Похожие презентации:
introduction_to_reactions_in_aqueous_solutions (1)
1.
GENERAL CHEMISTRYPrinciples and Modern Applications
PETRUCCI
HERRING
MADURA
TENTH EDITION
BISSONNETTE
Introduction to Reactions
in Aqueous Solutions
PHILIP DUTTON
UNIVERSITY OF WINDSOR
DEPARTMENT OF CHEMISTRY AND
BIOCHEMISTRY
5
2. Introduction to Reactions in Aqueous Solutions
CONTENTS6- 1
The Nature of Aqueous Solutions
6- 2
Precipitation Reactions
6- 3
Acid–Base Reactions
6- 4
Oxidation–Reduction Reactions:
Some General Principles
6- 5
Balancing Oxidation–Reduction
Equations
6- 6
Oxidizing and Reducing Agents
6- 7
Stoichiometry of Reactions in
Aqueous Solutions: Titrations
3. 5.1 The Nature of Aqueous Solutions
WaterInexpensive
Can dissolve a vast number of substances
Many substances dissociate into ions
Aqueous solutions are found everywhere
Seawater
Living systems
4.
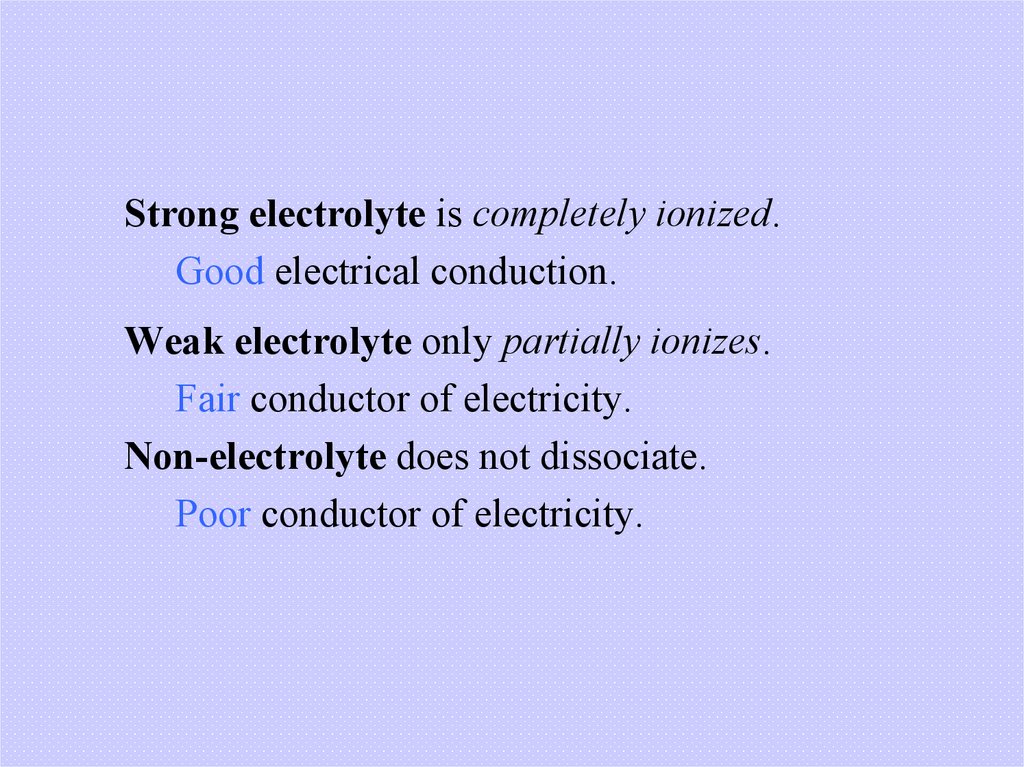
Strong electrolyte is completely ionized.Good electrical conduction.
Weak electrolyte only partially ionizes.
Fair conductor of electricity.
Non-electrolyte does not dissociate.
Poor conductor of electricity.
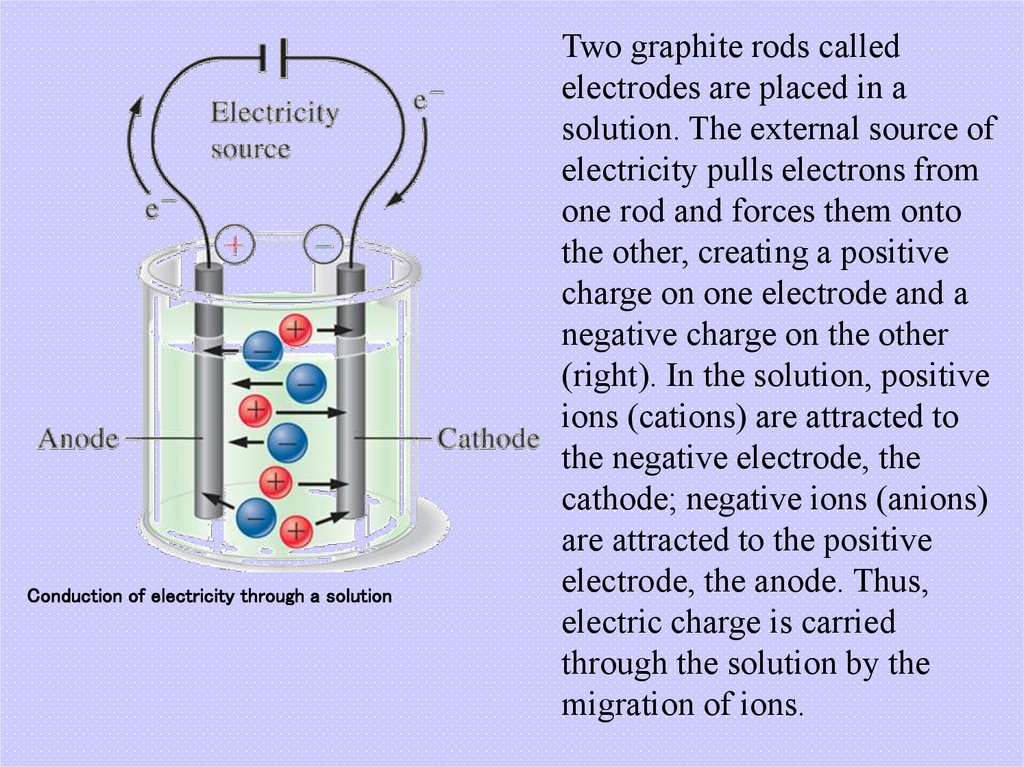
5. Conduction of electricity through a solution
Two graphite rods calledelectrodes are placed in a
solution. The external source of
electricity pulls electrons from
one rod and forces them onto
the other, creating a positive
charge on one electrode and a
negative charge on the other
(right). In the solution, positive
ions (cations) are attracted to
the negative electrode, the
cathode; negative ions (anions)
are attracted to the positive
electrode, the anode. Thus,
electric charge is carried
through the solution by the
migration of ions.
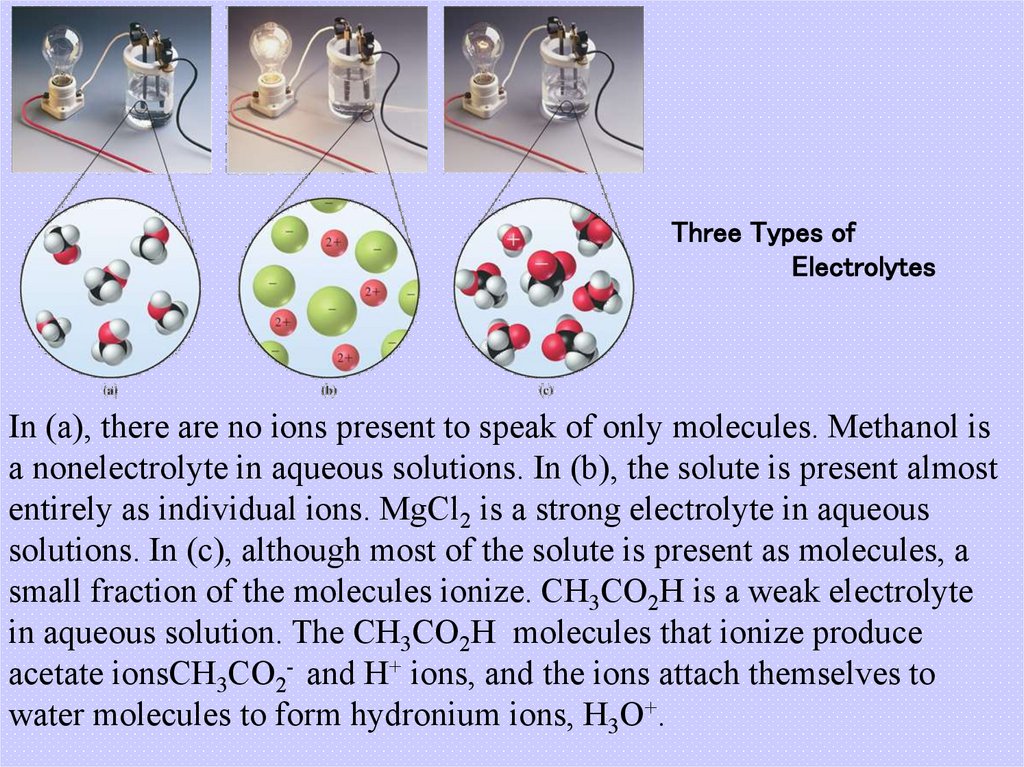
6. Three Types of Electrolytes
In (a), there are no ions present to speak of only molecules. Methanol isa nonelectrolyte in aqueous solutions. In (b), the solute is present almost
entirely as individual ions. MgCl2 is a strong electrolyte in aqueous
solutions. In (c), although most of the solute is present as molecules, a
small fraction of the molecules ionize. CH3CO2H is a weak electrolyte
in aqueous solution. The CH3CO2H molecules that ionize produce
acetate ionsCH3CO2- and H+ ions, and the ions attach themselves to
water molecules to form hydronium ions, H3O+.
7.
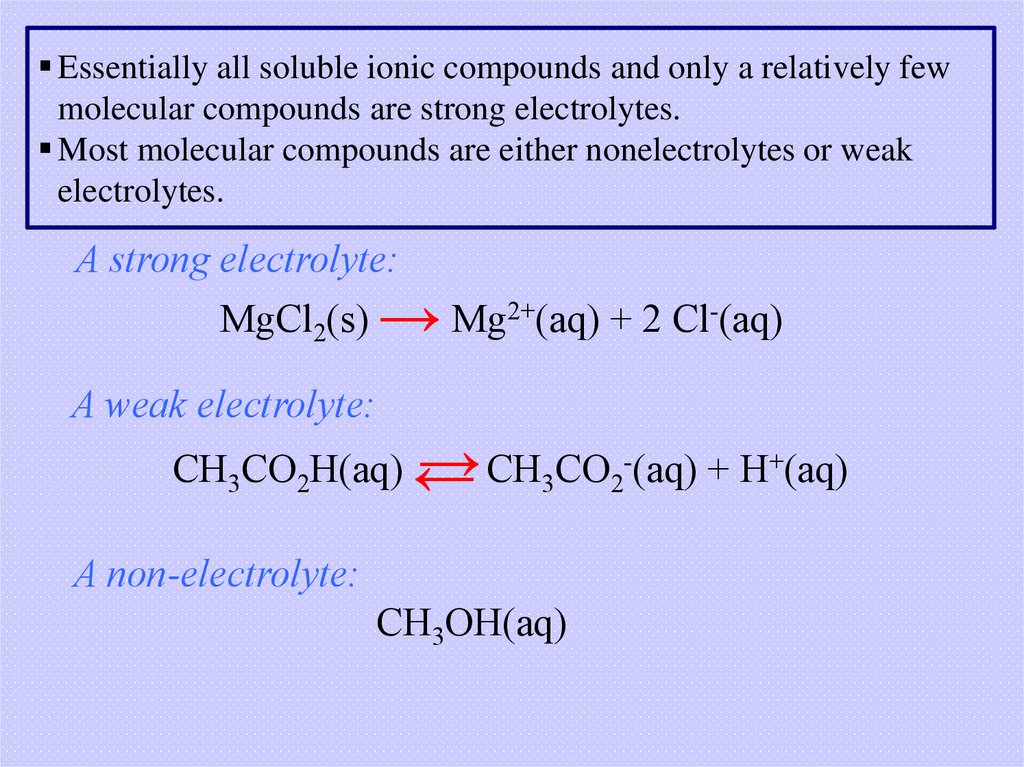
Essentially all soluble ionic compounds and only a relatively fewmolecular compounds are strong electrolytes.
Most molecular compounds are either nonelectrolytes or weak
electrolytes.
A strong electrolyte:
MgCl2(s) → Mg2+(aq) + 2 Cl-(aq)
A weak electrolyte:
→ CH3CO2-(aq) + H+(aq)
CH3CO2H(aq) ←
A non-electrolyte:
CH3OH(aq)
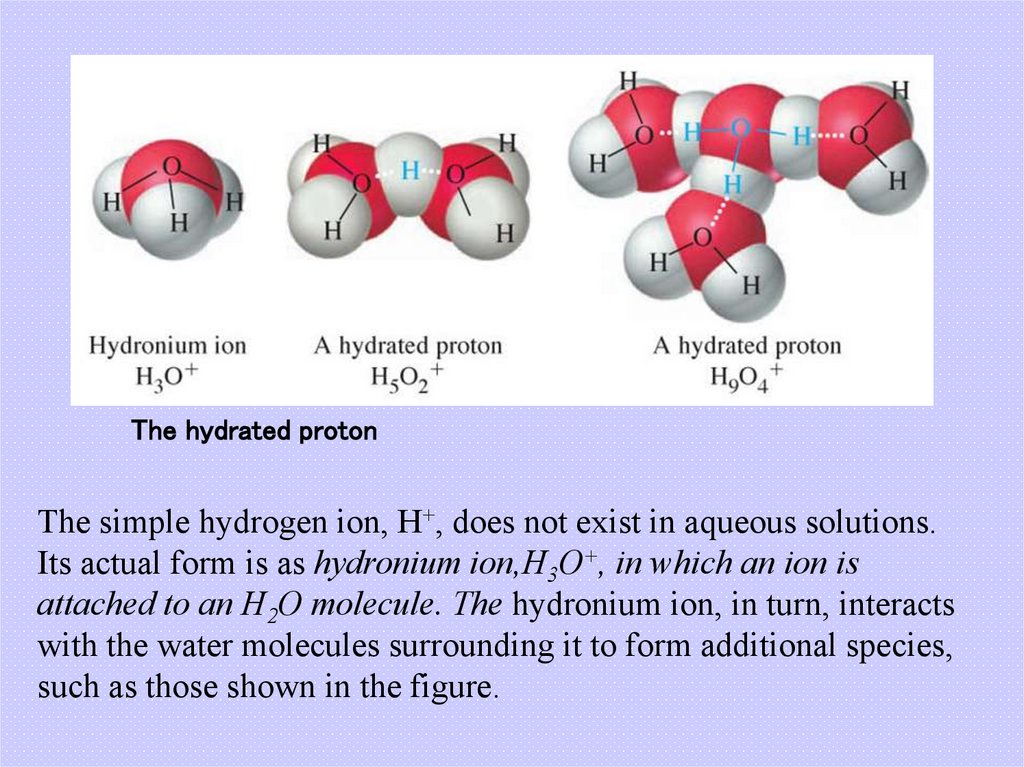
8. The hydrated proton
The simple hydrogen ion, H+, does not exist in aqueous solutions.Its actual form is as hydronium ion,H3O+, in which an ion is
attached to an H2O molecule. The hydronium ion, in turn, interacts
with the water molecules surrounding it to form additional species,
such as those shown in the figure.
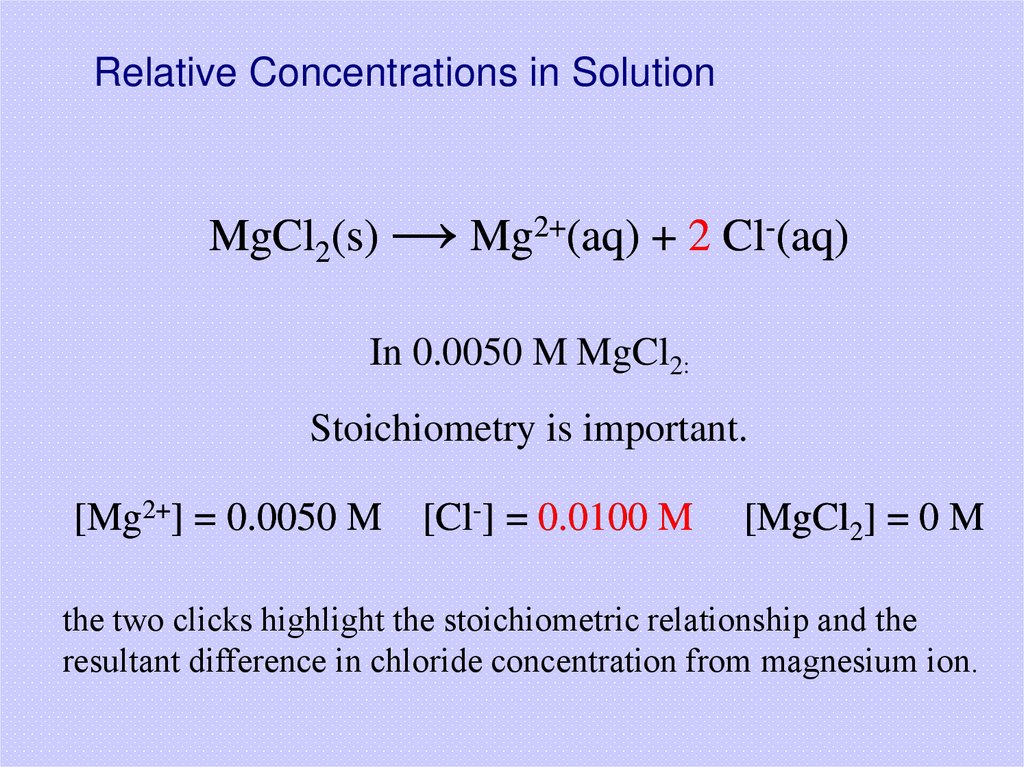
9. Relative Concentrations in Solution
MgCl2(s) → Mg2+(aq) + 2 Cl-(aq)In 0.0050 M MgCl2:
Stoichiometry is important.
[Mg2+] = 0.0050 M
[Cl-] = 0.0100 M
[MgCl2] = 0 M
the two clicks highlight the stoichiometric relationship and the
resultant difference in chloride concentration from magnesium ion.
10.
Examples:1. What are the aluminum and sulfate ion concentrations in 0.0165
M Al2(SO4)3
2. Na3PO4 is commonly used as a cleaning under the trade name
TSP. What are the ion concentrations in a 0.358 M solution of
TSP?
11. 5-2 Precipitation Reactions
Soluble ions can combine toform an insoluble
compound.
Precipitation occurs.
A test for the presence of
chloride ion in water.
Ag+(aq) + Cl-(aq) → AgCl(s)
Qualitative test for Cl- in tap water
The test involves the addition of
a few drops of AgNO3(aq) to tap
water. The formation of a
precipitate of AgCl(s) confirms
the presence of Cl-.
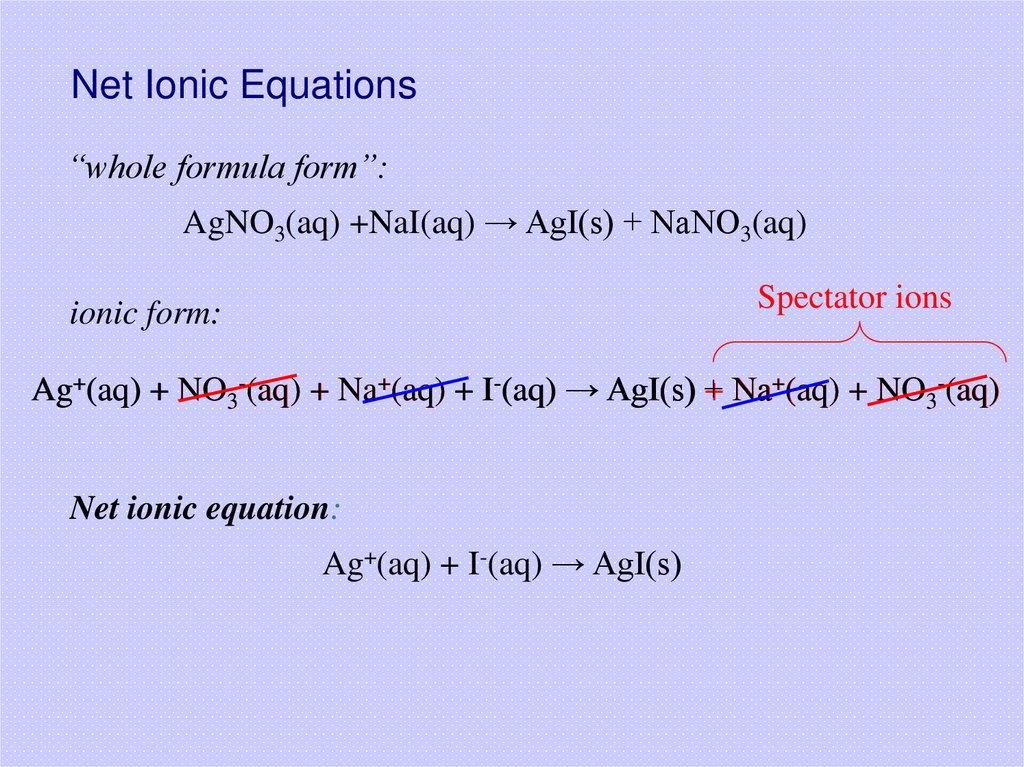
12. Net Ionic Equations
“whole formula form”:AgNO3(aq) +NaI(aq) → AgI(s) + NaNO3(aq)
Spectator ions
ionic form:
Ag+(aq) + NO3-(aq) + Na+(aq) + I-(aq) → AgI(s) +
+ Na
Na+(aq) + NO3-(aq)
Net ionic equation:
Ag+(aq) + I-(aq) → AgI(s)
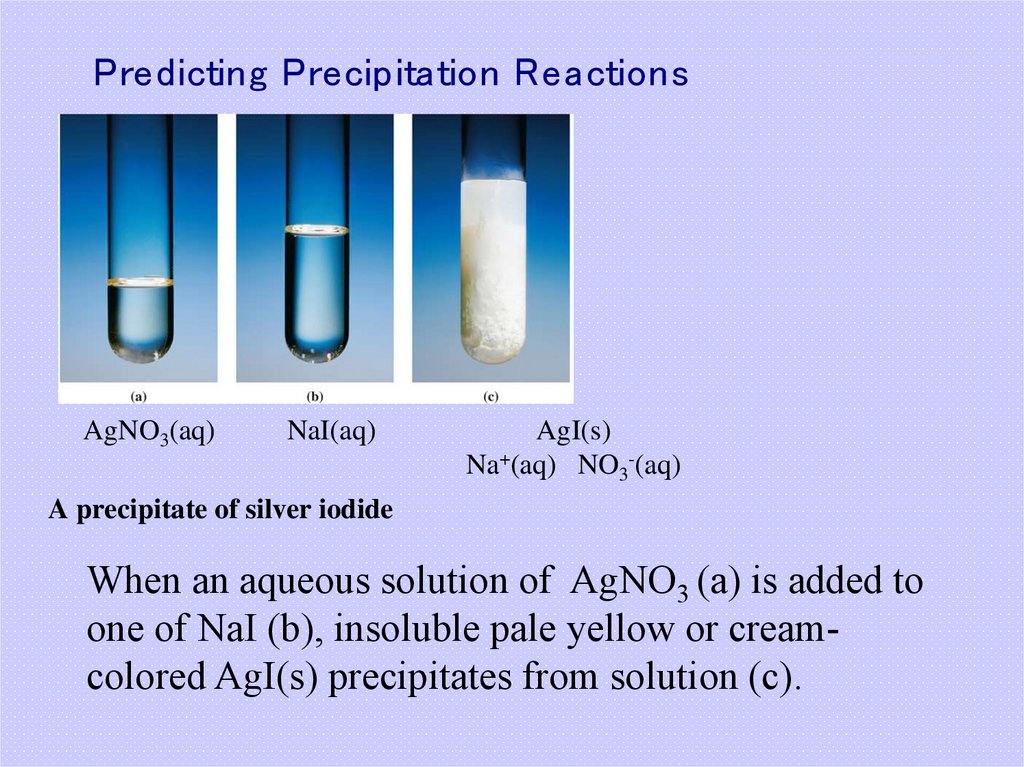
13. Predicting Precipitation Reactions
AgNO3(aq)NaI(aq)
AgI(s)
Na+(aq) NO3-(aq)
A precipitate of silver iodide
When an aqueous solution of AgNO3 (a) is added to
one of NaI (b), insoluble pale yellow or creamcolored AgI(s) precipitates from solution (c).
14.
Solubility Guidelines for Common Ionic Solids1. Salts of group 1 Cations (which some exceptions for Li+) and
the NH4+ cation are soluble
2. Nitrates, acetates and perchlorates are soluble
3. Salts of silver, lead and mercury (I) are insoluble/.
4. Chlorides, bromides and iodides are soluble.
5. Carbonates, phosphates, sulfides, oxides and hydroxides are
insoluble ( sulfides of group 2 cation and hydroxides of Ca+2,
Sr+2 and Ba+2 are slightly soluble
6. Sulfates are soluble except for those of calcium, strontium and
barium
15.
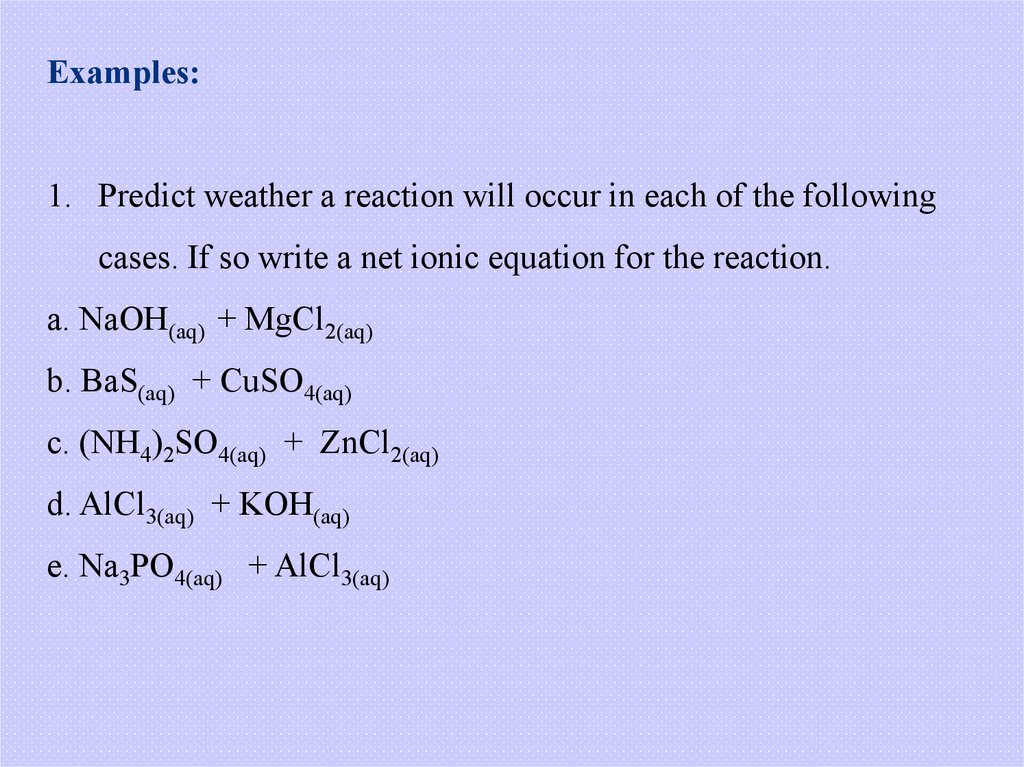
Examples:1. Predict weather a reaction will occur in each of the following
cases. If so write a net ionic equation for the reaction.
a. NaOH(aq) + MgCl2(aq)
b. BaS(aq) + CuSO4(aq)
c. (NH4)2SO4(aq) + ZnCl2(aq)
d. AlCl3(aq) + KOH(aq)
e. Na3PO4(aq) + AlCl3(aq)
16. 5-3 Acid-Base Reactions
An acid, a base, and an acid–base indicatorLatin acidus
Sour taste
Arabic al-qali
Bitter taste
Acid-Base theory
Svante Arrhenius 1884
Brønsted and Lowry 1923
Ideas about acids and bases (or alkalis) date back to ancient times. The
word acid is derived from the Latin acidus (sour).
Alkali (base) comes from the Arabic al-qali, referring to the ashes of
certain plants from which alkaline substances can be extracted.
The acid–base concept is a major theme in the history of chemistry. In
this section, we emphasize the view proposed by Svante Arrhenius
in 1884 but also introduce a more modern theory proposed in 1923
by Thomas Lowry and by Johannes Brønsted.
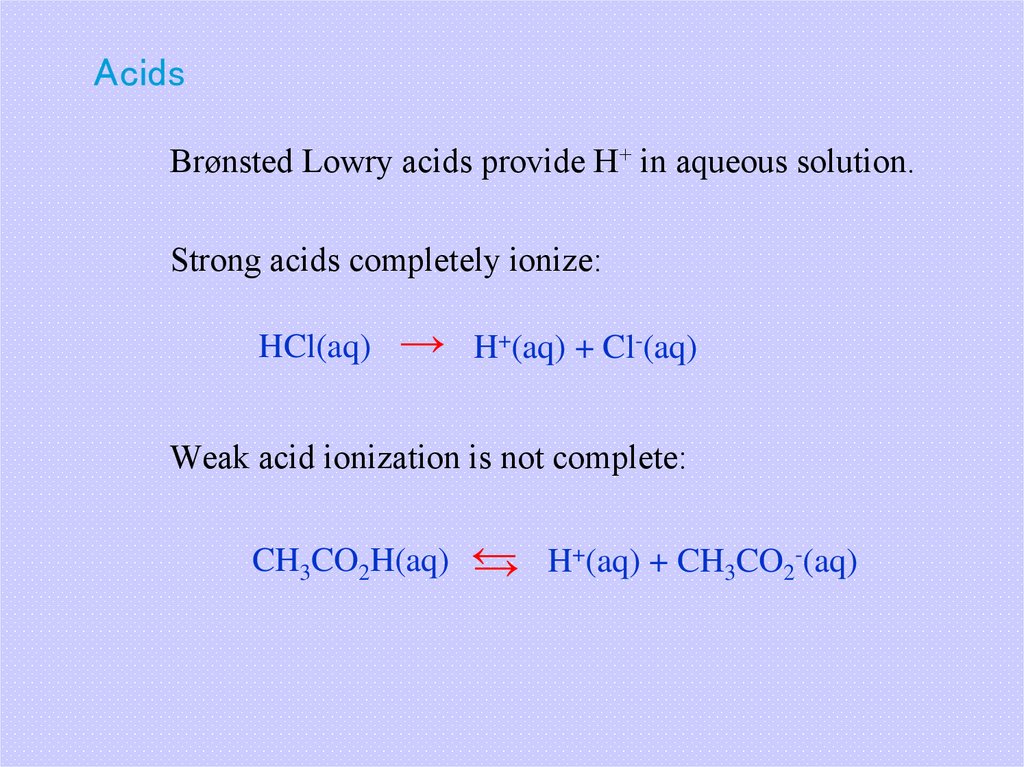
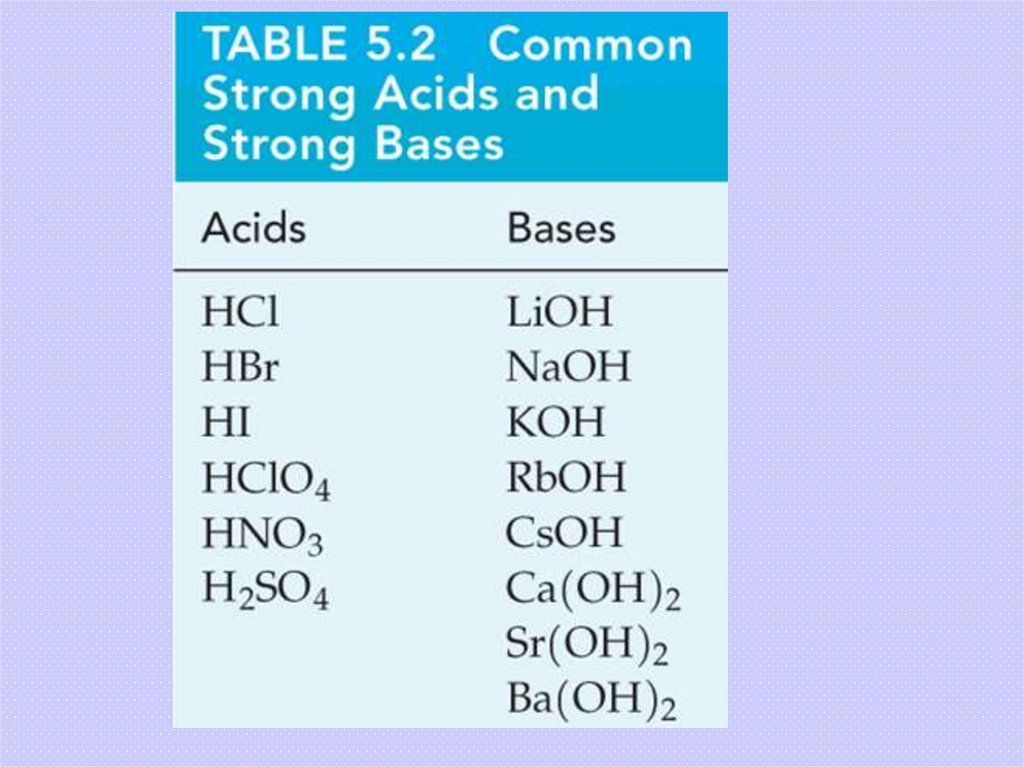
17. Acids
Brønsted Lowry acids provide H+ in aqueous solution.Strong acids completely ionize:
HCl(aq)
→ H+(aq) + Cl-(aq)
Weak acid ionization is not complete:
+(aq) + CH CO -(aq)
CH3CO2H(aq) ←
H
3
2
→
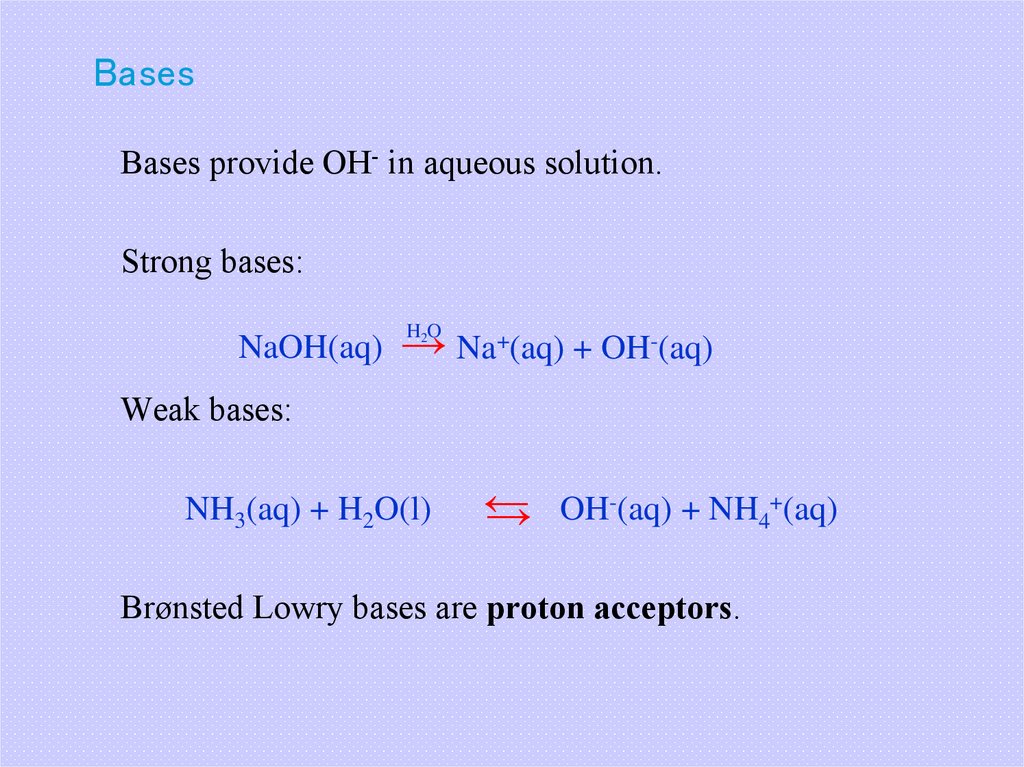
18. Bases
Bases provide OH- in aqueous solution.Strong bases:
NaOH(aq) → Na+(aq) + OH-(aq)
H2O
Weak bases:
NH3(aq) + H2O(l)
-(aq) + NH +(aq)
←
OH
4
→
Brønsted Lowry bases are proton acceptors.
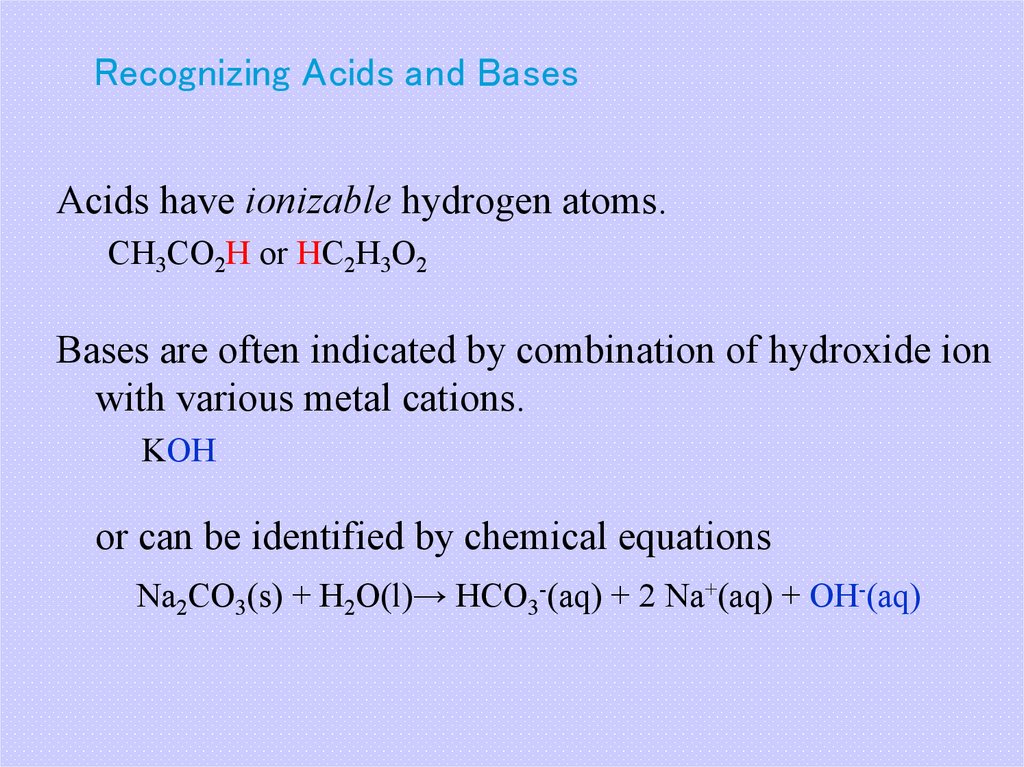
19. Recognizing Acids and Bases
Acids have ionizable hydrogen atoms.CH3CO2H or HC2H3O2
Bases are often indicated by combination of hydroxide ion
with various metal cations.
KOH
or can be identified by chemical equations
Na2CO3(s) + H2O(l)→ HCO3-(aq) + 2 Na+(aq) + OH-(aq)
20.
21. More Acid-Base Reactions
Milk of magnesiaMg(OH)2
Mg(OH)2(s) + 2 H+(aq) → Mg2+(aq) + 2 H2O(l)
Mg(OH)2(s) + 2 CH3CO2H(aq) →
Mg2+(aq) + 2 CH3CO2-(aq) + 2 H2O(l)
22.
Limestone and marble.CaCO3(s) + 2 H+(aq) → Ca2+(aq) + H2CO3(aq)
But: H2CO3(aq) → H2O(l) + CO2(g)
CaCO3(s) + 2 H+(aq) → Ca2+(aq) + H2O(l) + CO2(g)
23.
This marble statue has beeneroded by acid rain. Marble
consists primarily of CaCO3.
Acids react with and dissolve
marble through the reaction
described on the previous
slide
24.
25.
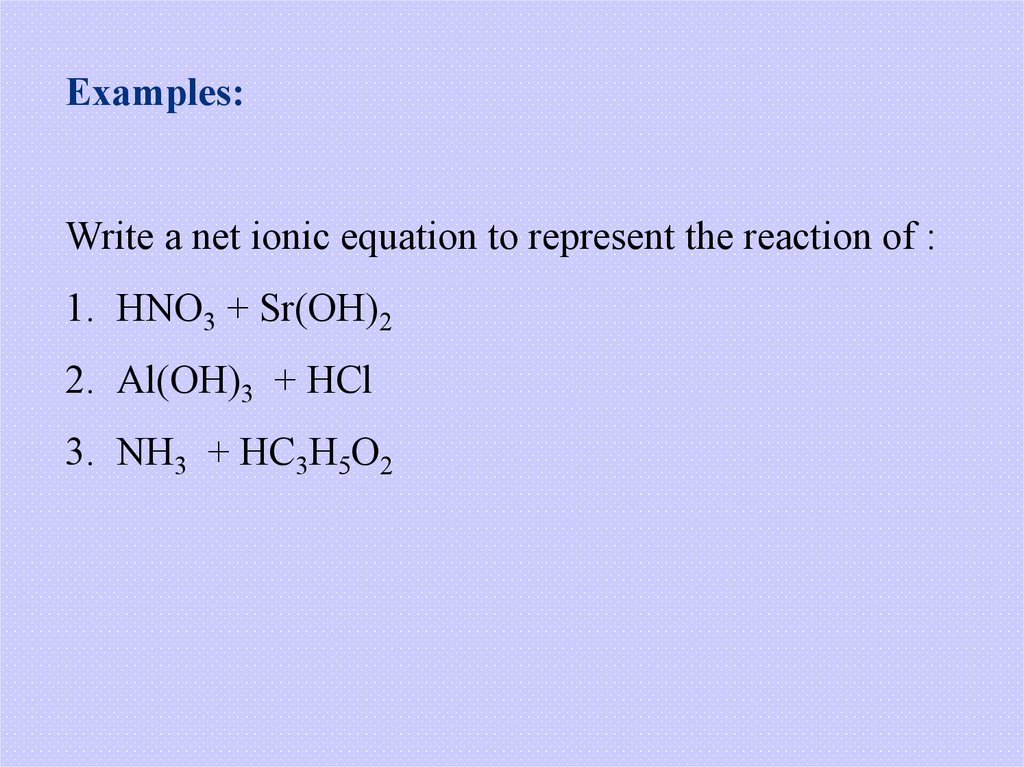
Examples:Write a net ionic equation to represent the reaction of :
1. HNO3 + Sr(OH)2
2. Al(OH)3 + HCl
3. NH3 + HC3H5O2
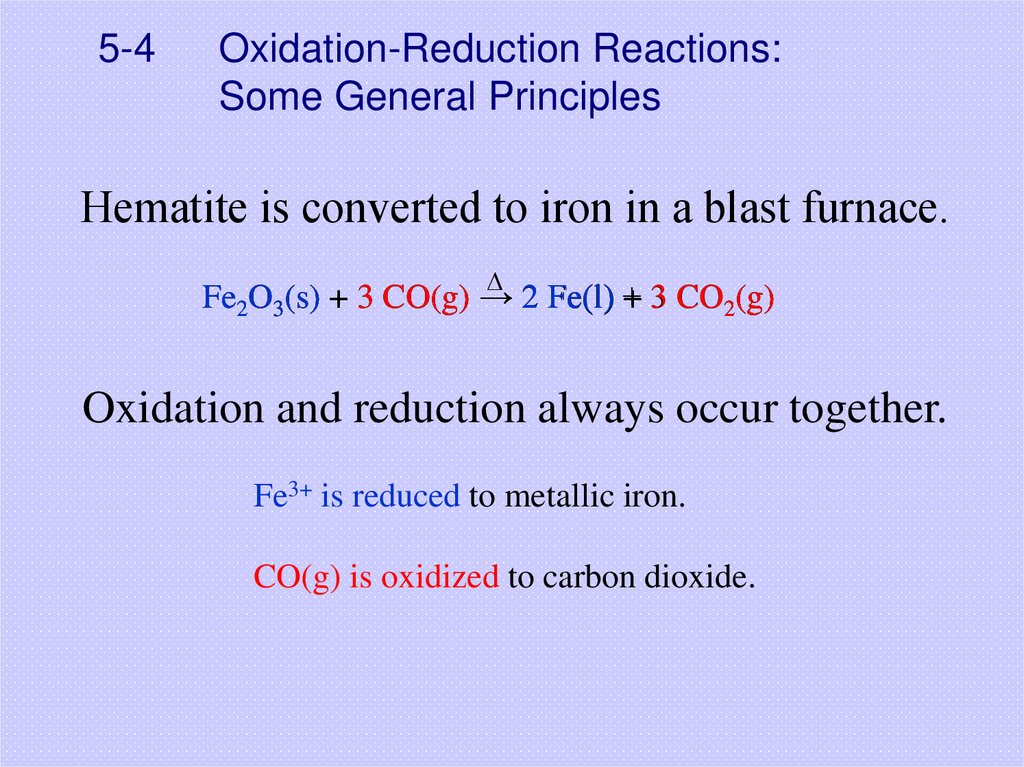
26. 5-4 Oxidation-Reduction Reactions: Some General Principles
Hematite is converted to iron in a blast furnace.D
Fe2O3(s) + 3 CO(g) → 22 Fe(l)
Fe(l) +
+ 33 CO
CO2(g)
Oxidation and reduction always occur together.
Fe3+ is reduced to metallic iron.
CO(g) is oxidized to carbon dioxide.
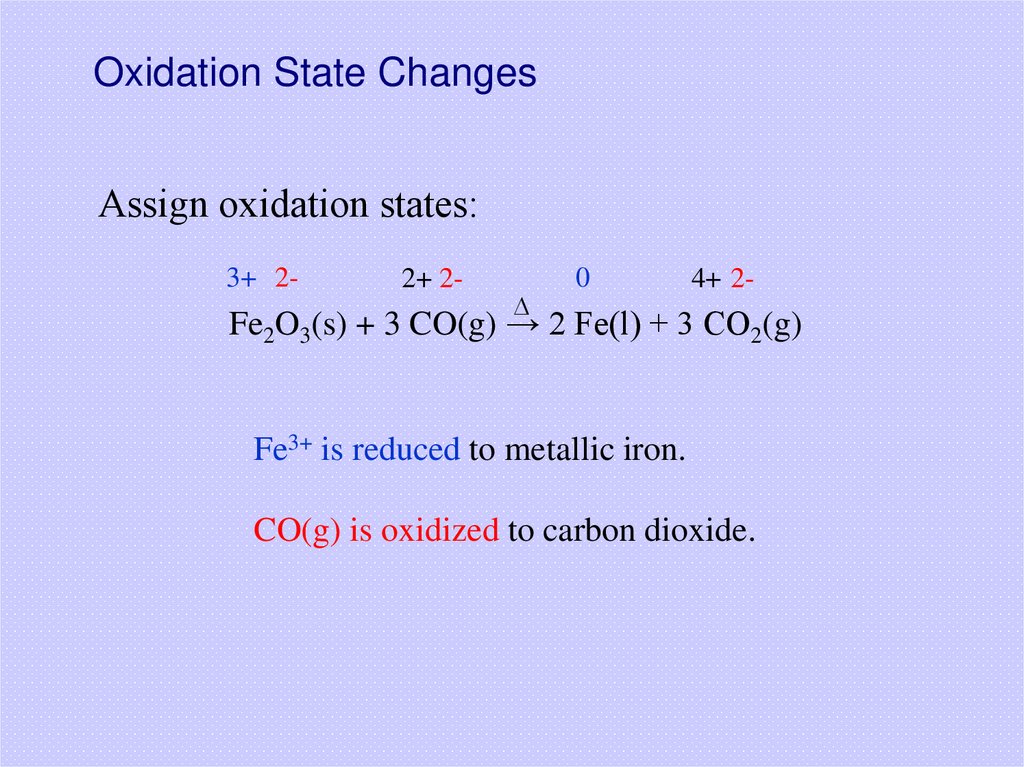
27. Oxidation State Changes
Assign oxidation states:3+ 2-
2+ 2-
D
0
4+ 2-
Fe2O3(s) + 3 CO(g) → 2 Fe(l) + 3 CO2(g)
Fe3+ is reduced to metallic iron.
CO(g) is oxidized to carbon dioxide.
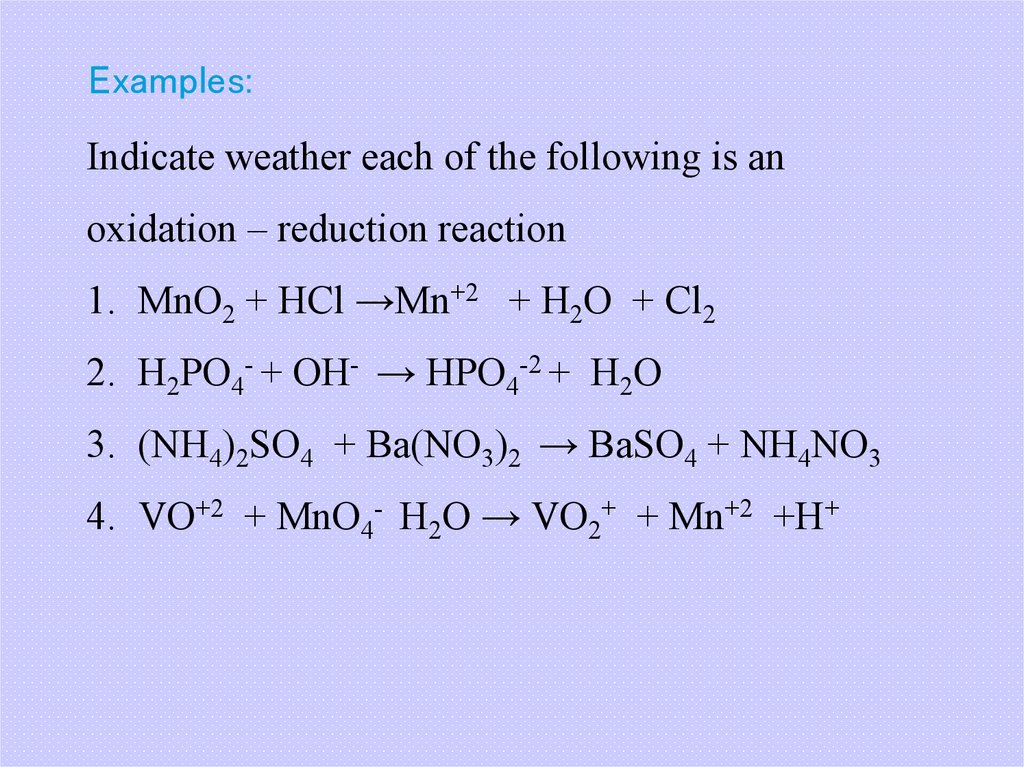
28. Examples:
Indicate weather each of the following is anoxidation – reduction reaction
1. MnO2 + HCl →Mn+2 + H2O + Cl2
2. H2PO4- + OH- → HPO4-2 + H2O
3. (NH4)2SO4 + Ba(NO3)2 → BaSO4 + NH4NO3
4. VO+2 + MnO4- H2O → VO2+ + Mn+2 +H+
29.
Fe2O3(s) + 2 Al(s) Al2O3(s) + 2 Fe(l)Iron atoms of iron(III) oxide give up O atoms to Al
atoms, producing Al2O3.
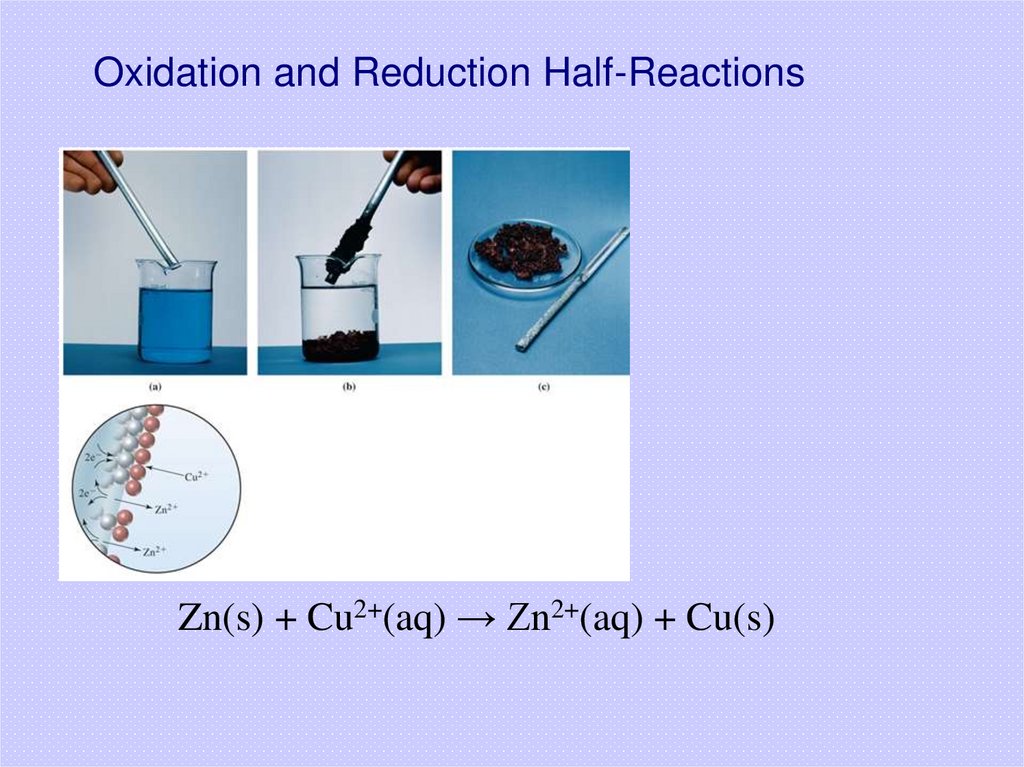
30. Oxidation and Reduction Half-Reactions
Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)31.
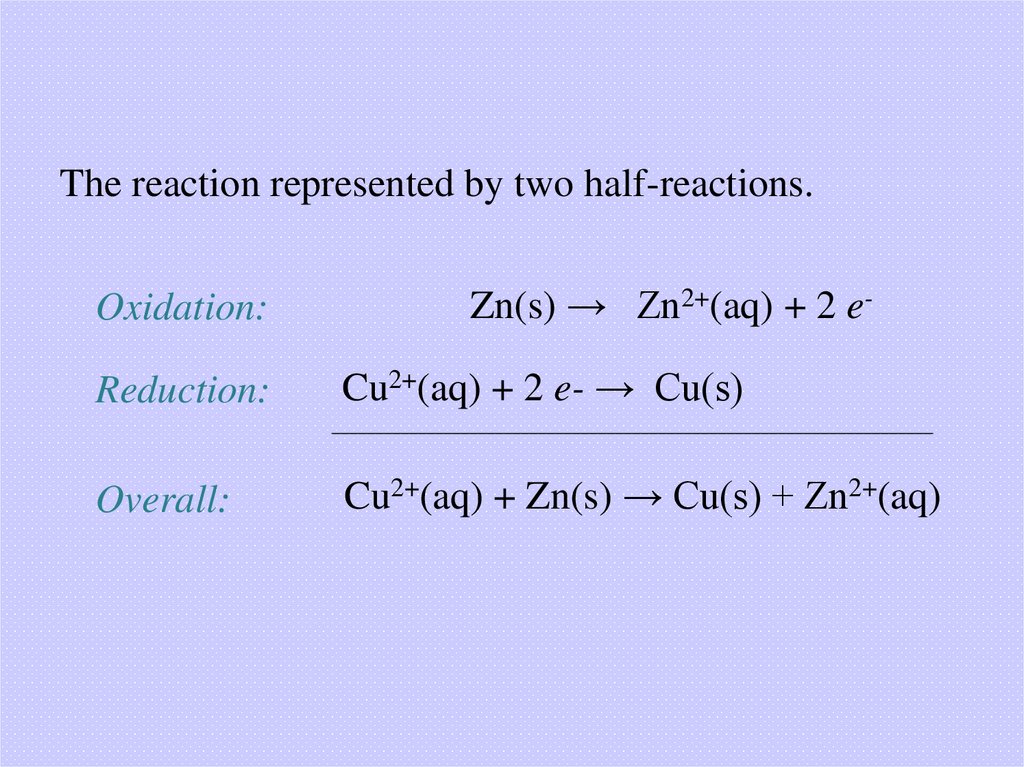
The reaction represented by two half-reactions.Oxidation:
Zn(s) → Zn2+(aq) + 2 e-
Reduction:
Cu2+(aq) + 2 e- → Cu(s)
Overall:
Cu2+(aq) + Zn(s) → Cu(s) + Zn2+(aq)
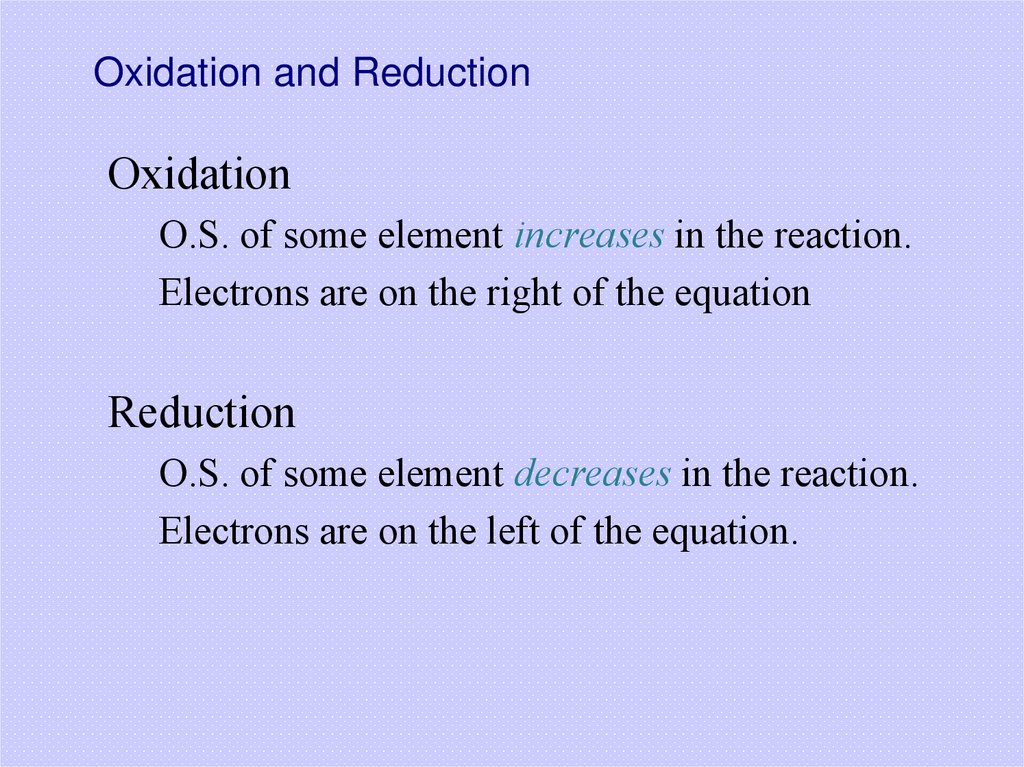
32. Oxidation and Reduction
OxidationO.S. of some element increases in the reaction.
Electrons are on the right of the equation
Reduction
O.S. of some element decreases in the reaction.
Electrons are on the left of the equation.
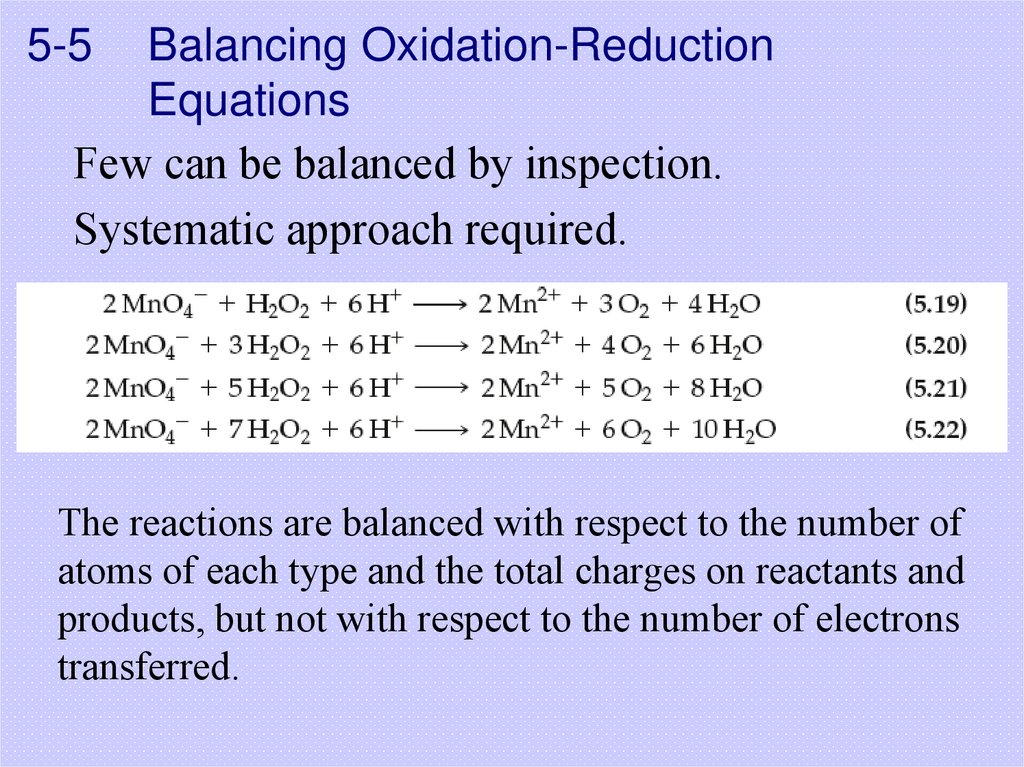
33. 5-5 Balancing Oxidation-Reduction Equations
Few can be balanced by inspection.Systematic approach required.
The reactions are balanced with respect to the number of
atoms of each type and the total charges on reactants and
products, but not with respect to the number of electrons
transferred.
34. The Half-Equation Method
• Write and balance separate half-equationsfor oxidation and reduction.
• Adjust coefficients in the two half-equations
so that the same number of electrons appear
in each half-equation.
• Add together the two half-equations
(canceling out electrons) to obtain the
balanced overall equation
35.
Balancing Equations for Redox Reactions in Acidic AqueousSolutions by the Half Equation Method:
1. Write the equations for the oxidation and reduction halfreaction
2. In each half equation
- Balance atoms of all the elements except H and O
- Balance oxygen by using H2O
- Balance hydrogen by using H+
- Balance charge by using electrons
3. If necessary, equalize the number of electrons in the
oxidation and reduction half- equations by multiplying one or
both half- equations by appropriate integers.
4. Add the half- equations, then cancel species common to
both sides of the overall equation.
5. Check that both numbers of atoms and charges balance
36.
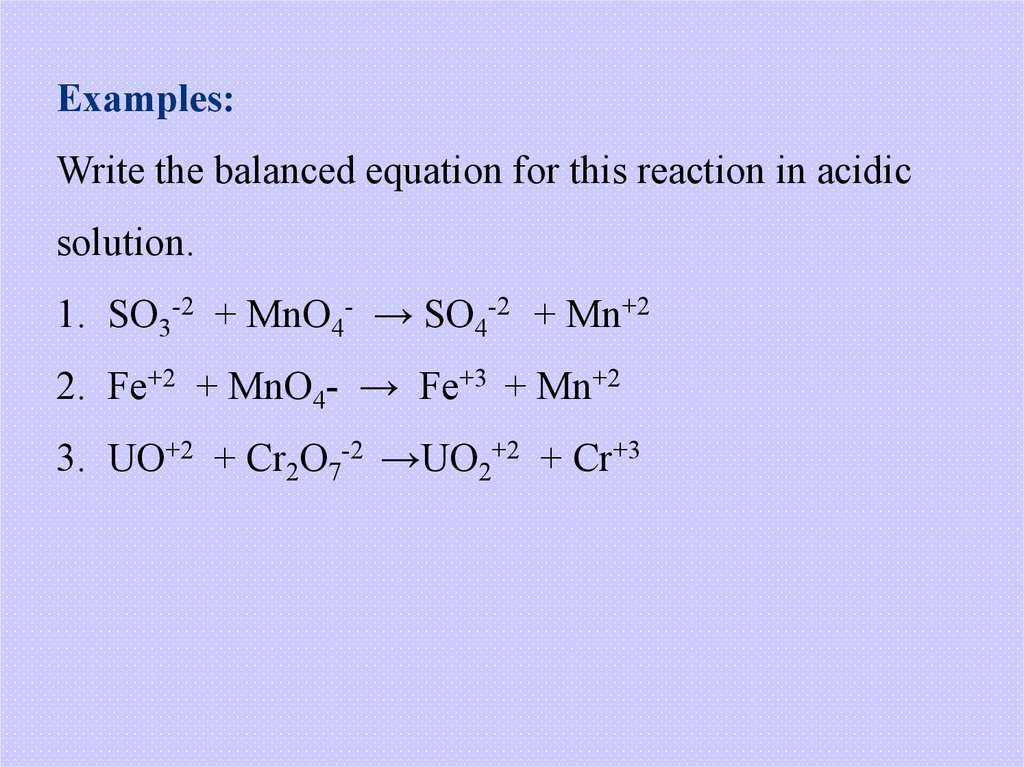
Examples:Write the balanced equation for this reaction in acidic
solution.
1. SO3-2 + MnO4- → SO4-2 + Mn+2
2. Fe+2 + MnO4- → Fe+3 + Mn+2
3. UO+2 + Cr2O7-2 →UO2+2 + Cr+3
37.
Balancing Equations for Redox Reactions in BasicAqueous Solutions by the Half Equation Method:
1. Balance the equation as if the reaction were
occurring in acidic medium by using the method for
acidic aqueous solutions summarized in acidic
aqueous.
2. Add a number of OH- ions equal to the number of
H+ ions to both sides of the overall equations.
3. Check that both numbers of atoms and charges
balance.
38.
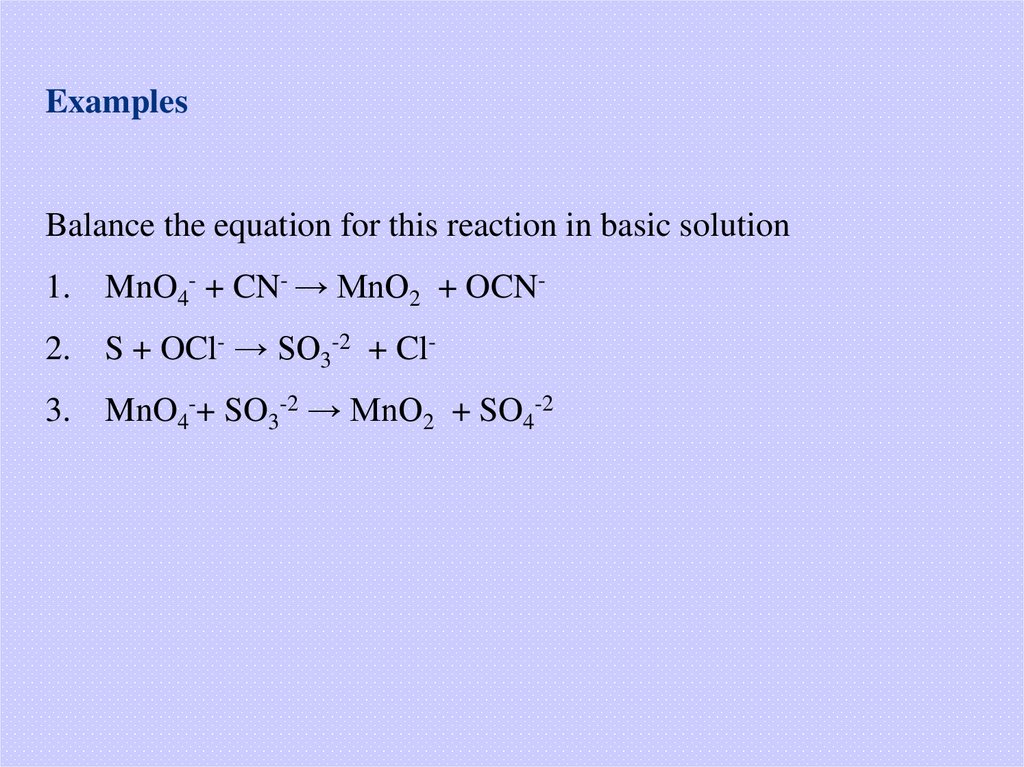
ExamplesBalance the equation for this reaction in basic solution
1.
MnO4- + CN- → MnO2 + OCN-
2.
S + OCl- → SO3-2 + Cl-
3.
MnO4-+ SO3-2 → MnO2 + SO4-2
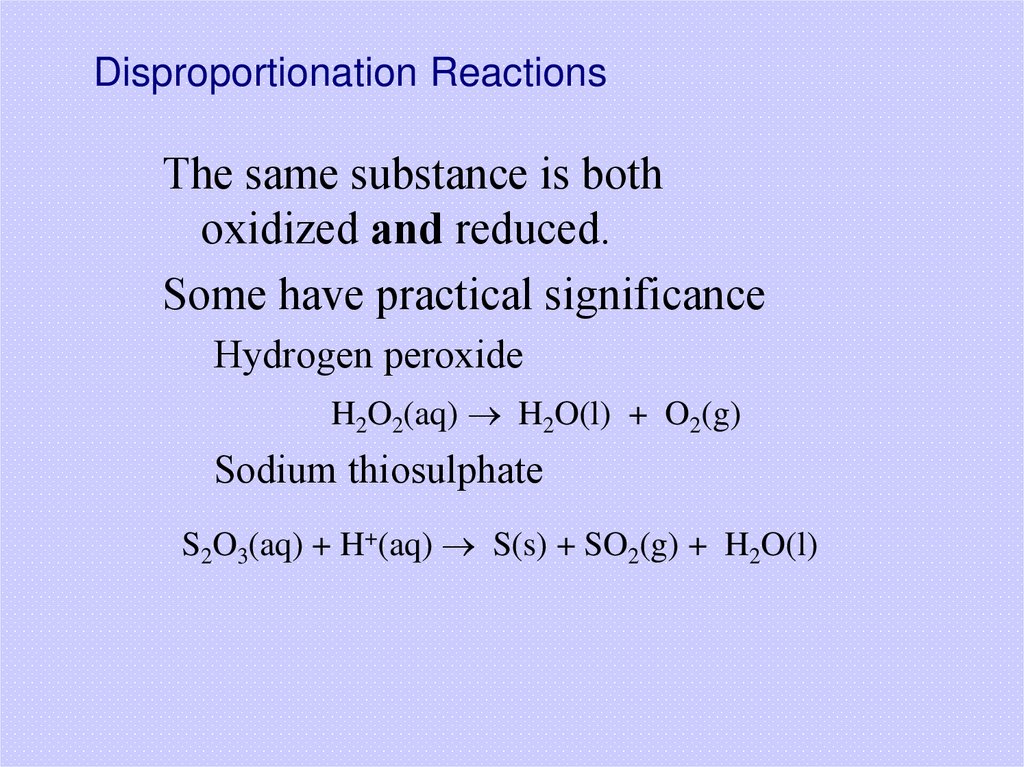
39. Disproportionation Reactions
The same substance is bothoxidized and reduced.
Some have practical significance
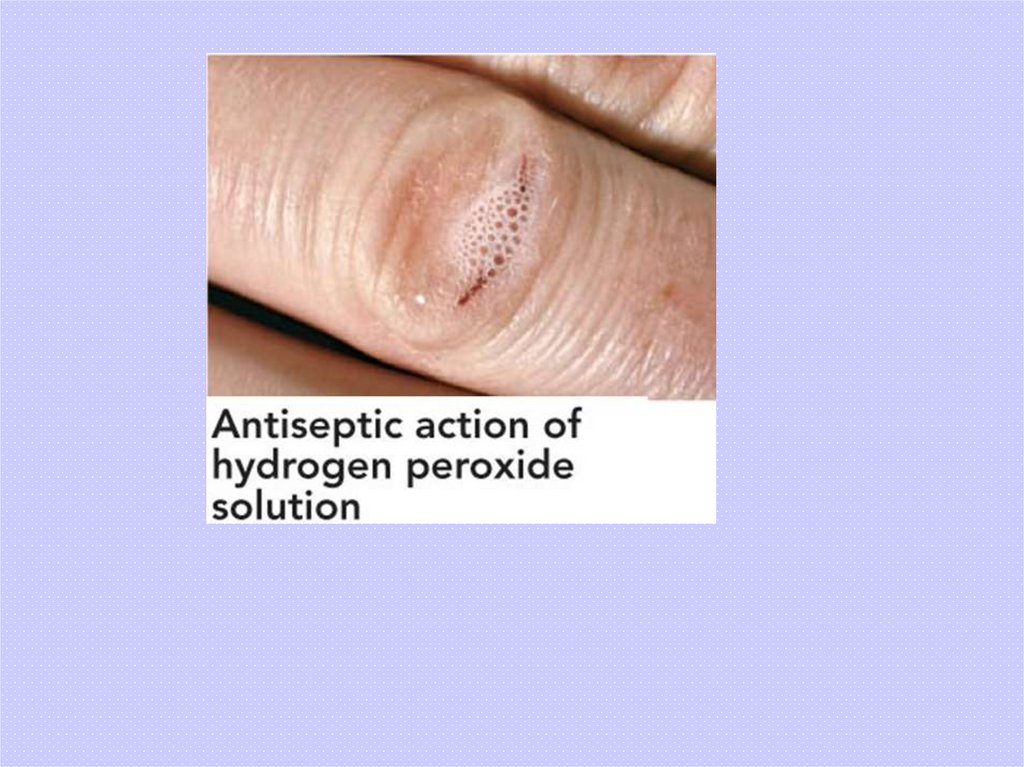
Hydrogen peroxide
H2O2(aq) H2O(l) + O2(g)
Sodium thiosulphate
S2O3(aq) + H+(aq) S(s) + SO2(g) + H2O(l)
40.
41.
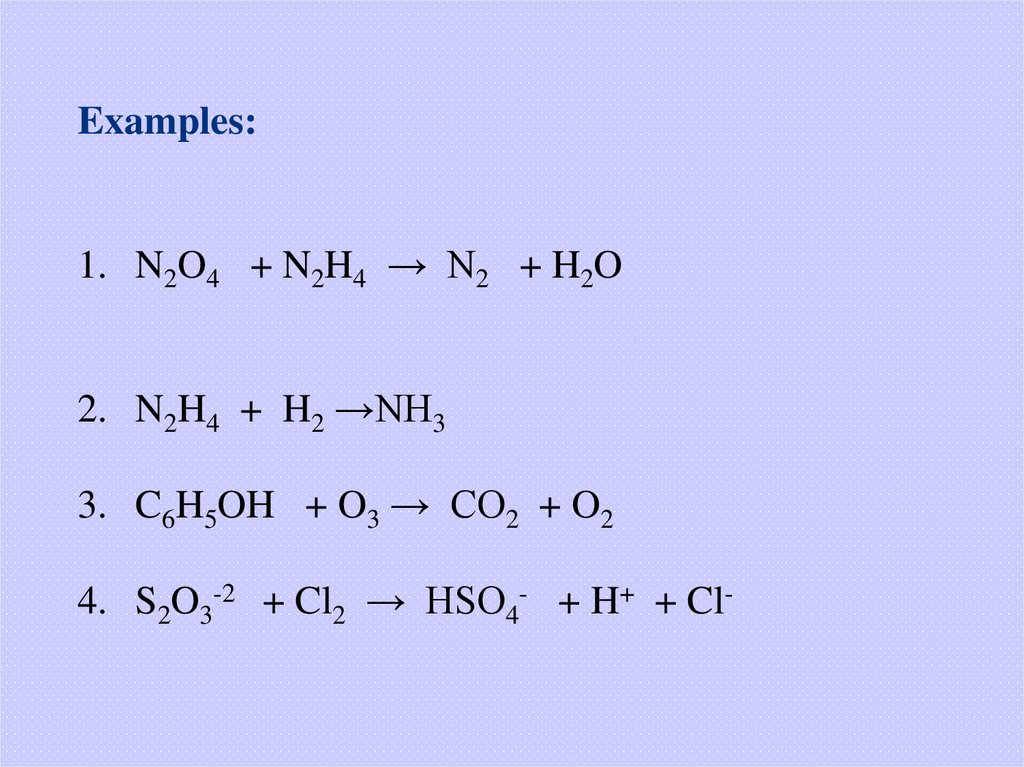
Examples:1. N2O4 + N2H4 → N2 + H2O
2. N2H4 + H2 →NH3
3. C6H5OH + O3 → CO2 + O2
4. S2O3-2 + Cl2 → HSO4- + H+ + Cl-
42. 5-6 Oxidizing and Reducing Agents.
An oxidizing agent (oxidant)• causes another substance to be oxidized
• contains an element whose oxidation state
decreases in a redox reaction
• gains electrons (electrons are found on the left
side of its half-equation)
• is reduced
43.
A reducing agent (reductant)• causes another substance to be reduced
• contains an element whose oxidation state
increases in a redox reaction
• loses electrons (electrons are found on the right
side of its half-equation)
• is oxidized
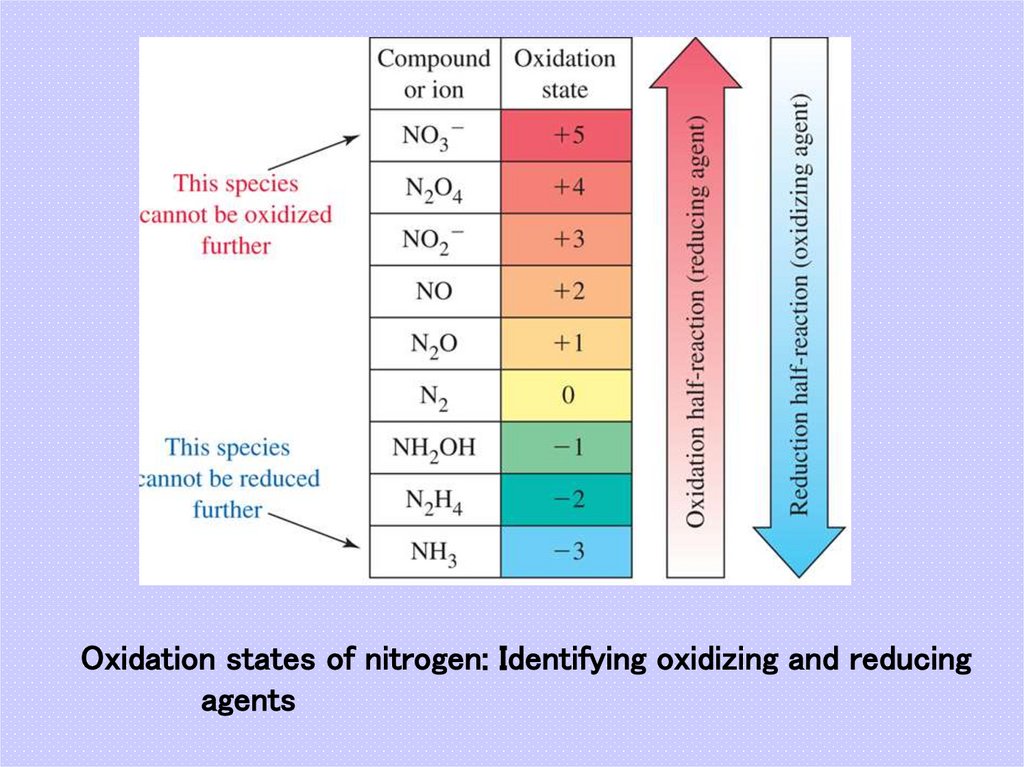
44. Oxidation states of nitrogen: Identifying oxidizing and reducing agents
45. Bleaching action of NaOCl(aq)
A red cloth becomes whitewhen immersed in NaOCl(aq),
which oxidizes the red pigment
to colorless products
Bleaching action of NaOCl(aq)
46.
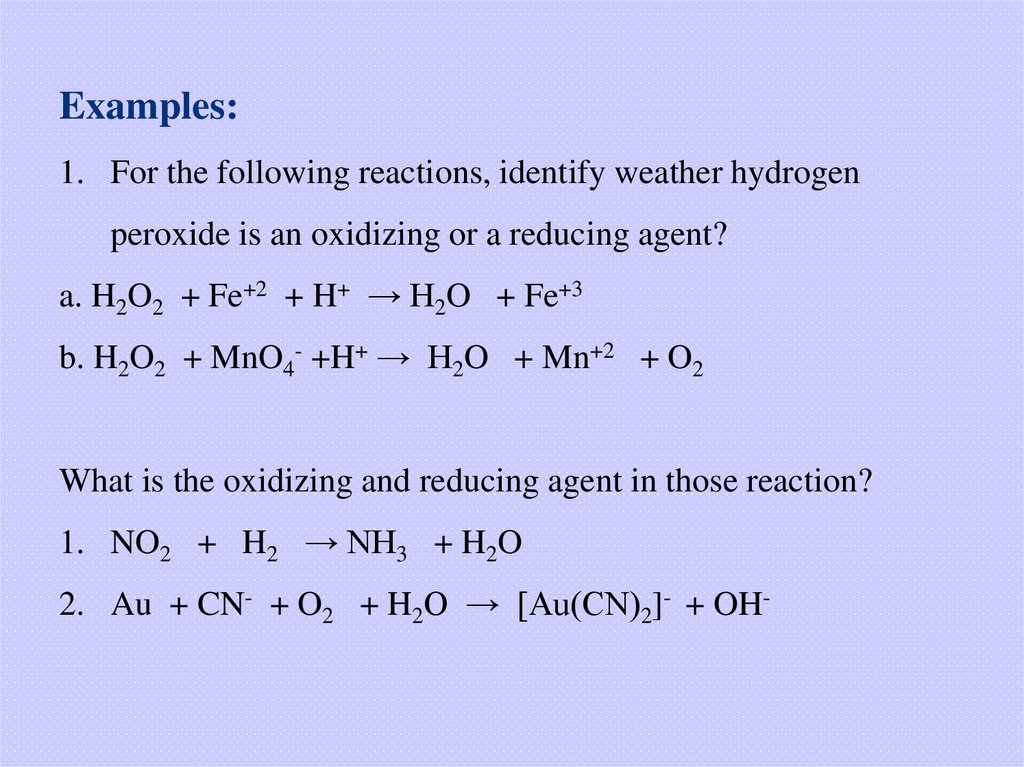
Examples:1. For the following reactions, identify weather hydrogen
peroxide is an oxidizing or a reducing agent?
a. H2O2 + Fe+2 + H+ → H2O + Fe+3
b. H2O2 + MnO4- +H+ → H2O + Mn+2 + O2
What is the oxidizing and reducing agent in those reaction?
1. NO2 + H2 → NH3 + H2O
2. Au + CN- + O2 + H2O → [Au(CN)2]- + OH-
47. 5-7 Stoichiometry of Reactions in Aqueous Solutions: Titrations.
TitrationCarefully controlled addition of one solution to
another.
Equivalence Point
Both reactants have reacted completely.
Indicators
Substances which change colour near an
equivalence point.
48.
Examples1.
The legal minimum acetic acid contain of vinegar is 4% by mass. A 5 ml
sample of a partıcular vinegar was titrated with 38 ml 0.1 M NaOH. Does
this sample exceed the minimum limit? (d: 1.01 g/ml)
2.
0.235 g sample of a solid that is 92.5% KOH, 7.5 Ba(OH)2 by mass
requires 45.6 ml of HCl solution for its titration. What is the molarity of
HCl?
3.
A piece of iron wire weighing 0.1568g is converted Fe+2 and requires
26.24 ml KMnO4 solution for this titration. What is the molarity of the
KMnO4?
4.
Anoter substance that may be used to standardize KMnO4 is sodium
oxalate. If 0.2482 g Na2C2O4 is dissolved in water and titrated with 23.68
ml KMnO4 what is the molarity of KMnO4?
49.
5.0 mLCH3CO2H
A few drops
phenolpthalein
Add 0.1000
M NaOH
The “endpoint”
(close to the equivalence point)
As long as the acid is in excess, the solution in the flask remains
colorless. When the acid has been neutralized, an additional drop of
NaOH(aq) causes the solution to become slightly basic. The
phenolphthalein indicator turns a
light pink. The first lasting appearance of the pink color is taken to be
the equivalence point of the titration.
50.
As it is added to the strongly Fe2+,acidic solution of Fe2+, the KMnO4is immediately decolorized as a result of reaction (5.27).
When all has been oxidized to additional has nothing left to oxidize
and the solution turns a distinctive pink. Even a fraction
of a drop of the beyond the equivalence point is sufficient to cause this
pink coloration. KMnO4
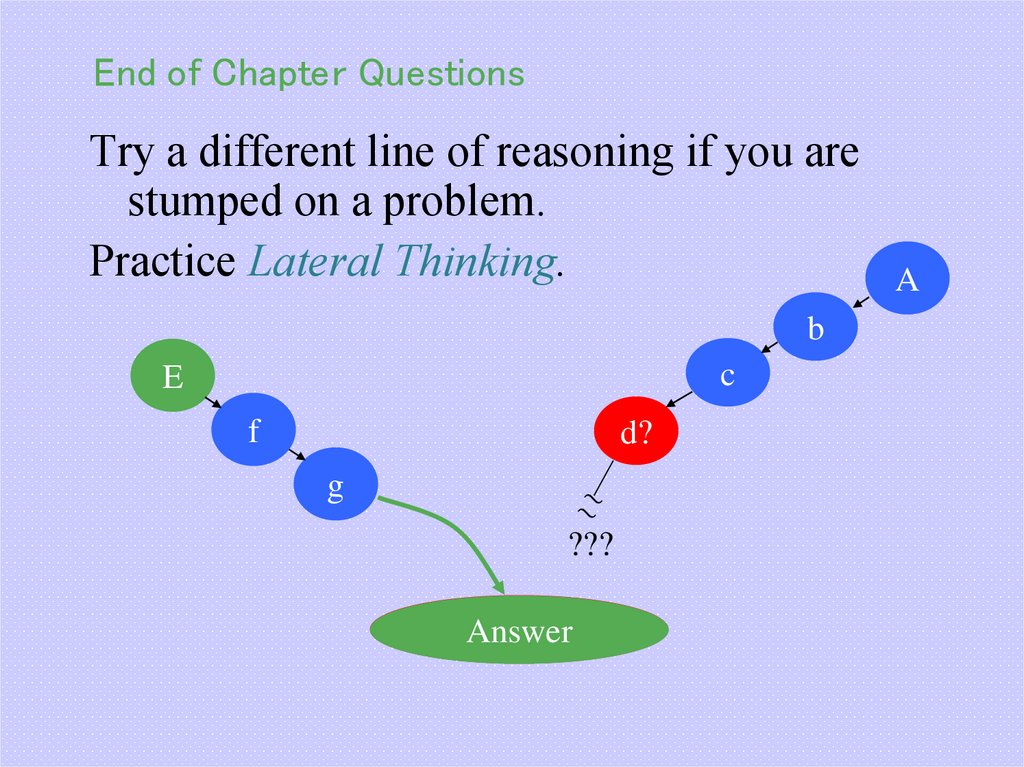
51. End of Chapter Questions
Try a different line of reasoning if you arestumped on a problem.
Practice Lateral Thinking.
b
c
E
f
d?
g
???
Answer
A






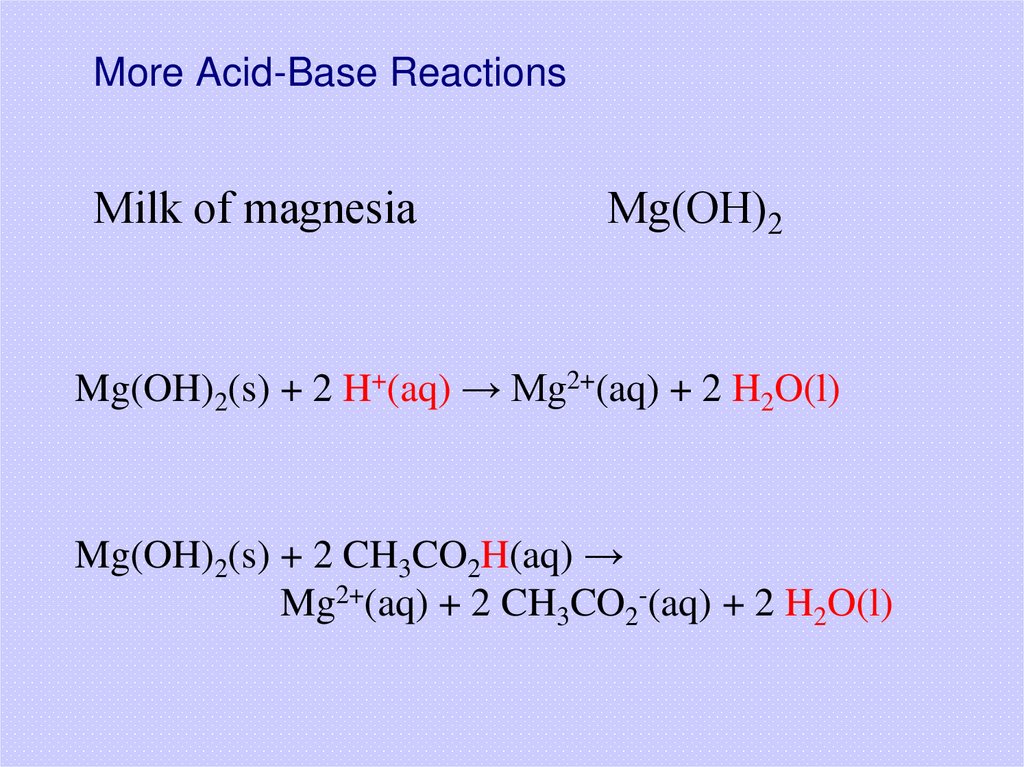
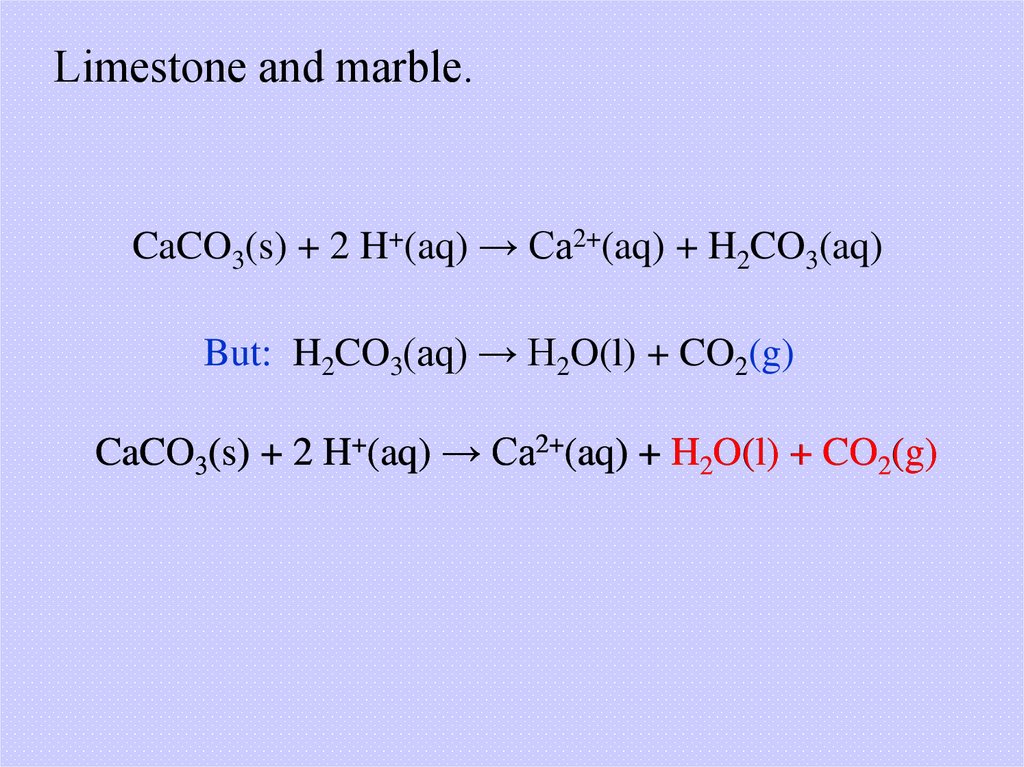











 Химия
Химия








