Похожие презентации:
TPP_Export_2026-01-29 (9)
1.
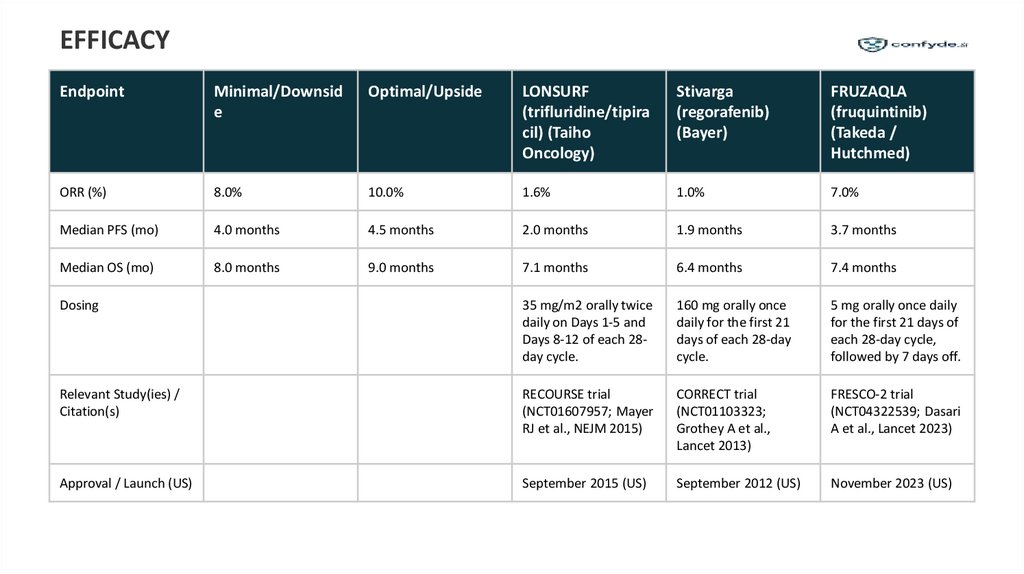
EFFICACYEndpoint
Minimal/Downsid
e
Optimal/Upside
LONSURF
(trifluridine/tipira
cil) (Taiho
Oncology)
Stivarga
(regorafenib)
(Bayer)
FRUZAQLA
(fruquintinib)
(Takeda /
Hutchmed)
ORR (%)
8.0%
10.0%
1.6%
1.0%
7.0%
Median PFS (mo)
4.0 months
4.5 months
2.0 months
1.9 months
3.7 months
Median OS (mo)
8.0 months
9.0 months
7.1 months
6.4 months
7.4 months
Dosing
35 mg/m2 orally twice
daily on Days 1-5 and
Days 8-12 of each 28day cycle.
160 mg orally once
daily for the first 21
days of each 28-day
cycle.
5 mg orally once daily
for the first 21 days of
each 28-day cycle,
followed by 7 days off.
Relevant Study(ies) /
Citation(s)
RECOURSE trial
(NCT01607957; Mayer
RJ et al., NEJM 2015)
CORRECT trial
(NCT01103323;
Grothey A et al.,
Lancet 2013)
FRESCO-2 trial
(NCT04322539; Dasari
A et al., Lancet 2023)
Approval / Launch (US)
September 2015 (US)
September 2012 (US)
November 2023 (US)
2.
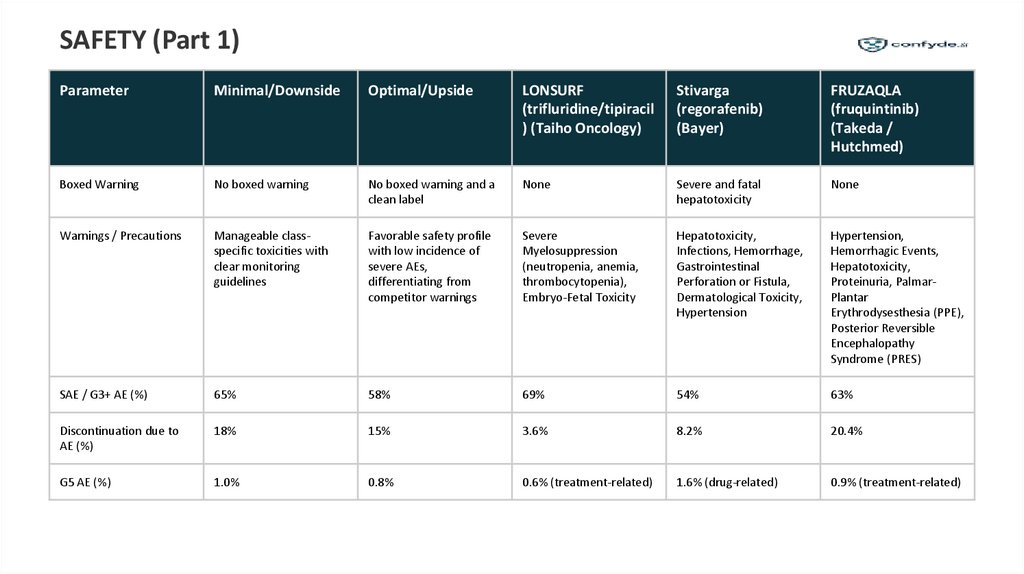
SAFETY (Part 1)Parameter
Minimal/Downside
Optimal/Upside
LONSURF
(trifluridine/tipiracil
) (Taiho Oncology)
Stivarga
(regorafenib)
(Bayer)
FRUZAQLA
(fruquintinib)
(Takeda /
Hutchmed)
Boxed Warning
No boxed warning
No boxed warning and a
clean label
None
Severe and fatal
hepatotoxicity
None
Warnings / Precautions
Manageable classspecific toxicities with
clear monitoring
guidelines
Favorable safety profile
with low incidence of
severe AEs,
differentiating from
competitor warnings
Severe
Myelosuppression
(neutropenia, anemia,
thrombocytopenia),
Embryo-Fetal Toxicity
Hepatotoxicity,
Infections, Hemorrhage,
Gastrointestinal
Perforation or Fistula,
Dermatological Toxicity,
Hypertension
Hypertension,
Hemorrhagic Events,
Hepatotoxicity,
Proteinuria, PalmarPlantar
Erythrodysesthesia (PPE),
Posterior Reversible
Encephalopathy
Syndrome (PRES)
SAE / G3+ AE (%)
65%
58%
69%
54%
63%
Discontinuation due to
AE (%)
18%
15%
3.6%
8.2%
20.4%
G5 AE (%)
1.0%
0.8%
0.6% (treatment-related)
1.6% (drug-related)
0.9% (treatment-related)
3.
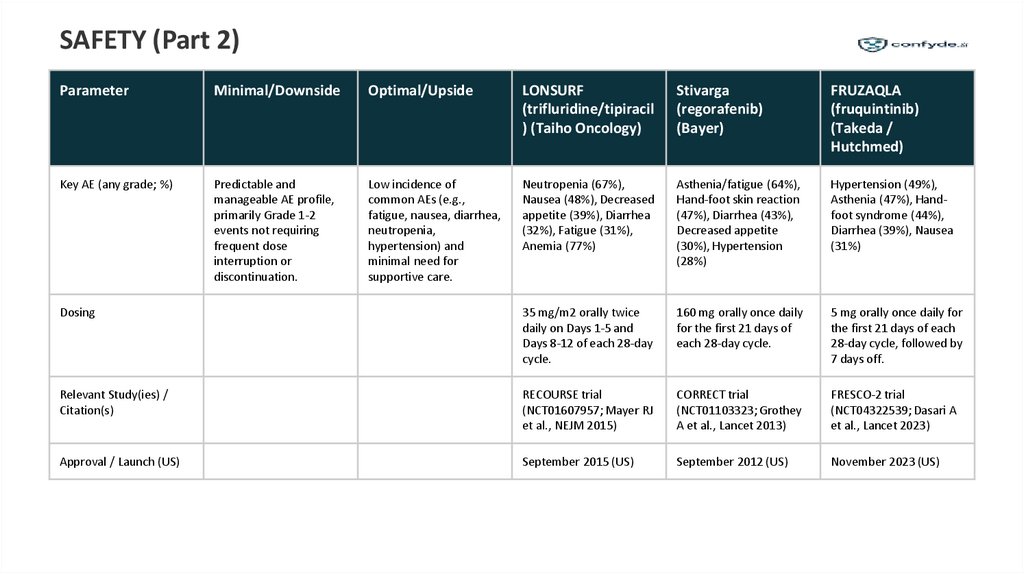
SAFETY (Part 2)Parameter
Minimal/Downside
Optimal/Upside
LONSURF
(trifluridine/tipiracil
) (Taiho Oncology)
Stivarga
(regorafenib)
(Bayer)
FRUZAQLA
(fruquintinib)
(Takeda /
Hutchmed)
Key AE (any grade; %)
Predictable and
manageable AE profile,
primarily Grade 1-2
events not requiring
frequent dose
interruption or
discontinuation.
Low incidence of
common AEs (e.g.,
fatigue, nausea, diarrhea,
neutropenia,
hypertension) and
minimal need for
supportive care.
Neutropenia (67%),
Nausea (48%), Decreased
appetite (39%), Diarrhea
(32%), Fatigue (31%),
Anemia (77%)
Asthenia/fatigue (64%),
Hand-foot skin reaction
(47%), Diarrhea (43%),
Decreased appetite
(30%), Hypertension
(28%)
Hypertension (49%),
Asthenia (47%), Handfoot syndrome (44%),
Diarrhea (39%), Nausea
(31%)
Dosing
35 mg/m2 orally twice
daily on Days 1-5 and
Days 8-12 of each 28-day
cycle.
160 mg orally once daily
for the first 21 days of
each 28-day cycle.
5 mg orally once daily for
the first 21 days of each
28-day cycle, followed by
7 days off.
Relevant Study(ies) /
Citation(s)
RECOURSE trial
(NCT01607957; Mayer RJ
et al., NEJM 2015)
CORRECT trial
(NCT01103323; Grothey
A et al., Lancet 2013)
FRESCO-2 trial
(NCT04322539; Dasari A
et al., Lancet 2023)
Approval / Launch (US)
September 2015 (US)
September 2012 (US)
November 2023 (US)