Похожие презентации:
Extraction of semivolatile organics from solid matrices
1.
Extraction of semivolatile organics from solid matricesMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Microwave-assisted extraction
Instrumentation
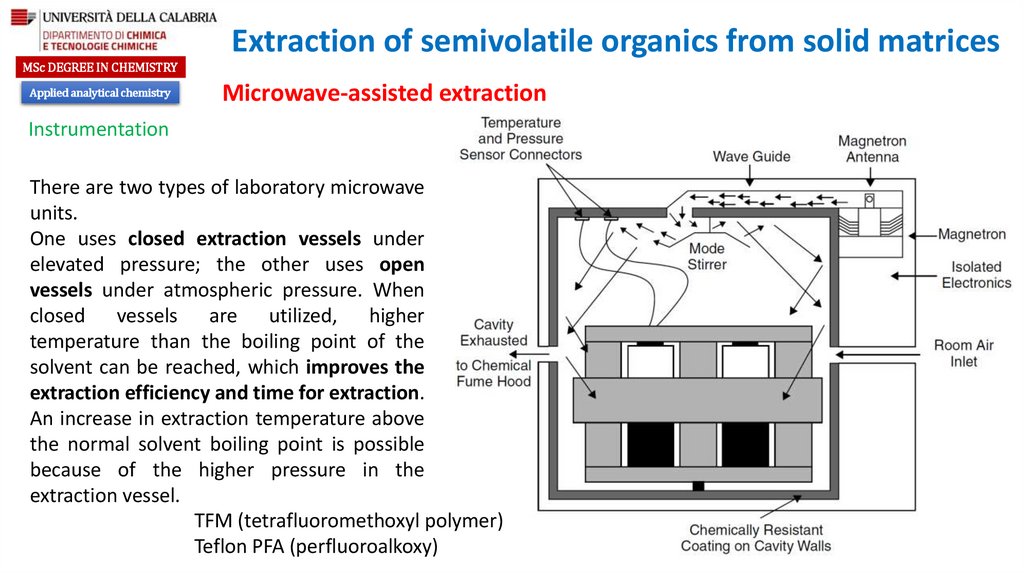
There are two types of laboratory microwave
units.
One uses closed extraction vessels under
elevated pressure; the other uses open
vessels under atmospheric pressure. When
closed vessels are utilized, higher
temperature than the boiling point of the
solvent can be reached, which improves the
extraction efficiency and time for extraction.
An increase in extraction temperature above
the normal solvent boiling point is possible
because of the higher pressure in the
extraction vessel.
TFM (tetrafluoromethoxyl polymer)
Teflon PFA (perfluoroalkoxy)
2.
Extraction of semivolatile organics from solid matricesMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Microwave-assisted extraction
Instrumentation
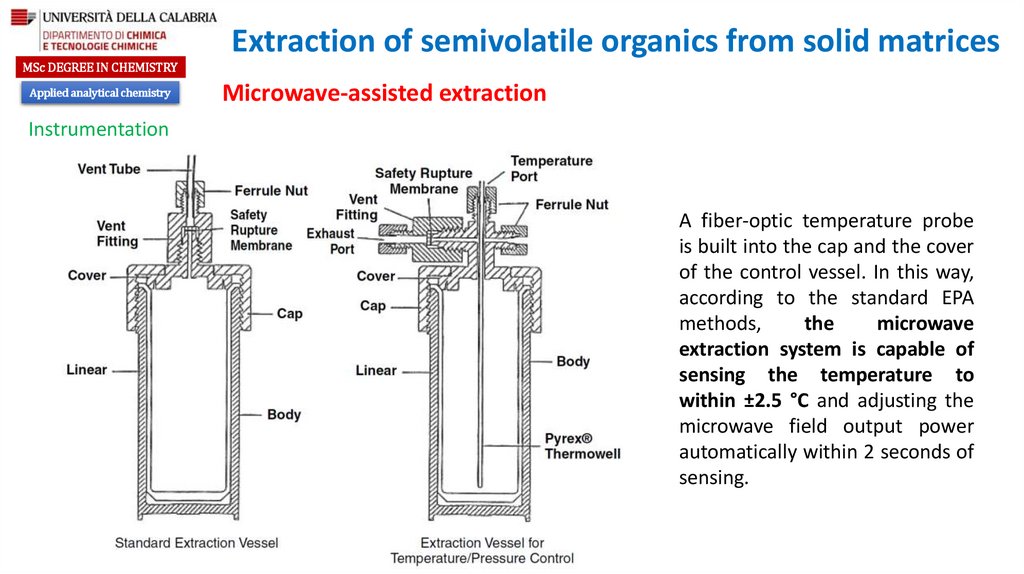
A fiber-optic temperature probe
is built into the cap and the cover
of the control vessel. In this way,
according to the standard EPA
methods,
the
microwave
extraction system is capable of
sensing the temperature to
within ±2.5 °C and adjusting the
microwave field output power
automatically within 2 seconds of
sensing.
3.
Extraction of semivolatile organics from solid matricesMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Microwave-assisted extraction
Instrumentation
This approach for an accurate control of temperature is indispensable when the method provide for a
temperature program of the microwave system. In this case, the system modulates the microwaves power
according to programmed temperatures, by receiving continuous feedback on accurate temperature detected
by the temperature probe.
On the other hand, if the extraction is carried out with a programmed microwave power, it is not necessary
to know the accurate temperature by means of the probe.
However, in both programmed temperature and programmed power it is absolutely necessary to activate the
temperature control by infrared radiation. This system allows for controlling the temperatures of all vessels
by sending an IR radiation to the bottom of each liner. In this way, while not obtaining an accurate
temperature, it can be assessed whether all the vessels are in the same operating conditions. Therefore, this
allows to repeat an extraction that took place, for example, at a lower temperature than all the other vessels
and understand the reason why the temperature in that vessel did not remain the same as the others.
4.
Extraction of semivolatile organics from solid matricesMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Microwave-assisted extraction
Instrumentation
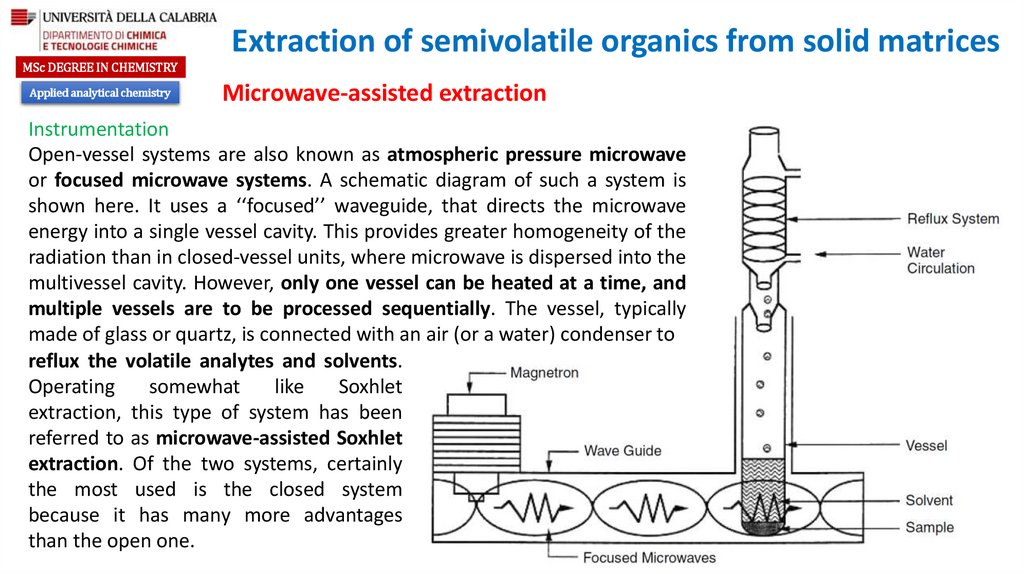
Open-vessel systems are also known as atmospheric pressure microwave
or focused microwave systems. A schematic diagram of such a system is
shown here. It uses a ‘‘focused’’ waveguide, that directs the microwave
energy into a single vessel cavity. This provides greater homogeneity of the
radiation than in closed-vessel units, where microwave is dispersed into the
multivessel cavity. However, only one vessel can be heated at a time, and
multiple vessels are to be processed sequentially. The vessel, typically
made of glass or quartz, is connected with an air (or a water) condenser to
reflux the volatile analytes and solvents.
Operating
somewhat
like
Soxhlet
extraction, this type of system has been
referred to as microwave-assisted Soxhlet
extraction. Of the two systems, certainly
the most used is the closed system
because it has many more advantages
than the open one.
5.
Extraction of semivolatile organics from solid matricesMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Microwave-assisted extraction
Optimization of extraction
The efficiency of MAE can be influenced by factors such as the choice of solvent, temperature, extraction
time, matrix effects, and water contents.
The proper choice of solvent is the key to successful extraction. In general, three types of solvent system can
be used in MAE: solvent(s) of high ’’, a mixture of solvents of high and low ’’, and a microwave transparent
solvent used with a sample of high ’’. The extracting selectivity and the ability of the medium to interact
with microwaves can be modulated by using mixtures of solvents. The solubility of the compounds of
interest in the solvent also must be considered in the solvent selection. Solvent penetration, interaction with
the sample matrix, and mass transfer kinetics of the process are also important in solvent selection. In some
cases, the matrix itself interacts with microwaves, while the surrounding solvent possesses a low dielectric
constant and thus remains cold. This situation could be advantageous for the extraction of thermosensitive
compounds, such as for the extraction of essential oils. Localized heating can lead to the expansion and
rupture of cell walls and is followed by the liberation of essential oils into the solvent.
6.
Extraction of semivolatile organics from solid matricesMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Microwave-assisted extraction
Optimization of extraction
Generally, recovery increases with the increase in temperature and then levels off after a certain point. For
thermally labile compounds, analyte degradation occurs at high temperatures and results in low recovery.
Excessively high temperatures lead to matrix decomposition in polymer extractions and should be avoided.
In general, pressure is not a critical parameter in MAE.
Regarding the extraction time, many microwave extractions can reach maximum recovery in 10 to 20
minutes. Longer extraction time is not necessary and may lead to the decomposition of thermolabile
analytes.
Matrix effects have been observed in MAE applications. Because water is a polar substance that can be
heated by microwave irradiation, it can often improve analyte recovery. In a study of PAHs extraction from
soil and sediments, sample moisture level showed significant influence on extraction efficiency, and 30%
water in the sample provided the highest recovery.
Advantages/Disadvantages
High efficiency is the major advantage of microwave extraction over conventional methods such as Soxhlet.
It can achieve the same recovery in a shorter time (20 to 30 minutes) and with less solvent (30 mL). The
throughput is high (up to 16 samples per hour for closed-vessel system). A disadvantage is represented by
the initial cost of the system.
7.
Extraction of volatile organics from solids and liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Introduction
Volatile organic compounds (VOCs) can be defined as organic compounds whose vapor
pressures are greater than or equal to 0.1 mmHg at 20 °C. The nature and concentration
of volatile compounds from a variety of raw samples (liquid or solid) can also be measured
by sampling the headspace of the material emitting these volatiles.
The analytical task becomes challenging when the analytes of interest are dissolved or
sorbed in a complex matrix such as soil, food, cosmetics, polymers, or pharmaceutical raw
materials. The challenge is to extract the analytes from this matrix reproducibly, and to
accurately determine their mass or concentration.
Static headspace extraction (SHE)
Dynamic headspace extraction (DHE) (purge and trap)
Solid-phase microextraction (SPME)
Membrane extraction
Liquid extraction, possibly combined with large-volume GC injection
8.
Extraction of volatile organics from solids and liquidsMSc DEGREE IN CHEMISTRY
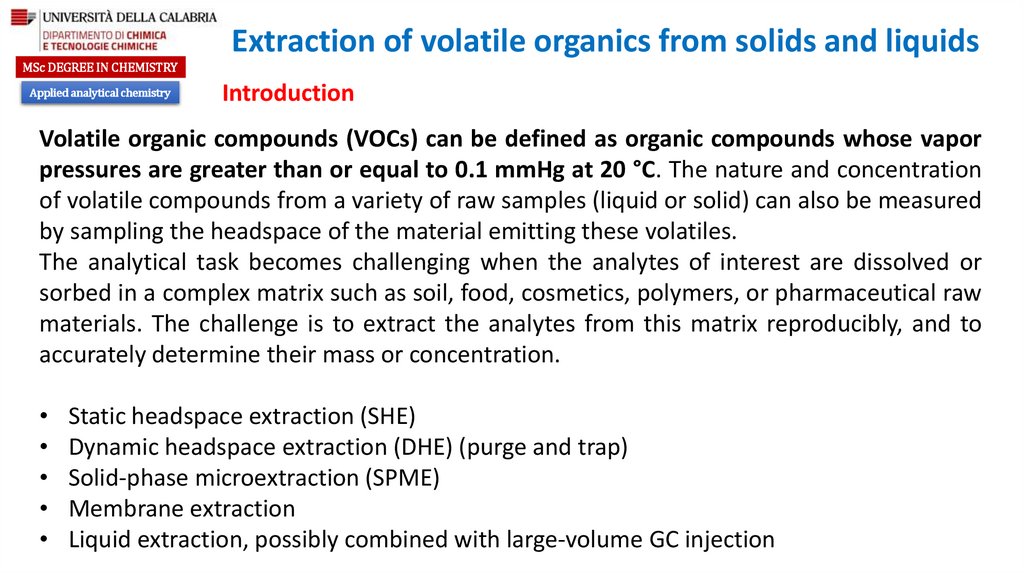
Applied analytical chemistry
Static headspace extraction
9.
Extraction of volatile organics from solids and liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Static headspace extraction
Sample preparation
The ease of initial sample preparation is one of the clear advantages of static headspace extraction. Often,
for qualitative analysis, the sample can be placed directly into the headspace vial, which is immediately
sealed, and analyzed with no additional preparation. However, for quantitation, it may be necessary to
understand and optimize the matrix effects to attain good sensitivity and, above all, accuracy. For
quantitative analysis of volatile compounds from solid particles, equilibrium between the analyte
concentration in the headspace and in the sample matrix must be reached in a sensible period of time,
typically a matter of minutes.
Two common approaches are crushing or grinding the sample and dissolving or dispersing the solid into a
liquid.
The choice of the approach must also be made on the basis of the type of quantitative analysis to be
conducted.
Analysis of methylene chloride in coated tablets. The sample preparation procedure calls for the
disintegration of 1 g of tablets in 20 mL of organic-free water via sonication. The solution is centrifuged after
sonication, and 2 mL of the supernatant solution is transferred to a HS vial and then analyzed by GC.
10.
Extraction of volatile organics from solids and liquidsMSc DEGREE IN CHEMISTRY
Static headspace extraction
Applied analytical chemistry
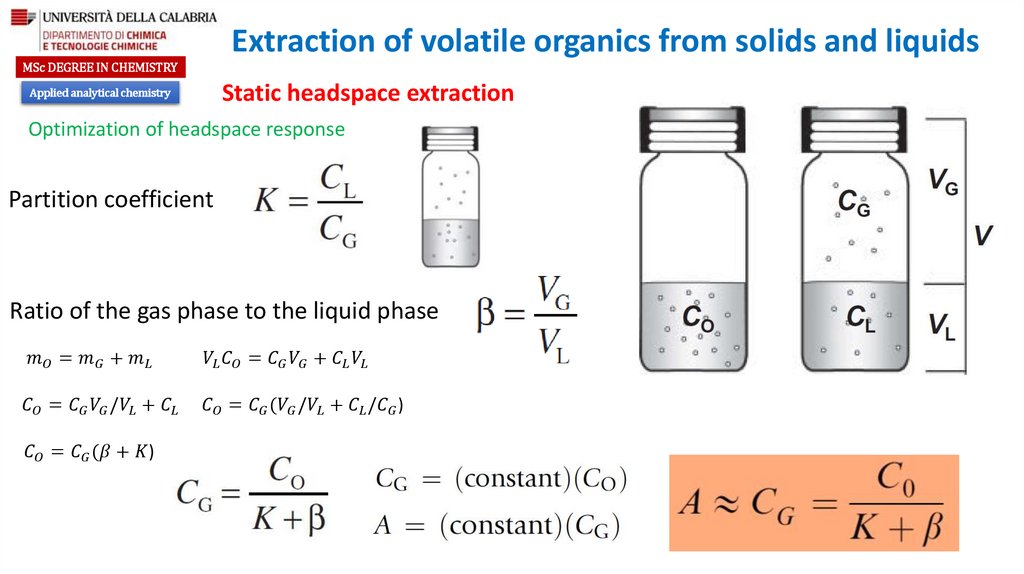
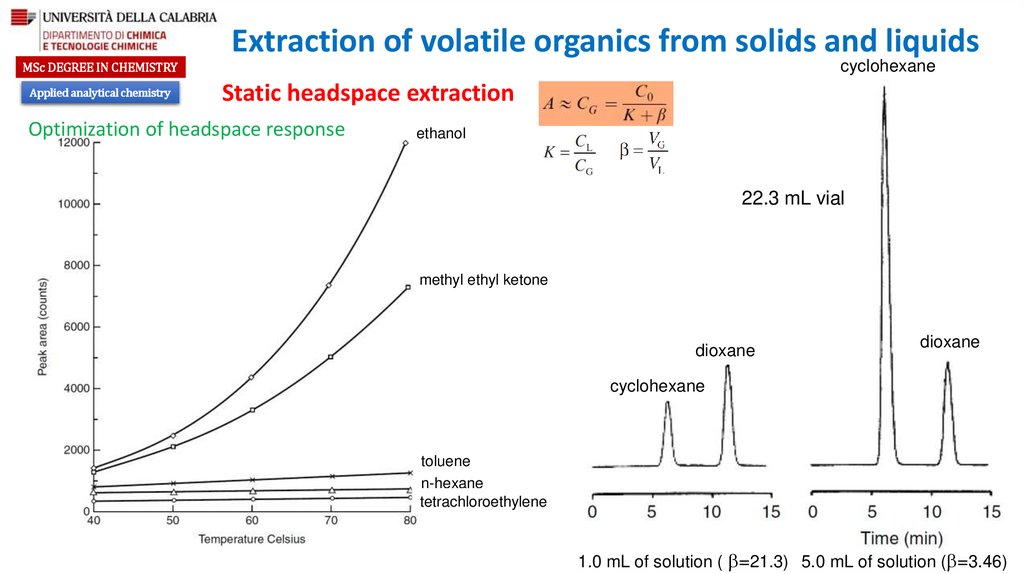
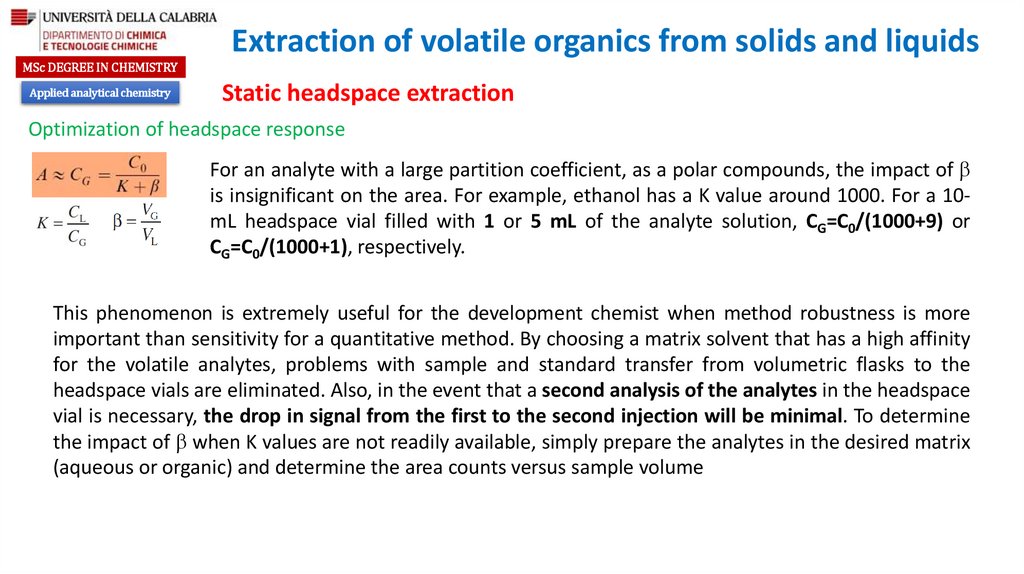
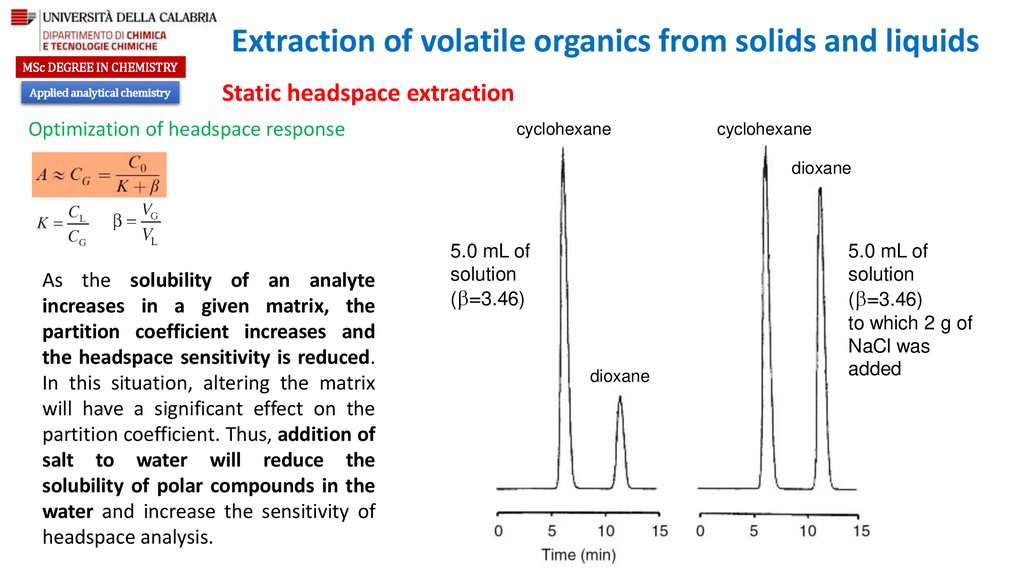
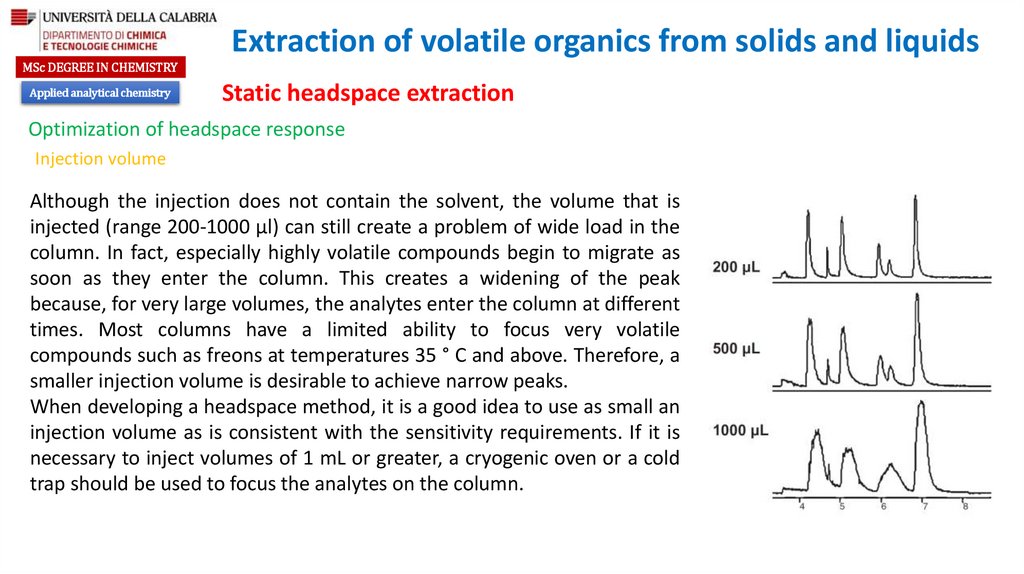
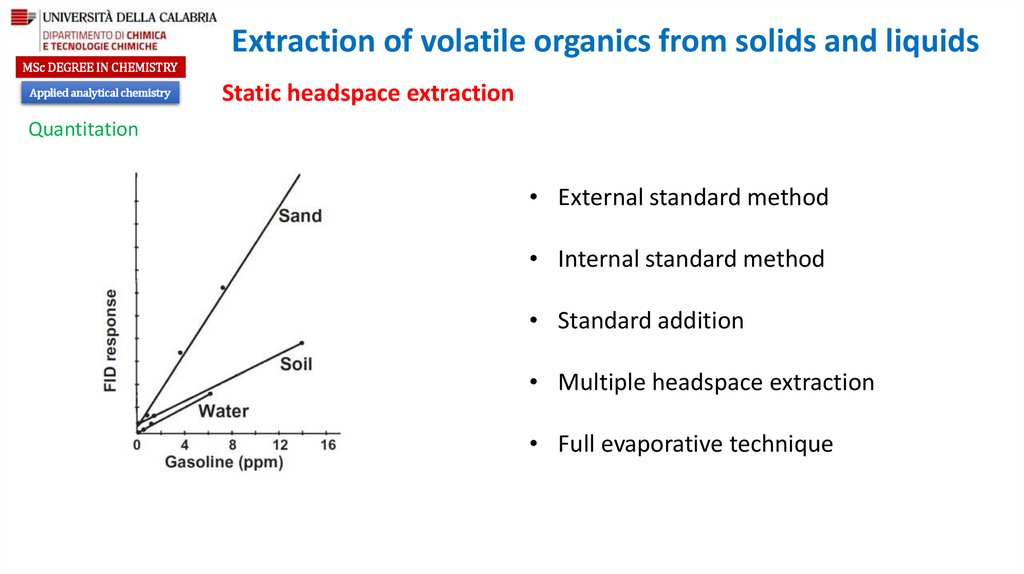
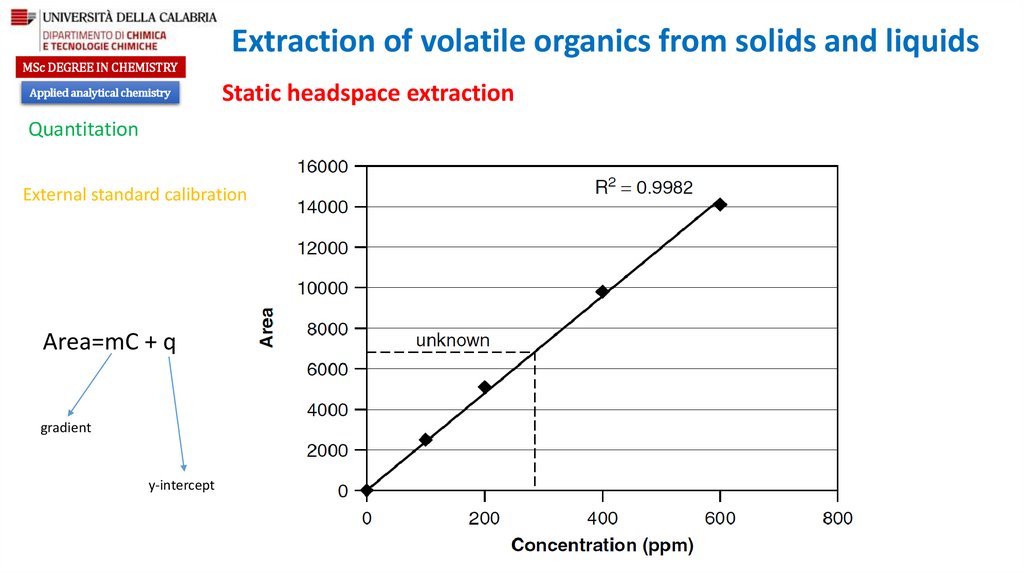
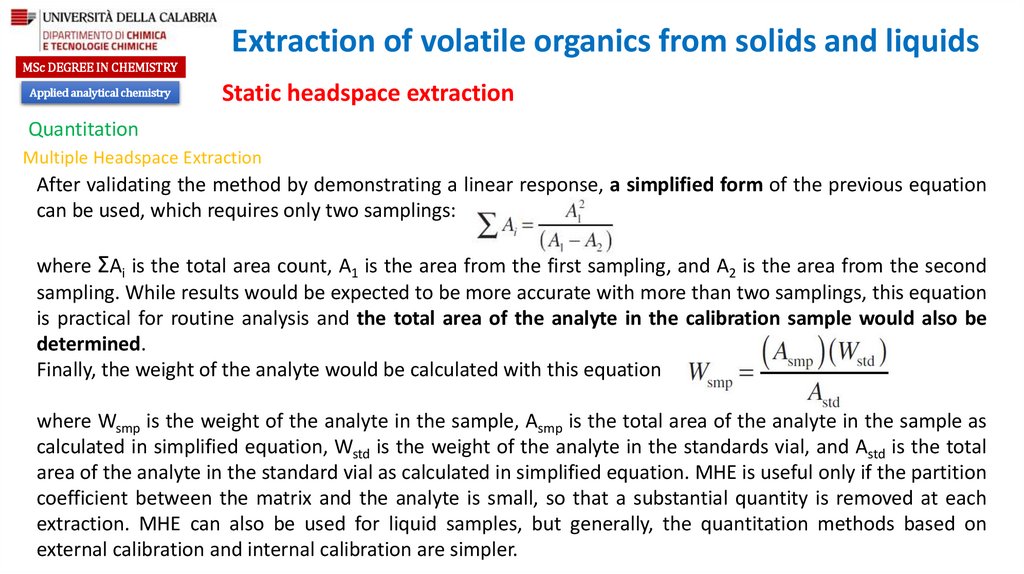
Optimization of headspace response
Partition coefficient
Ratio of the gas phase to the liquid phase


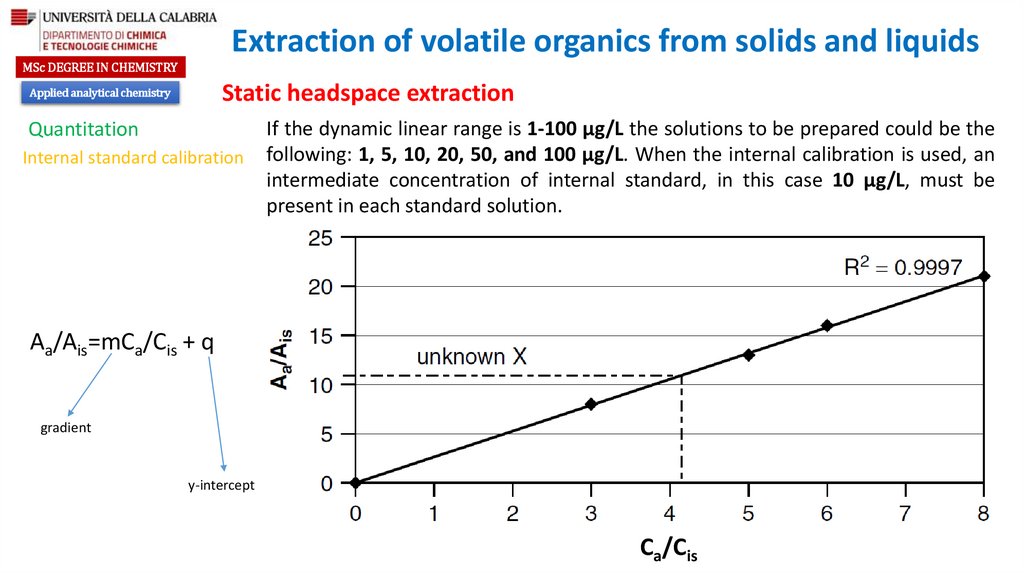





 Химия
Химия








