Похожие презентации:
Extraction of semivolatile organics from liquids. Solid Phase Microextraction (SPME)
1.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid Phase Microextraction (SPME)
Methodology
1) the sorbent, either an externally coated fiber or an internally coated tube, is exposed to the sample for a specified
period of time;
2) the sorbent is transferred to a device that interfaces with an analytical instrument for thermal desorption using GC or
for solvent desorption when using HPLC.
2.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Methodology
Solid Phase Microextraction (SPME)
3.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid Phase Microextraction (SPME)
Methodology
sample
4.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid Phase Microextraction (SPME)
Methodology
sample
Extraction time
5-40 min.
5.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid Phase Microextraction (SPME)
Methodology
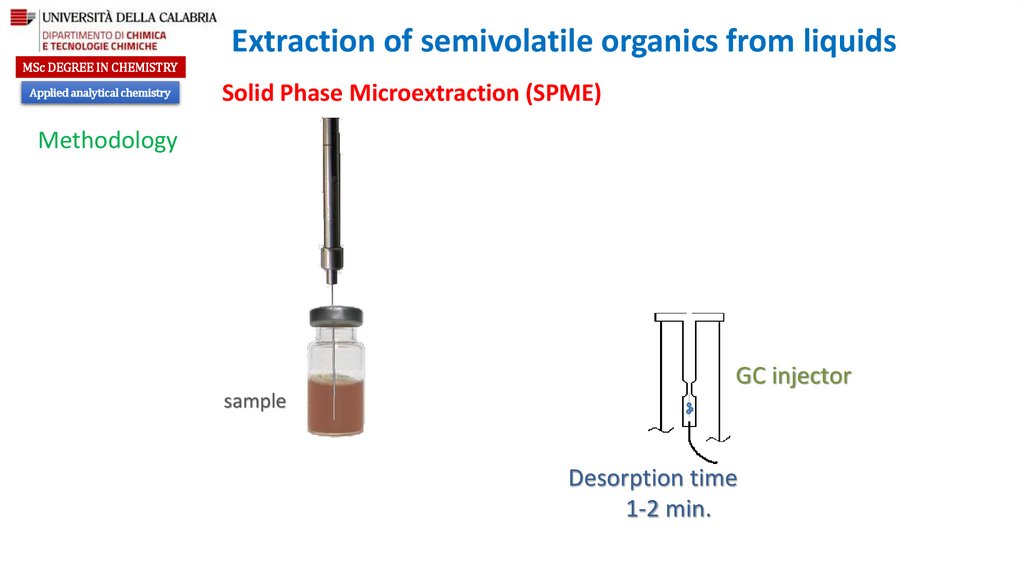
GC injector
sample
Desorption time
1-2 min.
6.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid Phase Microextraction (SPME)
Optimization of SPME analysis
A number of parameters must be selected for a successful SPME sample preparation.
Among these parameters are
(a) the type of fiber to be utilized, including chemical nature of the phase and film
thickness,
(b) the choice between solution (immersion) adsorption or headspace adsorption,
(c) adsorption time,
(d) temperature of the sample for analyte adsorption in the SPME fiber,
(e) the use of salting effect and evaluation of the presence of organic solvents in the
sample,
(f) the pH of the sample,
(g) desorption temperature (for GC analysis),
(h) desorption time.
7.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid Phase Microextraction (SPME)
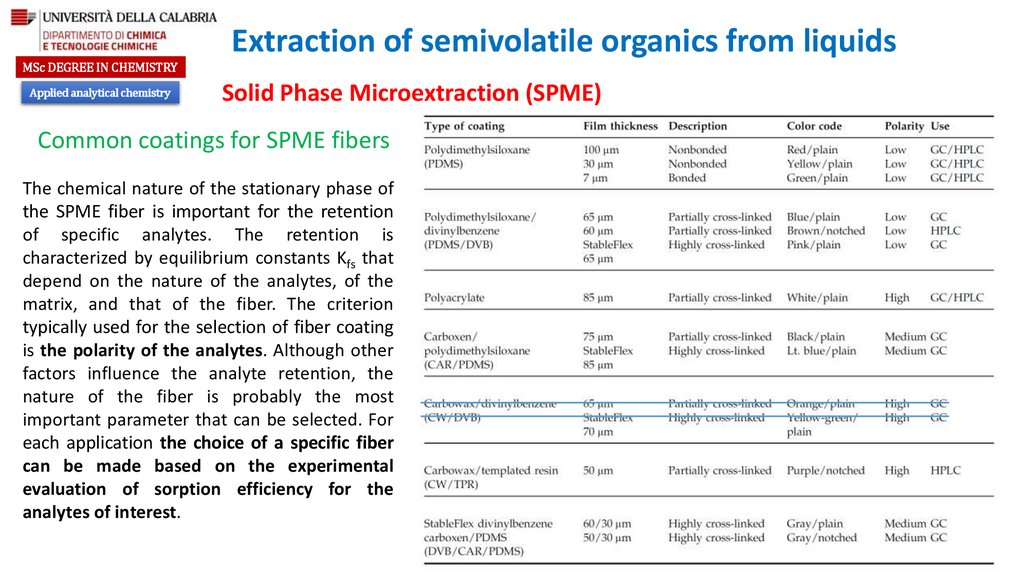
Common coatings for SPME fibers
The chemical nature of the stationary phase of
the SPME fiber is important for the retention
of specific analytes. The retention is
characterized by equilibrium constants Kfs that
depend on the nature of the analytes, of the
matrix, and that of the fiber. The criterion
typically used for the selection of fiber coating
is the polarity of the analytes. Although other
factors influence the analyte retention, the
nature of the fiber is probably the most
important parameter that can be selected. For
each application the choice of a specific fiber
can be made based on the experimental
evaluation of sorption efficiency for the
analytes of interest.
8.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid Phase Microextraction (SPME)
Common coatings for SPME fibers
Apolar, Single-Component Absorbent Phase
Polydimethylsiloxane (PDMS) is a single-component, nonpolar liquid absorbent phase coated on fused silica
commercially available in film thicknesses of 7, 30, and 100 µm. The PDMS phases can be used in conjunction with
analysis by GC or HPLC. The thickest coating, 100 µm, is used for more volatile compounds. The intermediate coating
level, 30 µm, is appropriate for use with nonpolar semivolatile organic compounds, while the smallest-diameter coating,
7 µm, is used when analyzing nonpolar, high-molecular-weight compounds. The use of PDMS fibers is restricted to a
sample pH between 2 and 11.
Polar, Single-Component Absorbent Phase
Polyacrylate (PA) is a single-component polar absorbent coating commercially available in a film thickness of 85 µm. The
sorbent is used with GC or HPLC analyses and is suitable for the extraction of polar semivolatile compounds.
Porous, Adsorbent, Blended Particle Phases (Multiple-component phases)
Divinylbenzene (DVB)
Carboxen (Car)
Polydimethylsiloxane (PDMS)
9.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid Phase Microextraction (SPME)
Common coatings for SPME fibers
Porous, Adsorbent, Blended Particle Phases (Multiple-component phases)
PDMS-DVB is a multiple-component bipolar solid sorbent coating. PDMS/DVB is commercially available in a film thickness
of 65 µm for SPME of volatile, amine, or nitroaromatic analytes for GC analyses or in a film thickness of 60 µm for SPME of
amines and polar compounds for final determination by HPLC. DVB is suspended in the PDMS phase.
Carboxen/PDMS is a multiple-component bipolar sorbent (75 or 85 µm thickness) used for SPME of gases and lowmolecular-weight compounds with GC analyses. Carboxen is suspended in the PDMS phase. Among the SPME fibers
currently available, the 85-µm Carboxen/PDMS sorbent is the best choice for extracting analytes having molecular weights
of less than 90, regardless of functional groups present. The Carboxen particles extract analytes by adsorption due to the
solid state of the sorbent.
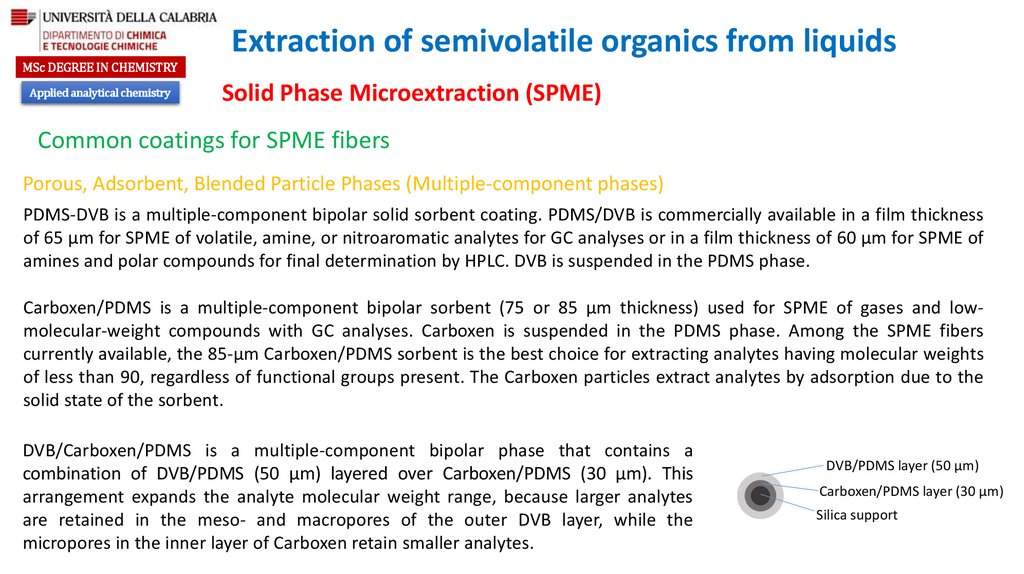
DVB/Carboxen/PDMS is a multiple-component bipolar phase that contains a
combination of DVB/PDMS (50 µm) layered over Carboxen/PDMS (30 µm). This
arrangement expands the analyte molecular weight range, because larger analytes
are retained in the meso- and macropores of the outer DVB layer, while the
micropores in the inner layer of Carboxen retain smaller analytes.
DVB/PDMS layer (50 µm)
Carboxen/PDMS layer (30 µm)
Silica support
10.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid Phase Microextraction (SPME)
Common coatings for SPME fibers
Overcoated fiber (PDMS/DVB/PDMS)
Recently a new fiber has been introduced on the market which is called overcoated fiber. This new fiber
consist in a PDMS/DVB overcoated by a PDMS layer. This overcoating serves as a barrier to protect the fiber
from the matrix, which makes it more physically robust and less prone to chemical fouling. High background
samples, such as many foods, pose a challenge to immersion SPME due to the presence of fats, sugars,
pigments, and other macromolecules. These can stick to the SPME fiber and reduce its usable life and/or be
transferred to the GC, where they may interfere with chromatographic analysis. Overcoating the fiber with
PDMS reduces these problems.
11.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid Phase Microextraction (SPME)
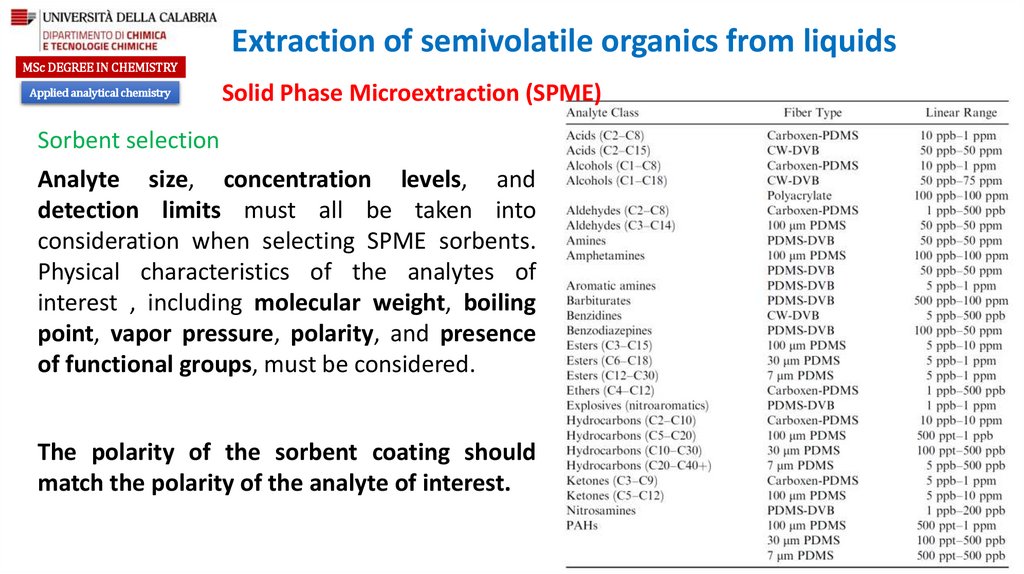
Sorbent selection
Analyte size, concentration levels, and
detection limits must all be taken into
consideration when selecting SPME sorbents.
Physical characteristics of the analytes of
interest , including molecular weight, boiling
point, vapor pressure, polarity, and presence
of functional groups, must be considered.
The polarity of the sorbent coating should
match the polarity of the analyte of interest.
12.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid Phase Microextraction (SPME)
Optimization of SPME analysis
The choice between solution adsorption or headspace adsorption
For compounds with higher solubility in water or for compounds with low volatility, the immersion
adsorption is usually preferred. The compounds with high volatility and low water solubility are typically
analyzed from the sample headspace. Another factor sometimes considered in the choice between immersion
and headspace is that fiber degradation after a number of analyses is usually higher for immersion mode.
Also, for very dirty samples, the adsorption from the solution must be avoided if possible.
When undesired matrix components can be adsorbed in the SPME fiber, the selectivity obtained as ratio
analyte/matrix adsorption could be more important than the actual amount of material adsorbed into the
fiber.
The sensitivity of the analytical instrumentation is also important in this decision. When ample sensitivity is
offered by the chromatographic instrument, the choice of the fiber and the adsorption method is based on
achieving the best “cleaning” of the sample and not necessarily on the highest amount of analyte collected
from the system.
13.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid Phase Microextraction (SPME)
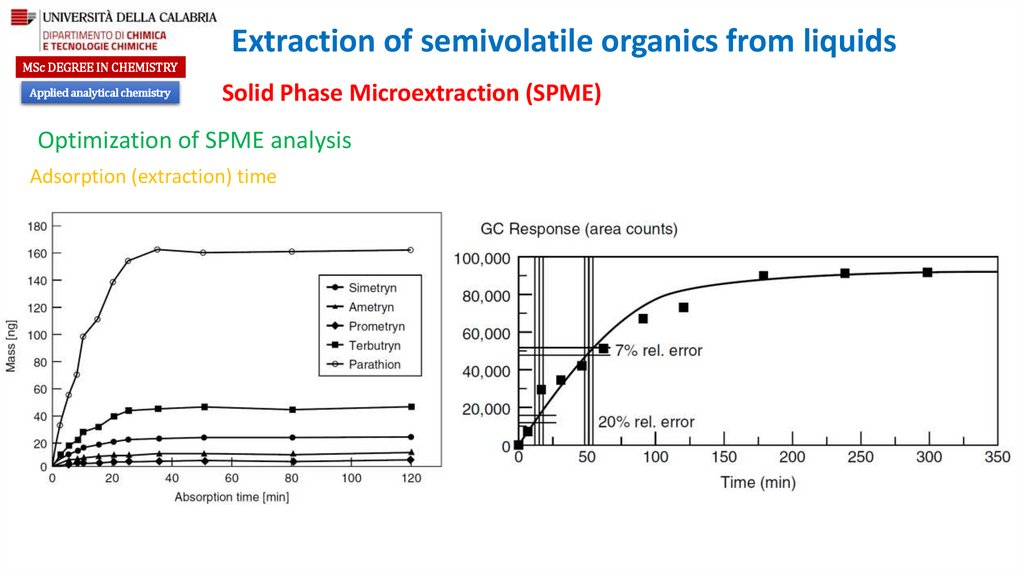
Optimization of SPME analysis
Adsorption (extraction) time
14.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid Phase Microextraction (SPME)
Optimization of SPME analysis
Adsorption (extraction) temperature
The optimization of the adsorption temperature is also important, because the process of desorption of the
analytes from the sample and the adsorption into the fiber are affected by temperature. This indicates that
the temperature during the adsorption process from solution must be kept constant. The temperature is a
critical parameter especially for the analyses carried out in the headspace mode.
The use of salting effect and evaluation of the presence of organic solvents in the sample
In order to modify the distribution constants when using water samples, the addition of an inorganic salt such
as NaCl has been proven useful in a number of cases by increasing the extraction of some organic compounds
by a salting-out effect. The addition of salt can be done for both solution extraction and for headspace
extraction.
While the increase in salinity favors the adsorption of organic compounds from aqueous solutions, the
presence of organic solvents in water has an opposite effect. The addition of a solvent of low polarity to the
sample may decrease significantly the partition into the fiber for a nonpolar analyte.
15.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid Phase Microextraction (SPME)
Optimization of SPME analysis
pH of the sample
The reason why extraction depends on pH is analogous to what we have discussed for SPE. In fact, if the
analytes to be extracted have an ionizable functional group (carboxylic acids, amines, etc.) the pH will affect
the distribution of the species present in solution. Also in this case, if the analytes are present as ions, they
will be extracted much less than when they are neutral and therefore the Kfs will be much smaller in the first
case than in the second.
Desorption temperature and desorption time
The desorption of the SPME fiber is typically done for a short period of time (about 1 min) at temperatures
recommended in literature for the specific fiber. Modifications of these parameters do not provide, in general,
a large variation in the results using SPME analysis.
16.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid Phase Microextraction (SPME)
Quantitative analysis with SPME
SPME quantitation is a quantitation of extraction under non-exhaustive conditions which is based on the
proportional relationship between the sorbed analyte and initial concentration.
A number of factors may influence the accuracy of the determination. The change in sample matrix may
affect Kfs, and therefore the quantitation of the same amount of a given analyte can lead to different
results in different matrices if the calibration is not performed for the same matrix. The method of external
calibration using a blank sample such that calibration standards have the same matrix as real samples is
typically successful. However, addition of analyte on the blank sample done using an organic solution may
strongly affect the results.
Calibration of the SPME technique can be based on internal calibration also using isotopically labeled
standards. This approach often assures the achievement of good analytical parameters. However, the use of
an internal standard in quantitative SPME determinations may also encounter problems because adsorption
of the internal standard into the fiber can be affected by the analyte concentration.
The application of standard addition method for quantitation in SPME can be used but in some cases it also
can be affected by some inaccuracies because of the nonlinearity of the calibration.
17.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid Phase Microextraction (SPME)
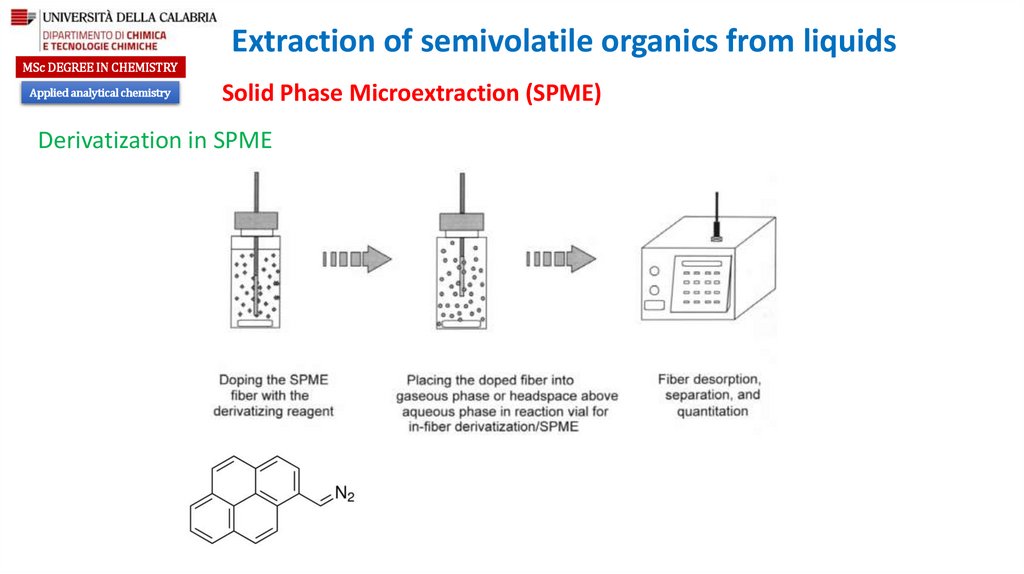
Derivatization in SPME
The compounds with functional groups
such as OH, COOH, SH, NH2, NH, or CONH
are polar and not very volatile and,
therefore, are not suitable for gas
chromatographic
analysis.
The
derivatization aims to the elimination of
the active hydrogens by the formation of
alkyl or aryl derivatives.
To achieve this, a large number of
reagents R-X can be used. In a simplified
approach it can be considered that R is
carrying a specific property and X a
specific reactivity, although the reactivity
of a reagent is influenced by both R and
X components of the molecule.
18.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid Phase Microextraction (SPME)
Derivatization in SPME
Loading the SPME
with the analytes
Placing the fiber loaded with the
analyte to the derivatizating
reagents for in-fiber derivatization
SPME
Fiber desorption,
separation, and
quantitation
19.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid Phase Microextraction (SPME)
Derivatization in SPME
20.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid Phase Microextraction (SPME)
Advantages of SPME
• Integrates extraction, concentration and sample introduction into a single step
• Enables solventless extraction, therefore is a “green” technique
• Suitable for analysis of semi-volatile and volatile compounds
• Fast and easy to use
• Is easily automated
• SPME sampling devices are portable, thereby enabling their use in field monitoring
• Facilitates unique investigations, such as extraction from very small samples
• Ensures high sensitivity
SPE
1 µg/L in real samples 1 mg/L in organic solution; injecting 1µL, the amount of injected analyte is 1 ng.
SPME
1 µg/L in real samples; recovery 10%; volume of real sample 10 mL the amount of injected analyte is 1 ng.
21.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Stir bar sorptive extraction(SBSE)
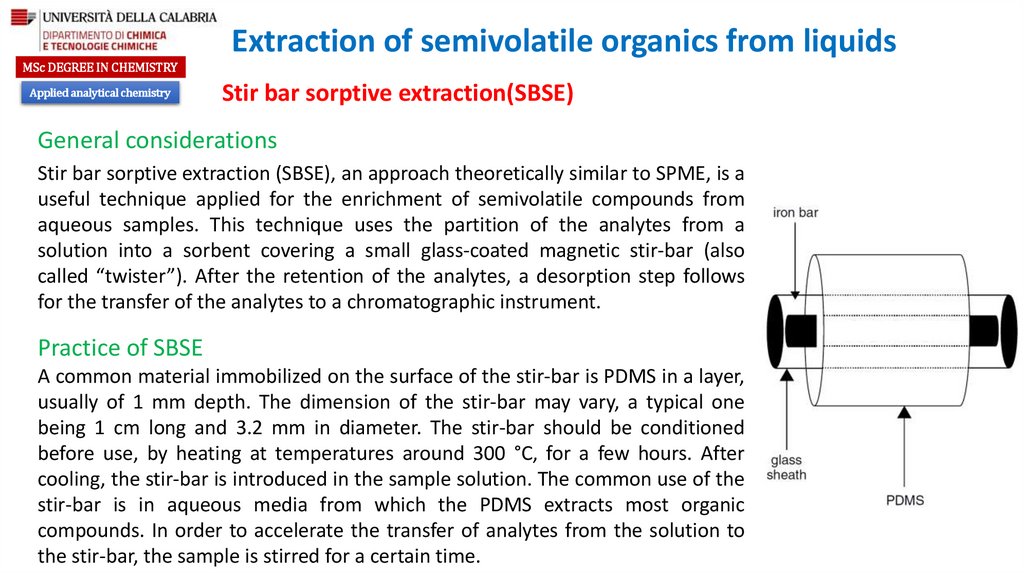
General considerations
Stir bar sorptive extraction (SBSE), an approach theoretically similar to SPME, is a
useful technique applied for the enrichment of semivolatile compounds from
aqueous samples. This technique uses the partition of the analytes from a
solution into a sorbent covering a small glass-coated magnetic stir-bar (also
called “twister”). After the retention of the analytes, a desorption step follows
for the transfer of the analytes to a chromatographic instrument.
Practice of SBSE
A common material immobilized on the surface of the stir-bar is PDMS in a layer,
usually of 1 mm depth. The dimension of the stir-bar may vary, a typical one
being 1 cm long and 3.2 mm in diameter. The stir-bar should be conditioned
before use, by heating at temperatures around 300 °C, for a few hours. After
cooling, the stir-bar is introduced in the sample solution. The common use of the
stir-bar is in aqueous media from which the PDMS extracts most organic
compounds. In order to accelerate the transfer of analytes from the solution to
the stir-bar, the sample is stirred for a certain time.
22.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Stir bar sorptive extraction(SBSE)
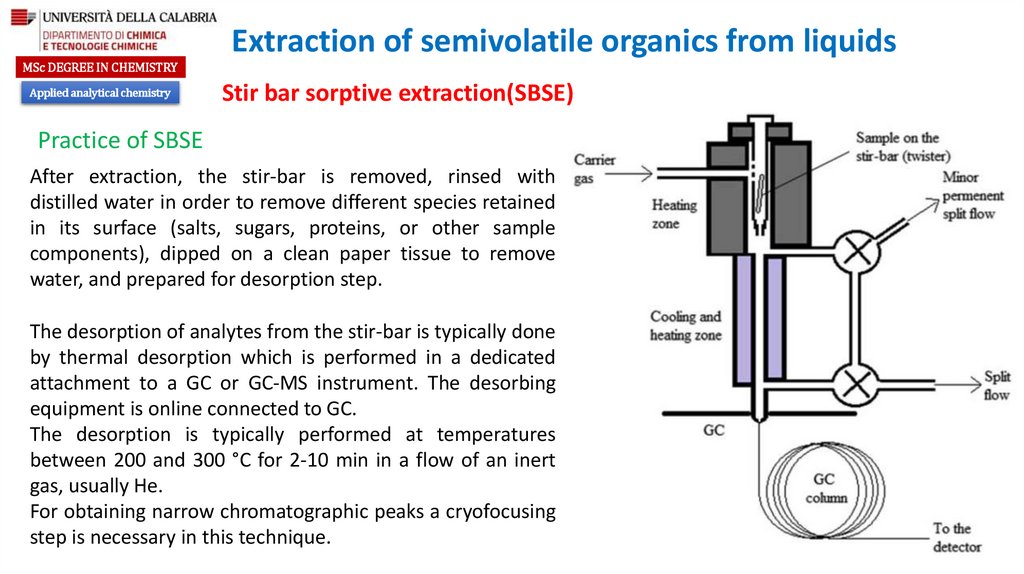
Practice of SBSE
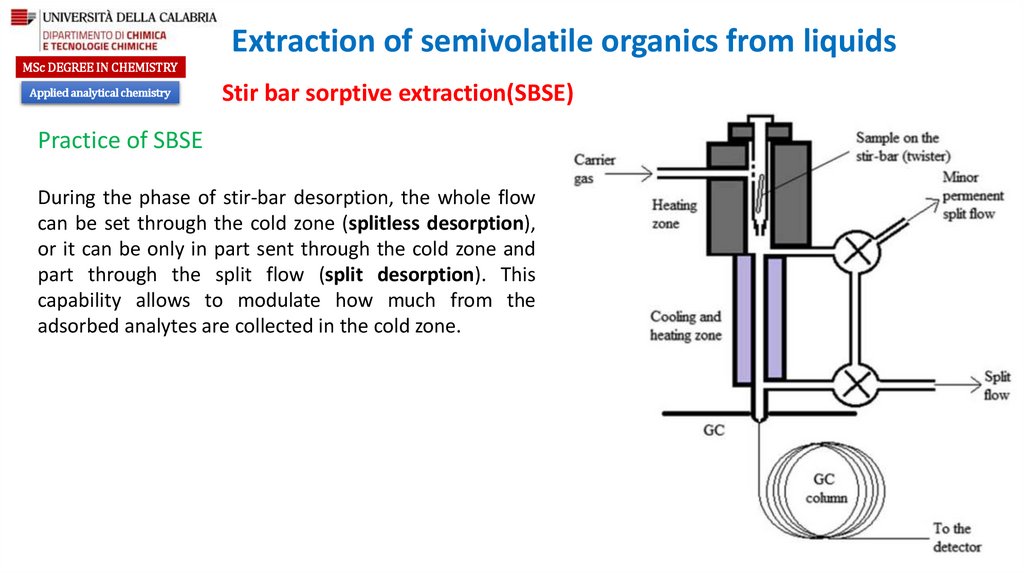
After extraction, the stir-bar is removed, rinsed with
distilled water in order to remove different species retained
in its surface (salts, sugars, proteins, or other sample
components), dipped on a clean paper tissue to remove
water, and prepared for desorption step.
The desorption of analytes from the stir-bar is typically done
by thermal desorption which is performed in a dedicated
attachment to a GC or GC-MS instrument. The desorbing
equipment is online connected to GC.
The desorption is typically performed at temperatures
between 200 and 300 °C for 2-10 min in a flow of an inert
gas, usually He.
For obtaining narrow chromatographic peaks a cryofocusing
step is necessary in this technique.
23.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Stir bar sorptive extraction(SBSE)
Practice of SBSE
During the phase of stir-bar desorption, the whole flow
can be set through the cold zone (splitless desorption),
or it can be only in part sent through the cold zone and
part through the split flow (split desorption). This
capability allows to modulate how much from the
adsorbed analytes are collected in the cold zone.
24.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Stir bar sorptive extraction(SBSE)
Practice of SBSE
Due to the increase in the quantity of PDMS coated on the stir-bar as compared to SPME, the quantification
limit can be increased up to 500-fold for a time of stirring of 30-60 min. This concentration process allows
determination at ppt level of concentration when GC-MS is further used for the analysis.
Solvent extraction of the stir-bar followed by the analysis of the extract can be performed for thermally labile
analytes, as well as for HPLC analysis. This procedure is based on immersion of the stir-bar in a stripping
solvent or a mixture of solvents, under mechanical shaking, specific temperature, or sonication, which
reextract the analytes from the polymeric layer of the stir-bar.
The most commonly used sorptive phase coating for the stir-bar is PDMS, which is known as a nonpolar
stationary phase in GC and SPME. PDMS is thermally stable up to 300 °C and allows the diffusion of the organic
compounds in their bulk. Other commercially available phases include poly(acrylate) and ethylene glycolsilicone. These phases are useful for the retention of more polar analytes but in general have a lower
mechanical resilience and can be utilized a fewer number of times compared to the PDMS stir-bar.
25.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Stir bar sorptive extraction(SBSE)
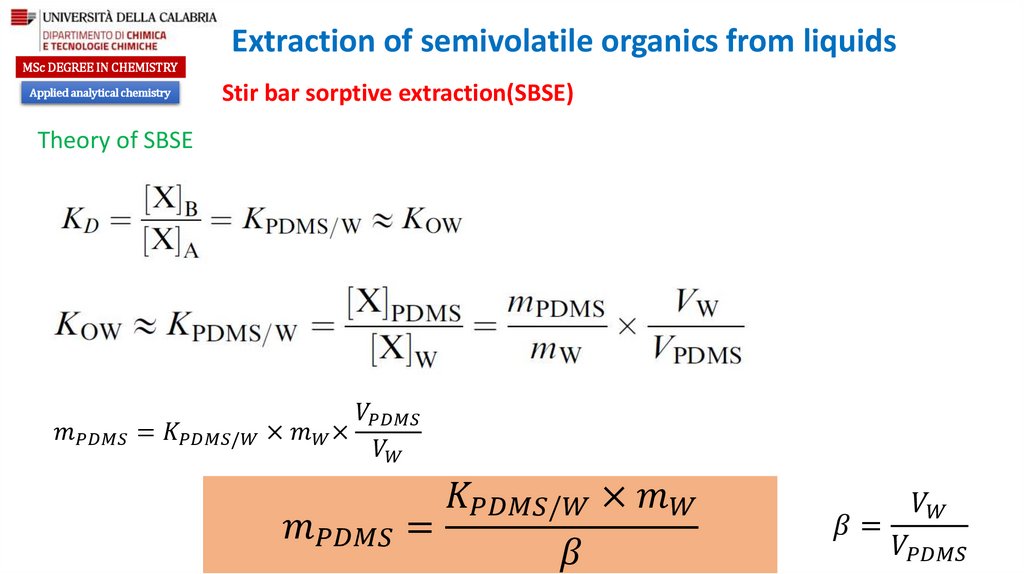
Theory of SBSE















 Химия
Химия








