Похожие презентации:
Chemical bonding and properties
1. Chemical Bonding and Properties
Wednesday, 17 December 2025Chemical Bonding and
Properties
Revision
2. Ionic Bonding
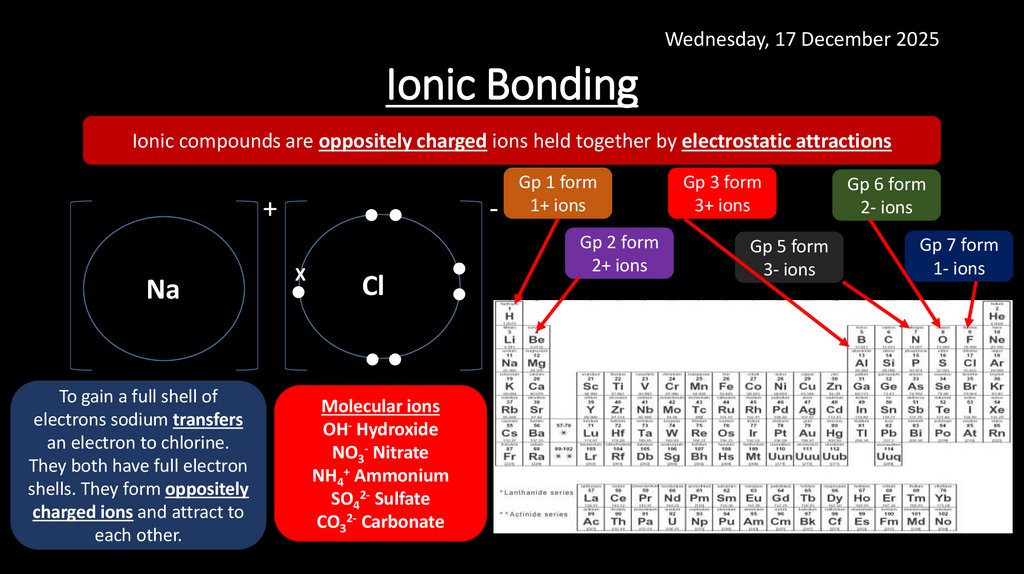
Wednesday, 17 December 2025Ionic Bonding
Ionic compounds are oppositely charged ions held together by electrostatic attractions
+
X
Cl
•
Na
-
•
•
To gain a full shell of
electrons sodium transfers
an electron to chlorine.
They both have full electron
shells. They form oppositely
charged ions and attract to
each other.
Molecular ions
OH- Hydroxide
NO3- Nitrate
NH4+ Ammonium
SO42- Sulfate
CO32- Carbonate
Gp 1 form
1+ ions
Gp 2 form
2+ ions
Gp 3 form
3+ ions
Gp 5 form
3- ions
Gp 6 form
2- ions
Gp 7 form
1- ions
3. Ionic Bonding
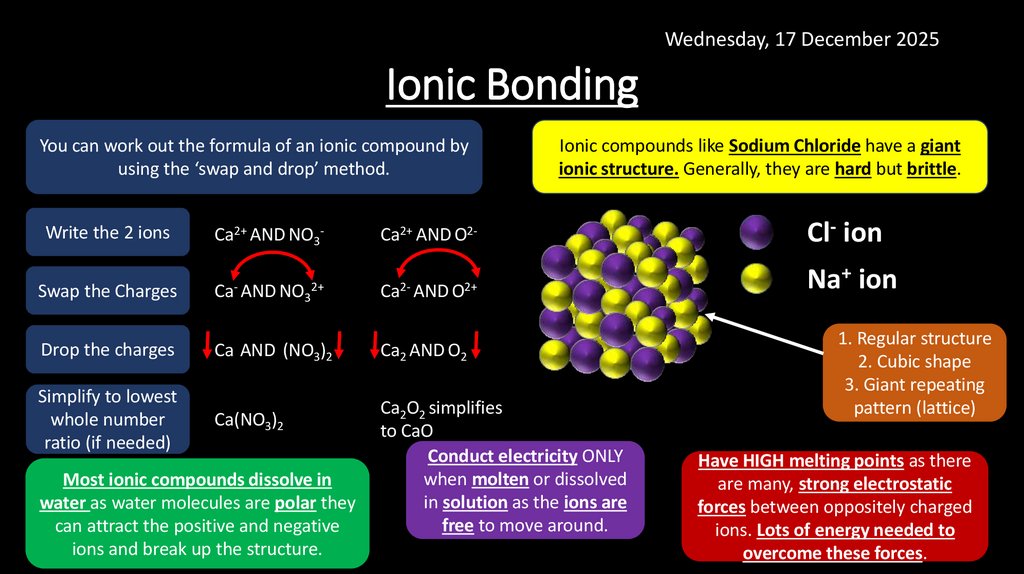
Wednesday, 17 December 2025Ionic Bonding
You can work out the formula of an ionic compound by
using the ‘swap and drop’ method.
Ionic compounds like Sodium Chloride have a giant
ionic structure. Generally, they are hard but brittle.
Write the 2 ions
Ca2+ AND NO3-
Ca2+ AND O2-
Cl- ion
Swap the Charges
Ca- AND NO32+
Ca2- AND O2+
Na+ ion
Drop the charges
Ca AND (NO3)2
Ca2 AND O2
Simplify to lowest
whole number
ratio (if needed)
Ca(NO3)2
Most ionic compounds dissolve in
water as water molecules are polar they
can attract the positive and negative
ions and break up the structure.
Ca2O2 simplifies
to CaO
Conduct electricity ONLY
when molten or dissolved
in solution as the ions are
free to move around.
1. Regular structure
2. Cubic shape
3. Giant repeating
pattern (lattice)
Have HIGH melting points as there
are many, strong electrostatic
forces between oppositely charged
ions. Lots of energy needed to
overcome these forces.
4. Covalent Bonding
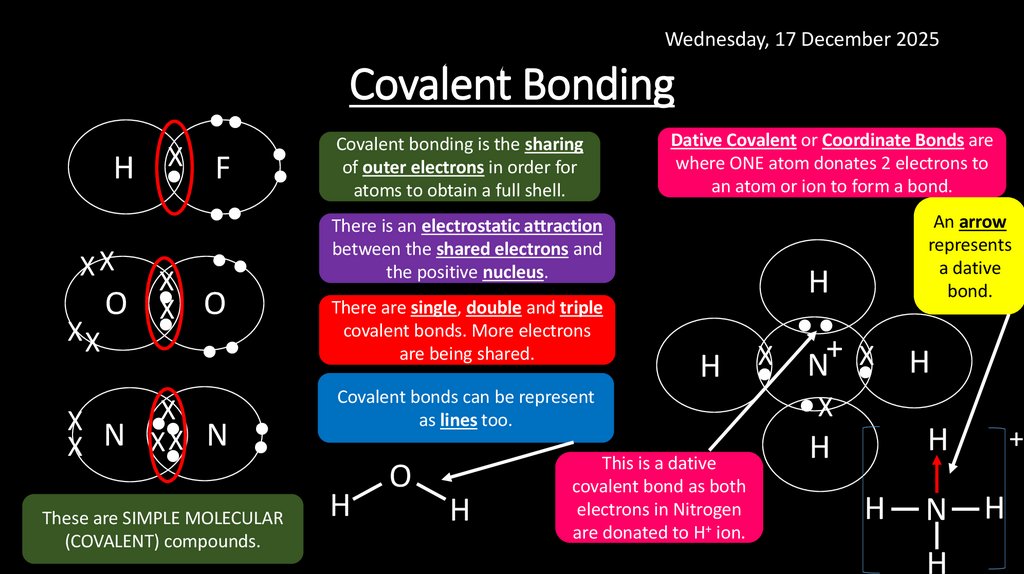
Wednesday, 17 December 2025•
H •X F
•
•
XX
XX
Covalent Bonding
There are single, double and triple
covalent bonds. More electrons
are being shared.
•
X
X
•X•X N
N
X
Dative Covalent or Coordinate Bonds are
where ONE atom donates 2 electrons to
an atom or ion to form a bond.
There is an electrostatic attraction
between the shared electrons and
the positive nucleus.
X
O •X O
Covalent bonding is the sharing
of outer electrons in order for
atoms to obtain a full shell.
These are SIMPLE MOLECULAR
(COVALENT) compounds.
Covalent bonds can be represent
as lines too.
H
O
H
An arrow
represents
a dative
bond.
H
•
+
H • N •X H
This is a dative
covalent bond as both
electrons in Nitrogen
are donated to H+ ion.
X
•X
H
H
H
N
H
+
H
5. Giant Covalent Structures
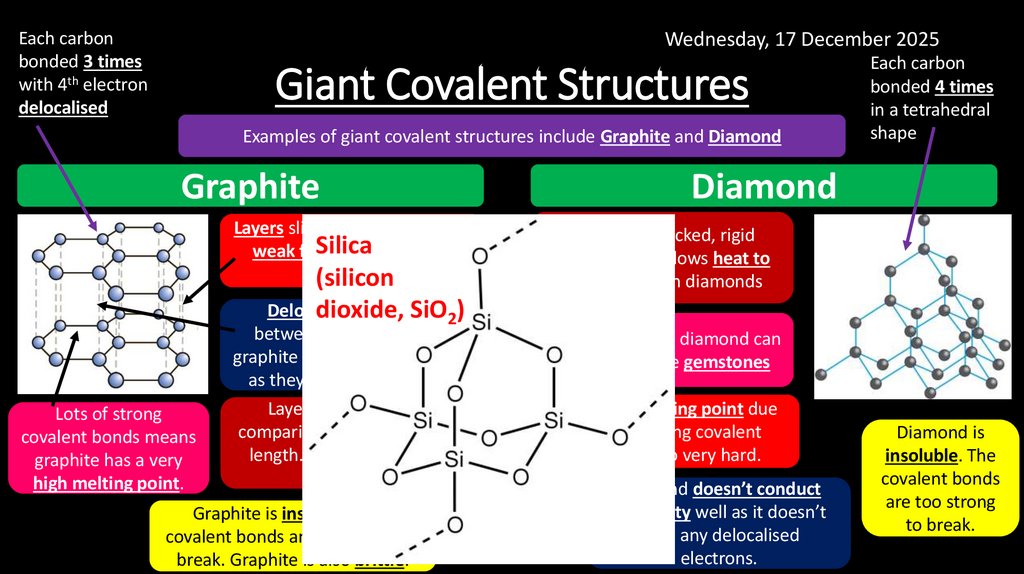
Wednesday, 17 December 2025Each carbon
bonded 3 times
with 4th electron
delocalised
Giant Covalent Structures
Examples of giant covalent structures include Graphite and Diamond
Graphite
Diamond
Layers slide easily as there are
Silica
weak forces
between the
layers.
(silicon
Lots of strong
covalent bonds means
graphite has a very
high melting point.
Delocalised
electrons
dioxide,
SiO2)
between the layers allow
graphite to conduct electricity
as they can carry a charge.
Layers are far apart in
comparison to covalent bond
length. This means it has a
low density.
Graphite is insoluble. The
covalent bonds are too strong to
break. Graphite is also brittle.
Each carbon
bonded 4 times
in a tetrahedral
shape
The tightly packed, rigid
arrangement allows heat to
conduct well in diamonds
Unlike graphite, diamond can
be cut to make gemstones
Very high melting point due
to many strong covalent
bonds. It also very hard.
C Harris - Allery Chemistry
Diamond doesn’t conduct
electricity well as it doesn’t
have any delocalised
electrons.
Diamond is
insoluble. The
covalent bonds
are too strong
to break.
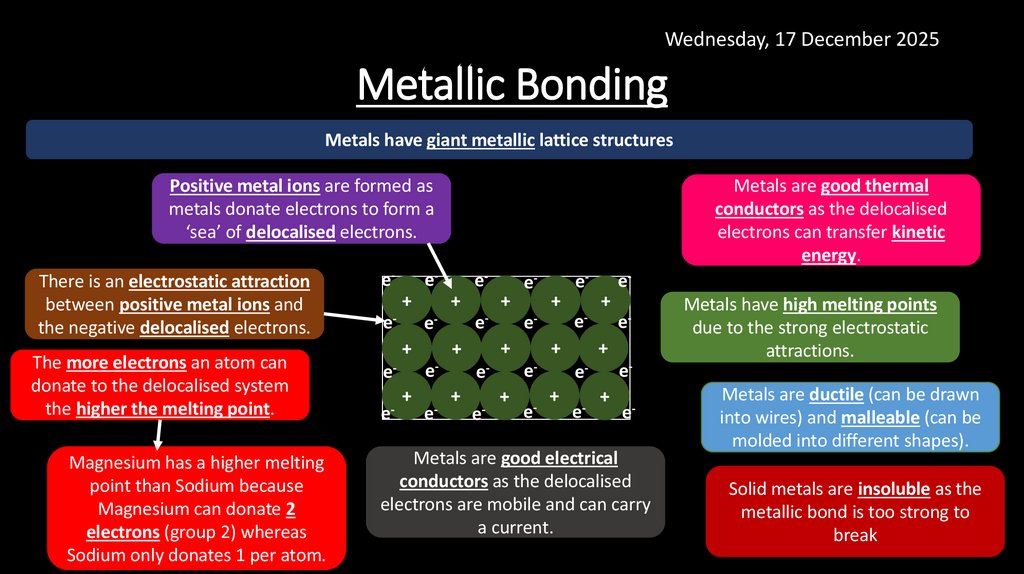
6. Metallic Bonding
Wednesday, 17 December 2025Metallic Bonding
Metals have giant metallic lattice structures
Positive metal ions are formed as
metals donate electrons to form a
‘sea’ of delocalised electrons.
There is an electrostatic attraction
between positive metal ions and
the negative delocalised electrons.
The more electrons an atom can
donate to the delocalised system
the higher the melting point.
Magnesium has a higher melting
point than Sodium because
Magnesium can donate 2
electrons (group 2) whereas
Sodium only donates 1 per atom.
e-
e-
e+
+
e-
+
e-
e-
+
+
e-
e-
e+
+
e-
+
e-
e-
e-
e-
+
e-
+
+
e-
e-
+
e-
e+
e-
Metals are good thermal
conductors as the delocalised
electrons can transfer kinetic
energy.
e-
e+
e-
+
e-
Metals are good electrical
conductors as the delocalised
electrons are mobile and can carry
a current.
Metals have high melting points
due to the strong electrostatic
attractions.
Metals are ductile (can be drawn
into wires) and malleable (can be
molded into different shapes).
Solid metals are insoluble as the
metallic bond is too strong to
break
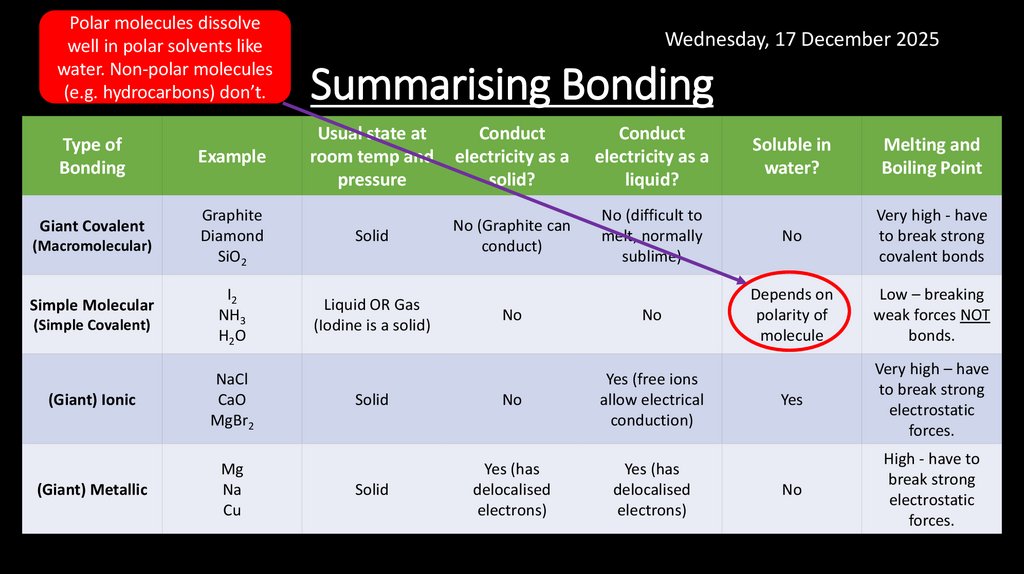
7. Summarising Bonding
Polar molecules dissolvewell in polar solvents like
water. Non-polar molecules
(e.g. hydrocarbons) don’t.
Type of
Bonding
Giant Covalent
(Macromolecular)
Simple Molecular
(Simple Covalent)
Wednesday, 17 December 2025
Summarising Bonding
Usual state at
room temp and
pressure
Conduct
electricity as a
solid?
Conduct
electricity as a
liquid?
Graphite
Diamond
SiO2
Solid
No (Graphite can
conduct)
I2
NH3
H2O
Liquid OR Gas
(Iodine is a solid)
Example
(Giant) Ionic
NaCl
CaO
MgBr2
(Giant) Metallic
Mg
Na
Cu
Soluble in
water?
Melting and
Boiling Point
No (difficult to
melt, normally
sublime)
No
Very high - have
to break strong
covalent bonds
No
No
Depends on
polarity of
molecule
Low – breaking
weak forces NOT
bonds.
Solid
No
Yes (free ions
allow electrical
conduction)
Yes
Very high – have
to break strong
electrostatic
forces.
Solid
Yes (has
delocalised
electrons)
Yes (has
delocalised
electrons)
No
High - have to
break strong
electrostatic
forces.
8.
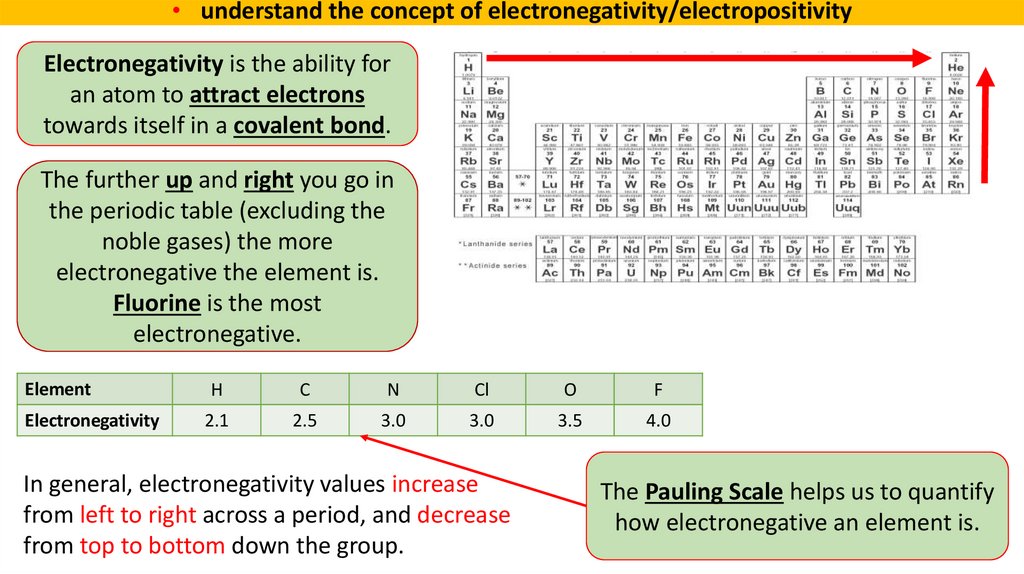
• understand the concept of electronegativity/electropositivityElectronegativity is the ability for
an atom to attract electrons
towards itself in a covalent bond.
The further up and right you go in
the periodic table (excluding the
noble gases) the more
electronegative the element is.
Fluorine is the most
electronegative.
Element
H
C
N
Cl
O
F
Electronegativity
2.1
2.5
3.0
3.0
3.5
4.0
In general, electronegativity values increase
from left to right across a period, and decrease
from top to bottom down the group.
The Pauling Scale helps us to quantify
how electronegative an element is.
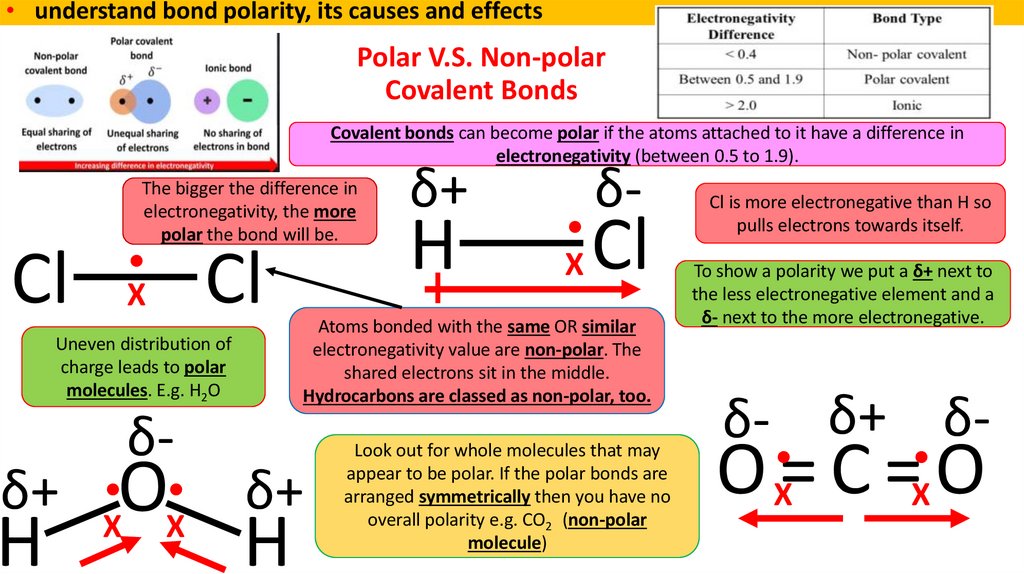
9. Polar V.S. Non-polar Covalent Bonds
• understand bond polarity, its causes and effectsPolar V.S. Non-polar
Covalent Bonds
Covalent bonds can become polar if the atoms attached to it have a difference in
electronegativity (between 0.5 to 1.9).
The bigger the difference in
electronegativity, the more
polar the bond will be.
Cl
Cl
X
Uneven distribution of
charge leads to polar
molecules. E.g. H2O
δ+
H
δ-
O
X X
H
X
δ-
Cl
Atoms bonded with the same OR similar
electronegativity value are non-polar. The
shared electrons sit in the middle.
Hydrocarbons are classed as non-polar, too.
δ+
H
δ+
Look out for whole molecules that may
appear to be polar. If the polar bonds are
arranged symmetrically then you have no
overall polarity e.g. CO2 (non-polar
molecule)
Cl is more electronegative than H so
pulls electrons towards itself.
To show a polarity we put a δ+ next to
the less electronegative element and a
δ- next to the more electronegative.
δ- δ+ δ-
O X= C =X O
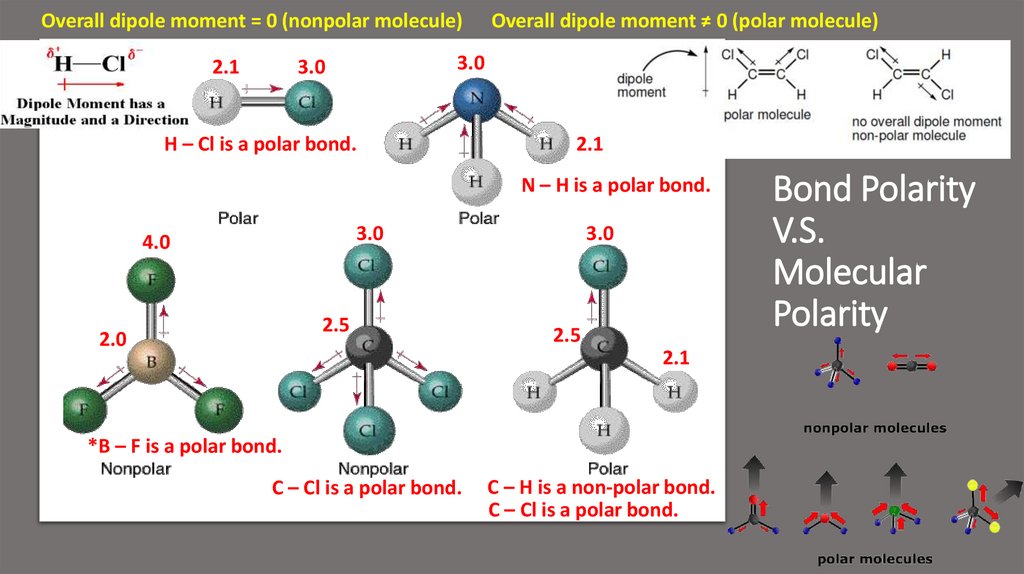
10. Bond Polarity V.S. Molecular Polarity
Overall dipole moment = 0 (nonpolar molecule)2.1
Overall dipole moment ≠ 0 (polar molecule)
3.0
3.0
H – Cl is a polar bond.
2.1
N – H is a polar bond.
3.0
4.0
2.5
2.0
3.0
2.5
2.1
*B – F is a polar bond.
C – Cl is a polar bond.
C – H is a non-polar bond.
C – Cl is a polar bond.
Bond Polarity
V.S.
Molecular
Polarity
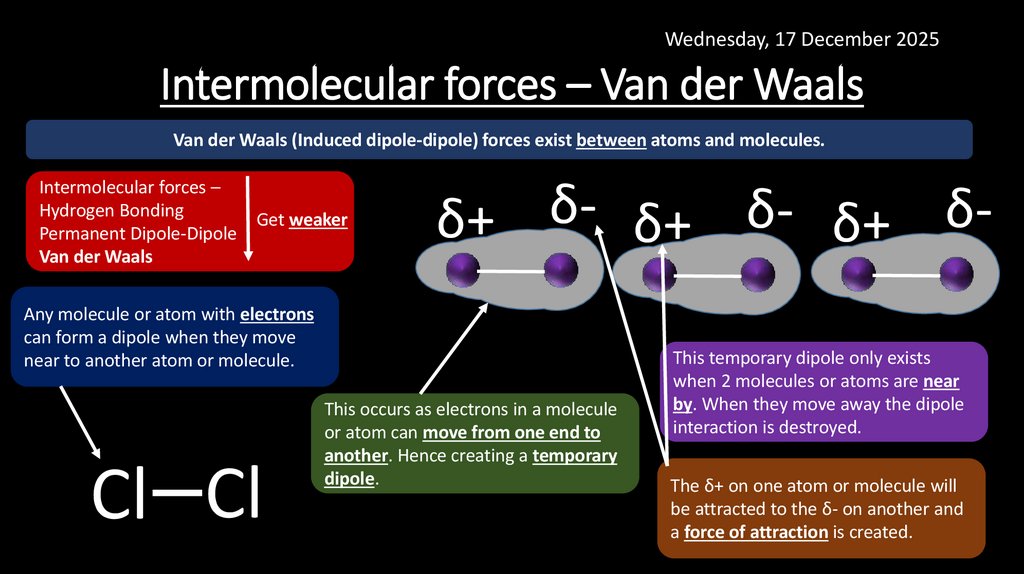
11. Intermolecular forces – Van der Waals
Wednesday, 17 December 2025Intermolecular forces – Van der Waals
Van der Waals (Induced dipole-dipole) forces exist between atoms and molecules.
Intermolecular forces –
Hydrogen Bonding
Permanent Dipole-Dipole
Van der Waals
Get weaker
δ+ δ- δ+ δ- δ+ δ-
Any molecule or atom with electrons
can form a dipole when they move
near to another atom or molecule.
Cl Cl
This occurs as electrons in a molecule
or atom can move from one end to
another. Hence creating a temporary
dipole.
This temporary dipole only exists
when 2 molecules or atoms are near
by. When they move away the dipole
interaction is destroyed.
The δ+ on one atom or molecule will
be attracted to the δ- on another and
a force of attraction is created.
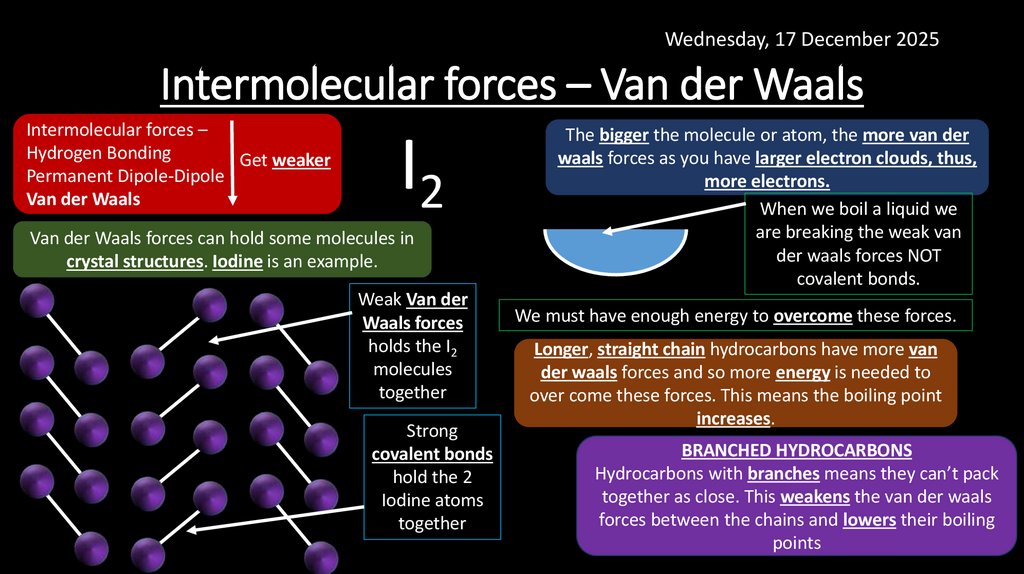
12. Intermolecular forces – Van der Waals
Wednesday, 17 December 2025Intermolecular forces – Van der Waals
Intermolecular forces –
Hydrogen Bonding
Get weaker
Permanent Dipole-Dipole
Van der Waals
I2
Van der Waals forces can hold some molecules in
crystal structures. Iodine is an example.
Weak Van der
Waals forces
holds the I2
molecules
together
Strong
covalent bonds
hold the 2
Iodine atoms
together
The bigger the molecule or atom, the more van der
waals forces as you have larger electron clouds, thus,
more electrons.
When we boil a liquid we
are breaking the weak van
der waals forces NOT
covalent bonds.
We must have enough energy to overcome these forces.
Longer, straight chain hydrocarbons have more van
der waals forces and so more energy is needed to
over come these forces. This means the boiling point
increases.
BRANCHED HYDROCARBONS
Hydrocarbons with branches means they can’t pack
together as close. This weakens the van der waals
forces between the chains and lowers their boiling
points
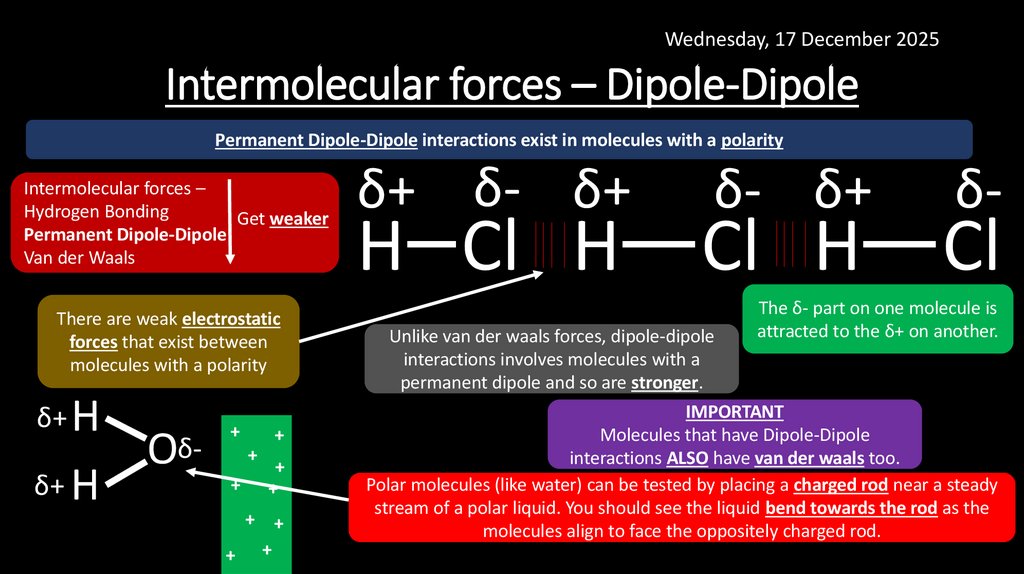
13. Intermolecular forces – Dipole-Dipole
Wednesday, 17 December 2025Intermolecular forces – Dipole-Dipole
Permanent Dipole-Dipole interactions exist in molecules with a polarity
Intermolecular forces –
Hydrogen Bonding
Get weaker
Permanent Dipole-Dipole
Van der Waals
There are weak electrostatic
forces that exist between
molecules with a polarity
δ+ H
δ+ H
Oδ-
+
+
+
+
+
+
+ +
+ +
δ+ δ- δ+
H Cl H
δ- δ+
Cl H
Unlike van der waals forces, dipole-dipole
interactions involves molecules with a
permanent dipole and so are stronger.
δ-
Cl
The δ- part on one molecule is
attracted to the δ+ on another.
IMPORTANT
Molecules that have Dipole-Dipole
interactions ALSO have van der waals too.
Polar molecules (like water) can be tested by placing a charged rod near a steady
stream of a polar liquid. You should see the liquid bend towards the rod as the
molecules align to face the oppositely charged rod.
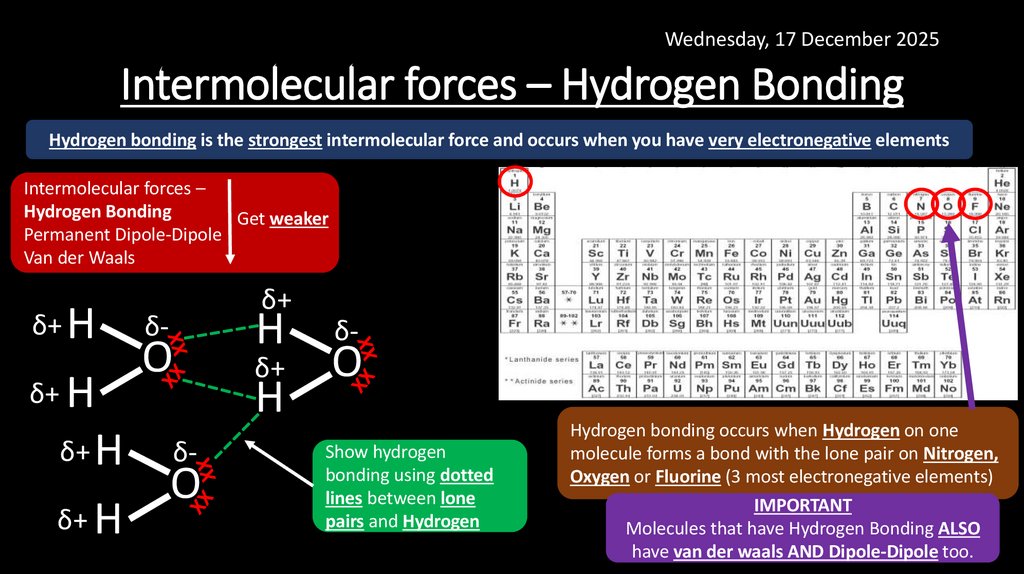
14. Intermolecular forces – Hydrogen Bonding
Wednesday, 17 December 2025Intermolecular forces – Hydrogen Bonding
Hydrogen bonding is the strongest intermolecular force and occurs when you have very electronegative elements
Intermolecular forces –
Hydrogen Bonding
Get weaker
Permanent Dipole-Dipole
Van der Waals
δ+ H
δ+ H
δ+ H
δ+ H
δ+
δ-
O
δ-
O
H δO
δ+
H
Show hydrogen
bonding using dotted
lines between lone
pairs and Hydrogen
Hydrogen bonding occurs when Hydrogen on one
molecule forms a bond with the lone pair on Nitrogen,
Oxygen or Fluorine (3 most electronegative elements)
IMPORTANT
Molecules that have Hydrogen Bonding ALSO
have van der waals AND Dipole-Dipole too.
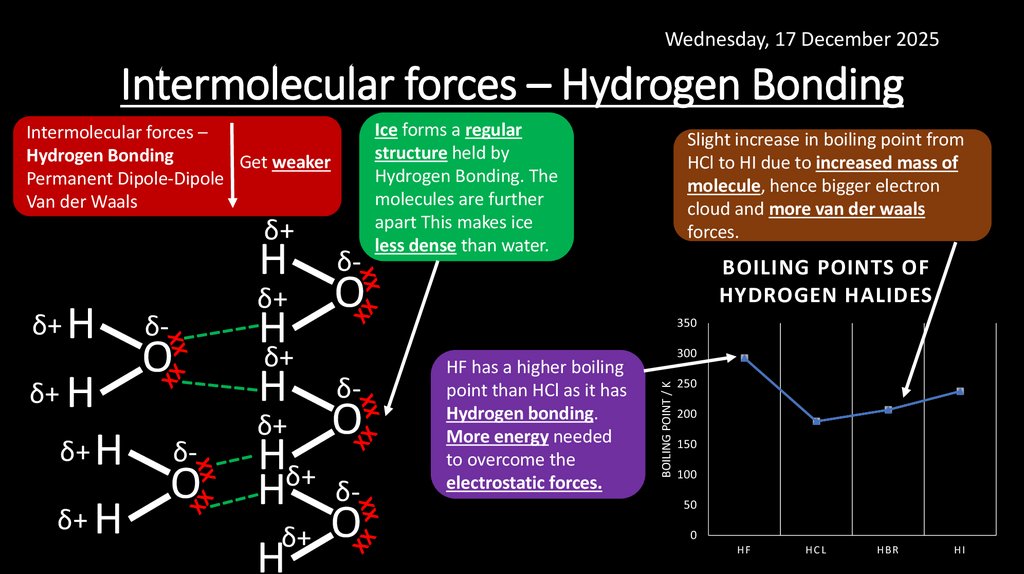
15. Intermolecular forces – Hydrogen Bonding
Wednesday, 17 December 2025Intermolecular forces – Hydrogen Bonding
δ+
δ+ H
δ+ H
δ+ H
δ+ H
δ-
O
δ-
O
H δO
δ+
H
δ+
H δδ+ O
Hδ+
H δδ+ O
H
Ice forms a regular
structure held by
Hydrogen Bonding. The
molecules are further
apart This makes ice
less dense than water.
Slight increase in boiling point from
HCl to HI due to increased mass of
molecule, hence bigger electron
cloud and more van der waals
forces.
BOILING POINTS OF
HYDROGEN HALIDES
350
HF has a higher boiling
point than HCl as it has
Hydrogen bonding.
More energy needed
to overcome the
electrostatic forces.
300
BOILING POINT / K
Intermolecular forces –
Hydrogen Bonding
Get weaker
Permanent Dipole-Dipole
Van der Waals
250
200
150
100
50
0
HF
HCL
HBR
HI
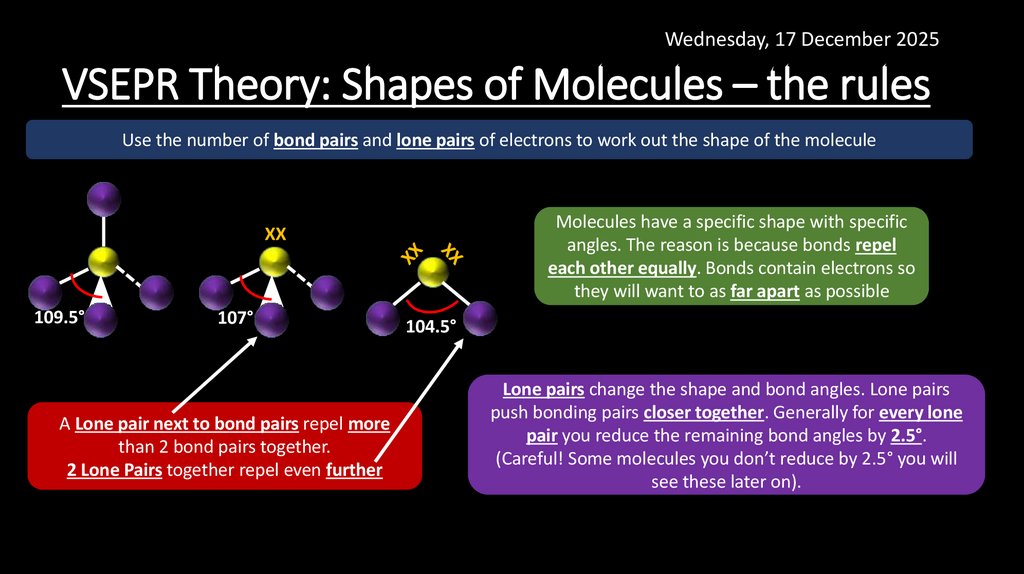
16. VSEPR Theory: Shapes of Molecules – the rules
Wednesday, 17 December 2025VSEPR Theory: Shapes of Molecules – the rules
Use the number of bond pairs and lone pairs of electrons to work out the shape of the molecule
Molecules have a specific shape with specific
angles. The reason is because bonds repel
each other equally. Bonds contain electrons so
they will want to as far apart as possible
XX
109.5°
107°
A Lone pair next to bond pairs repel more
than 2 bond pairs together.
2 Lone Pairs together repel even further
104.5°
Lone pairs change the shape and bond angles. Lone pairs
push bonding pairs closer together. Generally for every lone
pair you reduce the remaining bond angles by 2.5°.
(Careful! Some molecules you don’t reduce by 2.5° you will
see these later on).
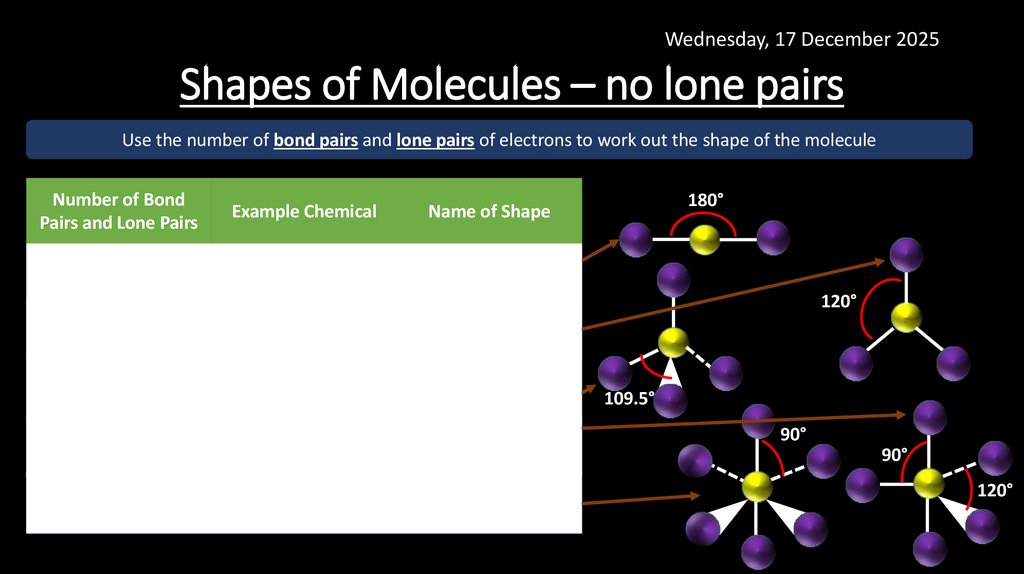
17. Shapes of Molecules – no lone pairs
Wednesday, 17 December 2025Shapes of Molecules – no lone pairs
Use the number of bond pairs and lone pairs of electrons to work out the shape of the molecule
Number of Bond
Pairs and Lone Pairs
Example Chemical
Name of Shape
BP = 2
LP = 0
BeCl2
Linear
BP = 3
LP = 0
BF3
Trigonal Planar
BP = 4
LP = 0
CH4
Tetrahedral
BP = 5
LP = 0
PCl5
Trigonal Bipyramidal
BP = 6
LP = 0
SF6
Octahedral
180°
120°
109.5°
90°
90°
120°
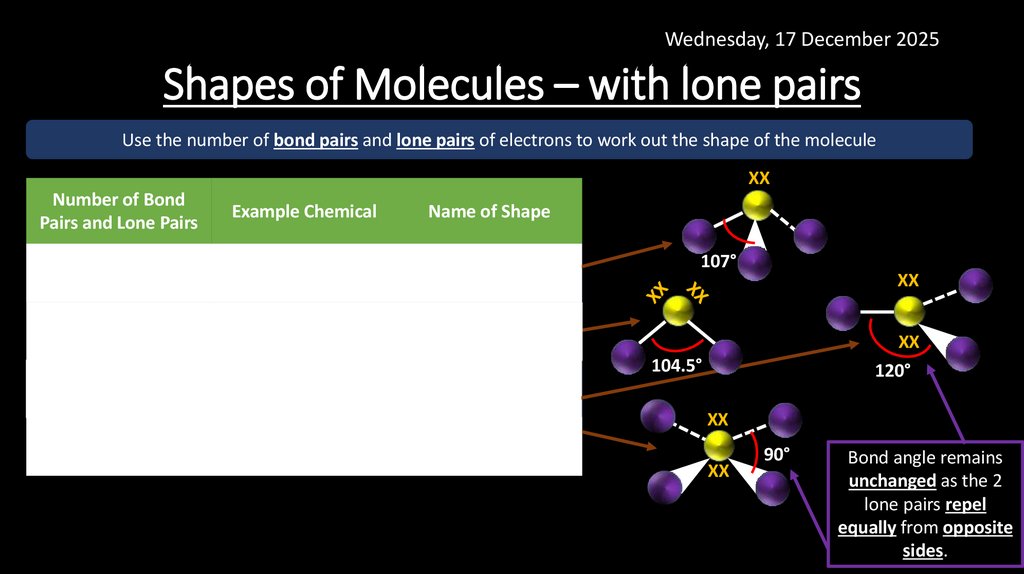
18. Shapes of Molecules – with lone pairs
Wednesday, 17 December 2025Shapes of Molecules – with lone pairs
Use the number of bond pairs and lone pairs of electrons to work out the shape of the molecule
XX
Number of Bond
Pairs and Lone Pairs
Example Chemical
Name of Shape
BP = 3
LP = 1
NH3
Pyramidal
BP = 2
LP = 2
H20
Bent
107°
XX
XX
120°
104.5°
BP = 3
LP = 2
ClF3
BP = 4
LP = 2
XeF4
Trigonal Planar
XX
Square Planar
XX
90°
Bond angle remains
unchanged as the 2
lone pairs repel
equally from opposite
sides.

 Химия
Химия








