Похожие презентации:
TPP_Export_2026-01-30
1.
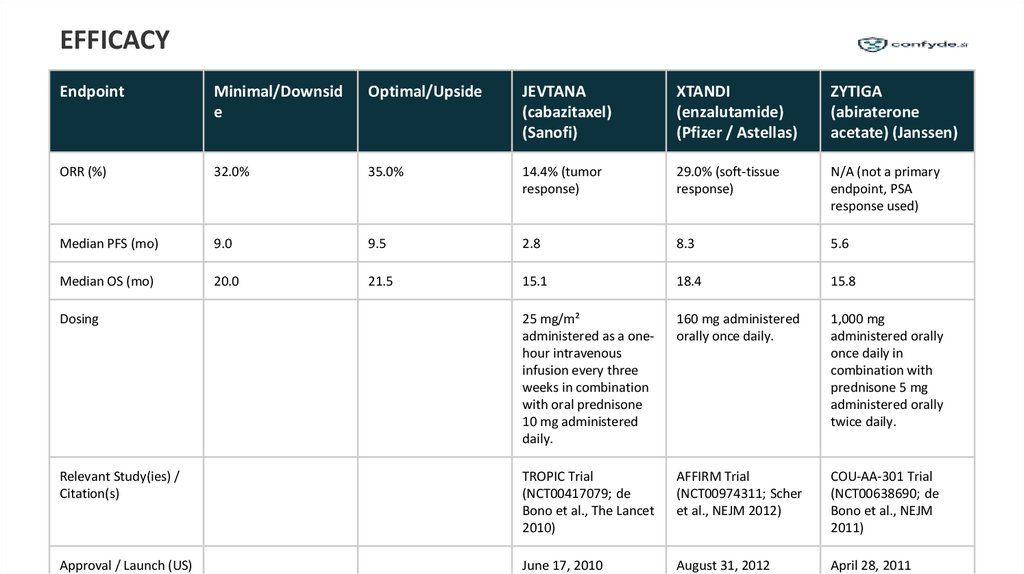
EFFICACYEndpoint
Minimal/Downsid
e
Optimal/Upside
JEVTANA
(cabazitaxel)
(Sanofi)
XTANDI
(enzalutamide)
(Pfizer / Astellas)
ZYTIGA
(abiraterone
acetate) (Janssen)
ORR (%)
32.0%
35.0%
14.4% (tumor
response)
29.0% (soft-tissue
response)
N/A (not a primary
endpoint, PSA
response used)
Median PFS (mo)
9.0
9.5
2.8
8.3
5.6
Median OS (mo)
20.0
21.5
15.1
18.4
15.8
Dosing
25 mg/m²
administered as a onehour intravenous
infusion every three
weeks in combination
with oral prednisone
10 mg administered
daily.
160 mg administered
orally once daily.
1,000 mg
administered orally
once daily in
combination with
prednisone 5 mg
administered orally
twice daily.
Relevant Study(ies) /
Citation(s)
TROPIC Trial
(NCT00417079; de
Bono et al., The Lancet
2010)
AFFIRM Trial
(NCT00974311; Scher
et al., NEJM 2012)
COU-AA-301 Trial
(NCT00638690; de
Bono et al., NEJM
2011)
Approval / Launch (US)
June 17, 2010
August 31, 2012
April 28, 2011
2.
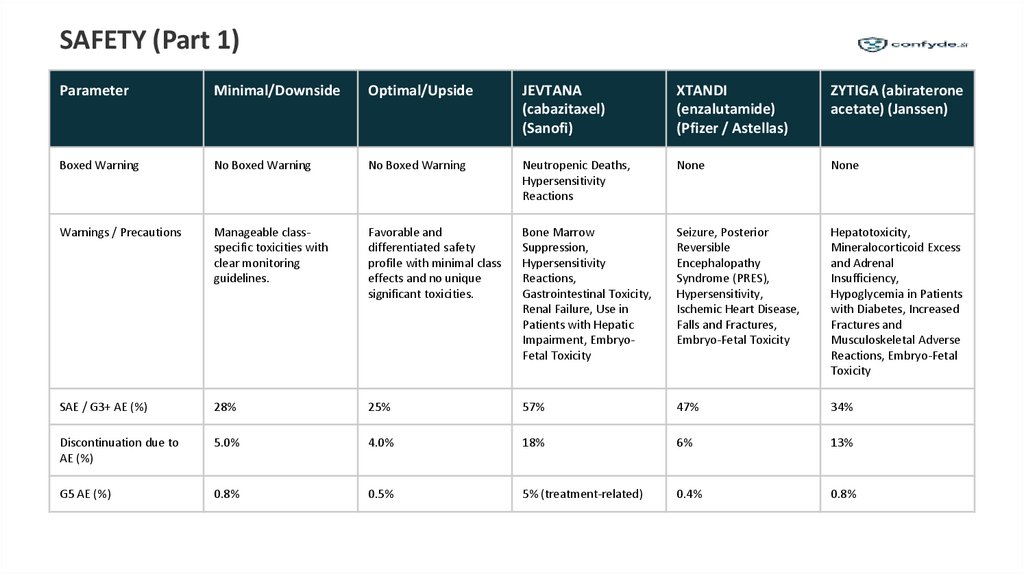
SAFETY (Part 1)Parameter
Minimal/Downside
Optimal/Upside
JEVTANA
(cabazitaxel)
(Sanofi)
XTANDI
(enzalutamide)
(Pfizer / Astellas)
ZYTIGA (abiraterone
acetate) (Janssen)
Boxed Warning
No Boxed Warning
No Boxed Warning
Neutropenic Deaths,
Hypersensitivity
Reactions
None
None
Warnings / Precautions
Manageable classspecific toxicities with
clear monitoring
guidelines.
Favorable and
differentiated safety
profile with minimal class
effects and no unique
significant toxicities.
Bone Marrow
Suppression,
Hypersensitivity
Reactions,
Gastrointestinal Toxicity,
Renal Failure, Use in
Patients with Hepatic
Impairment, EmbryoFetal Toxicity
Seizure, Posterior
Reversible
Encephalopathy
Syndrome (PRES),
Hypersensitivity,
Ischemic Heart Disease,
Falls and Fractures,
Embryo-Fetal Toxicity
Hepatotoxicity,
Mineralocorticoid Excess
and Adrenal
Insufficiency,
Hypoglycemia in Patients
with Diabetes, Increased
Fractures and
Musculoskeletal Adverse
Reactions, Embryo-Fetal
Toxicity
SAE / G3+ AE (%)
28%
25%
57%
47%
34%
Discontinuation due to
AE (%)
5.0%
4.0%
18%
6%
13%
G5 AE (%)
0.8%
0.5%
5% (treatment-related)
0.4%
0.8%
3.
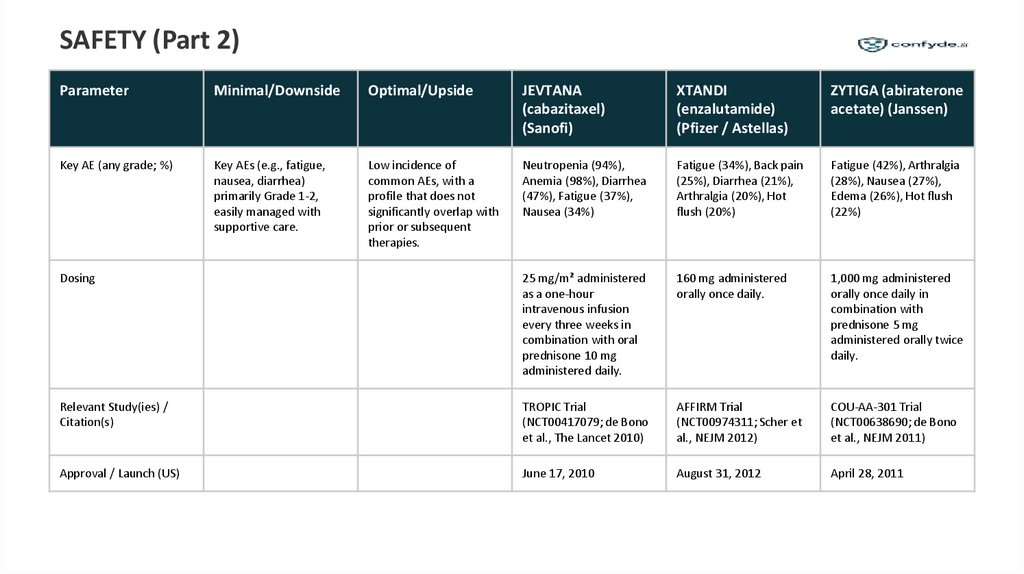
SAFETY (Part 2)Parameter
Minimal/Downside
Optimal/Upside
JEVTANA
(cabazitaxel)
(Sanofi)
XTANDI
(enzalutamide)
(Pfizer / Astellas)
ZYTIGA (abiraterone
acetate) (Janssen)
Key AE (any grade; %)
Key AEs (e.g., fatigue,
nausea, diarrhea)
primarily Grade 1-2,
easily managed with
supportive care.
Low incidence of
common AEs, with a
profile that does not
significantly overlap with
prior or subsequent
therapies.
Neutropenia (94%),
Anemia (98%), Diarrhea
(47%), Fatigue (37%),
Nausea (34%)
Fatigue (34%), Back pain
(25%), Diarrhea (21%),
Arthralgia (20%), Hot
flush (20%)
Fatigue (42%), Arthralgia
(28%), Nausea (27%),
Edema (26%), Hot flush
(22%)
Dosing
25 mg/m² administered
as a one-hour
intravenous infusion
every three weeks in
combination with oral
prednisone 10 mg
administered daily.
160 mg administered
orally once daily.
1,000 mg administered
orally once daily in
combination with
prednisone 5 mg
administered orally twice
daily.
Relevant Study(ies) /
Citation(s)
TROPIC Trial
(NCT00417079; de Bono
et al., The Lancet 2010)
AFFIRM Trial
(NCT00974311; Scher et
al., NEJM 2012)
COU-AA-301 Trial
(NCT00638690; de Bono
et al., NEJM 2011)
Approval / Launch (US)
June 17, 2010
August 31, 2012
April 28, 2011