Похожие презентации:
Antihistamine drugs
1.
Antihistamine drugsPrepared by: Gorelenkova Ekaterina and Golovin Alexey (151 group)
2. Histamine
Antihistamines - drugs that competitively block effects of histamine on corresponding receptor regions.The discovery and synthesis of histamine was a great achievement in harmacology, medicine, and
immunology.
Histamine is widely distributed in practically all tissues of mammals and is involved in various
physiological processes. The body’s reaction to histamine is characterized by contraction of smooth muscle,
signs of inflammation, constriction of vessels, and symptoms characteristic of shock. It is certain that
histamine plays a central role in allergic reactions, hypersensitivity reactions, and is part of the body’s
response mechanism in the inflammatory process.
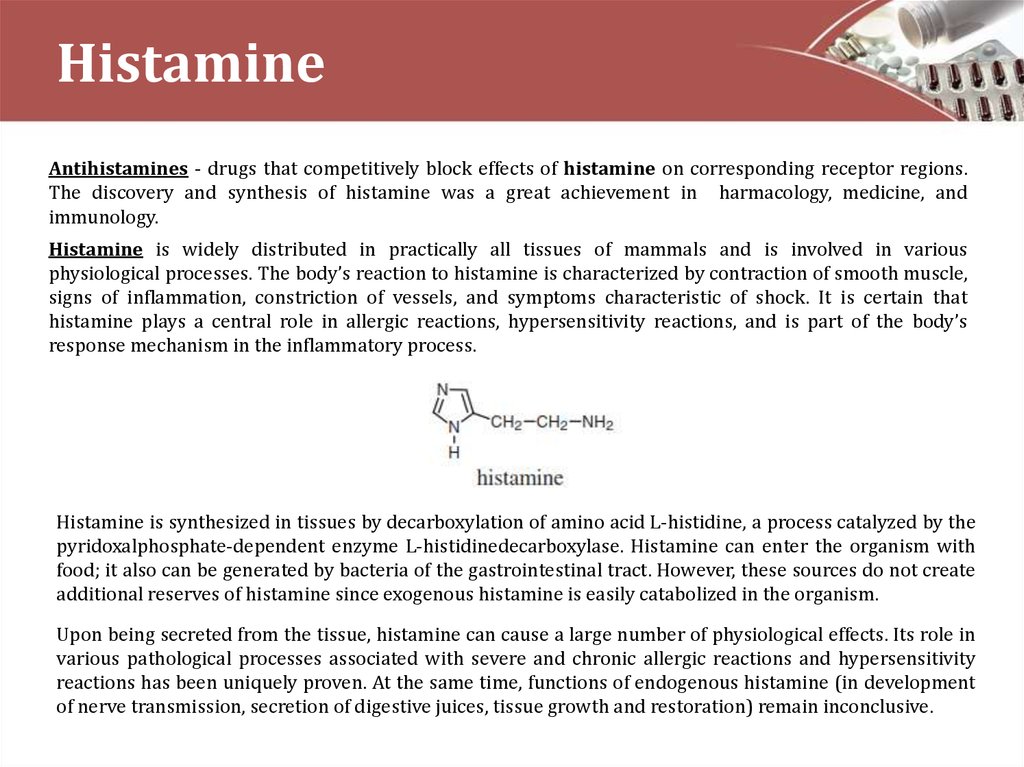
Histamine is synthesized in tissues by decarboxylation of amino acid L-histidine, a process catalyzed by the
pyridoxalphosphate-dependent enzyme L-histidinedecarboxylase. Histamine can enter the organism with
food; it also can be generated by bacteria of the gastrointestinal tract. However, these sources do not create
additional reserves of histamine since exogenous histamine is easily catabolized in the organism.
Upon being secreted from the tissue, histamine can cause a large number of physiological effects. Its role in
various pathological processes associated with severe and chronic allergic reactions and hypersensitivity
reactions has been uniquely proven. At the same time, functions of endogenous histamine (in development
of nerve transmission, secretion of digestive juices, tissue growth and restoration) remain inconclusive.
3. Histamine (2)
Despite the fact that a number of various factors can cause the release of endogenous histamine, it isbelieved that the most important reason is an immunological response of the organism.
Histamine also can be released from tissue stores in response to physical stimuli, effects of the so-called
histamine liberators, a number of chemical substances, various drugs, and toxins. There is a large class of
compounds that are capable of releasing histamine. They can be enzymes, toxins, morphine, d-tubocurarine,
and polymers such as dextran. Moreover, tissue damage such as trauma, bites, and stress can also cause a
release of histamine, and in all probability as a result, an endogenous polypeptide bradykinin is released.
Action of all of these listed substances as well as a number of others can facilitate formation of anaphylactic
reactions in the organism.
The main physiological effect of histamine is exhibited in the cardiovascular system, nonvascular smooth
musculature, and exocrine and adrenal glands. Its most important pharmacological effects are dilation of
veins and capillaries, increased permeability of capillaries, increased heart rate, contraction of nonvascular
smooth musculature (constriction of bronchi, gastrointestinal tract peristalsis), stimulation of gastric juice
secretion, and release of catecholamines from adrenal glands.
Two membrane-receptive binding sites called H1 and H2 receptors mediate the pharmacological effect of
histamine. H1 receptors are located in smooth muscle of vessels, and bronchial and gastrointestinal tract,
while H2 receptors are found in the walls of the stomach, myocardium, and certain vessels. Therefore, it is
very likely that contraction of nonvascular smooth muscle is an effect of activation of H1 receptors, while
secretion of digestive juice and increased heart rate are connected to activation of H2 receptors; and
dilation of vessels and increased permeability of capillaries is a result of combined activation of both types
of receptors.
4. Antihistamines. Classification
Release of histamine is blocked by various enzyme inhibitors and other substances.Antihistamine drugs are classified as antagonists of H1 and H2 receptors.
Quantitatively speaking H1 antagonists dominate. Moreover, the term antihistamine drug is associated
more with H1 antagonists. H2 blockers exhibit a specific effect on histamine receptive sites located in walls
of the stomach and they significantly increase secretion of hydrochloric acid.
Allergic illnesses are a complex collection of disturbances with chronic and severe effects ranging from
slight reddening, rashes, and runny nose to severe and even fatal anaphylaxis. It has been shown that
around 10% of the population may be prone to some form of allergy. Therapy directed toward removing the
source of allergen is not always successful. In a number of cases, the allergen itself is never found.
Therefore, symptomatic treatment using H1 antihistamines is carried out.
H1 antihistamines are clinically used in the treatment of histamine-mediated allergic conditions.
Specifically, these indications may include allergic rhinitis, allergic conjunctivitis, allergic dermatological
conditions (contact dermatitis), pruritus (atopic dermatitis, insect bites), anaphylactic or anaphylactoid
reactions – adjunct only nausea and vomiting, as well as sedation (first-generation H1 antihistamines).
Antihistamines can be administered topically (through the skin, nose, or eyes) or systemically, based on the
nature of the allergic condition.
5. Antihistamines. Classification (2)
First-generation H1 antihistamines are the oldest antihistaminergic drugs and are relatively inexpensiveand widely available. Representatives of first-generation H1 antihistamines are:
Ethanolamines (diphenhydramine was the prototypical agent in this group). Ethylenediamines, which
were the first group of clinically effective H1 antihistamines developed (pyrilamine).
Alkylamines
–
pheniramine,
chlorphenamine,
chlorpheniramine,
dexchlorphenamine,
brompheniramine.
Piperazines – compounds are structurally related to the ethylenediamines and to the ethanolamines:
hydroxyzine, meclizine.
Tricyclics – compounds which differ from the phenothiazine antipsychotics in the ring-substitution
and chain characteristics—promethazine, trimeprazine, cyproheptadine, azatadine.
Second-generation H1-receptor antagonists are newer drugs that are much more selective for peripheral H1
receptors in preference to the central nervous system (CNS) histaminergic and cholinergic receptors. This
selectivity significantly reduces the occurrence of adverse drug reactions compared with first-generation
agents, while still providing effective relief improved of allergic conditions The samples of secondgeneration H1-receptor antagonists are astemizole, fexofenadine, loratadine, mizolastine, terfenadine.
H2-receptor antagonists are drugs used to block the action of histamine on parietal cells in the stomach,
decreasing acid production by these cells. These drugs are used in the treatment of dyspepsia; however,
their use has waned since the advent of the more effective proton pump inhibitors. H2 antagonists are
clinically used in the treatment of acid-related gastrointestinal conditions. Specifically, these indications
may include peptic ulcer disease, gastroesophageal reflux disease, and dyspepsia.
The samples of H2-receptor antagonists are cimetidine, ranitidine, famotidine, nizatidine.
6. H1 antihistamine drugs
Antihistamine drugs were discovered at the end of the 1930s. By 1950, highly effectivehistamine antagonists tripelennamine and diphenhydramine were synthesized, which
triggered broad research in the area of synthesis of such drugs.
All of these compounds are reversible, competitive histamine H1 antagonists that do not
exhibit substantial activity with respect to H2 receptors. H1-receptor antagonists block
effects of histamine in different degrees in various organs or systems, and can protect the
organism from allergic and anaphylactic reactions. Despite the fact that there are minute
differences in relative activity of these drugs, they have comparable pharmacodynamic
properties and therapeutic use when viewed as a single group of drugs.
The most common H1 antihistamine drugs are structurally similar to histamine with a
substituted ethylamine side chain; however, they have two aromatic rings and can be formally represented by the general formula:
where Ar1 and Ar2 are carbocyclic or heterocyclic aromatic rings, one or both of which
can be separated from the X atom of carbon, and where X is oxygen, carbon, or nitrogen.
R1 and R2 represent alkyl substituents, usually methyl groups.
7. H1 antihistamine drugs (2)
H1 histamine receptor blockers can be grouped according to their chemical structures:ethanolamine derivatives (diphenhydramine, clemastine);
ethylenediamine derivatives (tripelennamine, pyrilamine);
alkylamines (chloropheniramine, dexchlorpheniramine, brompheniramine);
piperazines (cyclizine, meclizine, hydroxizine);
phenothiazines (promethazine, trimeprazine);
piperidines (cyproheptadine, diphenylpyraline);
others that do not belong to a specific chemical classification (terfenadine,
astemizole).
Their clinical efficacy and side effects differ significantly from group to group and from
patient to patient. These drugs prevent action of both endogenic and exogenic histamine.
They all act by competitively binding with H1 receptors. They are used for relieving
symptoms of allergic diseases (allergic rhinitis and other allergic reactions), for treating
anaphylactic reactions, for temporary relief of insomnia, as an adjuvant therapy for
treating parkinsonism and extrapyramidal disorders caused by antipsychotics, relieving
coughs due to colds, allergies, or other conditions, preventing and controlling nausea and
vomiting, as an adjuvant drug for analgesia of post-operational pain, and for preoperational sedation.
8. H1 antihistamine drugs. Aminoalkyl esters
DiphenhydramineDiphenhydramine, A,A-dimethyl-(diphenylmethoxy)ethylamine, is synthesized by a simple reaction of
benzhydrylbromide and 2-dimethylaminoethanol.
Diphenhydramine is one of the main representatives of antihistamine drugs that block H receptors. Besides
antihistamine activity, diphenhydramine exhibits a local anesthetic effect, relaxes smooth muscle, and has
sedative and soporific action.
Diphenhydramine is used for symptoms of allergies, for treating hives, hay fever, serum sickness, and other
allergic illnesses, and also as a sedative and soporific drug as an independent as well as in combination with
other drugs. Synonyms of this drug are dimedrol, benadryl, allergina, valdren, and many others.
9. H1 antihistamine drugs. Aminoalkyl esters (2)
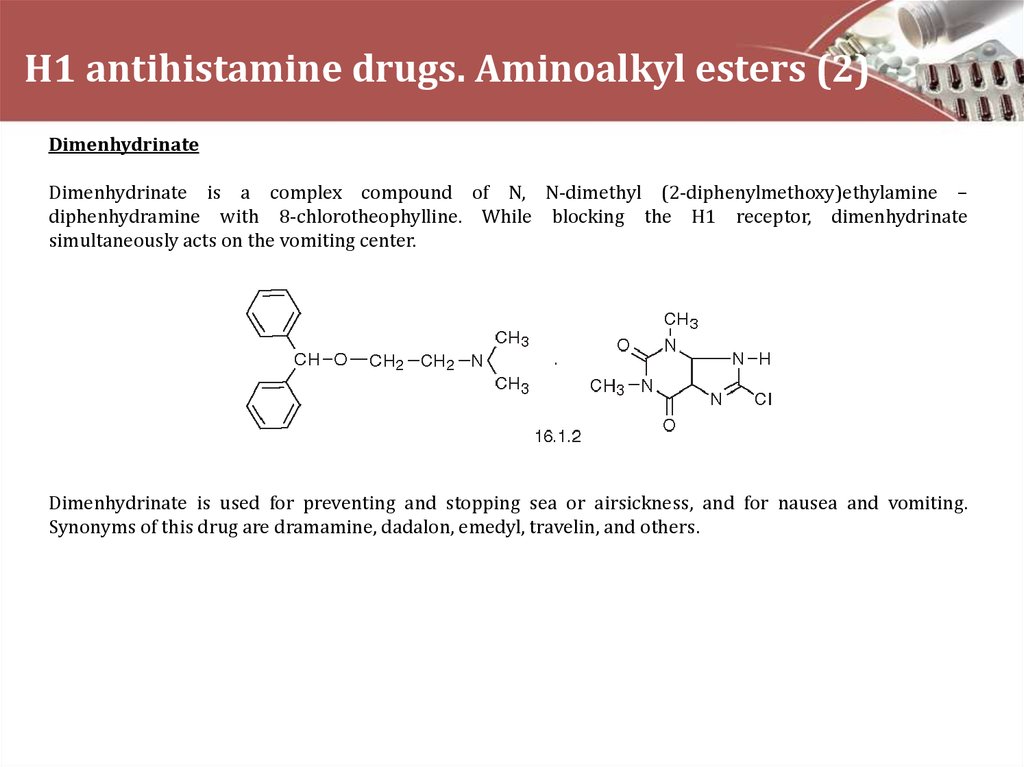
DimenhydrinateDimenhydrinate is a complex compound of N, N-dimethyl (2-diphenylmethoxy)ethylamine –
diphenhydramine with 8-chlorotheophylline. While blocking the H1 receptor, dimenhydrinate
simultaneously acts on the vomiting center.
Dimenhydrinate is used for preventing and stopping sea or airsickness, and for nausea and vomiting.
Synonyms of this drug are dramamine, dadalon, emedyl, travelin, and others.
10. H1 antihistamine drugs. Aminoalkyl esters (3)
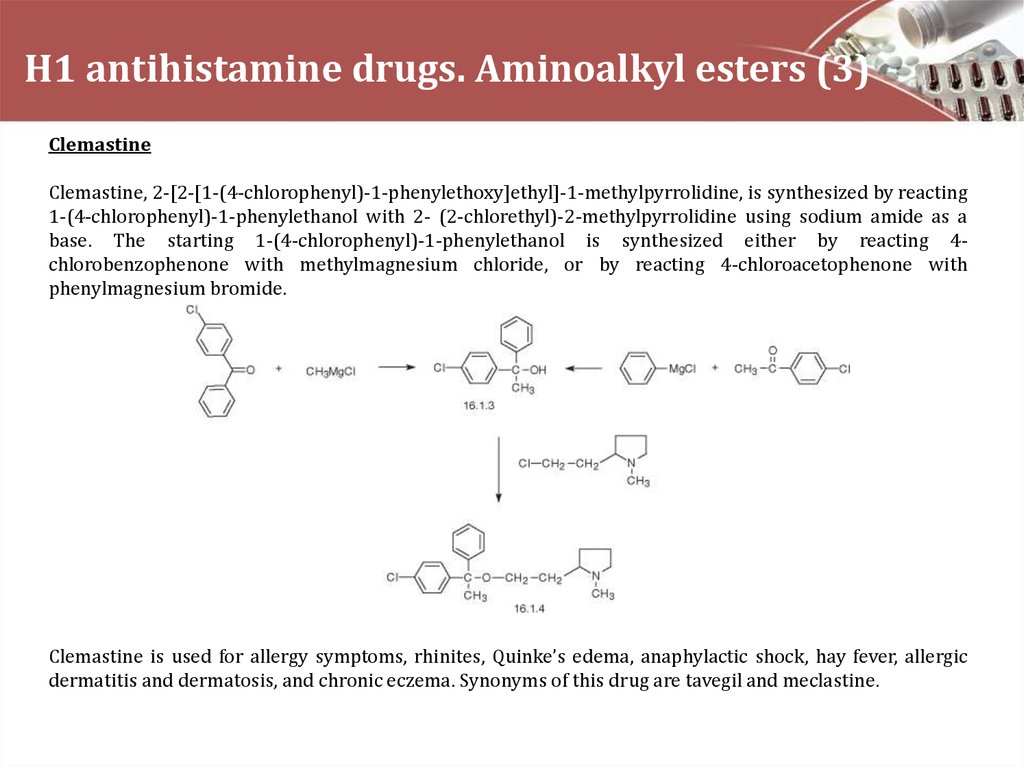
ClemastineClemastine, 2-[2-[1-(4-chlorophenyl)-1-phenylethoxy]ethyl]-1-methylpyrrolidine, is synthesized by reacting
1-(4-chlorophenyl)-1-phenylethanol with 2- (2-chlorethyl)-2-methylpyrrolidine using sodium amide as a
base. The starting 1-(4-chlorophenyl)-1-phenylethanol is synthesized either by reacting 4chlorobenzophenone with methylmagnesium chloride, or by reacting 4-chloroacetophenone with
phenylmagnesium bromide.
Clemastine is used for allergy symptoms, rhinites, Quinke’s edema, anaphylactic shock, hay fever, allergic
dermatitis and dermatosis, and chronic eczema. Synonyms of this drug are tavegil and meclastine.
11. H1 antihistamine drugs. Ethylenediamines
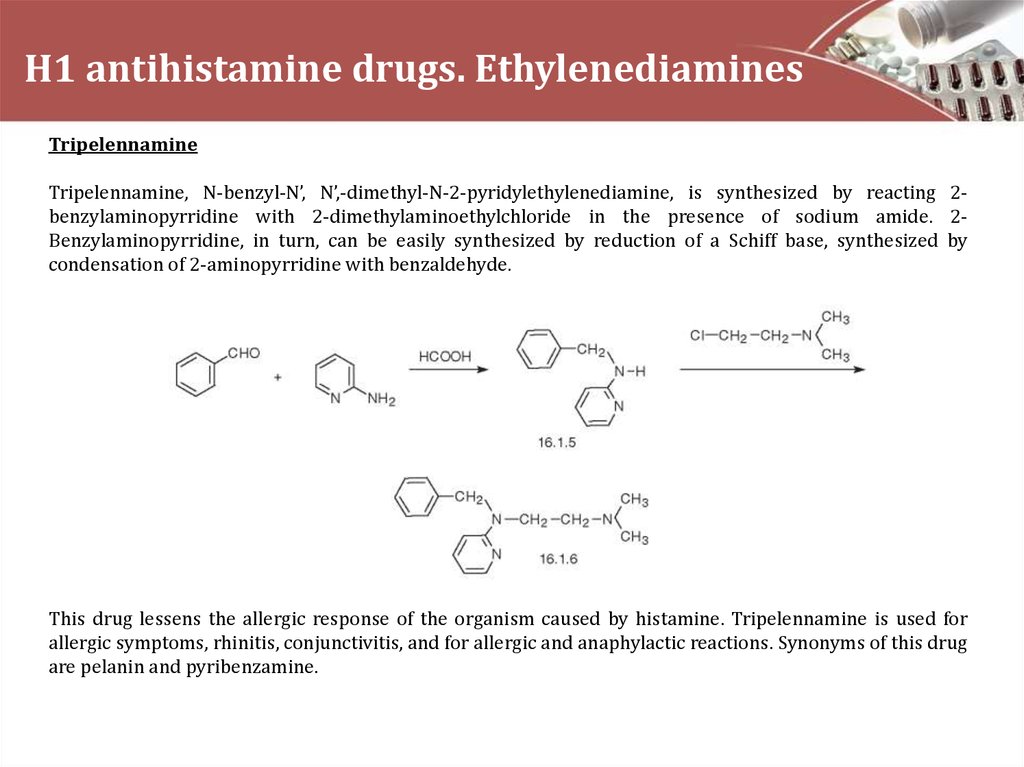
TripelennamineTripelennamine, N-benzyl-N’, N’,-dimethyl-N-2-pyridylethylenediamine, is synthesized by reacting 2benzylaminopyrridine with 2-dimethylaminoethylchloride in the presence of sodium amide. 2Benzylaminopyrridine, in turn, can be easily synthesized by reduction of a Schiff base, synthesized by
condensation of 2-aminopyrridine with benzaldehyde.
This drug lessens the allergic response of the organism caused by histamine. Tripelennamine is used for
allergic symptoms, rhinitis, conjunctivitis, and for allergic and anaphylactic reactions. Synonyms of this drug
are pelanin and pyribenzamine.
12. H1 antihistamine drugs. Ethylenediamines (2)
PyrilaminePyrilamine, N-(4-methoxybenzyl)-N',N'-dimethyl-N-2-pyridilethylenediamine, is synthesized in the same
manner, except using 2-(4-methoxybenzy- lamino)pyridine.
Pyrilamine is also used for allergy symptoms and rhinitis. Synonyms of this drug are vistosan, anthisan, and
triaminic.
13. H1 antihistamine drugs. Ethylenediamines (3)
ChloropyramineChloropyramine, N-(4-chlorobenzyl)-N',N'-dimethyl-N-2-pyridylethylenediamine, is synthesized in a
different manner, which is by reacting 2-brompyridine with N-(4-chlorobenzyl)-N',N'dimethylethylenediamine. N-(4-Chlorobenzyl)-N',N'-dimethylethylenediamine, in turn, is synthesized by
condensation of 4-chlorobenzaldehyde c N,N-dimethylethylenediamine with subsequent reduction of the
imine group.
Chloropyramine is used for allergic dermatosis, allergic rhinitis and conjunctivitis, for drug-induced
allergies, in the beginning stages of bronchial asthma, eczema, neurodermatitis, contact dermatitis, and
toxicodermia. It also exhibits a sedative effect. Synonyms of this drug are suprastin, chlortripelenamine, and
synopen.
14. H1 antihistamine drugs. Alkylamines
ChlorpheniramineChlorpheniramine, 3-(p-chlorophenyl)-3-(2-pyridyl)propyldimethylamine, is synthesized in two ways. The
first is from 4-chlorbenzylcyanide, which is reacted with 2-chlorpyridine in the presence of sodium amide to
form 4-chlorphenyl (2-pyridyl)acetonitrile. Alkylating this with 2-dimethylaminoethylchloride in the
presence of sodium amide gives -(4-chlorphenyl)- -cyano-N,N-dimethyl-2-pyridine-propanamine, the
hydrolysis and decarboxylation of which lead to chlorpheniramine.
The second way is from pyridine, which undergoes alkylation by 4-chlorobenzylchloride, giving 2-(4chlorobenzyl)pyridine. Alkylating this with 2-dimethylaminoethylchloride in the presence of sodium amide
gives chlorpheniramine.
Chlorpheniramine reduces the allergic response of the organism caused by histamine. It is used for allergy
symptoms, rhinitis, and also as an ingredient in numerous compositions with ephedrine and
pseudoephedrine, which are recommended for colds, upper respiratory tract infections, and allergic
rhinitis. Synonyms of this drug are chlortrimeton, histaspan, tripolon, and teldrin.
15. H1 antihistamine drugs. Alkylamines (2)
DexchlorpheniramineDexchlorpheniramine, d(+)-3-(p-chlorophenyl)-3-(2-pyridyl) propyldimethylamine, is synthesized by
separating the racemate obtained from the synthesis of chlorpheniramine using D-phenylsuccinic acid.
Activity of this drug is approximately twice that of chlorpheniramine. Dexchlorpheniramine is also used for
allergy symptoms, rhinitis, and dermatitis. A synonym of this drug is polaramin.
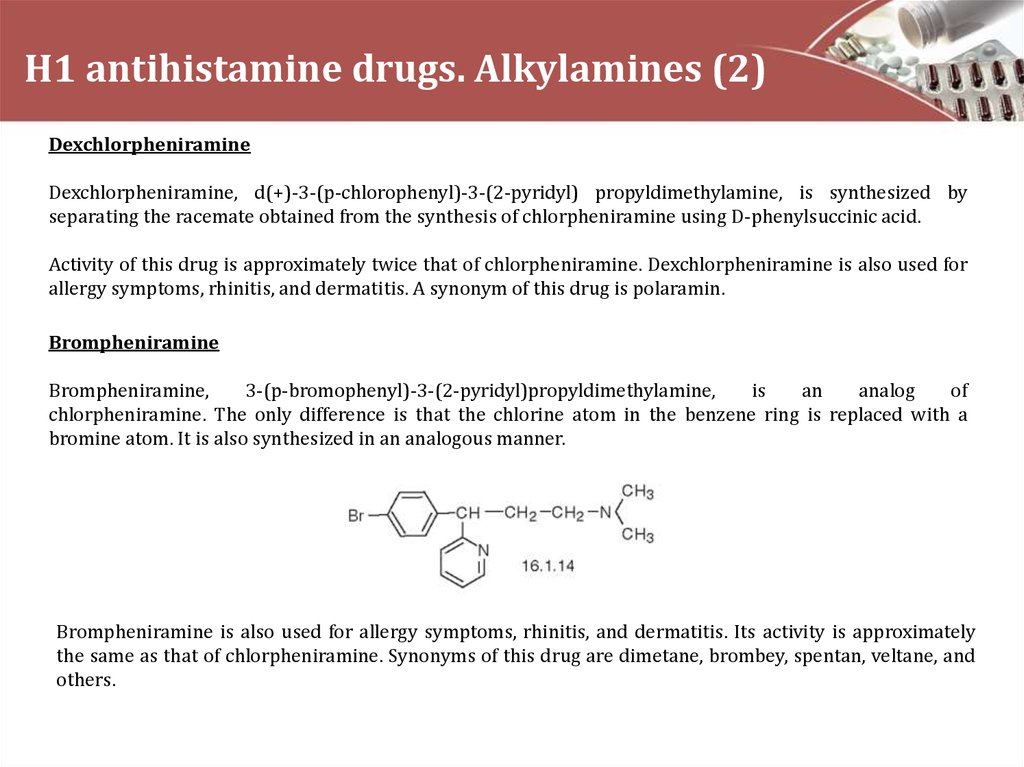
Brompheniramine
Brompheniramine,
3-(p-bromophenyl)-3-(2-pyridyl)propyldimethylamine,
is
an
analog
of
chlorpheniramine. The only difference is that the chlorine atom in the benzene ring is replaced with a
bromine atom. It is also synthesized in an analogous manner.
Brompheniramine is also used for allergy symptoms, rhinitis, and dermatitis. Its activity is approximately
the same as that of chlorpheniramine. Synonyms of this drug are dimetane, brombey, spentan, veltane, and
others.
16. H1 antihistamine drugs. Piperazines
CyclizineCyclizine, 1-(diphenylmethyl)-4-methylpiperazine, is synthesized by alkylating 1-methylpiperazine with
benzhydrylbromide .
Cyclizine exhibits antihistamine and anticholinergic action and is used for vomiting and diarrhea. The exact
mechanism of action is not known. Synonyms of this drug are marezine and migril.
Meclizine
Meclizine, 1-[(4-chlorphenyl)methyl]-4-[(3-methylphenyl)phenyl]piperazine, is synthesized by reductive
amination of a mixture of 3-methylbenzaldehyde with 1-(4-chlorbenzhydryl)piperazine using hydrogen
over Raney nickel.
Meclizine actively affects the vomiting center and is used for vomiting and diarrhea. Synonyms of this drug
are antivert, bonine, lamin, roclizin, and vertol.
17. H1 antihistamine drugs. Piperazines (2)
HydroxyzineHydroxyzine, 2-[2-[4-[(4-chlorophenyl)phenylmethyl]-1-piperazinyl]ethoxy]ethanol, is synthesized by
alkylating 1-(4-chlorbenzhydryl)piperazine with 2-(2-hydroxyethoxy)ethylchloride .
Hydroxyzine is an antihistamine drug with M-cholinoblocking properties and expressed action on the CNS.
It suppresses subcortical regions of the CNS including the limbic system and reticular formation. It
potentiates the effect of narcotic analgesics and exhibits sedative effects. It is used as a sympthomatic drug
for atopic dermatitis as a sedative drug before and after operational interventions, for preventing vomiting
and diarrhea, and for relieving agitation and emotional disorders. Synonyms of this drug are atarax, durrax,
and vistaril.
18. H1 antihistamine drugs. Phenothiazines
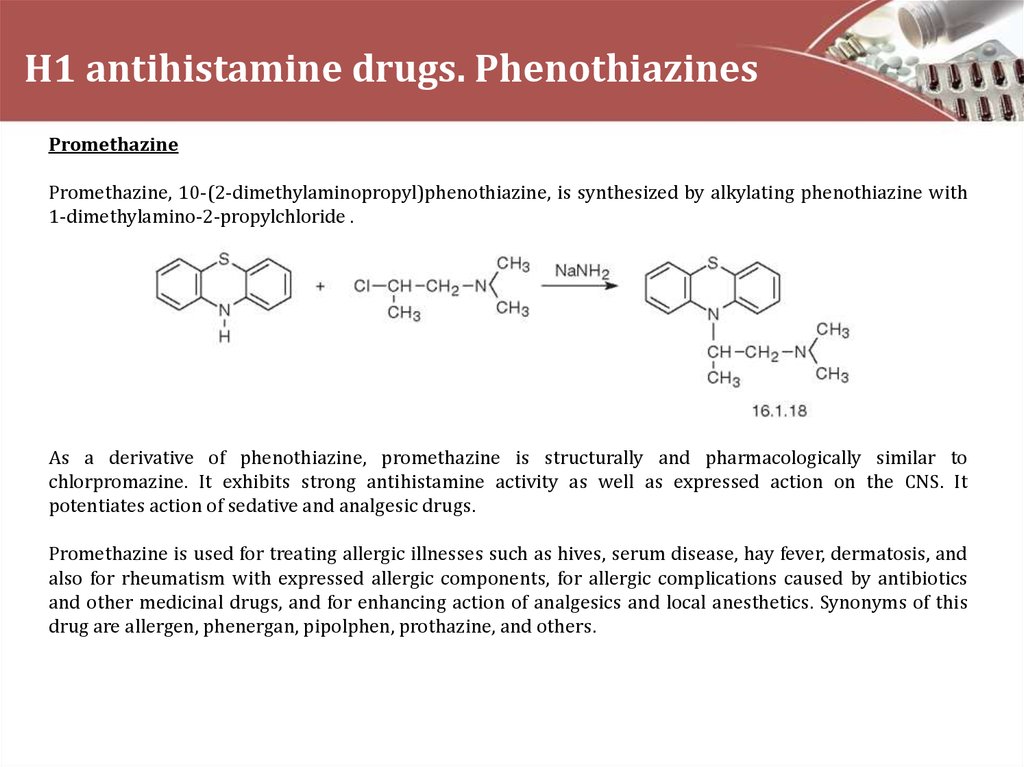
PromethazinePromethazine, 10-(2-dimethylaminopropyl)phenothiazine, is synthesized by alkylating phenothiazine with
1-dimethylamino-2-propylchloride .
As a derivative of phenothiazine, promethazine is structurally and pharmacologically similar to
chlorpromazine. It exhibits strong antihistamine activity as well as expressed action on the CNS. It
potentiates action of sedative and analgesic drugs.
Promethazine is used for treating allergic illnesses such as hives, serum disease, hay fever, dermatosis, and
also for rheumatism with expressed allergic components, for allergic complications caused by antibiotics
and other medicinal drugs, and for enhancing action of analgesics and local anesthetics. Synonyms of this
drug are allergen, phenergan, pipolphen, prothazine, and others.
19. H1 antihistamine drugs. Phenothiazines (2)
TrimeprazineTrimeprazine, 10-(3-dimethylamino-2-methylpropyl) phenothiazine,
phenothiazine with 1-dimethylamino-2-metyl- propylchloride [38].
is
synthesized
by
alkylating
Trimeprazine is used for treating itching during dermatitis of both allergic and nonallergic origin. A
synonym of this drug is temaril.
20. H1 antihistamine drugs. Piperidines
CyproheptadineCyproheptadine, 4-(dibenzo[a,d]cyclohepten-5-ylidene)-1-methylpiperidine, is synthesized by reacting 1methyl-4-magnesiumchloropiperidine with 5H-dibeno[a,d]cycloheptene-5-one, which forms carbinol, the
dehydration of which in an acidic medium leads to the formation of cyproheptadine.
Cyproheptadine has antianaphylactic activity that is associated with its ability to slow down the release of
histamine and other mediators from fat cells. It is mainly used for treating bronchial asthma attacks, allergic
bronchitis, rhinitis, and allergic skin reactions as well as in adjuvant therapy for anaphylactic reactions.
Synonyms of this drug are periactin and vimicon.
21. H1 antihistamine drugs. Piperidines (2)
TerfenadineTerfenadine, a-(4-tert-butylphenyl)-4-hydroxydiphenylmethyl)-1-piperidinebutanol, is synthesized in two
ways. According to the first, benzyl-4-magnesiumchloropiperidine is reacted with benzophenone, giving (1benzyl-4-piperidyl) diphenylcarbinol, which undergoes further debenzylation by reduction with hydrogen
using a palladium over carbon catalyst, giving (4-piperidyl)diphenylcarbinol. This product is alkylated by
either 1-(4-tert-butylphenyl)-4-chlorobutanol, which forms terfenadine, or by alkylating with (4-tertbutylphenyl-3-chloropropiophenone, which forms the product, the carbonyl group of which is reduced to an
alcohol group, thus giving the desired terfenadine.
22. H1 antihistamine drugs. Piperidines (3)
Terfenadine not only differs from the other antihistamine drugs in its chemical structure, but also in that itsaction begins within 1-2 h and last approximately 12 h, reaching its peak of action in 3-4 h. It is used for
relieving symptoms associated with seasonal allergic rhinitis and conjunctivitis, for angioneurotic edema
and allergic skin reaction, and also as an ingredient of complex therapy for bronchial asthma. Synonyms of
this drug are seldane, hystadin, trexil, and others.
Diphenylpyraline
Diphenylpyraline, 4-diphenylmethoxy-1-methylpiperidine, is synthesized by alkylating 4-hydroxy-1-methyl
piperidine with benzhydrylbromide.
Diphenylpyraline is an antihistamine drug with anticholinergic and sedative action.
It is intended for symptomatic treatment of seasonal allergies and allergic reactions as well as an adjuvant
drug in anaphylactic reaction therapy. Synonyms of this drug are arbid, timpil, histryl, hispril, and others.
23. H2 antihistamine drugs
H2-receptor antagonists almost completely block secretion of hydrochloric acid in the stomach in responseto most stimuli. These drugs play a major role in treating stomach ulcers associated with hypersecretion,
because they have the ability to reduce both the volume of stomach secretion and overall acidity as well as
pepsin activity.
Drugs of this kind are used for treating stomach and duodenum ulcers and hypersecretive conditions.
Traditional or H1 antihistamine drugs block many effects caused by histamine; however, it turns out that
they are not able to withstand events mediated by H2 receptors, in particular excess gastric juice secretion.
In 1977 an H2-receptor antagonist, cimetidine, was proposed, which revolutionized stomach ulcer
treatment. Later on, ranitidine was proposed, followed by drugs with minor structural and pharmacological
differences such as famotidine and nizatidine.
H2-receptor antagonists reversibly and competitively inhibit histamine action on H2 receptors. They are
pure antagonists since they do not affect H1 receptors, -adrenoreceptors, or muscarinic receptors.
Moreover, they do not have a significant effect on the synthesis, release, and biotransformation of histamine.
The structure of cimitidine is synthesized up of a methylimidazol ring with a sulfur containing side chain
with a cyanoguanidine group. It seemed that the presence of an imidazol ring in cimetidine contained in the
structure of histamine should be the determining factor in the exhibition of H2 blocking activity; however,
the formation of ranitidine, famotidine, and nizatidine, which contain furane and thiazol rings in place of an
imidazole ring showed the incorrectness of this suggestion.
24. H2 antihistamine drugs (2)
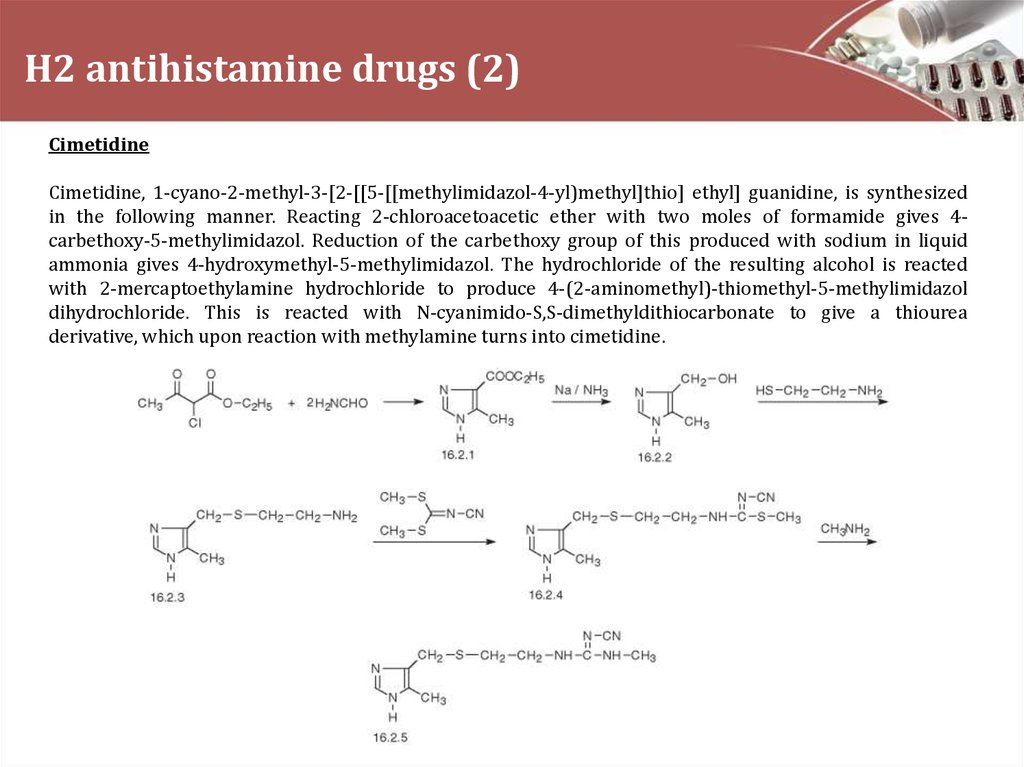
CimetidineCimetidine, 1-cyano-2-methyl-3-[2-[[5-[[methylimidazol-4-yl)methyl]thio] ethyl] guanidine, is synthesized
in the following manner. Reacting 2-chloroacetoacetic ether with two moles of formamide gives 4carbethoxy-5-methylimidazol. Reduction of the carbethoxy group of this produced with sodium in liquid
ammonia gives 4-hydroxymethyl-5-methylimidazol. The hydrochloride of the resulting alcohol is reacted
with 2-mercaptoethylamine hydrochloride to produce 4-(2-aminomethyl)-thiomethyl-5-methylimidazol
dihydrochloride. This is reacted with N-cyanimido-S,S-dimethyldithiocarbonate to give a thiourea
derivative, which upon reaction with methylamine turns into cimetidine.
25. H2 antihistamine drugs (3)
Cimetidine is a representative of first-generation antihistamine drugs that block H2 receptors. The mainpharmacological effect of cimetidine is the suppression of gastric juice secretion associated with H2
receptors of the stomach walls. It suppresses both basal and stimulated hydrochloric acid produced by food
as well as histamine and gastrine, which simultaneously lower pepsin activity.
Cimetidine is used for treating ulcer problems of the stomach and duodenum and for other conditions
accompanied by an elevation of acidity and excess secretion of gastric juice. It is used for preventing injuries
and the blood flow of the upper regions of the gastrointestinal tract. Synonyms of this drug are tagamet,
cinamet, and belomet.
26. H2 antihistamine drugs (4)
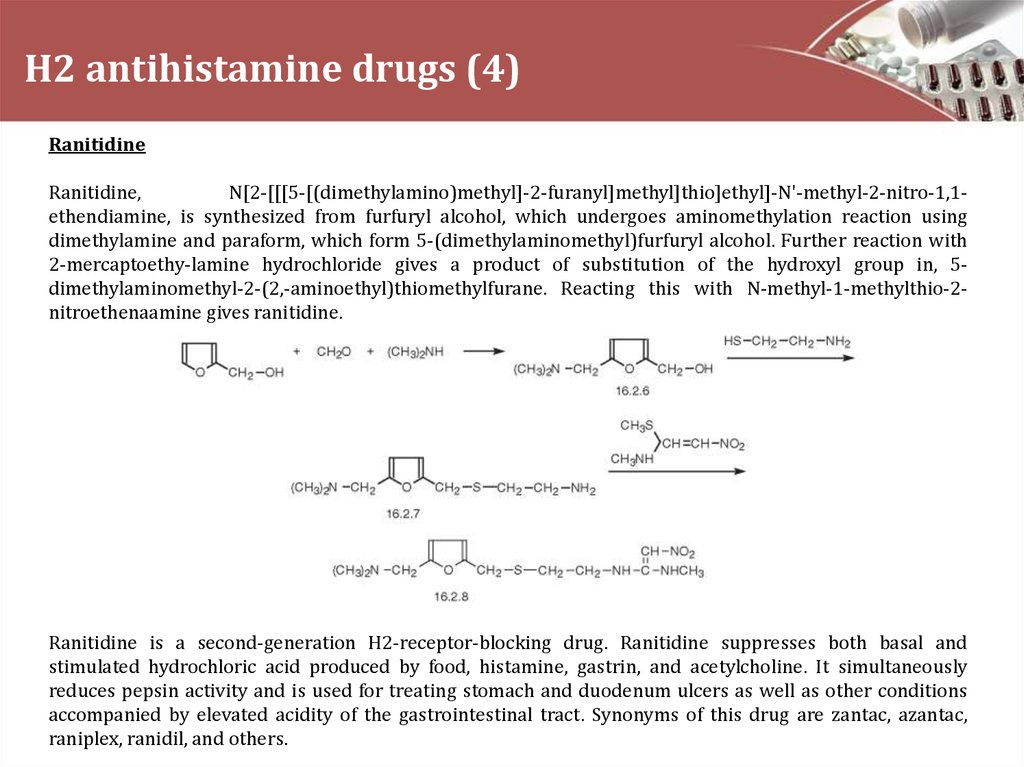
RanitidineRanitidine,
N[2-[[[5-[(dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-N'-methyl-2-nitro-1,1ethendiamine, is synthesized from furfuryl alcohol, which undergoes aminomethylation reaction using
dimethylamine and paraform, which form 5-(dimethylaminomethyl)furfuryl alcohol. Further reaction with
2-mercaptoethy-lamine hydrochloride gives a product of substitution of the hydroxyl group in, 5dimethylaminomethyl-2-(2,-aminoethyl)thiomethylfurane. Reacting this with N-methyl-1-methylthio-2nitroethenaamine gives ranitidine.
Ranitidine is a second-generation H2-receptor-blocking drug. Ranitidine suppresses both basal and
stimulated hydrochloric acid produced by food, histamine, gastrin, and acetylcholine. It simultaneously
reduces pepsin activity and is used for treating stomach and duodenum ulcers as well as other conditions
accompanied by elevated acidity of the gastrointestinal tract. Synonyms of this drug are zantac, azantac,
raniplex, ranidil, and others.
27. H2 antihistamine drugs (5)
FamotidineFamotidine, 3-[[[2-[(aminomethyl)amino]-4-thiazolyl] methyl]thio]-N-(aminosulfonyl)propanimidamide, is
synthesized from S'-(2-aminothiazol-4-yl- methyl)isothiourea, which is synthesized by reacting 1,3dichloroacetone with two molecules of thiourea, during which a thiazol ring is formed and the chlorine
atom is substituted, giving an intermediate 2-amino-5-chlormethylthiazol. Reacting this with 2-chlorpropionitrile gives S'-(2-aminothiazol-4-yl-methyl)-2-cyanoethane, which in turn is reacted with
benzoylizthiocyanate. The resulting benzoylthiourea derivative first undergoes S-methylation by
methyliodide and further cleaved by ammonia into 3-[[[2- (aminomethyl)amino] -4-thiazolyl]-methyl] thio]
ethylcyanide. Successive methanolysis of the nitrile group and subsequent reaction of the resulting
iminoether with sulfonamide gives famotidine.
28. H2 antihistamine drugs (6)
Like ranitidine, famotidine belongs to the group of second-generation H2-receptor-blocking drugs, and isused for treating stomach and duodenum ulcers and other conditions accompanied by elevated acidity of
the gastrointestinal tract. Synonyms of this drug are famodil, gastridin, pepcid, and others.
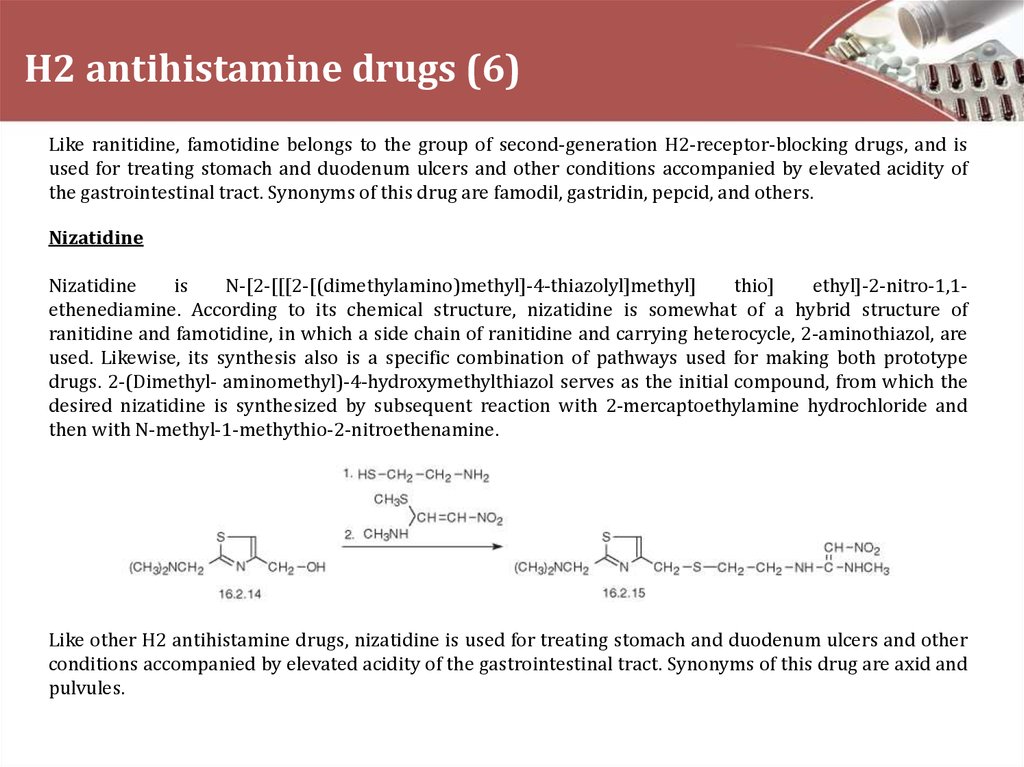
Nizatidine
Nizatidine
is
N-[2-[[[2-[(dimethylamino)methyl]-4-thiazolyl]methyl]
thio]
ethyl]-2-nitro-1,1ethenediamine. According to its chemical structure, nizatidine is somewhat of a hybrid structure of
ranitidine and famotidine, in which a side chain of ranitidine and carrying heterocycle, 2-aminothiazol, are
used. Likewise, its synthesis also is a specific combination of pathways used for making both prototype
drugs. 2-(Dimethyl- aminomethyl)-4-hydroxymethylthiazol serves as the initial compound, from which the
desired nizatidine is synthesized by subsequent reaction with 2-mercaptoethylamine hydrochloride and
then with N-methyl-1-methythio-2-nitroethenamine.
Like other H2 antihistamine drugs, nizatidine is used for treating stomach and duodenum ulcers and other
conditions accompanied by elevated acidity of the gastrointestinal tract. Synonyms of this drug are axid and
pulvules.



















 Медицина
Медицина








