Похожие презентации:
Application of nickel nanoparticles in diffusion bonding of stainless steel surfaces
1. Application of Nickel Nanoparticles in Diffusion Bonding of Stainless Steel Surfaces
Santosh Tiwari and Brian K. PaulSchool of Mechanical, Industrial and
Manufacturing Engineering
Oregon State University
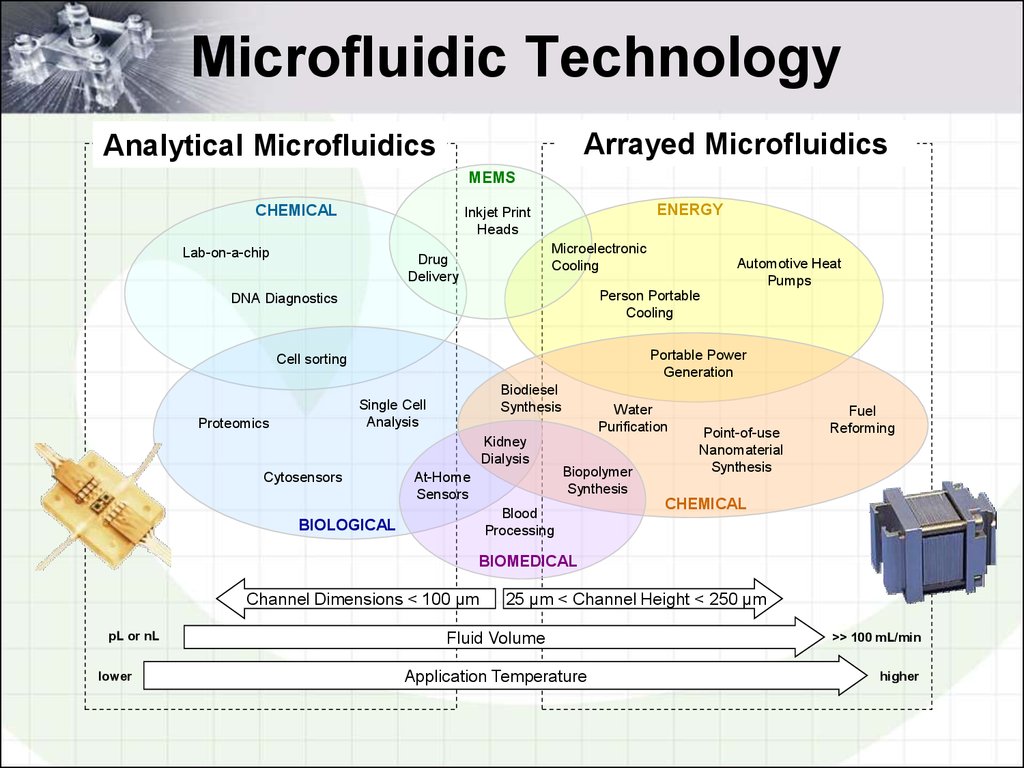
2. Microfluidic Technology
Arrayed MicrofluidicsAnalytical
Microfluidics
Micro Total Analysis
Systems (µTAS)
Micro Energy and Chemical Systems (MECS)
MEMS
CHEMICAL
ENERGY
Inkjet Print
Heads
Lab-on-a-chip
Microelectronic
Cooling
Drug
Delivery
Person Portable
Cooling
DNA Diagnostics
Portable Power
Generation
Cell sorting
Biodiesel
Synthesis
Single Cell
Analysis
Proteomics
Automotive Heat
Pumps
Kidney
Dialysis
Cytosensors
At-Home
Sensors
Water
Purification
Biopolymer
Synthesis
Blood
Processing
BIOLOGICAL
Point-of-use
Nanomaterial
Synthesis
Fuel
Reforming
CHEMICAL
BIOMEDICAL
Channel Dimensions < 100 µm
pL or nL
lower
25 µm < Channel Height < 250 µm
Fluid Volume
Application Temperature
>> 100 mL/min
higher
3. Emerging Industry
Fuel ProcessingChemical
Processing
Nanomateria
l Synthesis
Heating &
Cooling
Separation
s
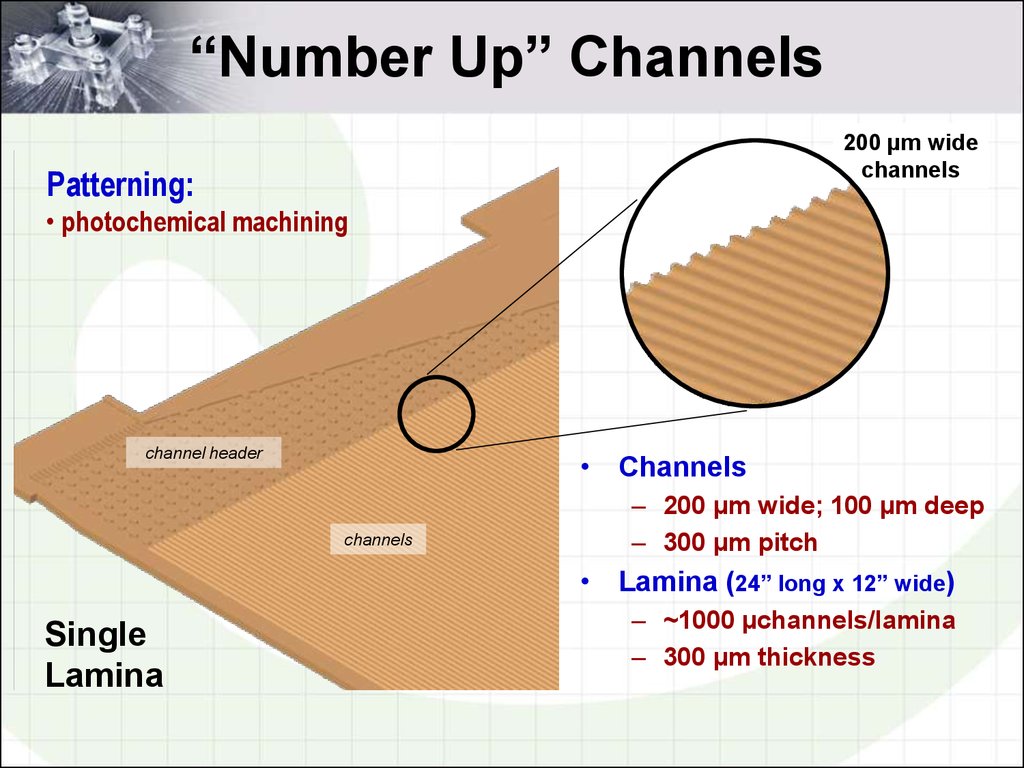
4. “Number Up” Channels
200 µm widechannels
Patterning:
• photochemical machining
channel header
• Channels
channels
– 200 µm wide; 100 µm deep
– 300 µm pitch
• Lamina (24” long x 12” wide)
Single
Lamina
– ~1000 µchannels/lamina
– 300 µm thickness
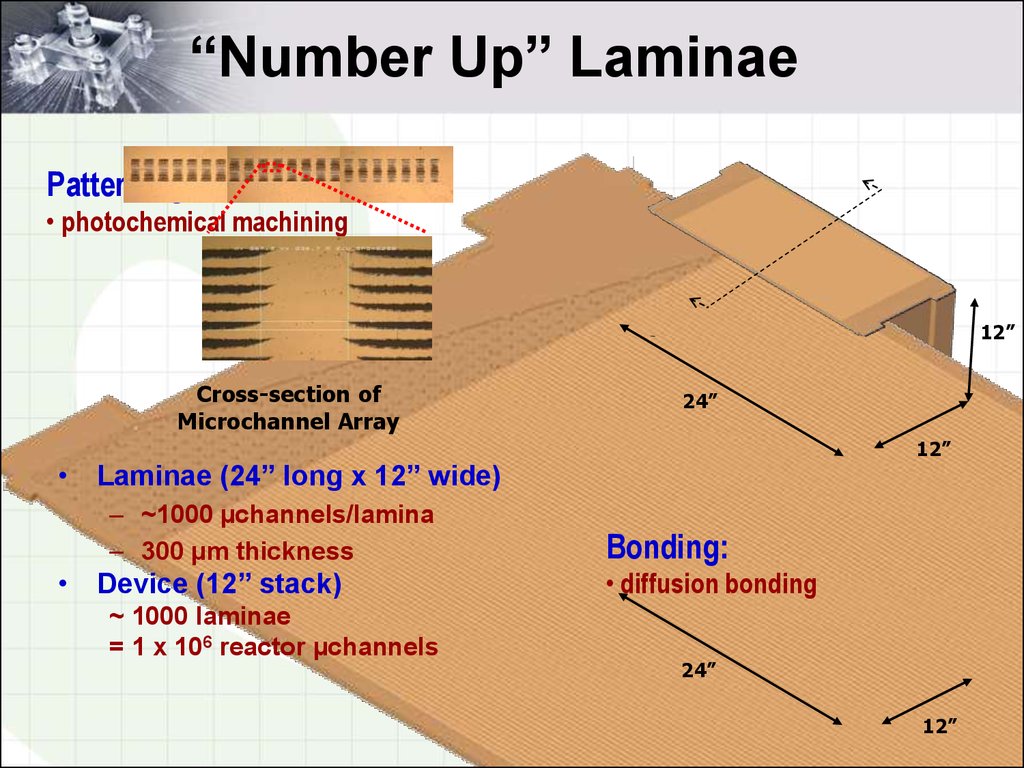
5. “Number Up” Laminae
Patterning:• photochemical machining
12”
Cross-section of
Microchannel Array
24”
12”
• Laminae (24” long x 12” wide)
– ~1000 µchannels/lamina
– 300 µm thickness
• Device (12” stack)
~ 1000 laminae
= 1 x 106 reactor µchannels
Bonding:
• diffusion bonding
24”
12”
6. Outline
Motivation and Objective
Approach
Results
Summary
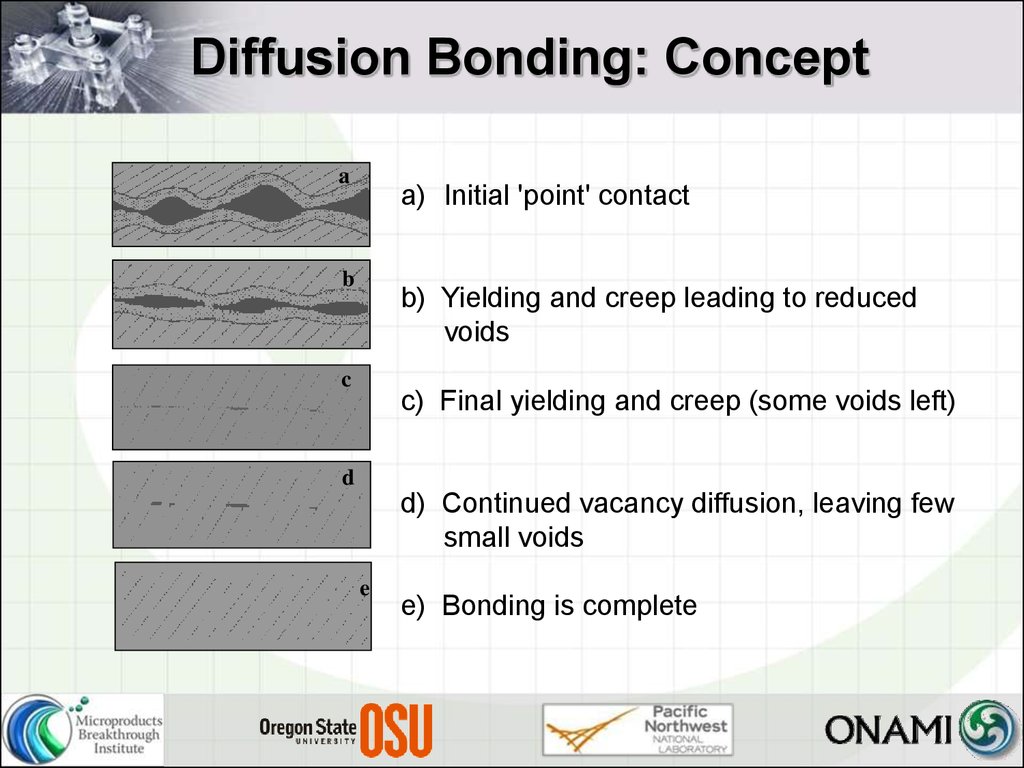
7. Diffusion Bonding: Concept
aa) Initial 'point' contact
b
b) Yielding and creep leading to reduced
voids
c
c) Final yielding and creep (some voids left)
d
d) Continued vacancy diffusion, leaving few
small voids
e
e) Bonding is complete
8. Diffusion Brazing of SS 316L
• Filler materials such as Ni, Cu, Au etc.• Nickel
– Almost 100 % solid solubility in Fe
– Good corrosion and wear resistance
– Compatible with stainless steel
• Temperature depressant materials (TDMs) like Si, B, P etc.
added to reduce the melting temperature
– Transient liquid phase bonding
• Adverse effect of TDMs
–
–
–
–
Formation of secondary phases
Bond strength and ductility ▼
Additional heat treatment cycle ~ up to 24 hrs
Time and Cost ▲
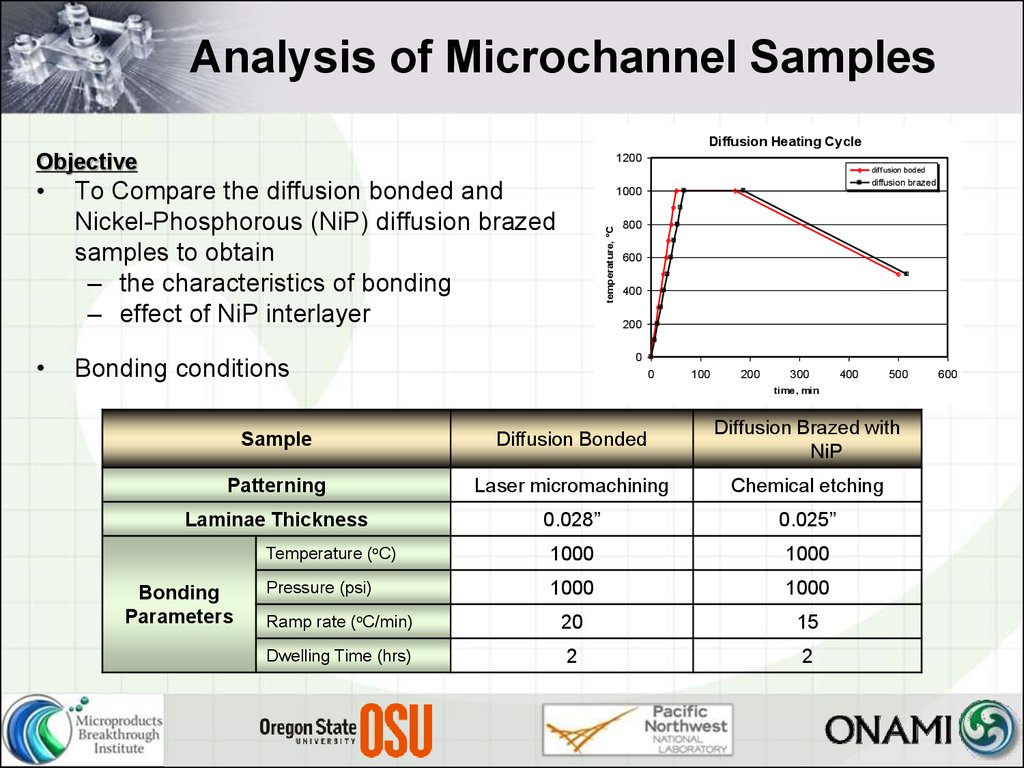
9. Analysis of Microchannel Samples
Diffusion Heating CycleObjective
1200
1000
diffusion boded
temperature, °C
To Compare the diffusion bonded and
Nickel-Phosphorous (NiP) diffusion brazed
samples to obtain
– the characteristics of bonding
– effect of NiP interlayer
diffusion brazed
800
600
400
200
0
Bonding conditions
0
100
200
300
400
500
time, min
Sample
Diffusion Bonded
Diffusion Brazed with
NiP
Patterning
Laser micromachining
Chemical etching
Laminae Thickness
0.028”
0.025”
Temperature (oC)
1000
1000
Pressure (psi)
1000
1000
Ramp rate (oC/min)
20
15
Dwelling Time (hrs)
2
2
Bonding
Parameters
600
10. Scanning Electron Microscopy
100 µm200 µm
SEM image of bond line for diffusion bonded sample
10 µm
two phases
present
intermetallic?
50 µm
SEM image of bond line for diffusion brazed sample
10 µm
11. Defect Quantification
Defects Quantificationµm, %
180
channel misregistration
warpage
void fraction (direct pressure transmission)
void fraction (in between channels)
160
140
120
100
80
60
40
20
0
Diffusion Bonded SS
Diffusion Brazed SS – NiP
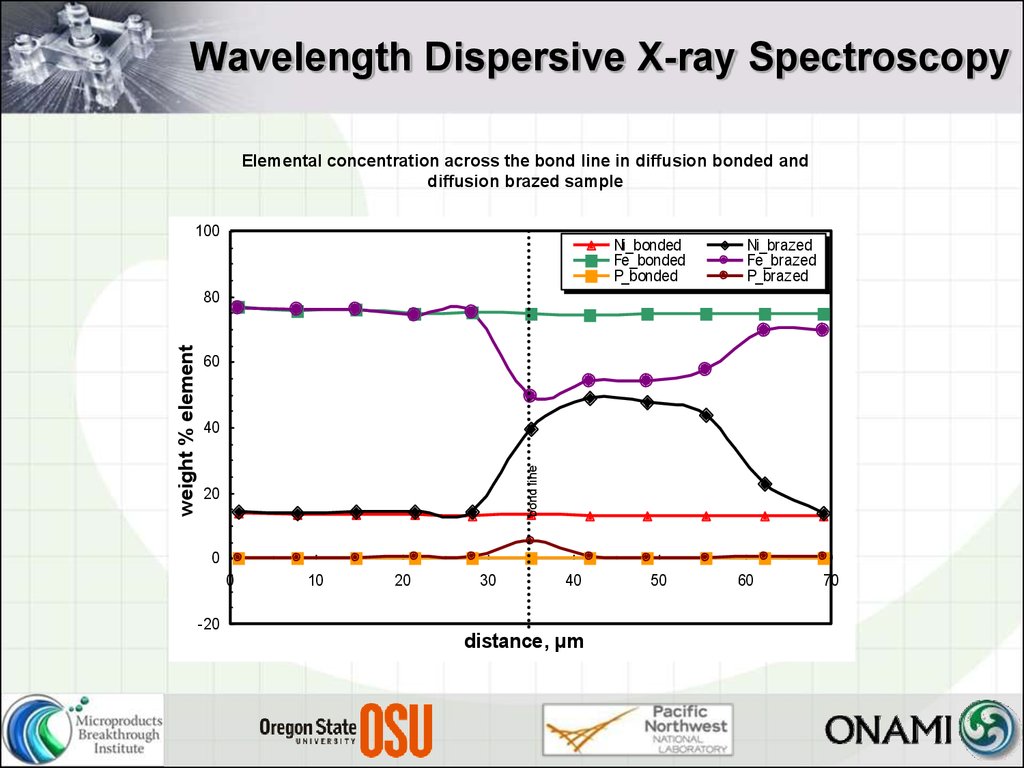
12. Wavelength Dispersive X-ray Spectroscopy
Elemental concentration across the bond line in diffusion bonded anddiffusion brazed sample
100
Ni_bonded
Fe_bonded
P_bonded
Ni_brazed
Fe_brazed
P_brazed
60
40
bond line
weight % element
80
20
0
0
-20
10
20
30
40
distance, µm
50
60
70
13. Effect of NP Size on Properties
AgAu
“As the size decreases beyond a critical value, due to the surface –to-volume
ratio, the melting temperature decreases and becomes size dependent”
Nano Al : 2nm (200oC) and 9nm (660oC)
Generally, critical value is ~10nm
Nanoscale Materials in Chemistry, Wiley, 2001
Q Jiang, Materials chemistry and physics, v. 83, 2003, pp. 225-227
14. Role of Nanoparticles
• Nano-sized particles– exhibit lower melting temperature than the bulk material
– lower activation energy required to liberate atoms from the
surface
– tremendously high surface area causing higher diffusion rate
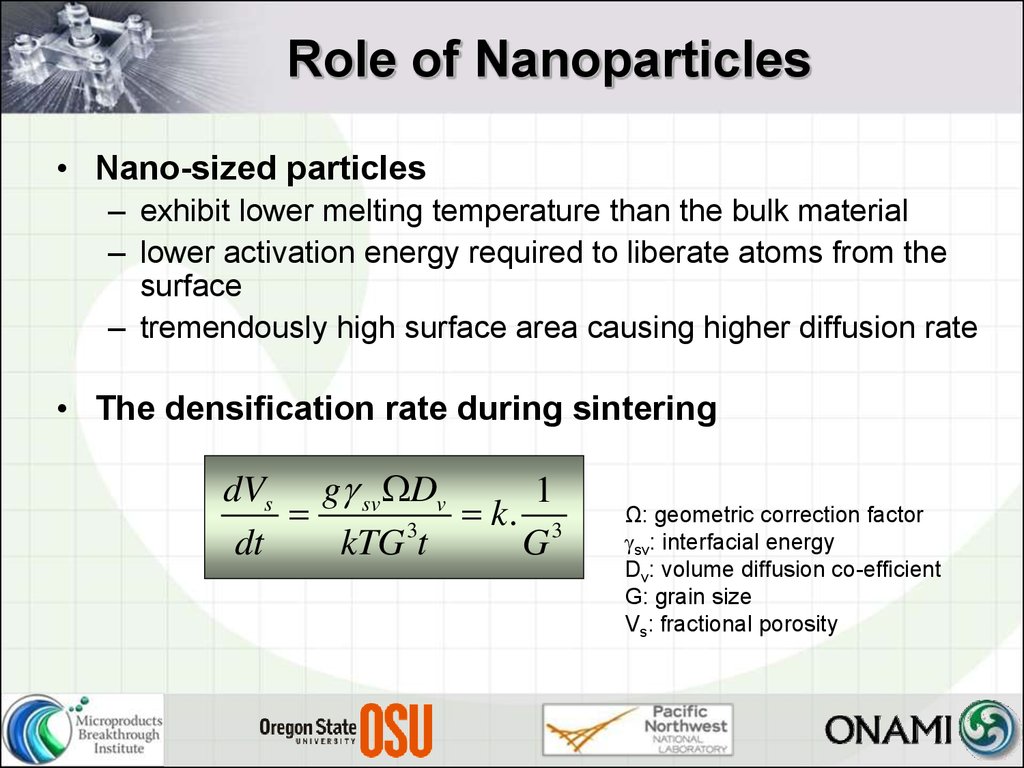
• The densification rate during sintering
dVs g sv Dv
1
k. 3
3
dt
kTG t
G
Ω: geometric correction factor
sv: interfacial energy
Dv: volume diffusion co-efficient
G: grain size
Vs: fractional porosity
15. Outline
Motivation and Objective
Approach
Results
Summary
16. Objective and Protocol
Objectives• to compare NiNP-brazed samples with diffusion bonded and NiP
diffusion brazed samples
• to investigate the microstructural evolution and bond strength of
the stainless steel shims bonded using a Ni NP interlayer
Sample Preparation
• Materials
– Stainless steel 316L shims of 1.0 mm thickness (1”x1”)
– Suspension: Nicrobraz binder mixed with Ni nanoparticles
• Processing
–
–
–
–
Laser machining and deburring
Coating of NiNPs: ~5 µm thick
Drying: 200°C for 30 min
Diffusion bonding
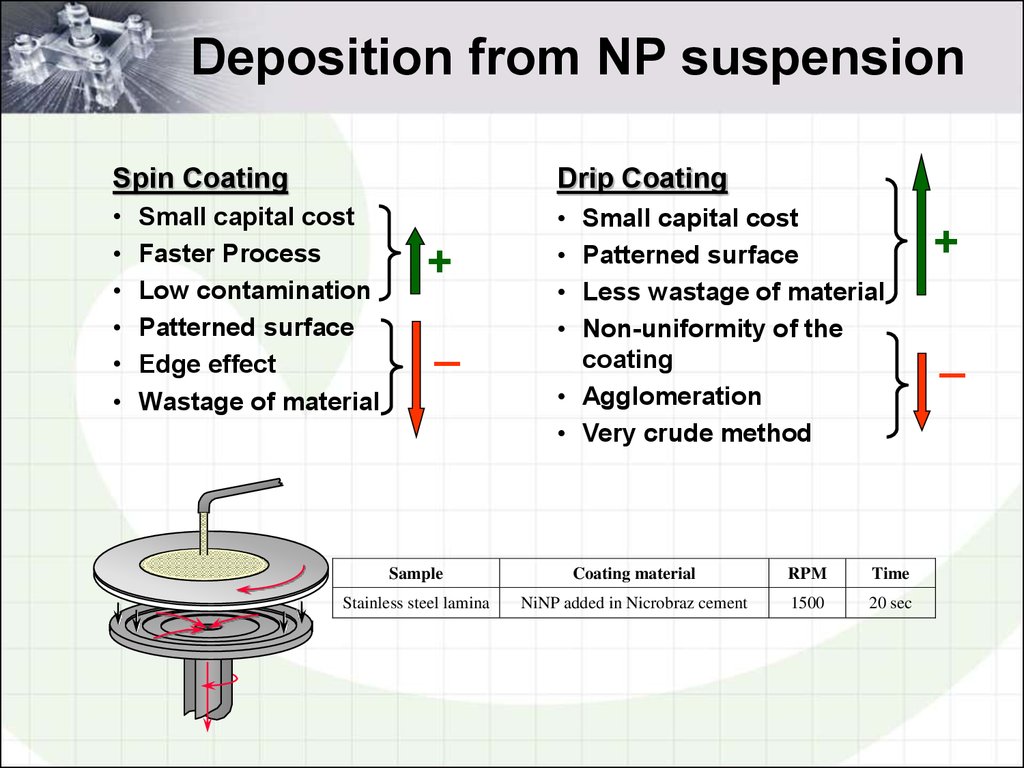
17. Deposition from NP suspension
Spin CoatingDrip Coating
Small capital cost
Faster Process
Low contamination
Patterned surface
Edge effect
Wastage of material
+
_
Small capital cost
Patterned surface
Less wastage of material
Non-uniformity of the
coating
• Agglomeration
• Very crude method
Sample
Coating material
RPM
Time
Stainless steel lamina
NiNP added in Nicrobraz cement
1500
20 sec
+
_
18. Nicrobraz Binder
• A commercially available water based binder (WallColmonoy Corporation)
–
–
–
–
Low viscosity: better for deposition
Readily wets the surface of clean metal substrates
Excellent adherence and a relatively short drying time
Low content of binder material to minimize outgassing
during the bonding cycle
– All binding material volatilizes by 540°C leaving behind
the compact layer of particles
– No residue remains on the parts after brazing, when
using nickel-based filler metals
• Ideally suited for application of nickel-based
brazing filler metals
19. Film Characterization
ab
200 µm
SEM images of the (a) coated and (b) dried (200°C, 30 min) nickel
nanoparticles film on SS substrate
(a) Continuous and uniform film
(b) Nanoparticle film (50 to 100 nm dia.) implying that
high diffusion rate still achievable at relatively
lower temperatures
20. Experimental Design
Independent variablesDependent variables
Void fraction
Warpage
Bonding temperature
Bonding pressure
Bonding time
Surface condition
Bonding environment
sample
temperature (°C)
Pressure (psi)
Time (min)
SS
1000
1000
120
SS-NiP
1000
1000
120
SS – NiNP interlayer
750
1000
60
SS – NiNP interlayer
750
1000
120
SS – NiNP interlayer
800
1000
60
SS – NiNP interlayer
800
1000
120
SS – NiNP interlayer
900
1000
60
SS – NiNP interlayer
900
1000
120
SS – NiNP interlayer
1000
1000
60
SS – NiNP interlayer
1000
1000
120
21. Outline
Motivation and Objective
Approach
Results
Summary
22. Bonded and Brazed Samples
ab
c
20 µm
10 µm
20 µm
(a) diffusion bonded SS at 1000°C, 2 hrs (b) NiP diffusion brazed at 1000°C, 2 hrs and (c)
NiNP diffusion brazed SS at 1000°C, 2 hrs
Surface etched with “Aqua-Regia” (3HCl + HNO3)
Evidence of phase change!
23. Experimental Design
MaterialsNicrobraz cement with NiNP
Diffusion cycles at different tempratures
1000
Solution Preparation
30 min ultrasonic stirring
30 min electromagnetic stirring
900
800
Spin Coating
1500 rpm, 20 sec
Diffusion Bonding
700°C - 900°C, 1000psi,
60 - 120 min
temperature, °C
700
600
500
400
300
200
700°C, 60 min
800°C, 60 min
900°C, 60 min
100
700°C, 120 min
800°C, 120 min
900°C, 120 min
0
Characterization
SEM
Process flow chart for bonding of
SS with NiNP interlayer
0
50
100
150
tim e, m in
200
250
300
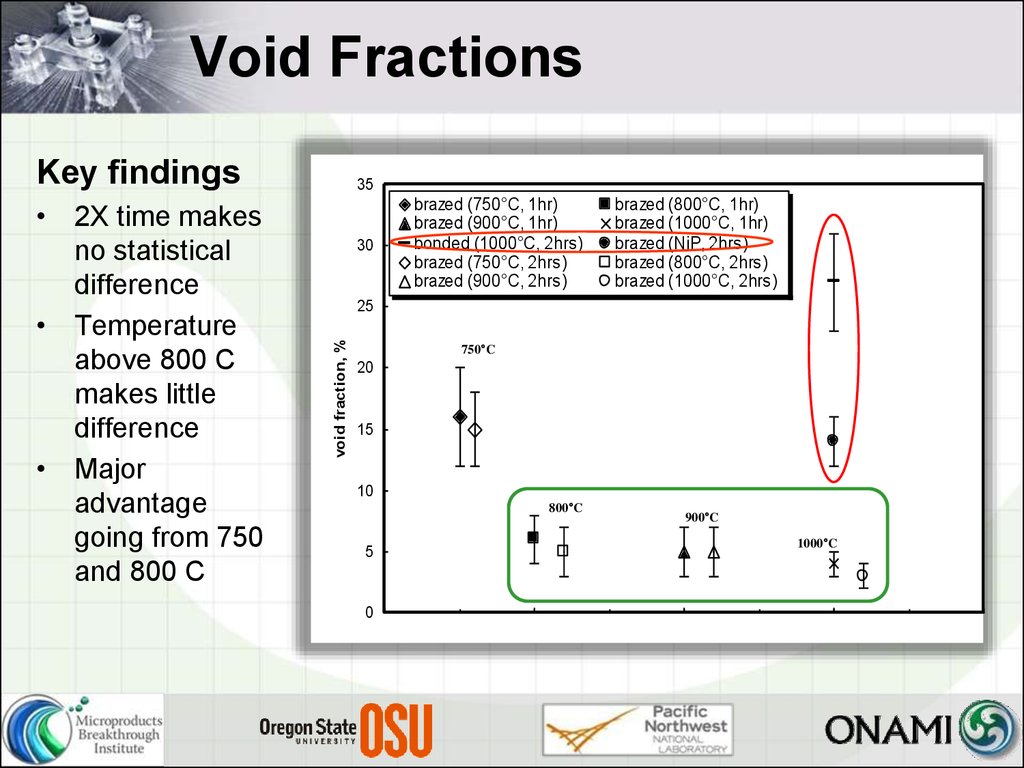
24. Void Fractions
Key findings30
brazed (750°C, 1hr)
brazed (900°C, 1hr)
bonded (1000°C, 2hrs)
brazed (750°C, 2hrs)
brazed (900°C, 2hrs)
brazed (800°C, 1hr)
brazed (1000°C, 1hr)
brazed (NiP, 2hrs)
brazed (800°C, 2hrs)
brazed (1000°C, 2hrs)
25
void fraction, %
• 2X time makes
no statistical
difference
• Temperature
above 800 C
makes little
difference
• Major
advantage
going from 750
and 800 C
35
750°C
20
15
10
800°C
5
0
900°C
1000°C
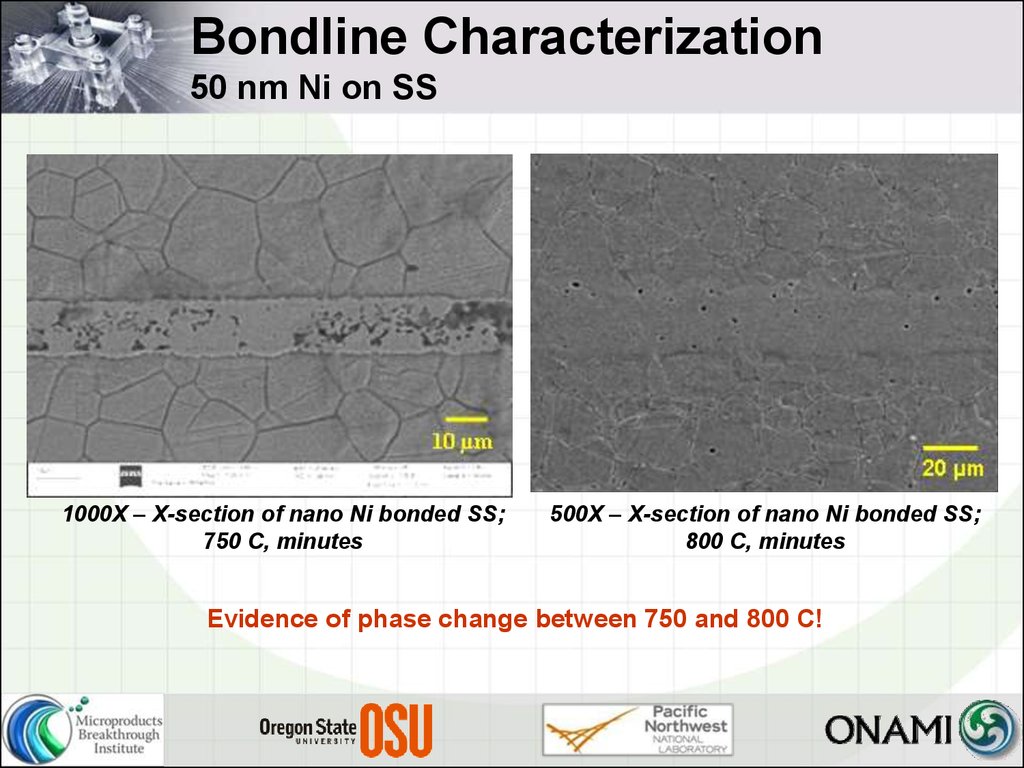
25. Bondline Characterization 50 nm Ni on SS
1000X – X-section of nano Ni bonded SS;750 C, minutes
500X – X-section of nano Ni bonded SS;
800 C, minutes
Evidence of phase change between 750 and 800 C!
26. Summary
• A 50 nm+ dia. nickel nanoparticle (NiNP)interlayer has been shown to:
– lower the bonding temperature for diffusion brazing
– eliminate the use of melting temperature depressants
• NiNP-brazing yielded
– low void fractions
– no deleterious secondary phases
– expected require less time at lower temperature than
conventional diffusion techniques
• 50 nm+ dia. NiNPs appear to have gone through
phase change between 750 and 800 C
• Currently evaluating shear strength of joints
27. Acknowledgments
This research is sponsored by theNational Science Foundation CTS.














 Английский язык
Английский язык Промышленность
Промышленность







