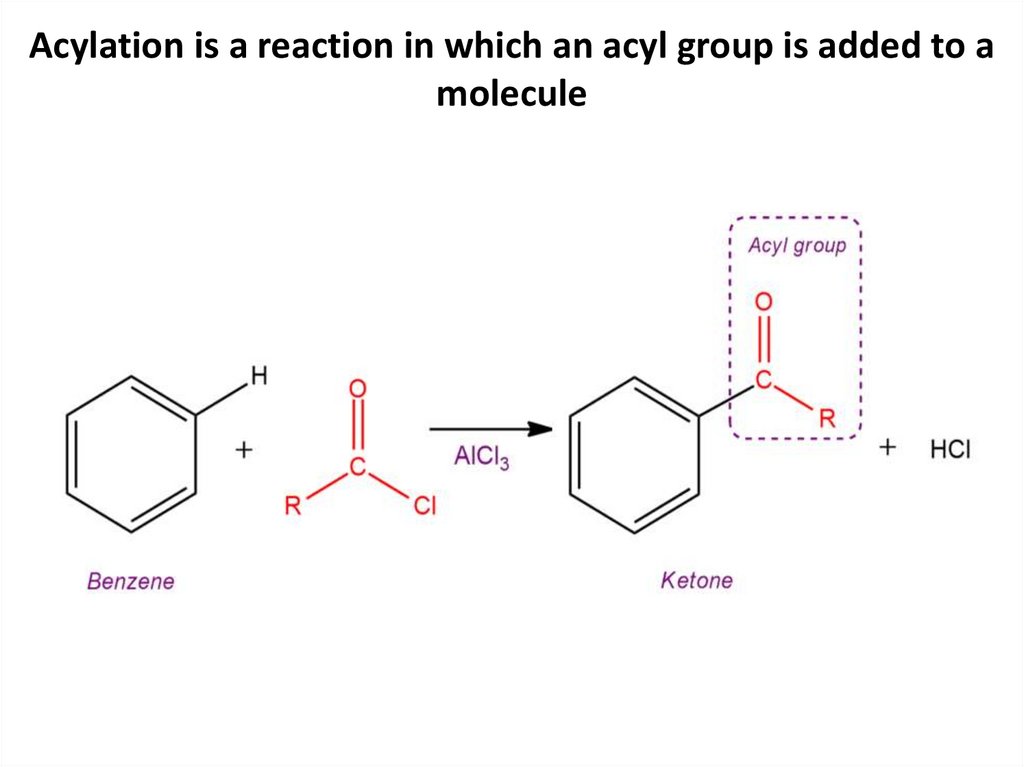
Acylation is a reaction in which an acyl group is added to amolecule
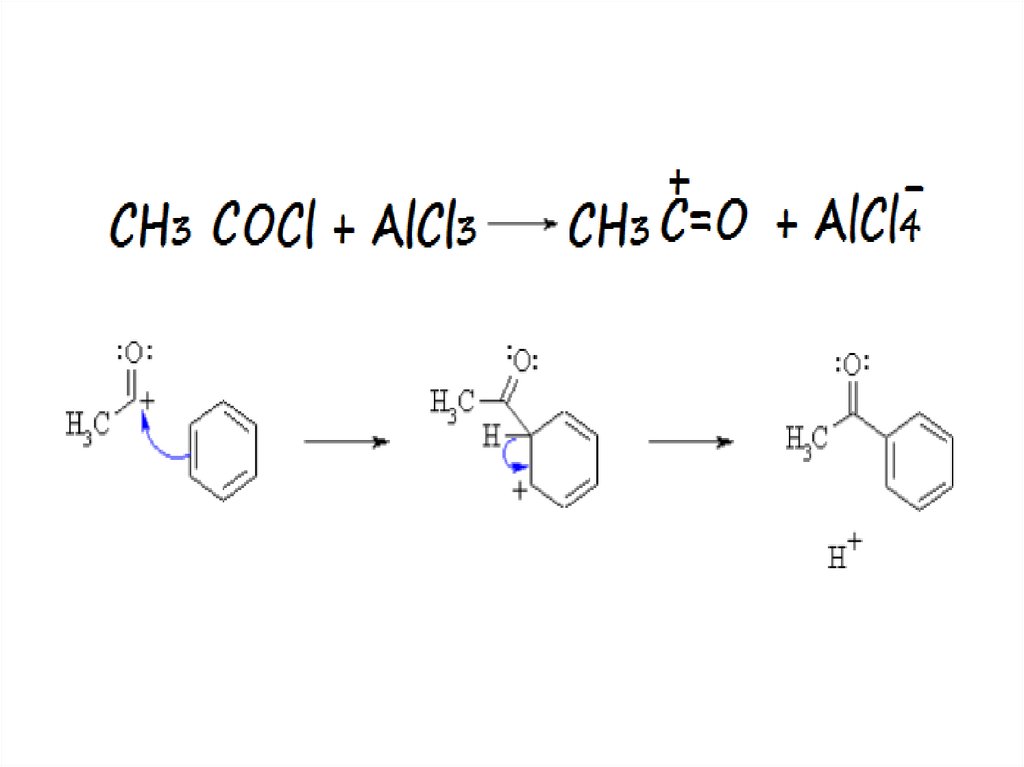
16.
17.
understand the role of acylation in the manufacture of aspirinI understand the role of acylation in the manufacture of aspirin
18.
Reflection• What has been learned
• What remained unclear
• What is necessary to work on