Похожие презентации:
Microflora and sanitary-indicative bacteria of the soil, water, air the methods of studying
1. MICROFLORA AND SANITARY-INDICATIVE BACTERIA OF THE SOIL, WATER, AIR THE METHODS OF STUDYING
MICROFLORA AND SANITARYINDICATIVE BACTERIA OFTHE SOIL, WATER, AIR
THE METHODS OF STUDYING
2.
Microorganisms are widespread. Microbes aredistributed everywhere in the environment
surrounding us. They are found in the
Soil
Water
Air
Plants
Animals
Food products
In the human body and on the surface of the
human body
3.
The environment is a transmission factor ofinfectious diseases. Potentially pathogenic and
pathogenic microorganisms get to environment
mainly, in 2 ways:
1) fecal (with excrement from the intestine)
2) airborne (with droplets of mucus from the
respiratory tract)
Thus sanitary-microbiological investigations are
performed for study and evaluation of different
objects for determination of their epidemic
potential.
4.
Sanitary microbiology is a science thatstudies the microflora of the environment
and its harmful effect on the human body.
Methods for sanitary-microbiological
investigation include:
1) determination of a total microbial
contamination
2)detection and titration of sanitary-indicative
microorganisms
3)detection of pathogenic microorganisms
and/or their metabolites
5.
Direct detection of pathogenic microorganismsin the different objects of environment, in general,
is complicated because of their small quantity,
their temporarily staying in the environment and
the duration and laboriousness of methods for
their determination.
Thus indirect methods of detection of microbial
contamination are used:
1) total microbial contamination as indicator of
intensity of contamination by organic substances;
2) contamination by sanitary- indicative
microorganisms.
6.
Total viable count (TVC) is used for evaluationof total microbial contamination.
TVC is the number of microbes in 1 ml of
water, 1 g of soil , in 1 m3 of air.
7.
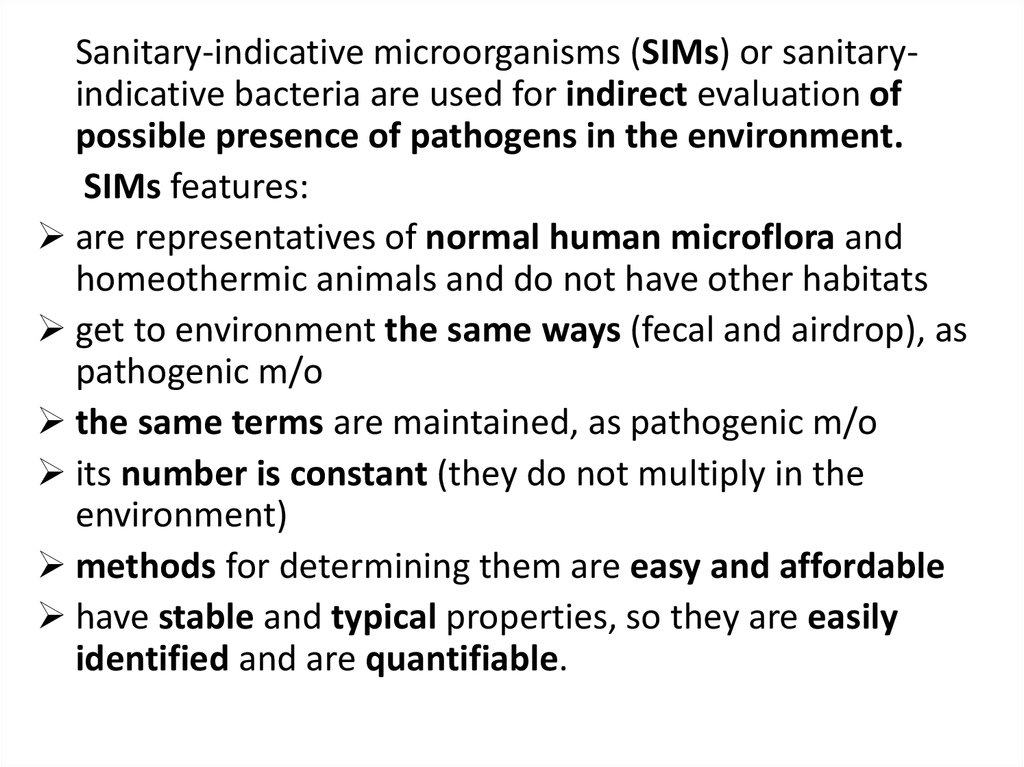
Sanitary-indicative microorganisms (SIMs) or sanitaryindicative bacteria are used for indirect evaluation ofpossible presence of pathogens in the environment.
SIMs features:
are representatives of normal human microflora and
homeothermic animals and do not have other habitats
get to environment the same ways (fecal and airdrop), as
pathogenic m/o
the same terms are maintained, as pathogenic m/o
its number is constant (they do not multiply in the
environment)
methods for determining them are easy and affordable
have stable and typical properties, so they are easily
identified and are quantifiable.
8.
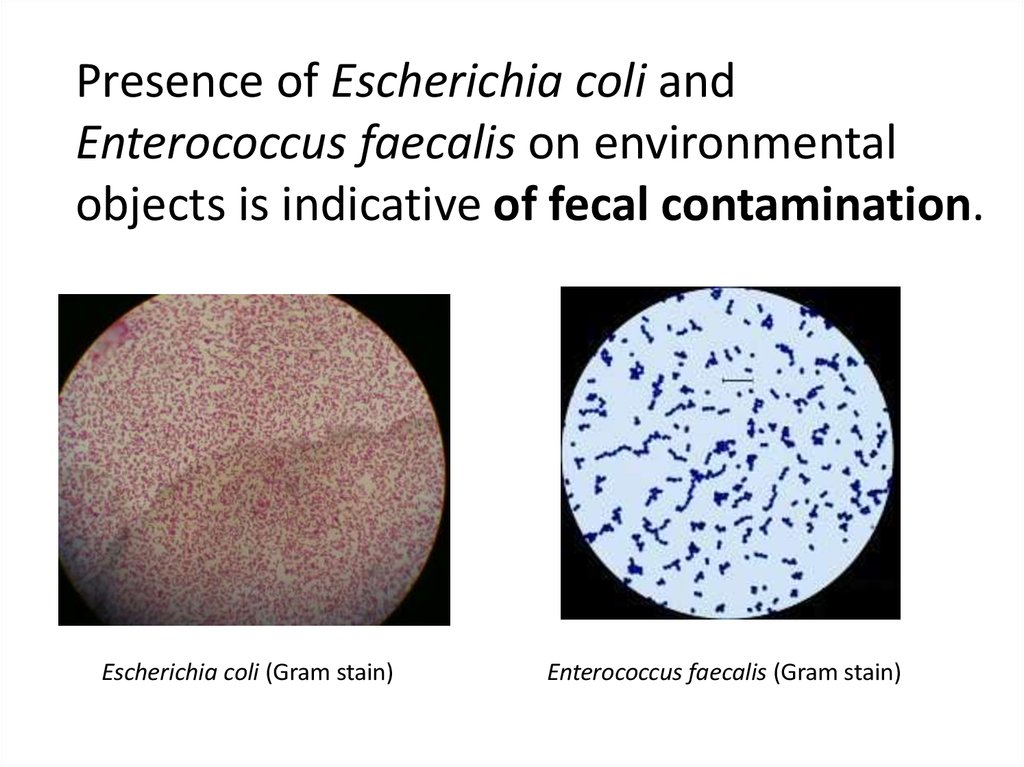
Presence of Escherichia coli andEnterococcus faecalis on environmental
objects is indicative of fecal contamination.
Escherichia coli (Gram stain)
Enterococcus faecalis (Gram stain)
9.
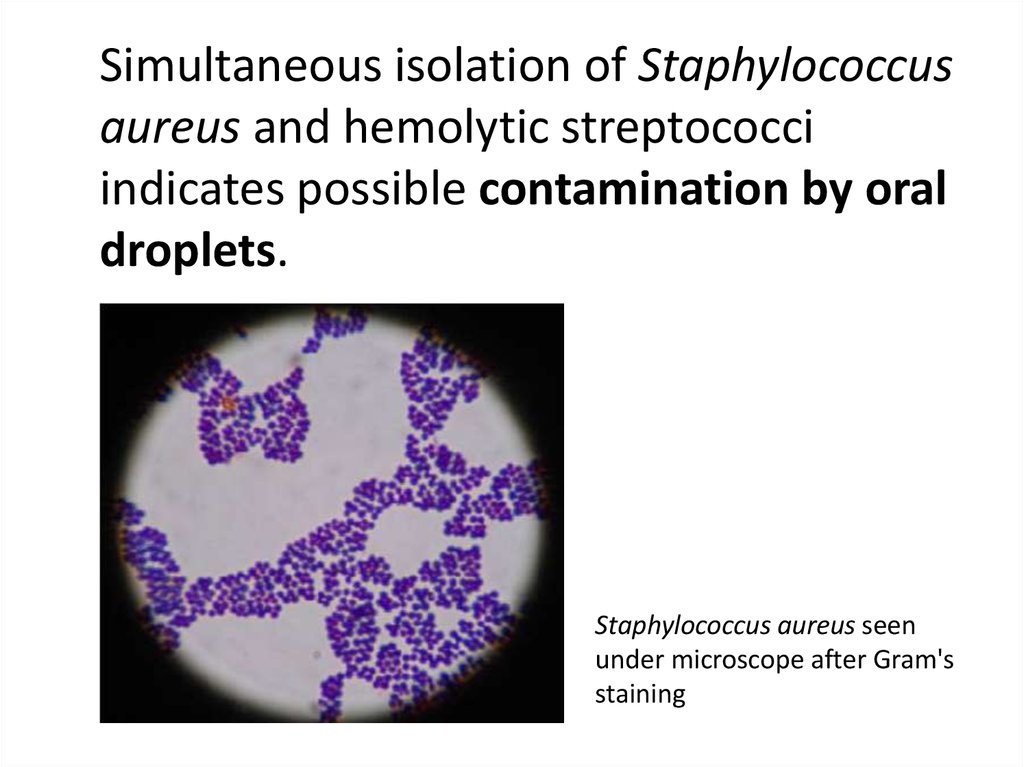
Simultaneous isolation of Staphylococcusaureus and hemolytic streptococci
indicates possible contamination by oral
droplets.
Staphylococcus aureus seen
under microscope after Gram's
staining
10.
If the amount of SIMs increases inenvironmental objects, the probability of the
presence of pathogenic and opportunistic
microbes in them increases. For different
objects there are specific SIMs.
Presence of sanitary-indicative microorganisms
is measured by titer and index.
The titer is a minimal mass (in g) or volume (in
ml), where else are detected SIMs.
The index is the amount of SIMs contained in a
1 l of water, 1 g of soil, 1 m3 of air.
11. WATER MICROFLORA
Pseudomonas fluorescens, Micrococcus roseusetc., are among the specific aquatic aerobic
microorganisms. Anaerobic bacteria are very
rarely found in water.
Pseudomonas fluorescens (Gram stain)
Micrococcus roseus (Gram stain)
12.
The microflora of rivers depends on thedegree of pollution and the quality of
purification of sewage waters flowing into
river beds. Microorganisms are widespread
in the waters of the seas and oceans. They
have been found at different depths (37009000 m).
13.
https://dornsife.usc.edu/labs/laketyrrell/research/14.
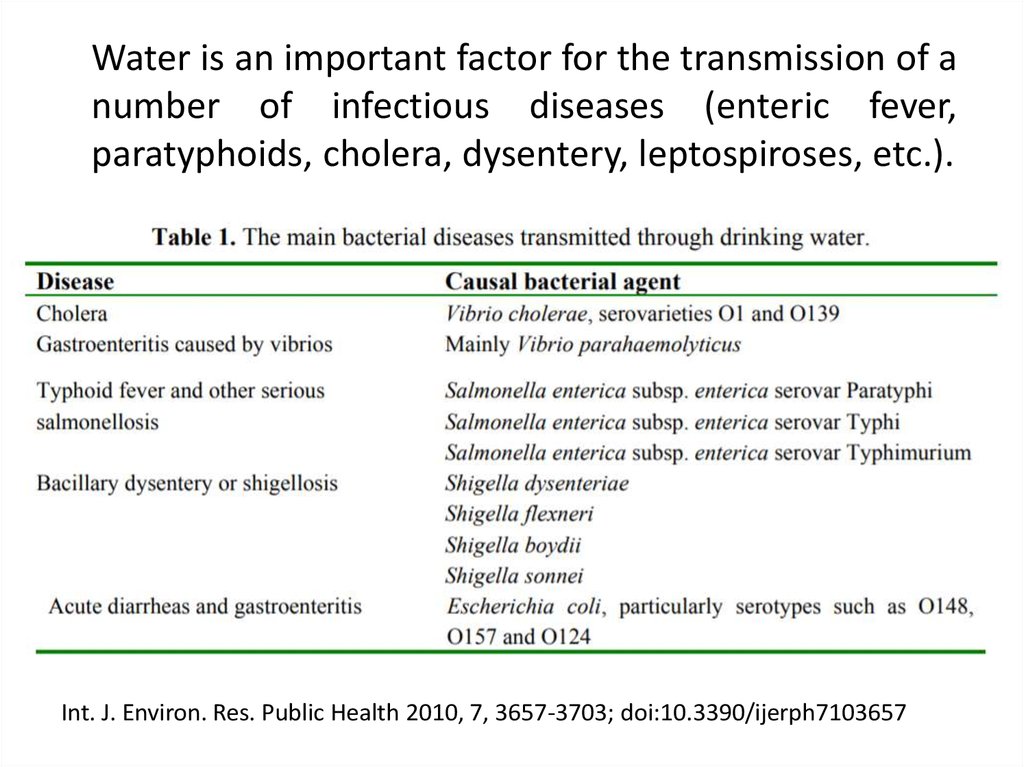
Water is an important factor for the transmission of anumber of infectious diseases (enteric fever,
paratyphoids, cholera, dysentery, leptospiroses, etc.).
Int. J. Environ. Res. Public Health 2010, 7, 3657-3703; doi:10.3390/ijerph7103657
15. How do we monitor the sanitary quality of water?
There are many kinds of pathogens that might betransmitted in water. These include bacteria, viruses
and protozoa. Each type of bacterium, virus or
protozoa requires a different test. Many of these
tests are expensive because they require special
materials or equipment or are time-consuming. It is
impractical to monitor water quality for every
pathogen on a routine basis. We should explore tap
(drinking) water, swimming pool water, the water of
open reservoirs, sewage waters, purified water for
preparation of medicines, distilled water for the
preparation of sterile solutions (injections, eye
drops).
16.
The sanitary - bacteriological investigation of waterincludes:
1) determination of total viable count in 1 ml of water
2) determination of coliform bacteria, as indicator of
fecal pollution (they live in the intestine,
representatives of normal human intestine microflora)
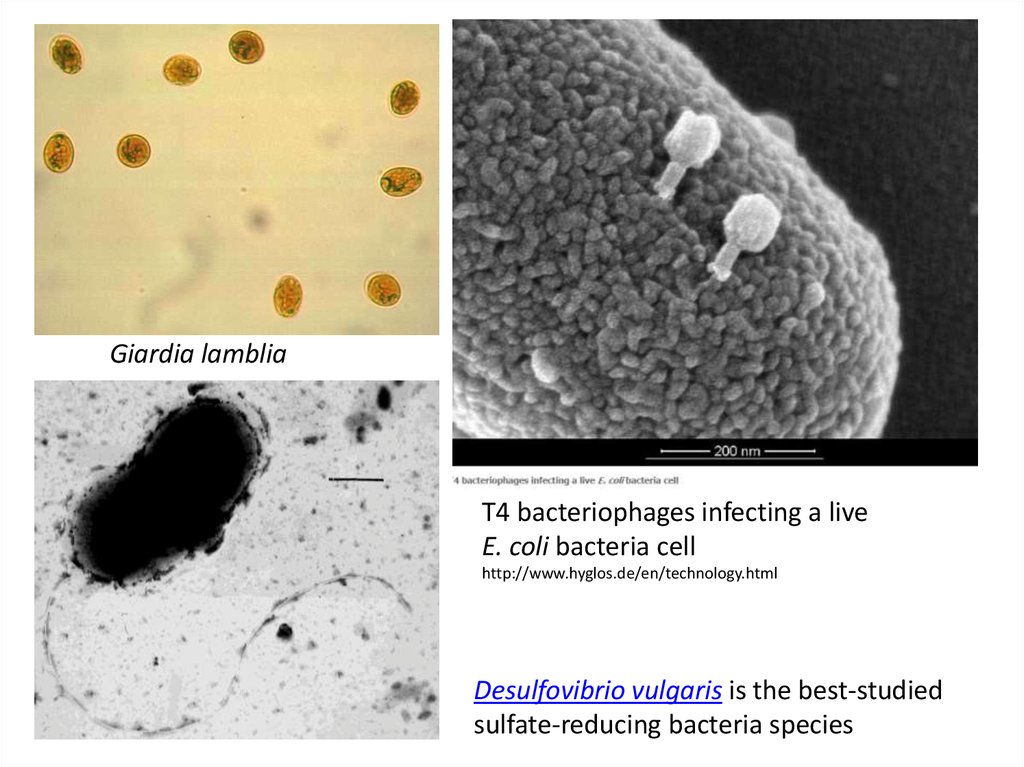
3) presence of spores of sulphite-reducing bacteria and
cysts of Giardia lamblia
4) presence of bacteriophages of E. coli
5) detection of pathogenic microbes in case of
epidemiological necessity
Due to the enormous sanitary-epidemiological role of
water in relation to the intestinal group of diseases, it
became necessary to work out rapid indicator methods
for revealing coliform and pathogenic bacteria in water.
17.
Giardia lambliaT4 bacteriophages infecting a live
E. coli bacteria cell
http://www.hyglos.de/en/technology.html
Desulfovibrio vulgaris is the best-studied
sulfate-reducing bacteria species
18.
WATER TVC DETERMINATION1. Sampling: 500 ml (tap water and purified water), 20
ml (water for injection), 100 ml (river water).
2. 1 ml of water is seeded in at least 2 Petri dishes
according to Koch's deep method on MPA.
3. Incubation: 37 ° C, 24 hours.
4. Calculation: count the number of colonies on both
plates, add up and divide by
The result is expressed in CFU(colony forming units) /
ml.
Take into account only those Petri dishes, where no
more than 300 colonies have grown.
19.
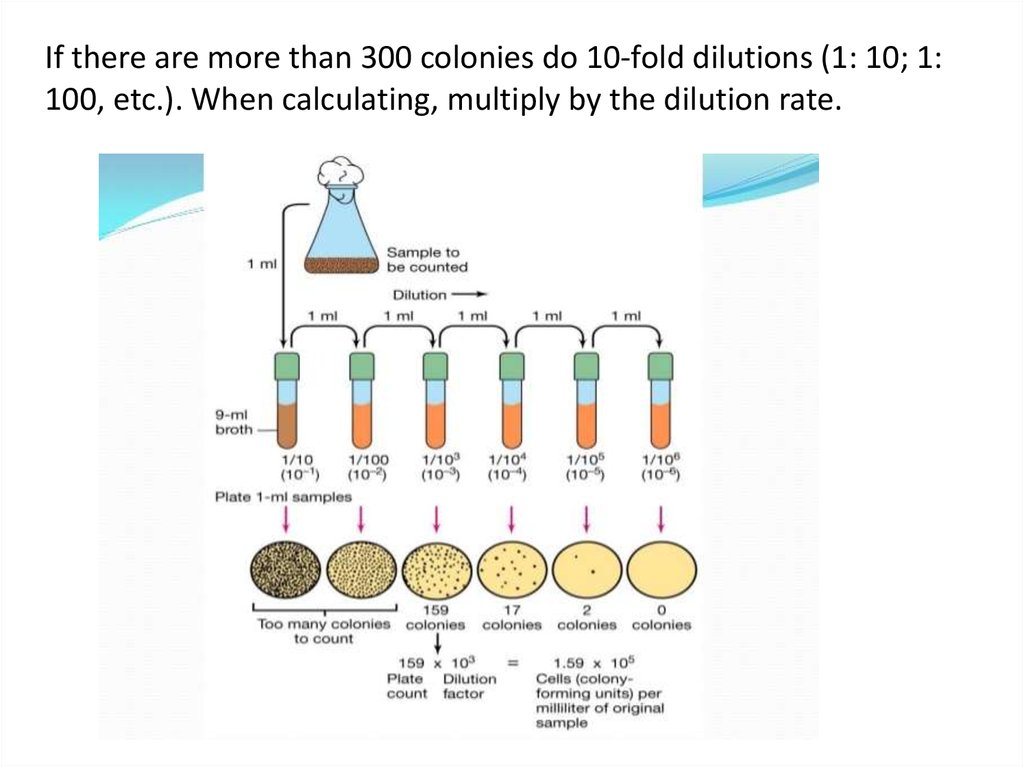
If there are more than 300 colonies do 10-fold dilutions (1: 10; 1:100, etc.). When calculating, multiply by the dilution rate.
20.
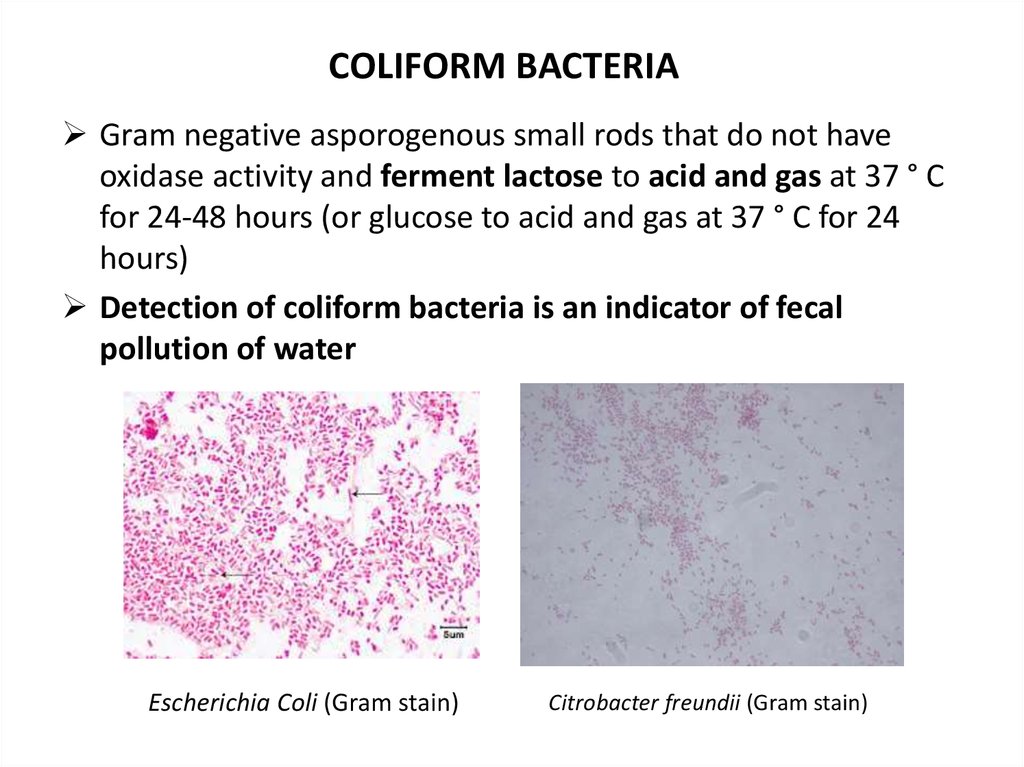
COLIFORM BACTERIAGram negative asporogenous small rods that do not have
oxidase activity and ferment lactose to acid and gas at 37 ° C
for 24-48 hours (or glucose to acid and gas at 37 ° C for 24
hours)
Detection of coliform bacteria is an indicator of fecal
pollution of water
Escherichia Coli (Gram stain)
Citrobacter freundii (Gram stain)
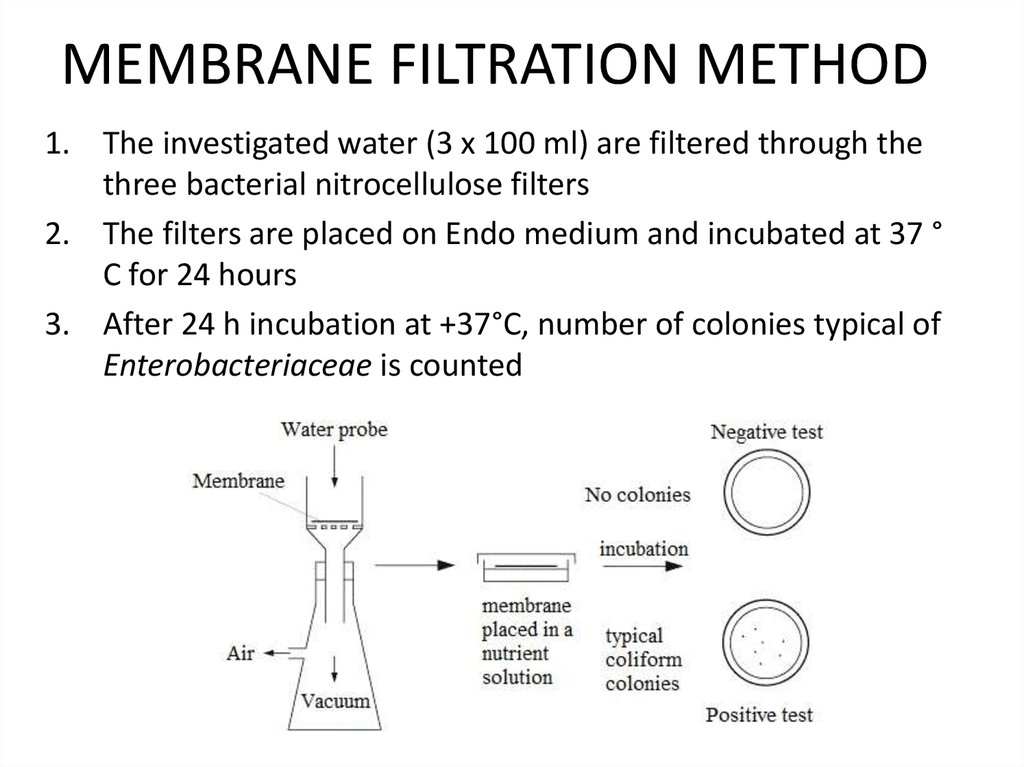
21. MEMBRANE FILTRATION METHOD
1. The investigated water (3 x 100 ml) are filtered through thethree bacterial nitrocellulose filters
2. The filters are placed on Endo medium and incubated at 37 °
C for 24 hours
3. After 24 h incubation at +37°C, number of colonies typical of
Enterobacteriaceae is counted
22. MEMBRANE FILTRATION METHOD
4. From 2 to 3 red-colored colonies are used for preparation of smearand Gram stain, followed by oxidase test allowing to distinguish
Escherichia spp., Citrobacter spp., Enterobacter spp. and other
Enterobacteriaceae from Pseudomonas spp. and other oxidasepositive non-fermenters which might be present in water.
5. For that purpose, filter with grown colonies (do not turn over! ) is
transferred with forceps to filter paper disk wetted with
dimethyl—n-phenyldiamine. Presence of oxidase will lead to
development of blue coloration of colony.
6. After that, 2 or 3 colonies, which did not change color, are
inoculated into semi-solid medium with 0.5% of glucose (lactose),
followed by 24 h incubation at +37°C. In case of presence of
formation of gas, you make a conclusion about the detection of
coliform bacteria. Then number of red colonies is counted and
coliform index is determined.
23. MEMBRANE FILTRATION METHOD
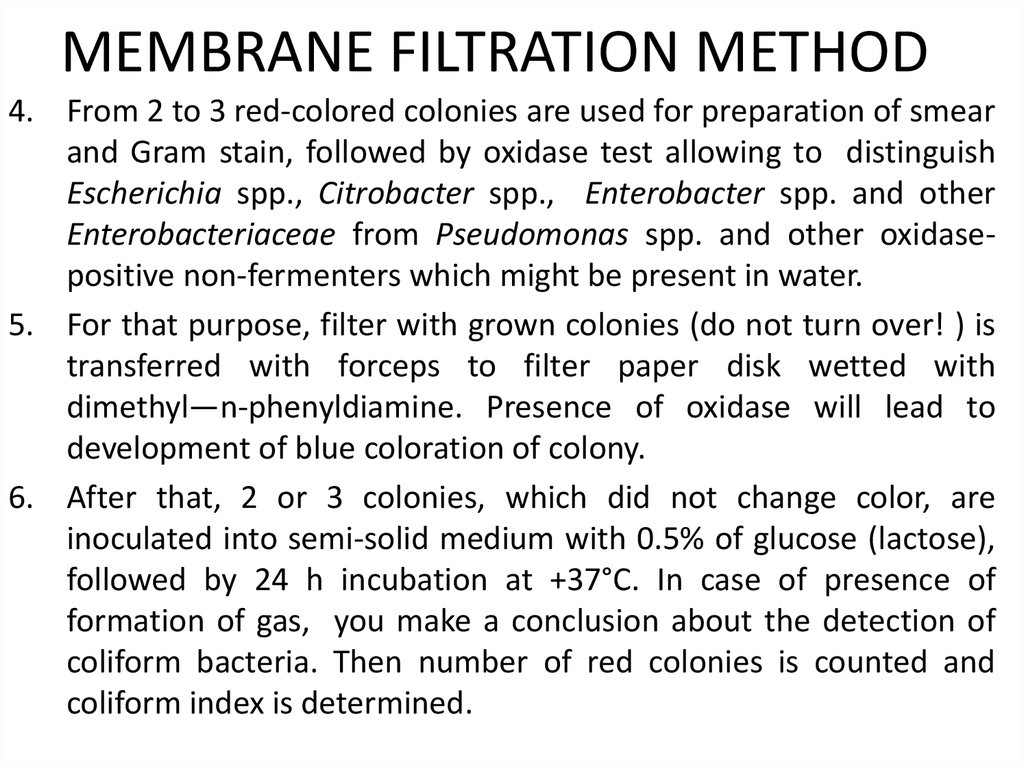
7.The index of CFU (colony forming units) of coliforms in 100 ml water is
calculated according to the following formula:
where X - coliforms CFU in 100 ml; V - total volume (300 ml) of water
filtered through the 3 filters; a - the total number of colonies of coliforms
grown on 3 filters.
7. If coliform bacteria are absent in all three samples of water of 100 ml,
then the water accords to the requirements of microbial purity.
8. If coliform bacteria are detected in at least in one sample in a 100 ml the
water does not accord to the requirements of microbial purity
9. In large settlements drinking water is being tested daily.
10. In the case of repeated detection of coliform bacteria, pathogenic
microbes are determined.
24. What are the standards for drinking water?
The USEPA issued revised Primary Drinking WaterStandards in mid-1994. These standards address the source
of water quality. The Primary Standards. If this test is used,
and the sampling agency tests more than 40 samples, no
more than 5% of those samples may test positive for total
coliforms. If fewer than 40 samples are used, no more than
1 sample may test positive. In addition, the maximum
contaminant levels, which vary with treatment technique,
are specified for Giardia lamblia, Legionella (the bacterium
which causes Legionnaire's disease) and viruses. The USEPA
Safe Drinking Water Hotline provides more information.
That number is 1-800-426- 4791.
The best way to ensure water safety - protection of water
sources from microbial contamination!
25. STANDARDS
The drinking water should not have more than50 microbes in 1 ml.
The microbial number in water of open
reservoirs can be up 1000.
26. Soil Microflora
Soil fertility depends not only on thepresence
of
inorganic
and
organic
substances, but also on the presence of
various species of microorganisms which
influence the qualitative composition of the
soil. Due to nutrients and moisture in the soil
the number of microbes in 1 g of soil reaches
a colossal number — from 200 million
bacteria in clayey soil to 5 thousand million in
black soil.
27.
Soil microflora consists bacteria (nitrifying,nitrogen-fixing, denitrifying), cellulose-splitting
and sulfur bacteria, pigmented microbes fungi,
protozoa, etc.
28.
The greatest amount of microbes (1 000000per cubic cm) is found in the top layer of soil
at a depth of 5-15 cm. In deeper layers (1.5-5
m) individual microbes are found. However,
they have been discovered at a depth of 17.5
m in artesian water.
29.
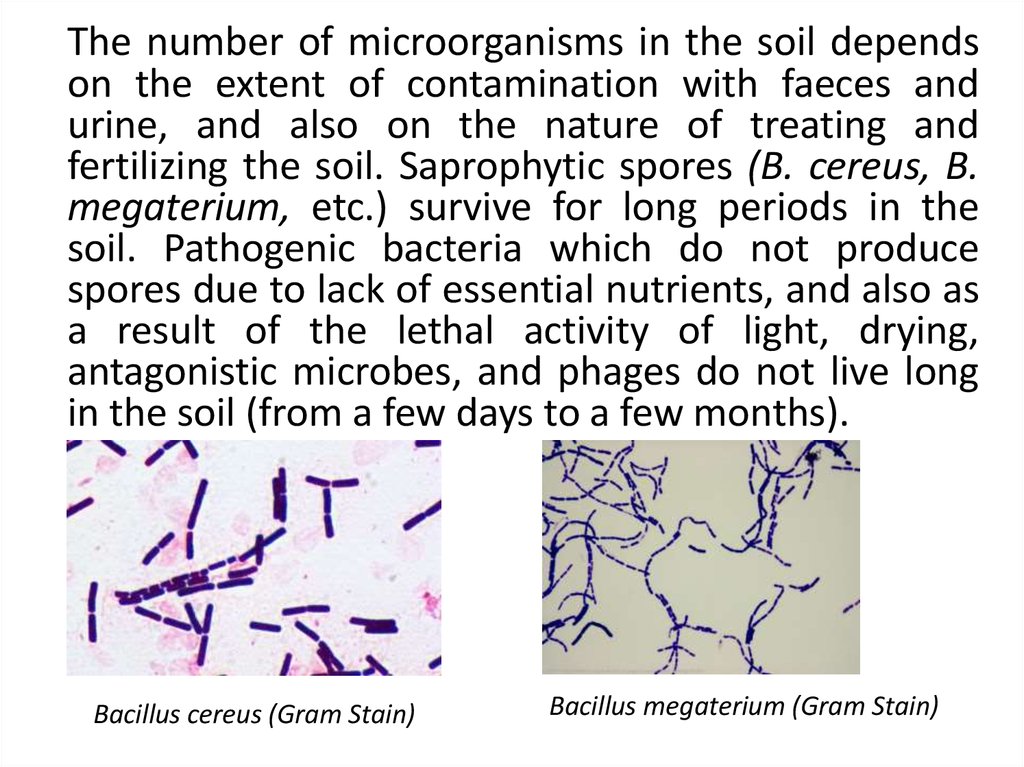
The number of microorganisms in the soil dependson the extent of contamination with faeces and
urine, and also on the nature of treating and
fertilizing the soil. Saprophytic spores (B. cereus, B.
megaterium, etc.) survive for long periods in the
soil. Pathogenic bacteria which do not produce
spores due to lack of essential nutrients, and also as
a result of the lethal activity of light, drying,
antagonistic microbes, and phages do not live long
in the soil (from a few days to a few months).
Bacillus cereus (Gram Stain)
Bacillus megaterium (Gram Stain)
30.
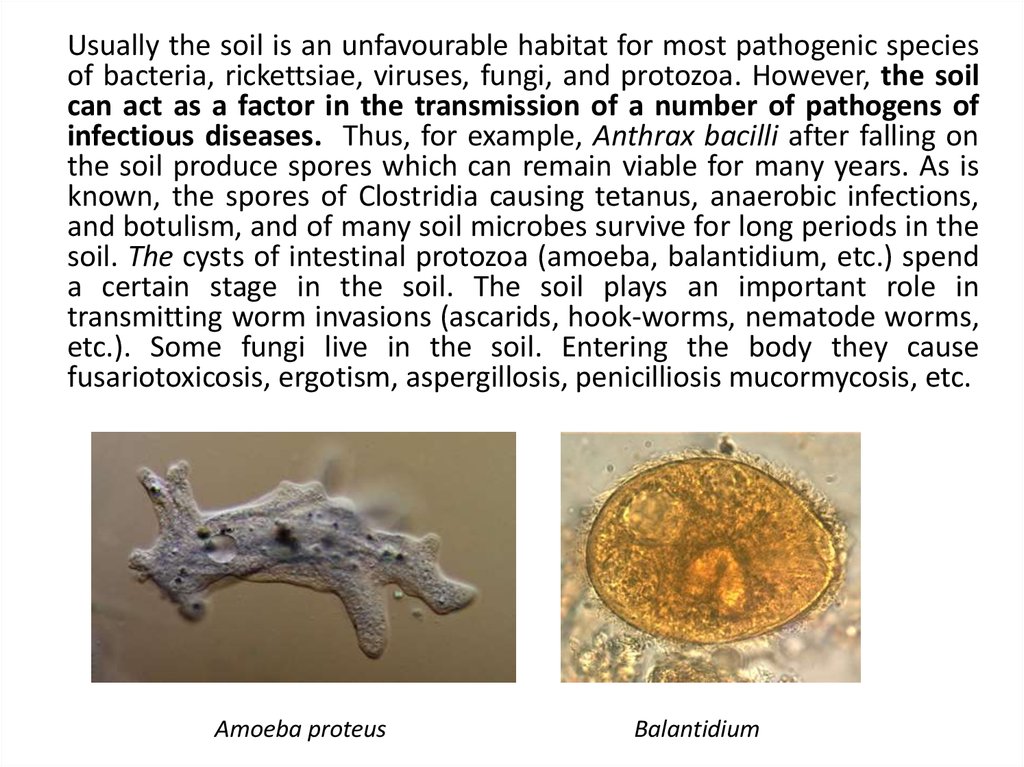
Usually the soil is an unfavourable habitat for most pathogenic speciesof bacteria, rickettsiae, viruses, fungi, and protozoa. However, the soil
can act as a factor in the transmission of a number of pathogens of
infectious diseases. Thus, for example, Anthrax bacilli after falling on
the soil produce spores which can remain viable for many years. As is
known, the spores of Clostridia causing tetanus, anaerobic infections,
and botulism, and of many soil microbes survive for long periods in the
soil. The cysts of intestinal protozoa (amoeba, balantidium, etc.) spend
a certain stage in the soil. The soil plays an important role in
transmitting worm invasions (ascarids, hook-worms, nematode worms,
etc.). Some fungi live in the soil. Entering the body they cause
fusariotoxicosis, ergotism, aspergillosis, penicilliosis mucormycosis, etc.
Amoeba proteus
Balantidium
31.
Microbiological Investigation of SoilTaking
into
consideration
the
definite
epidemiological role played by the soil in
spreading some infectious diseases of animals
and man, sanitary-microbiological evaluation of
soil is performed.
The sanitary - bacteriological investigation of soil
includes:
1) a total quantity of saprophytes bacteria in 1 g
of soil
2) presence of sanitary-indicative bacteria as
indicator of fecal contamination
32.
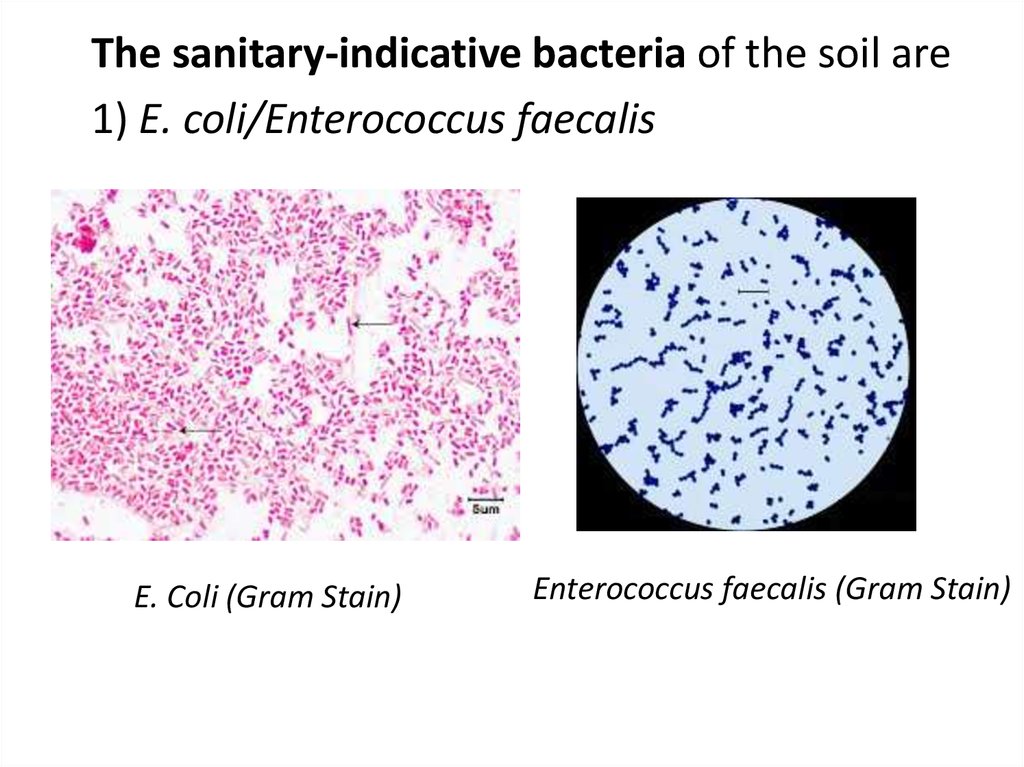
The sanitary-indicative bacteria of the soil are1) E. coli/Enterococcus faecalis
E. Coli (Gram Stain)
Enterococcus faecalis (Gram Stain)
33.
The sanitary-indicative bacteria of the soil are2) Citrobacter spp. /Enterobacter spp.
34.
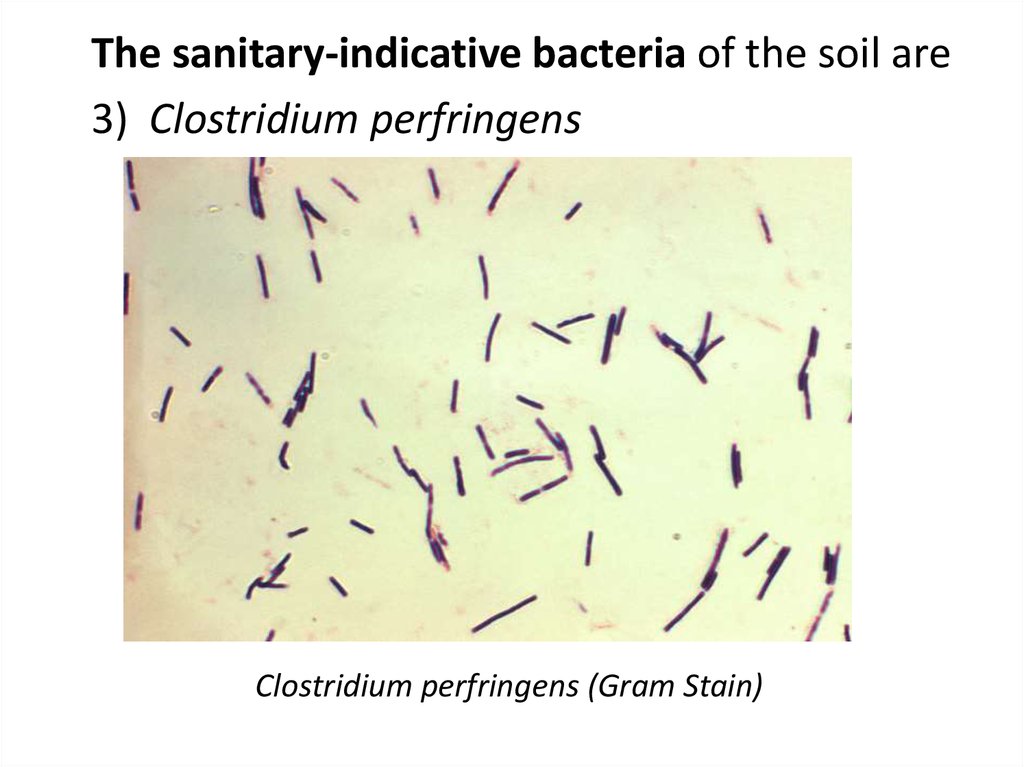
The sanitary-indicative bacteria of the soil are3) Clostridium perfringens
Clostridium perfringens (Gram Stain)
35.
More accurate evaluation is performed usingcoli-index — number of Enterobacteriaceae
(so called coliform bacteria) found in 1 g of
soil
perfringens-titer - mass of soil in which 1 cell
C. perfringens is found.
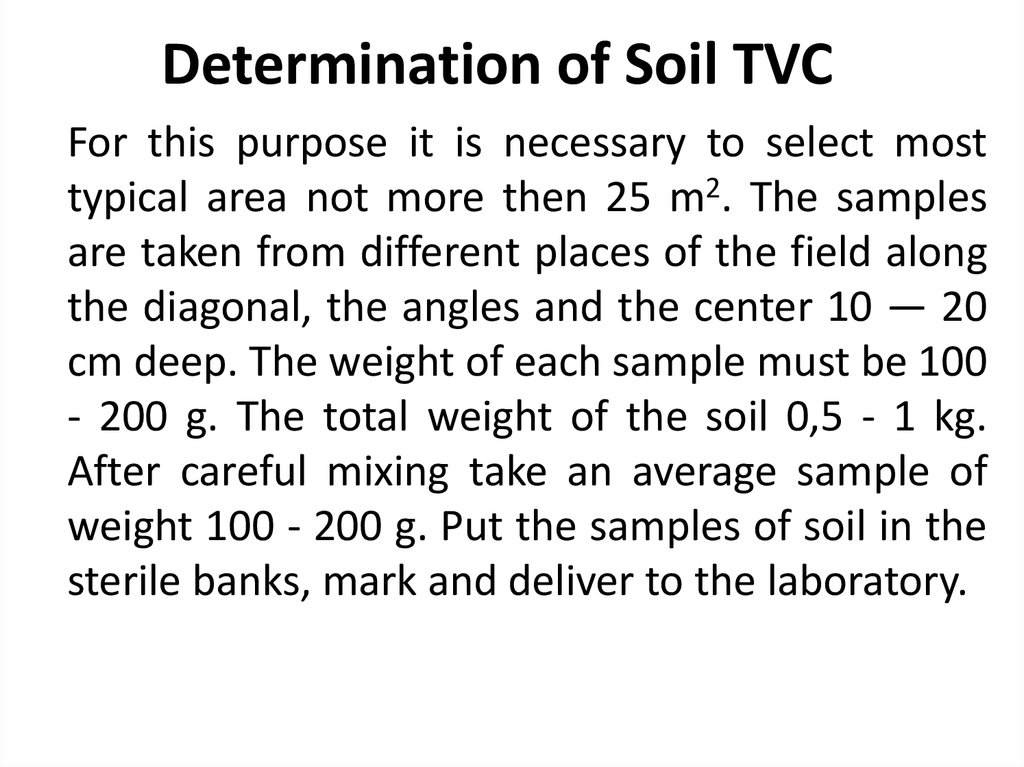
36. Determination of Soil TVC
For this purpose it is necessary to select mosttypical area not more then 25 m2. The samples
are taken from different places of the field along
the diagonal, the angles and the center 10 — 20
cm deep. The weight of each sample must be 100
- 200 g. The total weight of the soil 0,5 - 1 kg.
After careful mixing take an average sample of
weight 100 - 200 g. Put the samples of soil in the
sterile banks, mark and deliver to the laboratory.
37. Determination of Soil TVC
1. Prepare 10-fold dilutions (1:10, 1: 100, etc.) in an isotonicsterile solution of sodium chloride.
2. Make seeding of the soil dilutions on MPA (for bacteria) and on
Saburo medium (for fungi): 1 ml in the depth of agar or 0.1 ml on
the surface of agar.
3. Incubation: at 24 ° C (for fungi) and 37 ° C (for bacteria).
After incubation at optimal temperature count the colonies on
the plates (1 colony=1 cell). The number of cells in 1 g of soil is
calculated, taking into account:
- the weight of each sample;
- the rate of dilution;
- the volume of seeding.
38. Determination of Perfringens-titer
1. Seeding onto the Wilson-Blair medium: blackcolonies are formed and the gas breaks up
the medium
2. Calculation: maximal dilution, where there
are signs of growth of Clostridium
perfringens.
39. AIR MICROFLORA
The composition of the microbes of the air isquite variable. Then more dust, smoke, and soot
in the air, the greater the number of microbes.
Each particle of dust or smoke is able to adsorb
on its surface numerous microbes. The number of
microbes in the air varies from a few specimens to
many tens of thousands per 1 m3. Depending on
the time of the year, the composition and the
amount of microflora change. If the total amount
of microbes in winter is accepted as 1, then in
spring it will be 1.7, in summer— 2 and in autumn
— 1.2.
40.
The number of microbes in factories and homes isassociated closely with the sanitary hygienic conditions
of the building. At poor ventilation and natural lighting
and if the premises are not properly cleaned, the
number of microbes increases.
Pathogenic species of microbes (Pyogenic Cocci,
Tubercle Bacilli, Anthrax Bacilli, bacteria of tularaemia,
rickettsia of Q-fever, etc.) may be found in the
surroundings of sick animals and humans, infected
arthropods and insects, and in dust. The causative
agents of influenza, measles, scarlet fever, diphtheria,
whooping cough, meningococcal infections, tonsillitis,
acute catarrhs of the respiratory tract, tuberculosis,
smallpox, pneumatic plague, and other diseases can be
transmitted through the air together with droplets of
mucus and sputum during sneezing, coughing, and
talking.
41. Pathogenic Species of Microbes
Mycobacterium tuberculosis (Gram Stain)Anthrax bacilli (Gram Stain)
Francisella tularensis (Gram Stain)
42.
The air is an unfavourable medium for microbes.The absence of nutrient substances, the presence
of moisture, optimal temperature, the lethal
activity of sunlight, and desiccation do not create
conditions for keeping microbes viable and most
of them perish. However, the relatively short
period during which the microbes are in air is
quite enough to bring about the transmission of
pathogenic bacteria and viruses from sick to
healthy persons, and to cause extensive
epidemics of diseases such as influenza.
43.
The laboratory investigation of air is carriedout to determine the qualitative and
quantitative composition of its microflora.
This is achieved by using simple and complex
methods. For a more accurate investigation of
microbial contents of the air special apparatus
are used.
At present Streptococcus viridans serves as
sanitary indices for the air of closed buildings,
and haemolytic streptococci and pathogenic
staphylococci are a direct epidemiological
hazard.
44.
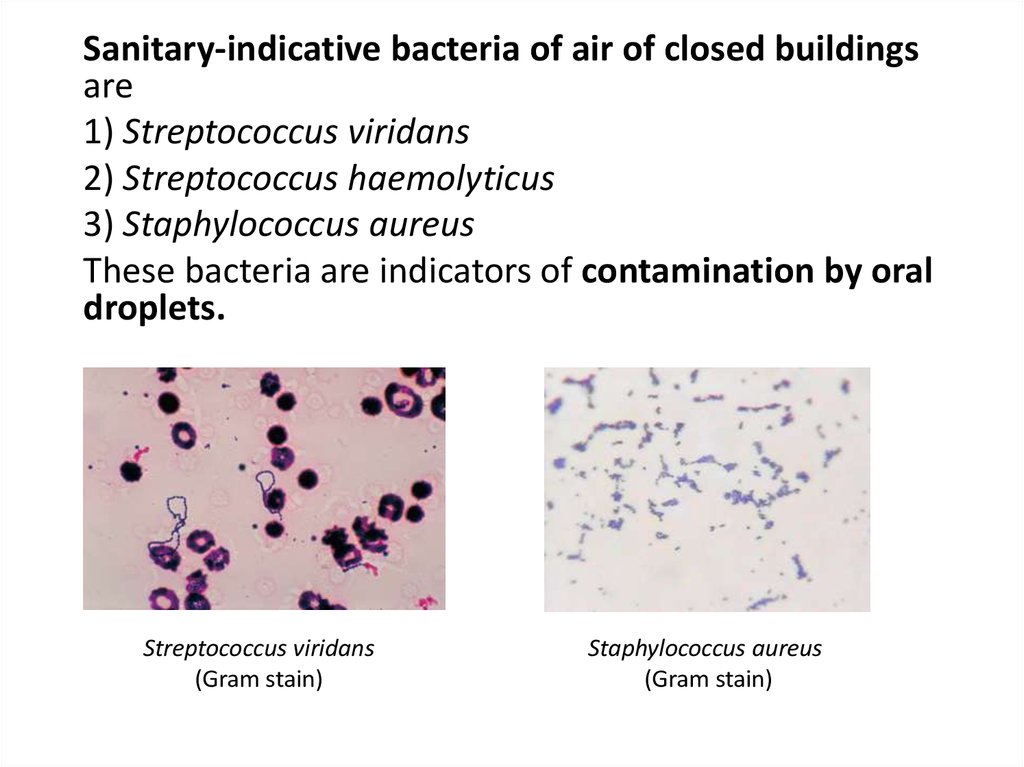
Sanitary-indicative bacteria of air of closed buildingsare
1) Streptococcus viridans
2) Streptococcus haemolyticus
3) Staphylococcus aureus
These bacteria are indicators of contamination by oral
droplets.
Streptococcus viridans
(Gram stain)
Staphylococcus aureus
(Gram stain)
45.
Microbiological Investigation of the AirThe sanitary - bacteriological investigation of
air includes:
1) determination the total viable count (TVC)
in 1 m3 of the air
2) presence of sanitary-indicative bacteria —
Str. viridans, Str. haemolyticus , S. aureus.
For taking the samples sedimentation and
aspiration methods are used.
46. Plate method (sedimentation method)
The Petri’s dishes with meat-peptone agar or another special nutrientmedia for staphylococci and streptococci, for example blood agar, yolksalt agar are used. They are opened and are stayed in investigated room.
Term of exposition depends on prospective quantity of microbes in the
air. With a plenty of microorganisms a plate is opened for 5-10 minutes
to detect a total microbial number, with a little - for 20 — 40 minutes for
detection of cocci.
Then the dishes put into thermostat at 37 °C for 24 hrs. After incubation
all colonies are accounted (for determination of total number of
microorganisms). Number of grown colonies
indicates degree of air contamination.
According to Omeliansky’s data in 5 minutes on a surface of 100 cm2 so
many microbes sedimentate, as they present in 10 L of air. For example,
on the dish surface with MPA after 5 minute exposure 32 colonies have
grown. It is necessary to calculate amount of microbes which are
present in 1 nr3 of the air, applying the Omeliansky’s formula. The plate
has 100 cm2 . 32 colonies of microbes contain in 10 L of the air, and in 1
m3 (1000 л) there will be (32 • 1000): 10 = 3200.
47. ASPIRATION METHOD
Krotov’s apparatus is used for bacteriological airresearch. It give us the possibility to let pass 50 -100 L
of air with a speed of 25 L per minute through clinoid
chink in the special glass above the open dish with
MPA. The rotation of Petry’s dish (1 rotation/sec)
provides uniform dispersion of microorganisms on all
surface of a medium. Then dish is incubated in a
thermostat at 37 °C for 18-24 hrs.
Krotov’s apparatus
for bacteriological air
research
48. ASPIRATION METHOD
For example, 250 colonies are revealed on thesurface of dish after 2-minutes exposure with a
25 1/min speed. Thus the number of microbes (x)
in 1 m3 of the air is: x = (250 • 1000): 50 = 5000.
There are a number of soft
for automatic counting:
Colony-Counter
OpenCFU
CellCounter etc.
49. Determination of Staphylococci and Streptococci
Using Krotov’s apparatus 250 L of air areseeded on the surface of open Petri dish with
yolk-salt agar for staphylococci and with blood
agar for streptococci. Then dishes are
incubated in a thermostat at 37 °C for 18-24
hrs. After incubation growing up colonies are
accounted and the number of staphylococci or
streptococci in 1 m3 of the air is calculated.
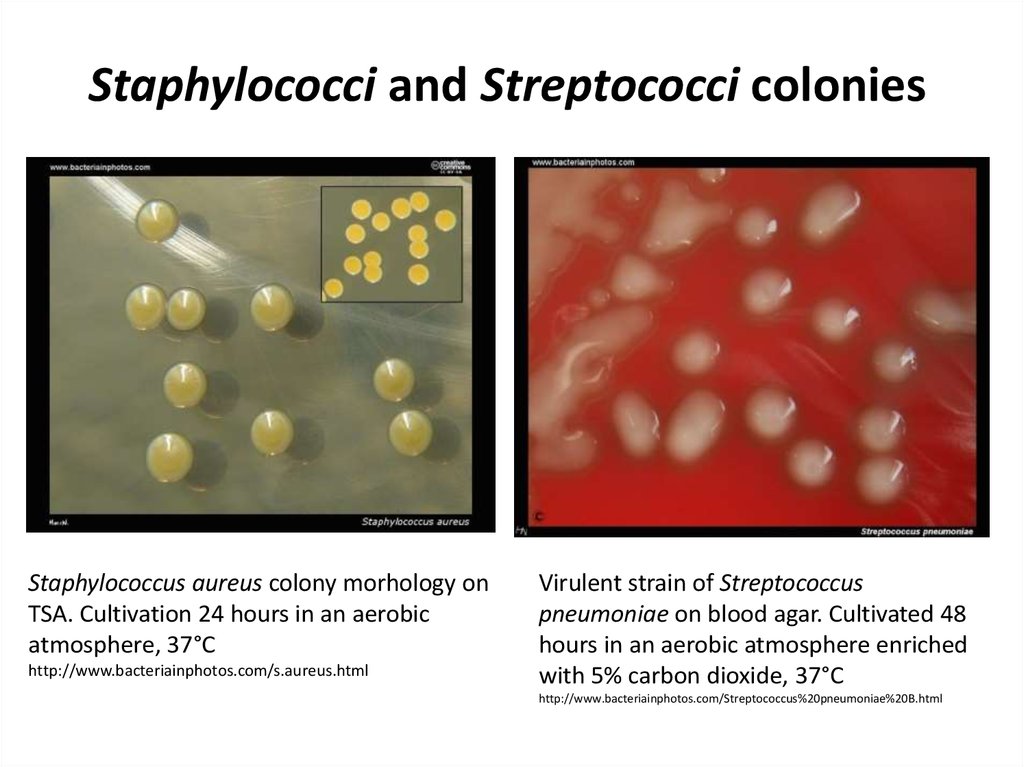
50. Staphylococci and Streptococci colonies
Staphylococcus aureus colony morhology onTSA. Cultivation 24 hours in an aerobic
atmosphere, 37°C
http://www.bacteriainphotos.com/s.aureus.html
Virulent strain of Streptococcus
pneumoniae on blood agar. Cultivated 48
hours in an aerobic atmosphere enriched
with 5% carbon dioxide, 37°C
http://www.bacteriainphotos.com/Streptococcus%20pneumoniae%20B.html
51.
To the air environment of pharmacies stricthygienic requirements are imposed, which is
reflected in normative documents.
Sources of air pollution pharmacies:
Visitors
Employees
Infected material (recipes, dishes, packaging
material)
Poor-quality medicinal plant raw materials.
The permissible standards of the microbial
number of air in various pharmacy premises have
also been developed.



























 Экология
Экология








