Похожие презентации:
Радиоспектроскопические методы исследования, часть 3
1. Радиоспектроскопические методы исследования 3
2.
HO
H
H3C
H3C
H
N
HH
N H
HH
H
H
H
S
O
H
2
3.
+13
S
O
CH3
12
11
0.38
10
4.05 2.08
9
8
0.81
7
6
Chemical Shift (ppm)
0.97
5
3.757
0.71
9.610
0.72
7.990
7.969
7.825
O
H3 C
10.882
HH
N Me
H
O
1.296
H
3.309
HO
11.929
H
H
H
5.955
5.925
5.276
5.246
O
H
H
H
N
7.804
7.531
7.380
7.283
7.264
HH
NH
3.169
O H
H
+
S
N
O
H
2.778
2.490
DMSO-d6
3.59 3.35
4
3
3
6.00
2
1
0
4.
2.4902.778
DMSO-d6
O H
H
+
S
N
O
H
HH
3.309
O
+
HH
O
H3 C
1.296
3.35
3.5
H
HO
N Me
H
3.169
3.757
3.59
4.0
H
H
H
H
H
3.10
3.0
2.5
Chemical Shift (ppm)
6.00
2.0
1.5
NH
1.0
4
O
O
CH3
H
N
S
5.
1.968.1
8.0
7.9
7.8
4.05
1.97
7.7
7.6
7.5
Chemical Shift (ppm)
HH
2.08
7.4
7 .2 8 3
7 .8 2 5
O
7 .3 0 3
7 .3 8 0
H
H
7 .3 6 1
7 .3 9 9
7 .5 5 2
7 .5 3 1
7 .8 0 4
7 .8 4 3
HH
H
H
H
7.3
7 .2 6 4
7 .2 2 7
7 .2 0 8
7 .1 9 0
7 .1 6 4
7 .1 2 87 .1 4 6
7 .5 8 0
7 .8 6 4
7 .9 9 0
7 .9 6 9
O
O
+
N
H
+
N Me
H
2.03
5
H
S
H
HO
O
H3 C
NH
H
1.01
7.2
O
H
N
S
O
CH3
1.00
7.1
6.
11.92910.882
O
HH
O
H
0.72
12.5
12.0
0.71
11.5
11.0
Chemical Shift (ppm)
+
HH
N Me
H
O
H3 C
0.38
10.5
10.0
H
HO
9.610
H
H
H
H
H
O
+
N
9.5
6
NH
S
H
H
O
O
CH3
H
N
S
7.
O5.955
5.925
HH
O
H
H
H
H
H
H
H
HO
+
HH
N Me
H
NH
S
H
O
H3 C
H
O
H
N
S
O
CH3
0.81
6.2
6.1
6.0
5.276
5.246
Угол Ф в диапазоне
от 169 до 177°
O
+
N
0.97
5.9
5.8
5.7
5.6
5.5
Chemical Shift (ppm)
5.4
5.3
5.2
7
5.1
5.0
8.
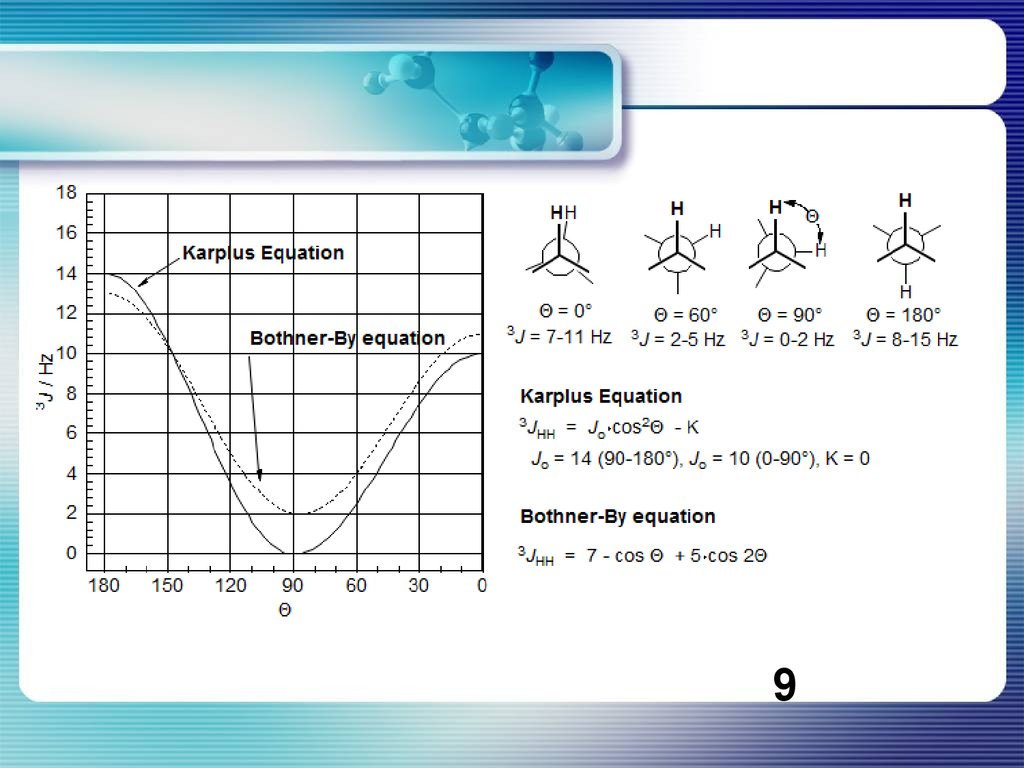
Уравнение Карплуса-КонрояКривая КК чественно хорошо согласуются с расчетами, проведенными для
фрагмента H—С—С—H. Однако эксперимент показывает, что
значения 3Jнн для ф = 0 и 180° в общем на 2-4 Гц больше, чем
рассчитанные. Поэтому для уравнения были предложены эмпирические
постоянные A = 7, B = - 1 и С = 5. Кривая Карплуса — Конроя объясняет
ряд важных закономерностей. Например, в олефиновых системах спинспиновое взаимодействие между граис-протонами всегда больше, чем
между цис-протонами. Поэтому легко различать цис- и транс-изомеры. Для
1,2-дизамещенных этанов справедливо соответствующее соотношение
Jгош < Jтранс.
8
9.
910.
The Karplus Equation for 3JHH (H-Csp3-sp3C-H) is:3
J = 7.8 - 1.0 cos(phi) + 5.6 cos(2*phi)
This basic form of the Karplus equation does not correct for the
electronegativity of the substituents.The Altona equations for
vicinal 3JHH (H-Csp3-sp3C-H) are:
3
J = p1 cos2(f) + p2 cos(f) + p3 + S li (p4 + p5 cos2(ei f + p6 |li|))
where the sum is over the four substituents. The order of substitution
around each carbon makes a difference. The direction coefficient, e i, is
+1 for S1 and S3 and -1 for S2 and S4. The electronegativity of the
substituents includes the "beta effect" and is given by:
li = (Ca -CH) + p7 S ( Cb -CH)
where Ca is the Huggin's electronegativity of the directly
attached a atom, CH is the electronegativity of hydrogen, and the sum
is over the b atoms that are attached to the a atom. The substituent
electronegativity for each attached group is listed under the
substituent name. The coefficients have also been modified to use
10
11.
The Diez, Altona, Donders equation is:3
J = c00 + c01 S li + c10 cos(f) + (c20 + c21 S li) cos(2f) + (s211 S ei l2i)
sin(2f)
The coefficients for the Diez, Altona, Donders equations with chemical
groups are:
c00 = 7.82 , c01 =-0.79 , c10 = -0.78 , c20 = 6.54 , c21 = -0.64 , s211 =
0.70
Please see: L. A. Donders, F. A. A. M. de Leeuw, C. Altona, "Relationship
Between Proton-Proton NMR Coupling Constants and Substituent
Electronegativities IV. An Extended Karplus Equation Accounting for
Interactions Between Substituents and its Application to Coupling
Constant Data Calculated by the Extended Huckel Method," Magn.
Reson. Chem., 1989, 27, 556-563.
11
12.
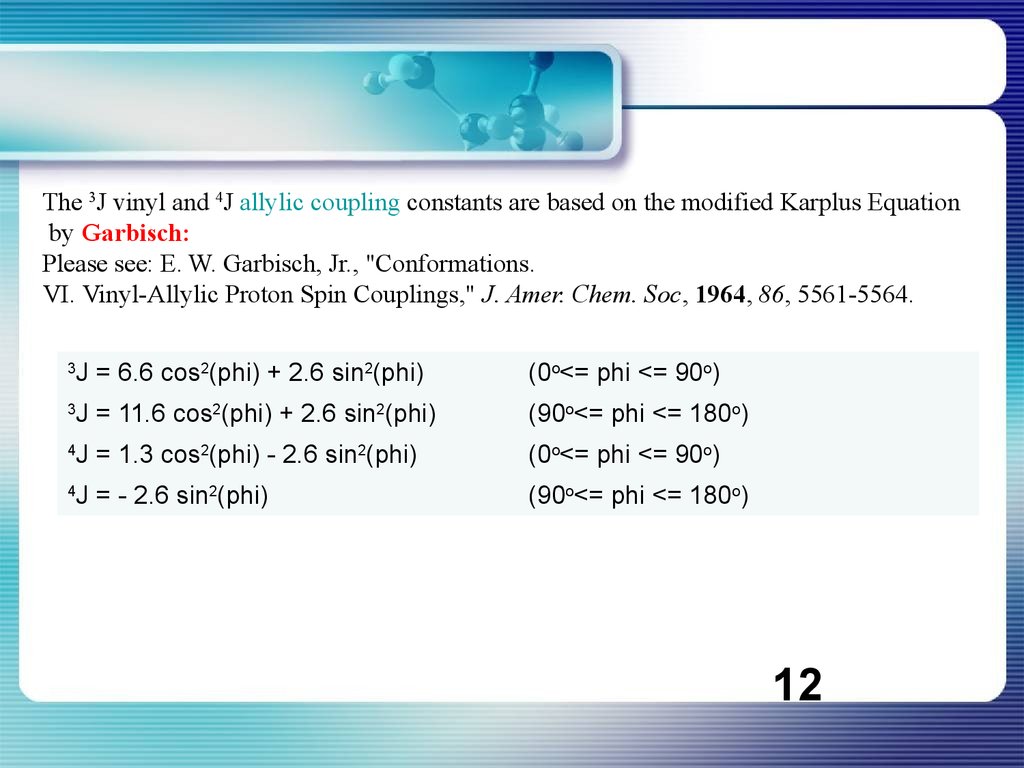
The 3J vinyl and 4J allylic coupling constants are based on the modified Karplus Equationby Garbisch:
Please see: E. W. Garbisch, Jr., "Conformations.
VI. Vinyl-Allylic Proton Spin Couplings," J. Amer. Chem. Soc, 1964, 86, 5561-5564.
3
J = 6.6 cos2(phi) + 2.6 sin2(phi)
(0o<= phi <= 90o)
3
J = 11.6 cos2(phi) + 2.6 sin2(phi)
(90o<= phi <= 180o)
4
J = 1.3 cos2(phi) - 2.6 sin2(phi)
(0o<= phi <= 90o)
4
J = - 2.6 sin2(phi)
(90o<= phi <= 180o)
12
13.
http://www.colby.edu/chemistry/NMR/scripts/altona/altona.html13
14.
1415.
109
H HH
H N
N
H H
2.07
8
2.490
N
2.126.33
1.00
7
6
1.01
5
Chemical Shift (ppm)
1.10
4
3
2.069
3.322
O H
S
3.648 3.332
3.097
2.894
2.525
7.321
H
7.299
7.229
7.003
6.996
6.005
5.984
4.595
4.562
4.345
4.324
4.098
3.835
3.807
8.079
8.064
7.715
7.576
S
DMSO-d6
3.79 1.05
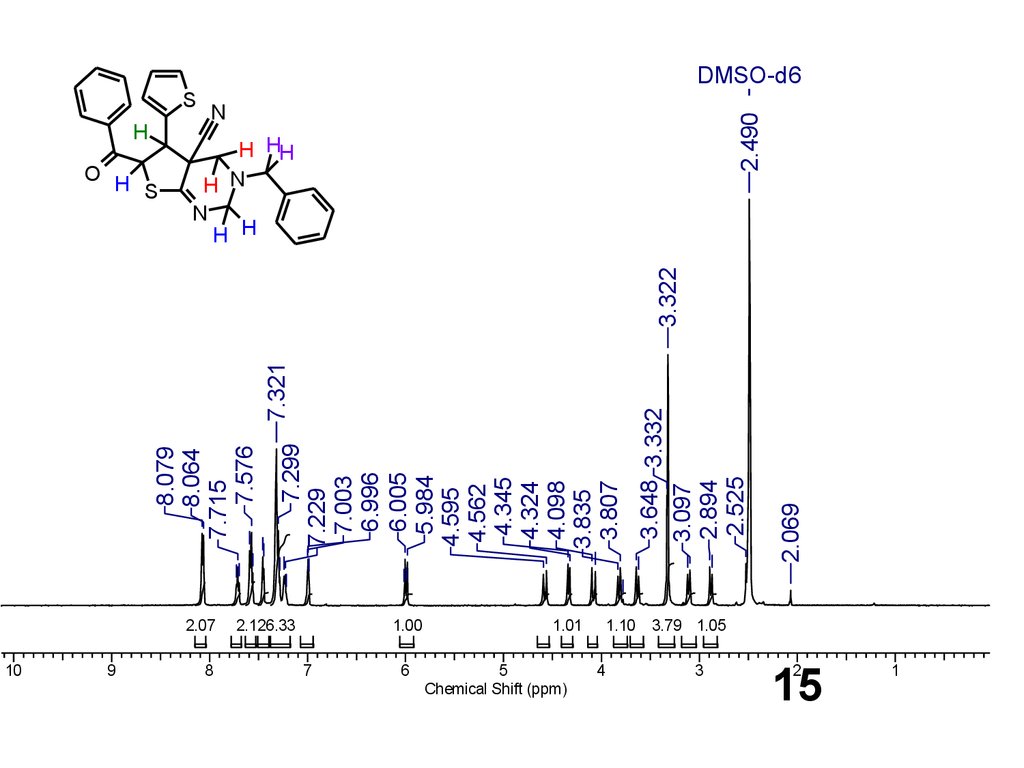
15
2
1
16.
1.001.01
4.5
1.01
1.10
4.0
Chemical Shift (ppm)
3.332
3.322
H HH
H N
N
H H
1.07
3.79
3.5
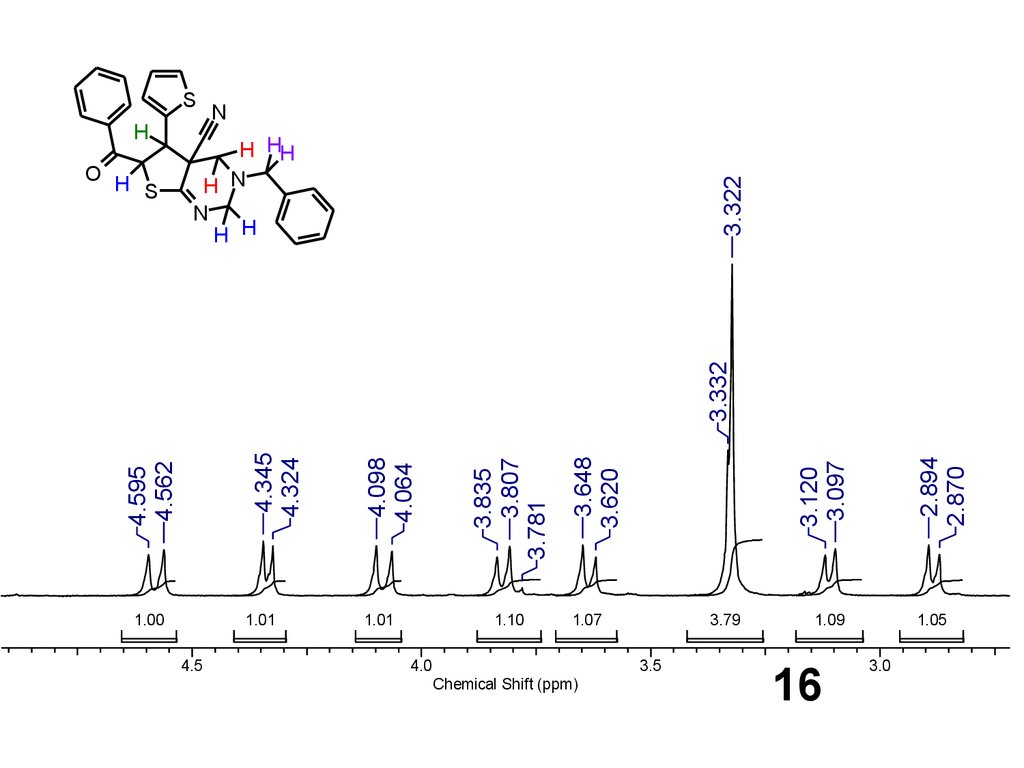
16
2.894
2.870
3.120
3.097
3.648
3.620
3.835
3.807
3.781
O H
S
4.098
4.064
H
4.345
4.324
4.595
4.562
S
N
1.09
1.05
3.0
17.
2.078.5
1.08 2.12 1.17
8.0
6.33
7.5
7.0
Chemical Shift (ppm)
6.005
5.984
O H
S
7.321
H HH
H N
N
H H
6.014
H
7.730
7.715
7.700 7.591
7.576
7.460
7.450
7.299
7.284
7.242
7.229
7.003
6.996
6.986
8.079
8.064
S
N
1.01
6.5
17
1.00
6.0
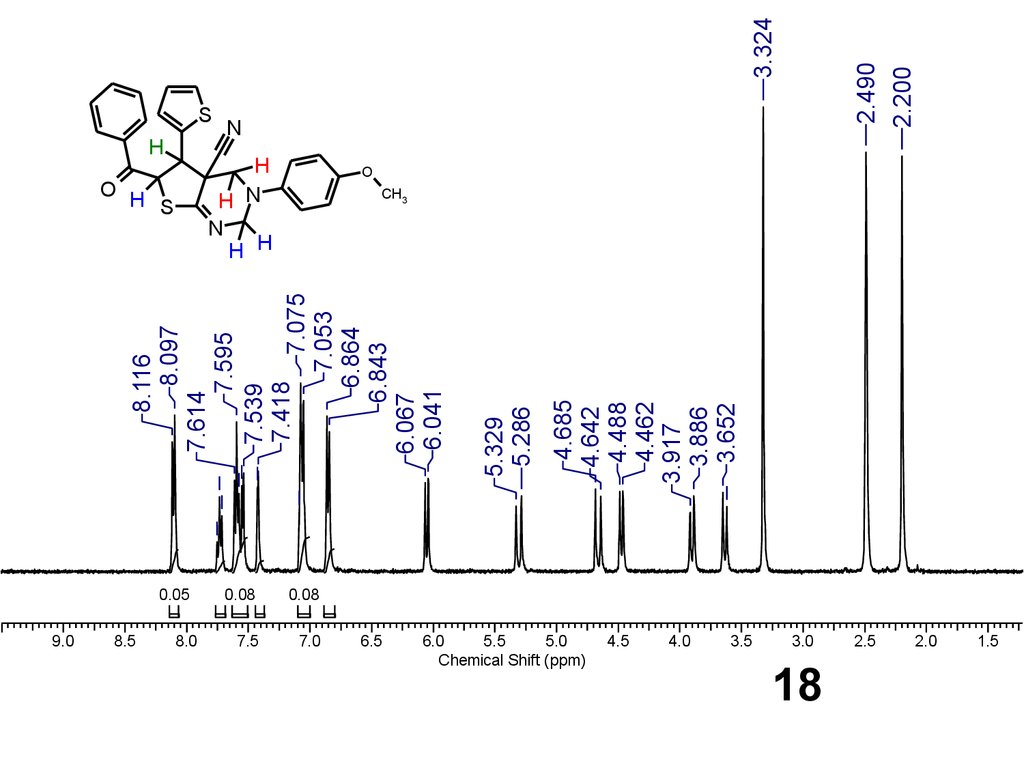
18.
O HS
9.0
8.5
H
H N
N
H H
0.05
8.0
0.08
0.08
7.5
7.0
6.5
4.685
4.642
4.488
4.462
3.917
3.886
3.652
H
5.329
5.286
8.116
8.097
7.614
7.595
7.539
7.418
7.075
7.053
6.864
6.843
6.067
6.041
S
6.0
5.5
5.0
Chemical Shift (ppm)
4.5
4.0
3.5
3.324
2.490
2.200
N
O
CH3
3.0
18
2.5
2.0
1.5
19.
6.05.5
5.0
4.5
4.0
3.621
3.652
3.886
O H
S
H
H N
N
H H
3.917
H
4.685
4.642
4.488
4.462
5.329
5.286
6.067
6.041
S
N
O
CH3
19
3.5
20.
0.058.3
8.2
8.1
0.03
8.0
7.9
7.8
7.7
O
0.08
0.03
7.6
7.5
7.4
Chemical Shift (ppm)
6.864
6.843
CH3
7.088
7.752
7.734
7.715
8.116
8.097
O H
S
H
H N
N
H H
7.614
7.595
7.575
7.553
7.539
7.426
7.418
H
N
7.075
7.053
S
0.08
7.3
7.2
7.1
0.05
7.0
20
6.9
6.8
6.7
21.
OMeH
H
H
H
H
H
O
H
N
N
H
S
+H
N
H
H
21
22.
2223.
2324.
Thanks for yourpatience and attention
24


















 Физика
Физика Химия
Химия








