Похожие презентации:
Solid waste biotreatment. Ethanol production from lignocellulosic materials. Lecture 4
1.
BIOPROCESS TECHNOLOGYDr. TERESA FERNANDEZ ALDAMA
“SAMARA UNIVERSITY”
1
2.
LECTURE No. 4. SOLID WASTEBIOTREATMENT/Ethanol production
from lignocellulosic materials (I)
2
3.
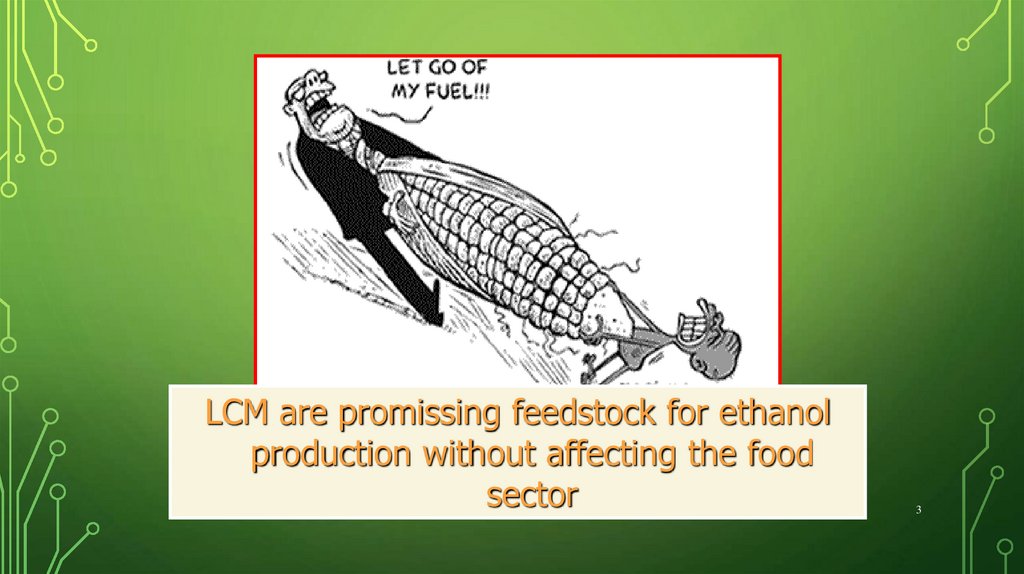
LCM are promissing feedstock for ethanolproduction without affecting the food
sector
3
4.
ObjectiveTo describe components of lignocellulosic
materials, their characteristics and how to
prepare them for ethanol production.
4
5.
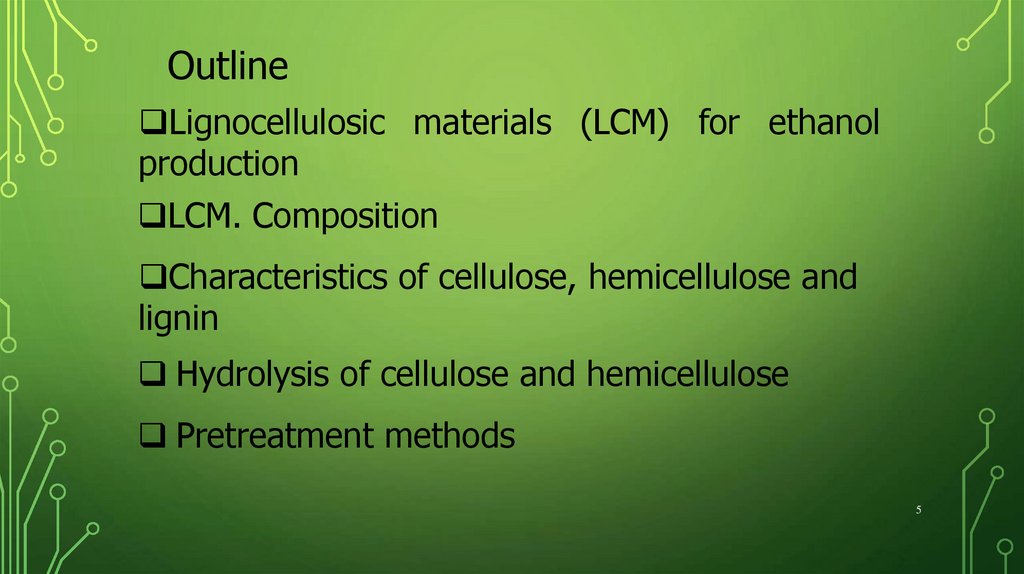
OutlineLignocellulosic materials (LCM) for ethanol
production
LCM. Composition
Characteristics of cellulose, hemicellulose and
lignin
Hydrolysis of cellulose and hemicellulose
Pretreatment methods
5
6.
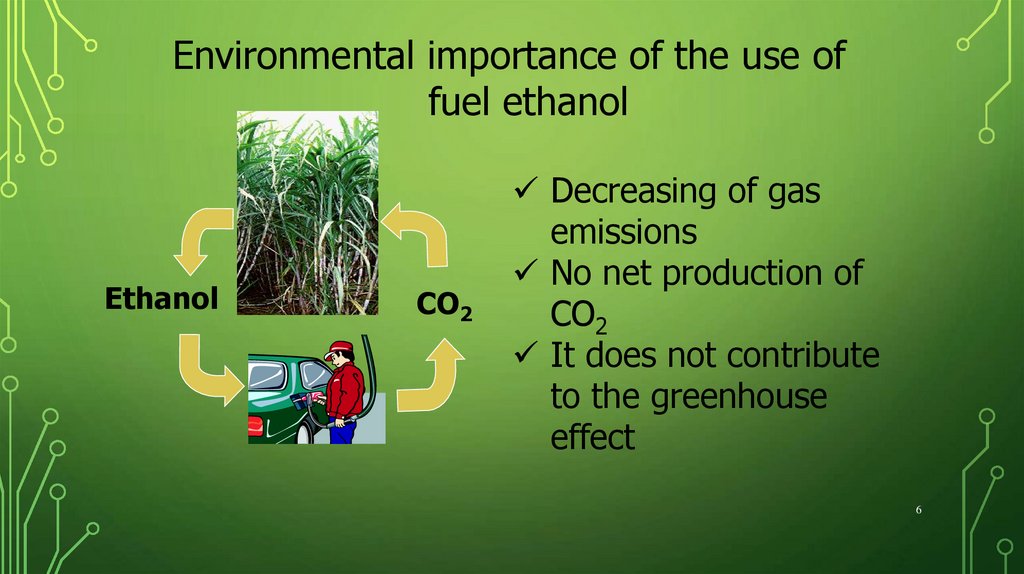
Environmental importance of the use offuel ethanol
Ethanol
CO2
Decreasing of gas
emissions
No net production of
CO2
It does not contribute
to the greenhouse
effect
6
7.
Lignocellulosic materialsForest residues
Agricultural and food industry residues
Municipal solid wastes (Recycled paper)
Energy crops
7
8.
Sugarcane bagasseCassava stems
Rice husks
Peanut shells
8
9.
Alternative for production of ethanolSecond generation
biofuel
9
10.
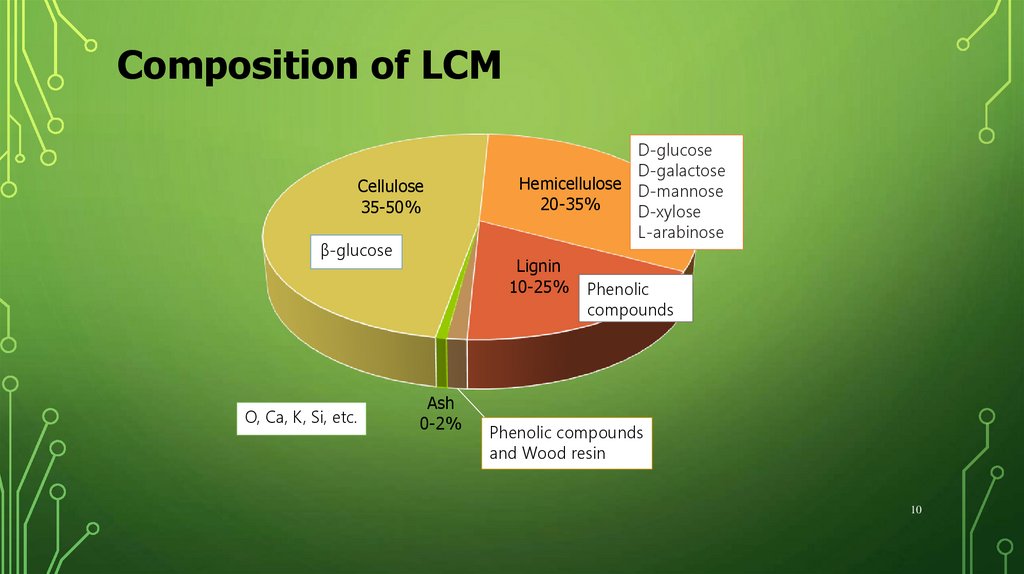
Composition of LCMCellulose
35-50%
β-glucose
O, Ca, K, Si, etc.
D-glucose
D-galactose
Hemicellulose D-mannose
20-35%
D-xylose
L-arabinose
Lignin
10-25%
Ash
0-2%
Phenolic
compounds
Extractives
Phenolic
compounds
1-5%resin
and Wood
10
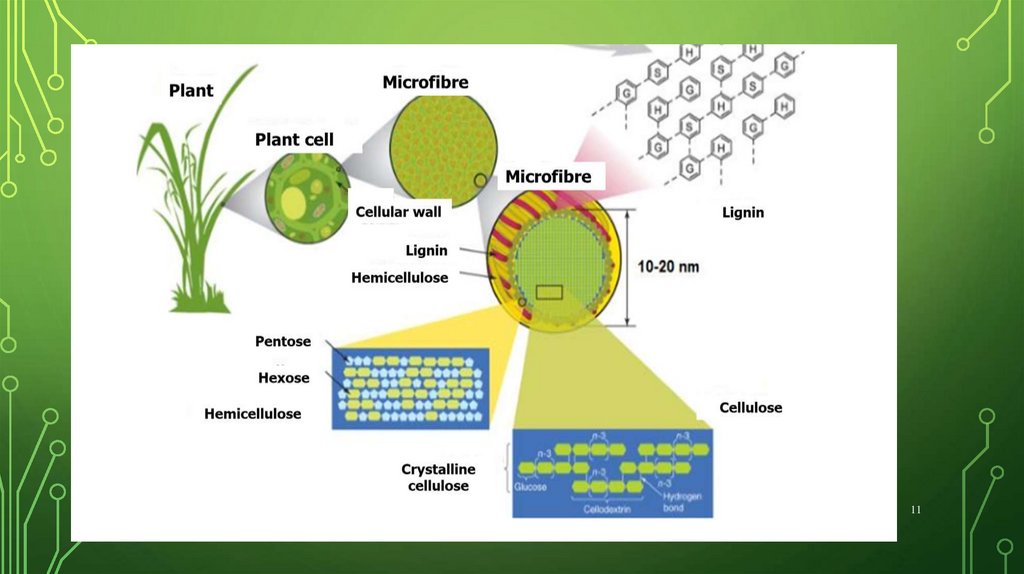
11.
1112.
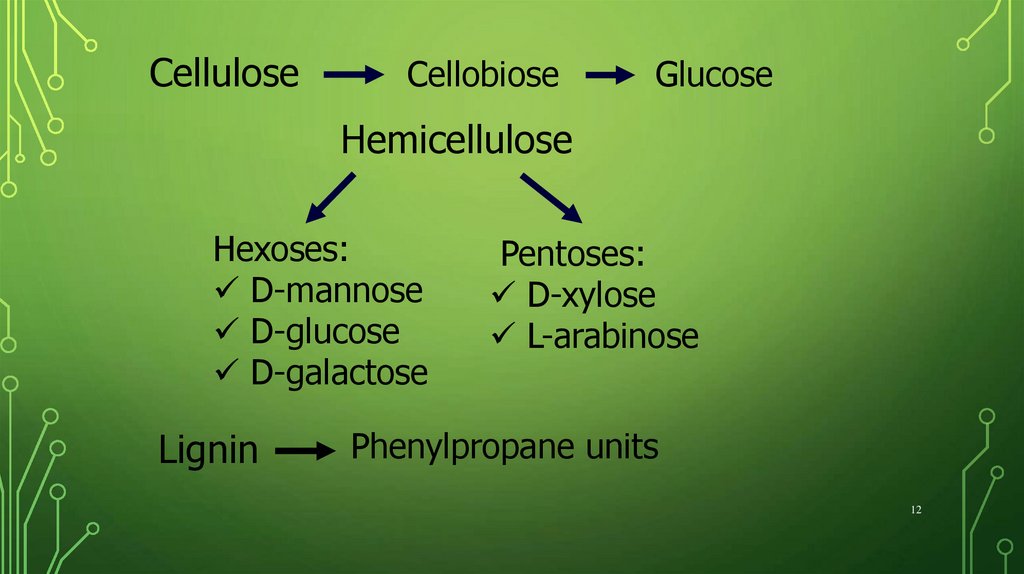
CelluloseCellobiose
Glucose
Hemicellulose
Hexoses:
D-mannose
D-glucose
D-galactose
Lignin
Pentoses:
D-xylose
L-arabinose
Phenylpropane units
12
13.
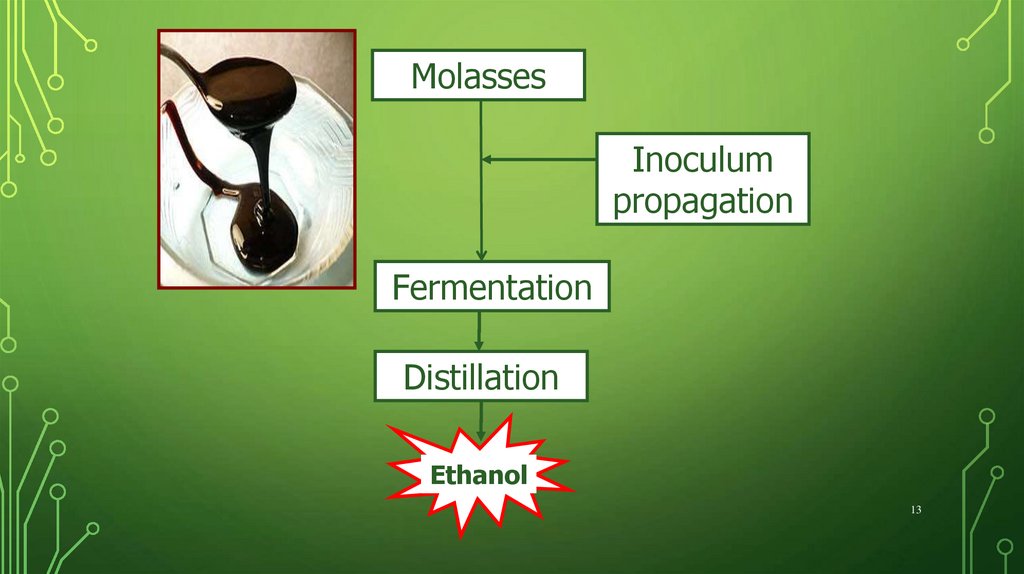
MolassesInoculum
propagation
Fermentation
Distillation
Ethanol
13
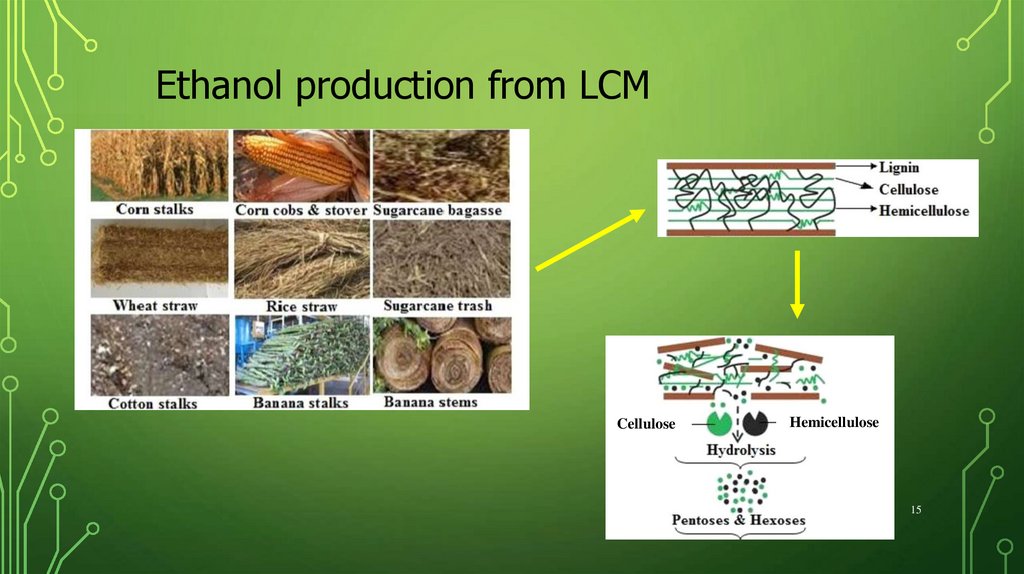
14.
Ethanol production from LCMEthanol
Hydrolysis
14
15.
Ethanol production from LCMCellulose
Hemicellulose
15
16.
Hydrolysis of celluloseAcid hydrolysis
With concentrated acids
With diluted acids
Enzymatic hydrolysis
With cellulases and hemicellulases
16
17.
Acid hydrolysisH2SO4 at high concentration:
☺
☺
☺
High sugar yields
Use of low temperature
Few by-products
☹ Equipment corrosion
☹ Need of expensive drying the raw material
☹ High acid recovery costs
☹ Incrustation (Neutralization)
17
18.
Acid hydrolysisDilute-acid Hydrolysis
☺ Lower requirement of acid (compared with
the previous one)
☹ High temperatures
☹ Sugar degradation
☹ Formation of by-products
☹ Equipment corrosion
18
19.
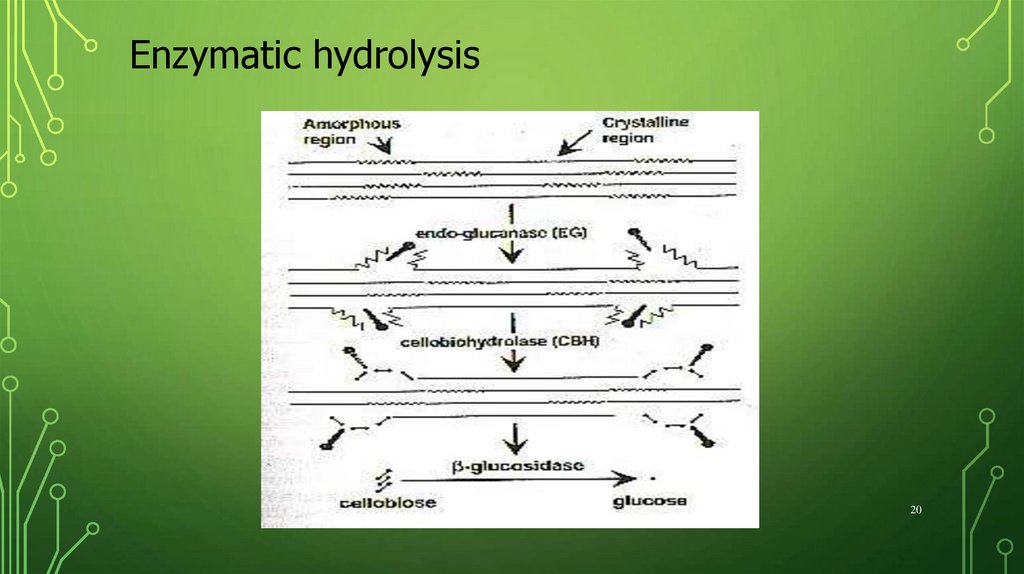
Enzymatic hydrolysisBacterial cellulases (Clostridium and Bacillus)
Fungal cellulases (Trichoderma, Aspergillus,
Penicillium)
Endoglucanase (EC 3.2.1.4)
Cellobiohydrolase (EC 3.2.1.91)
-glicosidase (EC 3.2.1.21)
19
20.
Enzymatic hydrolysis20
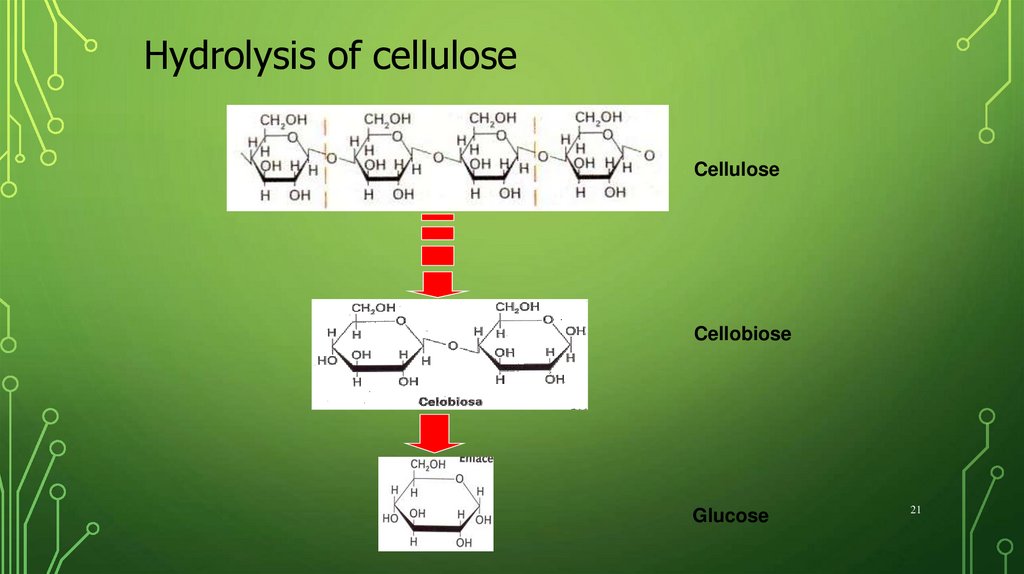
21.
Hydrolysis of celluloseCellulose
Cellobiose
Glucose
21
22.
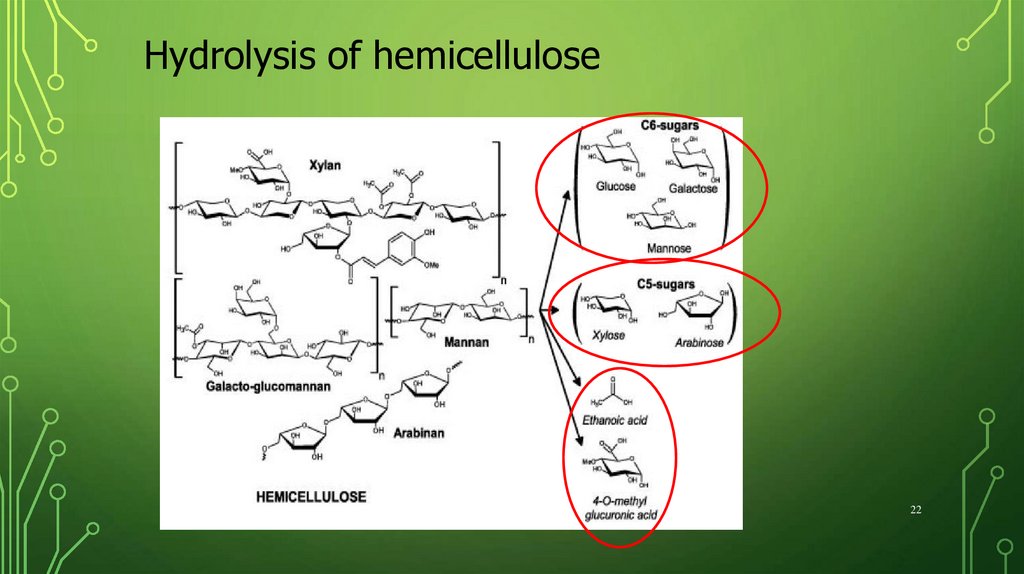
Hydrolysis of hemicellulose22
23.
Enzymatic hydrolysis☺ High conversion yield
90-100% for cellulose
70-75% for hemicelluloses
☺ No sugar degradation
☹ High cost of enzymes
☹ Slow process
23
24.
Enzymatic hydrolysisThe cellulases must be able to:
Reach and adsorb onto cellulose
surface (Accessibility)
Find, or make, reactive ends of
cellulose chain (Reactivity)
24
25.
Enzymatic hydrolysis☹ Low accessibility (Association with
hemicelluloses and lignin)
☹ Low reactivity (High degree of crystallinity)
PRETREATMENT
25
26.
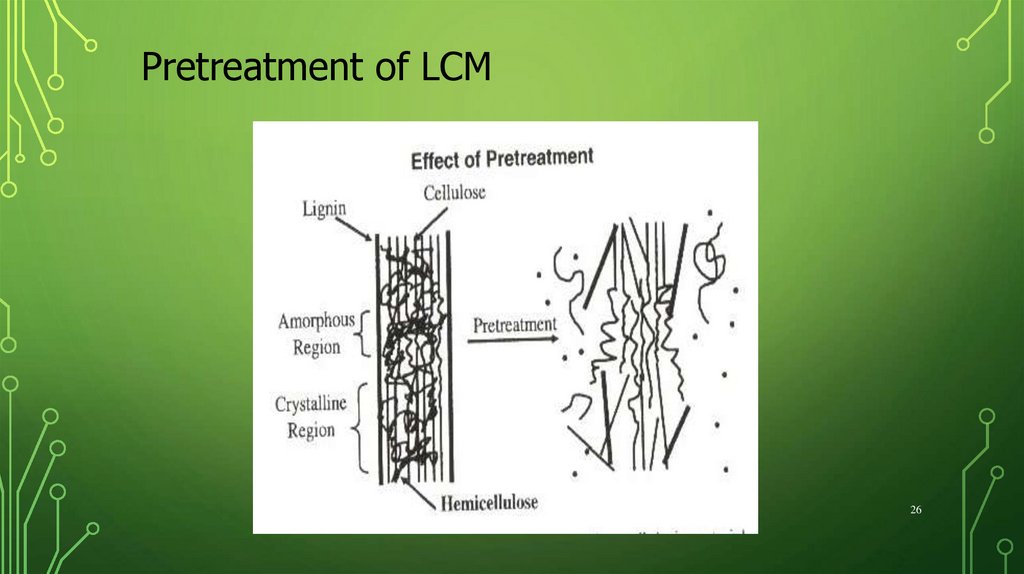
Pretreatment of LCM26
27.
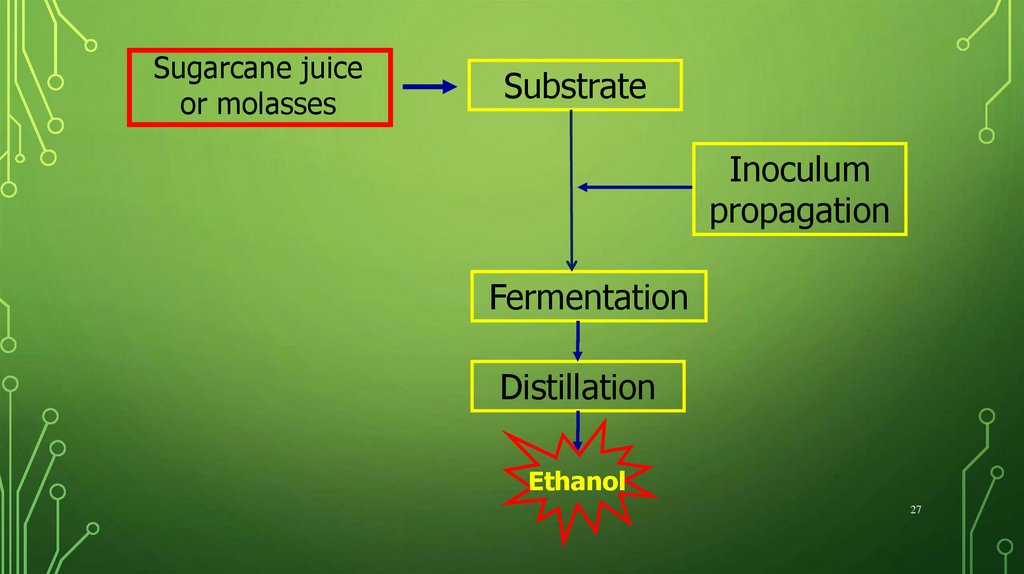
Sugarcane juiceor molasses
Substrate
Inoculum
propagation
Fermentation
Distillation
Ethanol
27
28.
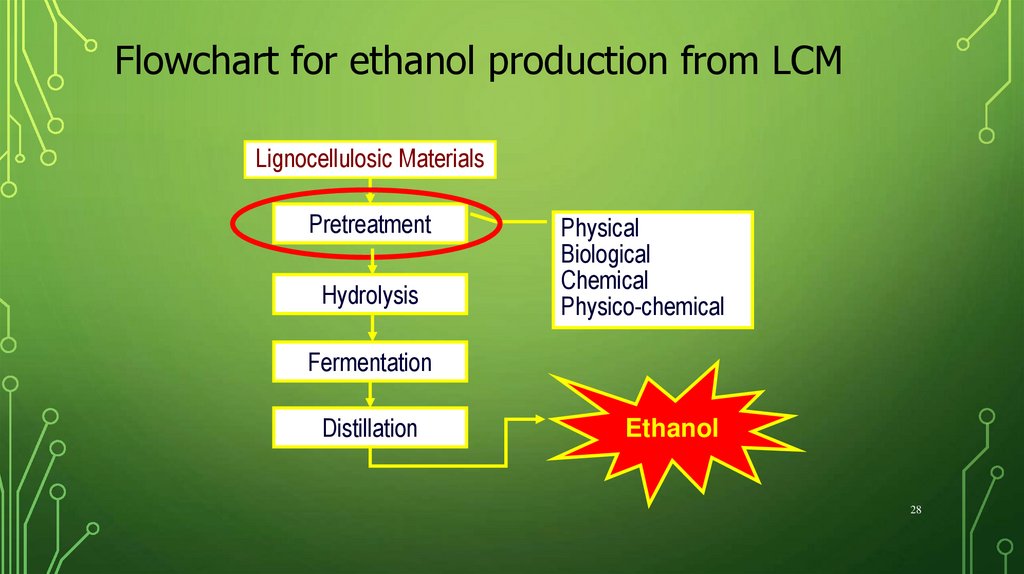
Flowchart for ethanol production from LCMLignocellulosic Materials
Pretreatment
Hydrolysis
Physical
Biological
Chemical
Physico-chemical
Fermentation
Distillation
Ethanol
28
29.
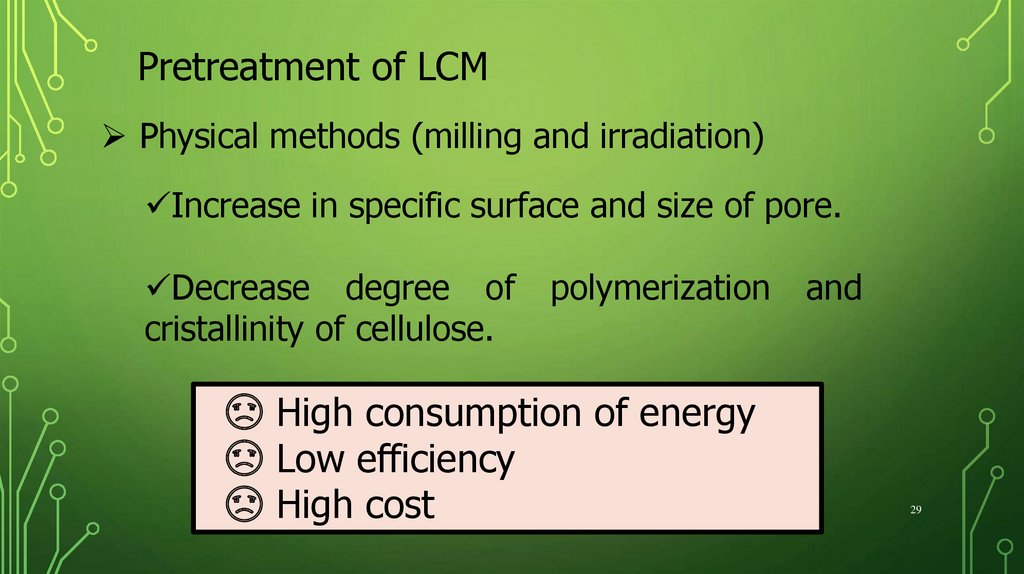
Pretreatment of LCMPhysical methods (milling and irradiation)
Increase in specific surface and size of pore.
Decrease degree of
cristallinity of cellulose.
polymerization
☹ High consumption of energy
☹ Low efficiency
☹ High cost
and
29
30.
Pretreatment of LCMBiological methods: decrease the degree of
polymerization and crystallinity of cellulose.
☹ Slow
☹ High cost
30
31.
Pretreatment of LCMChemical and physico-chemical methods
Delignification
Reduction of degree of polymerization and
crystallinity
Degradation of hemicellulose
Increase of the surface area and porosity
31
32.
Pretreatment of LCMChemical and physico-chemical methods
Steam explosion
Wet oxidation
Dilute-acid prehydrolysis
Autohydrolysis
Liquid hot water
Ammonia fiber explosion
☺ Efficient
☺ Low cost
32
33.
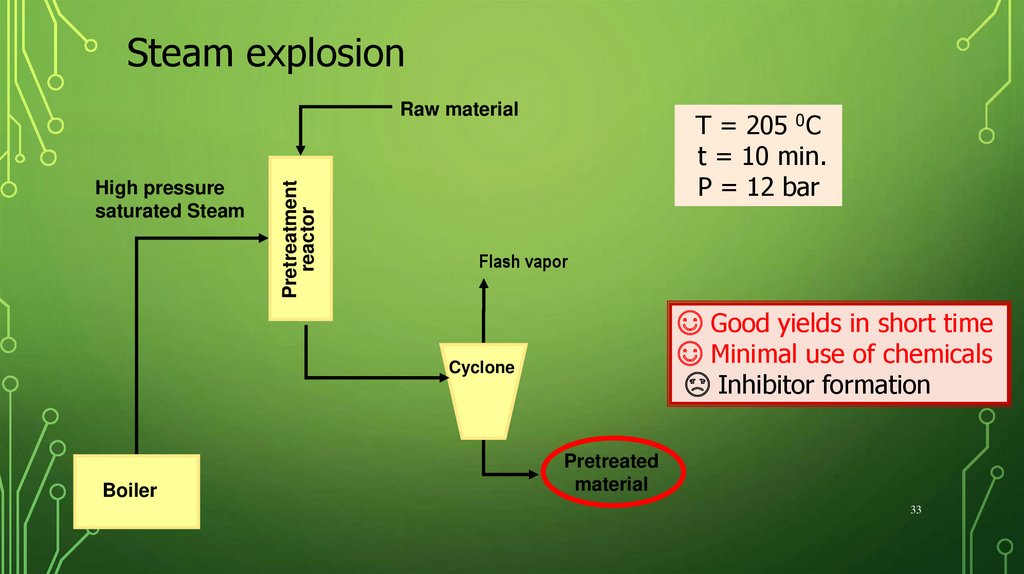
Steam explosionHigh pressure
saturated Steam
Pretreatment
reactor
Raw material
T = 205 0C
t = 10 min.
P = 12 bar
Flash vapor
☺ Good yields in short time
☺ Minimal use of chemicals
☹ Inhibitor formation
Cyclone
Boiler
Pretreated
material
33
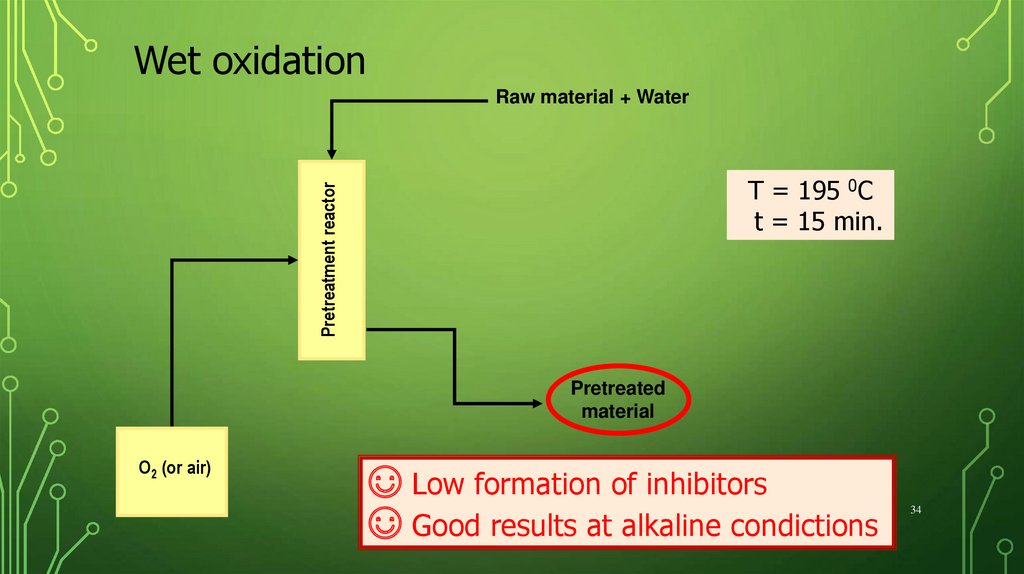
34.
Wet oxidationRaw material + Water
Pretreatment reactor
T = 195 0C
t = 15 min.
Pretreated
material
O2 (or air)
☺ Low formation of inhibitors
☺ Good results at alkaline condictions
34
35.
Control questions1. Describe the composition of lignocellulosic materials.
2. What characteristics distinguish cellulose from
hemicelluloses?
3. Mention the hydrolysis methods that can be used to obtain
fermentable sugars. Explain one of them.
4. Why is necessary to pretreat lignocellulosic materials before
their use for ethanol production.
5. Describe one pretreatment that can be used before
hydrolysis of lignocellulosic materials. Specify the most
important goals to achieve an efficient pretreatment?
35
36.
Suggested literature- Harmsen P., Huijgen W., Bermudez L., Bakker R. Literature
review of physical and chemical pretreatment processes for
lignocellulosic biomass. Biosynergy, September 2010, Report
1184. ISBN 978-90-8585-757-0.
- Zoghlami A., Paës G. Lignocellulosic Biomass:
Understanding Recalcitrance and Predicting Hydrolysis.
Frontiers in Chemistry, 2019, Volume 7, Article 874.
- Galbe M., Zacchi G. A review of the production of ethanol
from softwood. Appl Microbiol Biotechnol, 2002, 59:618–628.
36
37.
THANK YOU FOR YOURATTENTION!
37
















 Химия
Химия Промышленность
Промышленность








