Похожие презентации:
Pathophysiology of the metabolic syndrome
1.
Pathophysiology of themetabolic syndrome
2.
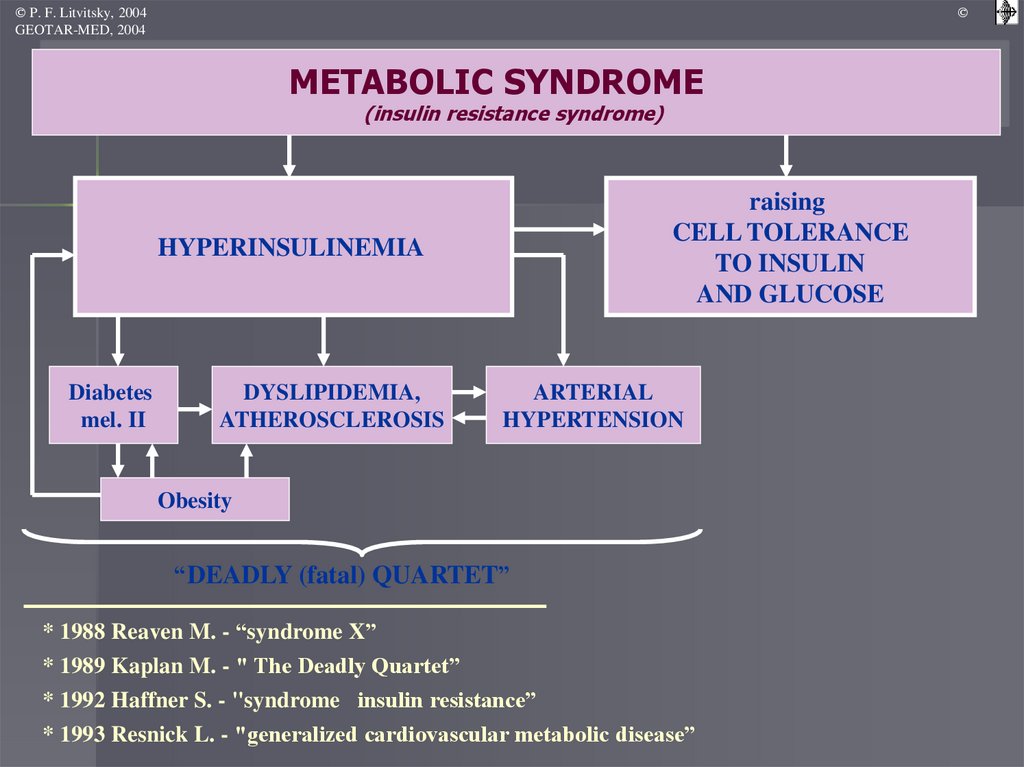
Metabolic syndrome (insulinresistance syndrome)
complex of metabolic, hormonal and
clinical disorders that are risk factors
for the development of cardiovascular
diseases, which are based on insulin
resistance and compensatory
hyperinsulinemia
3.
EpidemiologyIn industrial countries the prevalence is 10-20% in
the population over 30 years age
It is more common in men, while in women its
frequency increases in the menopausal period.
Accelerates the development and progression of
atherosclerotic vascular diseases, which occupy the
first place among the causes of death in the
population of developed countries.
Among patients with metabolic syndrome, the
mortality rate from CHD (coronary heart disease) is
23 times higher than in the general population
4.
© P. F. Litvitsky, 2004GEOTAR-MED, 2004
©
METABOLIC SYNDROME
(insulin resistance syndrome)
raising
CELL TOLERANCE
TO INSULIN
AND GLUCOSE
HYPERINSULINEMIA
Diabetes
mel. II
DYSLIPIDEMIA,
ATHEROSCLEROSIS
ARTERIAL
HYPERTENSION
Obesity
“DEADLY (fatal) QUARTET”
* 1988 Reaven M. - “syndrome X”
* 1989 Kaplan M. - " The Deadly Quartet”
* 1992 Haffner S. - "syndrome insulin resistance”
* 1993 Resnick L. - "generalized cardiovascular metabolic disease”
5.
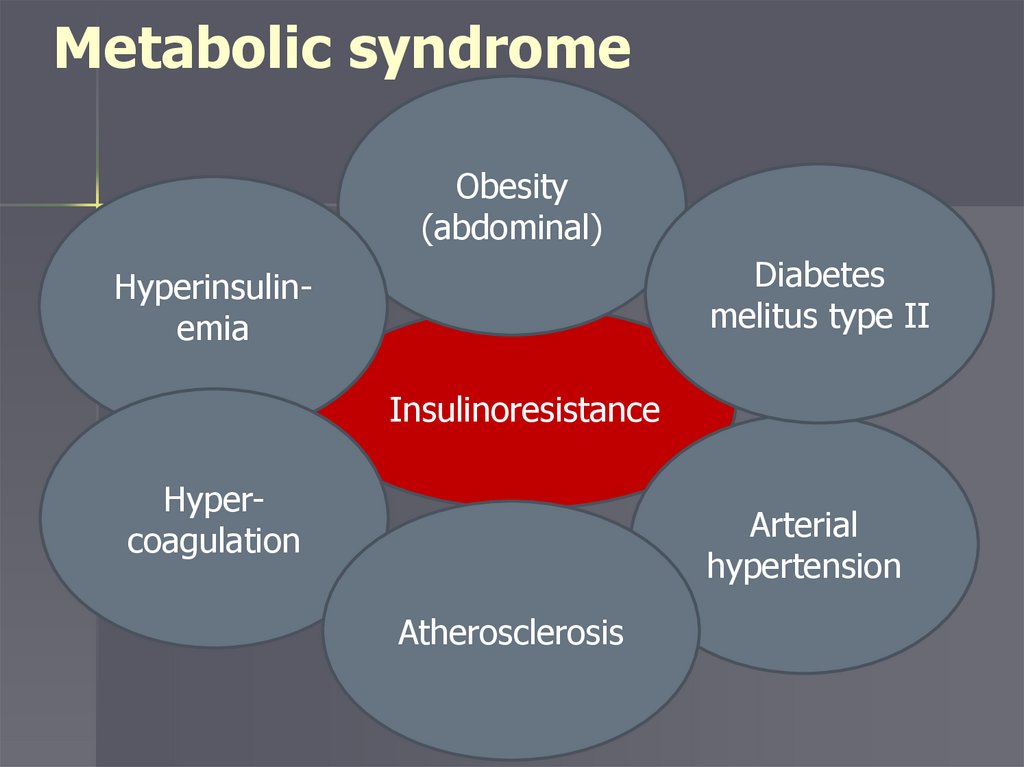
Metabolic syndromeObesity
(abdominal)
Diabetes
melitus type II
Hyperinsulinemia
Insulinoresistance
Hypercoagulation
Arterial
hypertension
Atherosclerosis
6.
Main manifestations of themetabolic syndrome
abdominal-visceral obesity
insulin resistance and hyperinsulinemia
dyslipidemia (lipid triad - ↑LDL, ↑ triglyceride, ↓HDL)
arterial hypertension
impaired glucose tolerance/type 2 diabetes mellitus
early atherosclerosis/Ischemic heart disease
hemostatic disorders
hyperuricemia and gout
microalbuminuria
7.
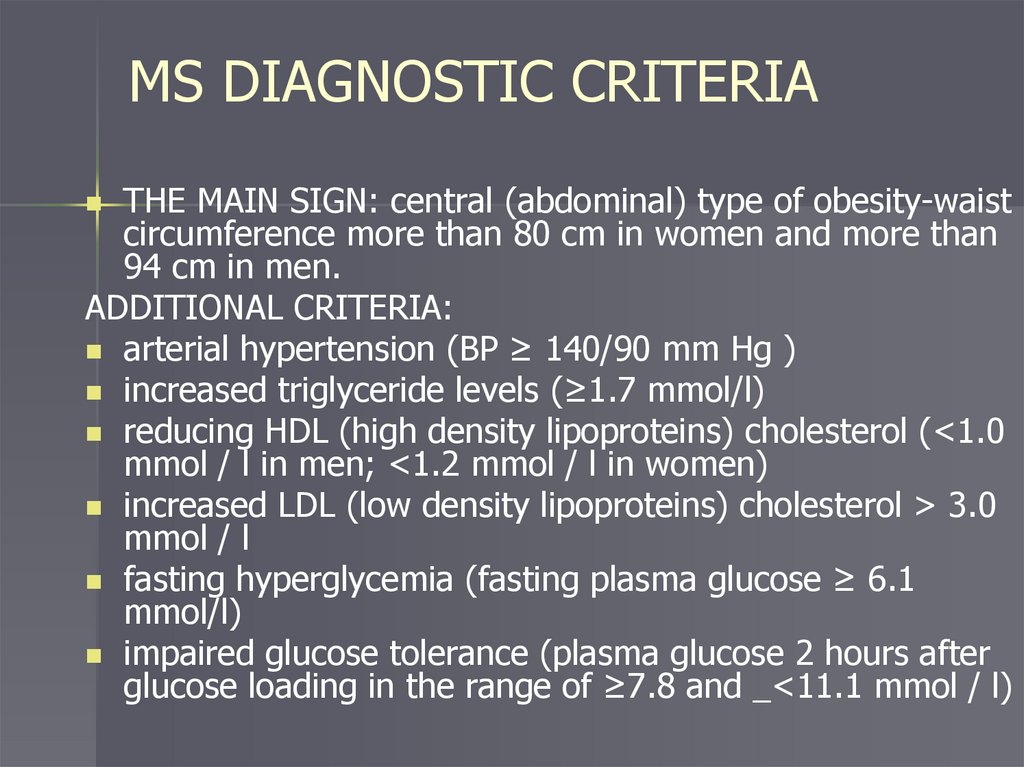
MS DIAGNOSTIC CRITERIATHE MAIN SIGN: central (abdominal) type of obesity-waist
circumference more than 80 cm in women and more than
94 cm in men.
ADDITIONAL CRITERIA:
arterial hypertension (BP ≥ 140/90 mm Hg )
increased triglyceride levels (≥1.7 mmol/l)
reducing HDL (high density lipoproteins) cholesterol (<1.0
mmol / l in men; <1.2 mmol / l in women)
increased LDL (low density lipoproteins) cholesterol > 3.0
mmol / l
fasting hyperglycemia (fasting plasma glucose ≥ 6.1
mmol/l)
impaired glucose tolerance (plasma glucose 2 hours after
glucose loading in the range of ≥7.8 and _<11.1 mmol / l)
8.
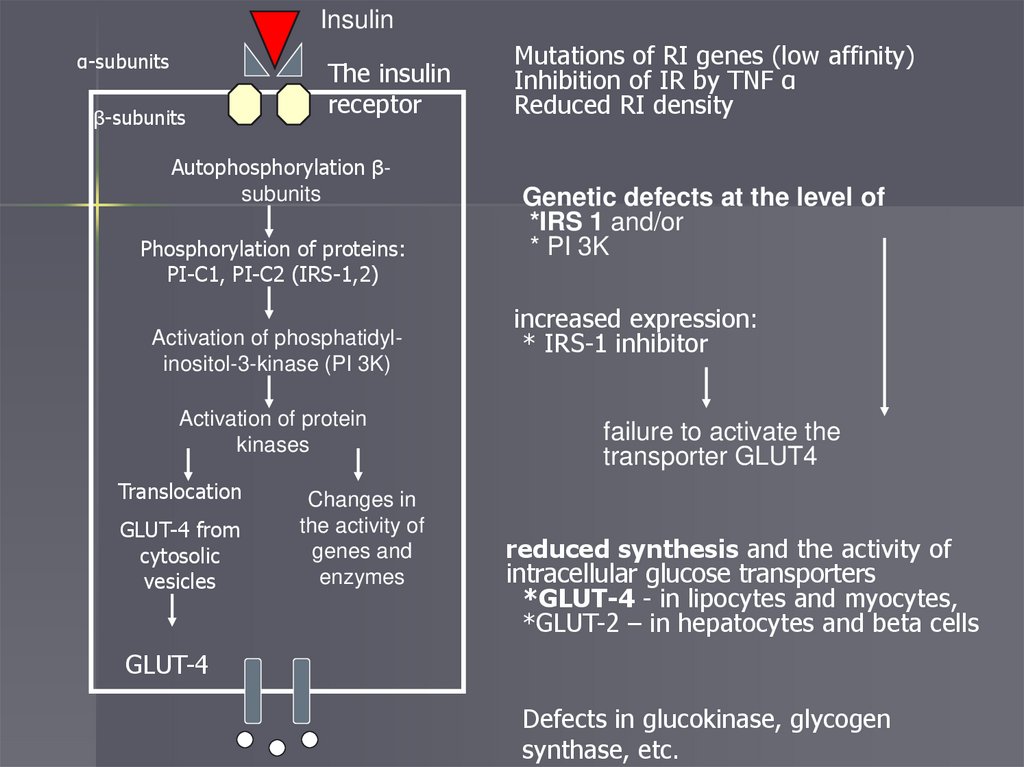
Insulin resistancea condition characterized by an
insufficient biological response of cells
to insulin when it is sufficiently
concentrated in the blood
Currently, IR is more often associated
with a violation of the action of insulin
at the post-receptor level
9.
Insulinα-subunits
β-subunits
The insulin
receptor
Autophosphorylation βsubunits
Phosphorylation of proteins:
PI-C1, PI-C2 (IRS-1,2)
Activation of phosphatidylinositol-3-kinase (PI 3K)
Activation of protein
kinases
Translocation
GLUT-4 from
cytosolic
vesicles
Changes in
the activity of
genes and
enzymes
Mutations of RI genes (low affinity)
Inhibition of IR by TNF α
Reduced RI density
Genetic defects at the level of
*IRS 1 and/or
* PI 3K
increased expression:
* IRS-1 inhibitor
failure to activate the
transporter GLUT4
reduced synthesis and the activity of
intracellular glucose transporters
*GLUT-4 - in lipocytes and myocytes,
*GLUT-2 – in hepatocytes and beta cells
GLUT-4
Defects in glucokinase, glycogen
synthase, etc.
10.
Impaired glucose toleranceand type 2 diabetes
11.
Stages of developmentI stage – initial insulin resistance
II stage – severe insulin resistance,
relative insulin insufficiency, impaired
glucose tolerance
III stage – reduced insulin secretion and
obvious diabetes
12.
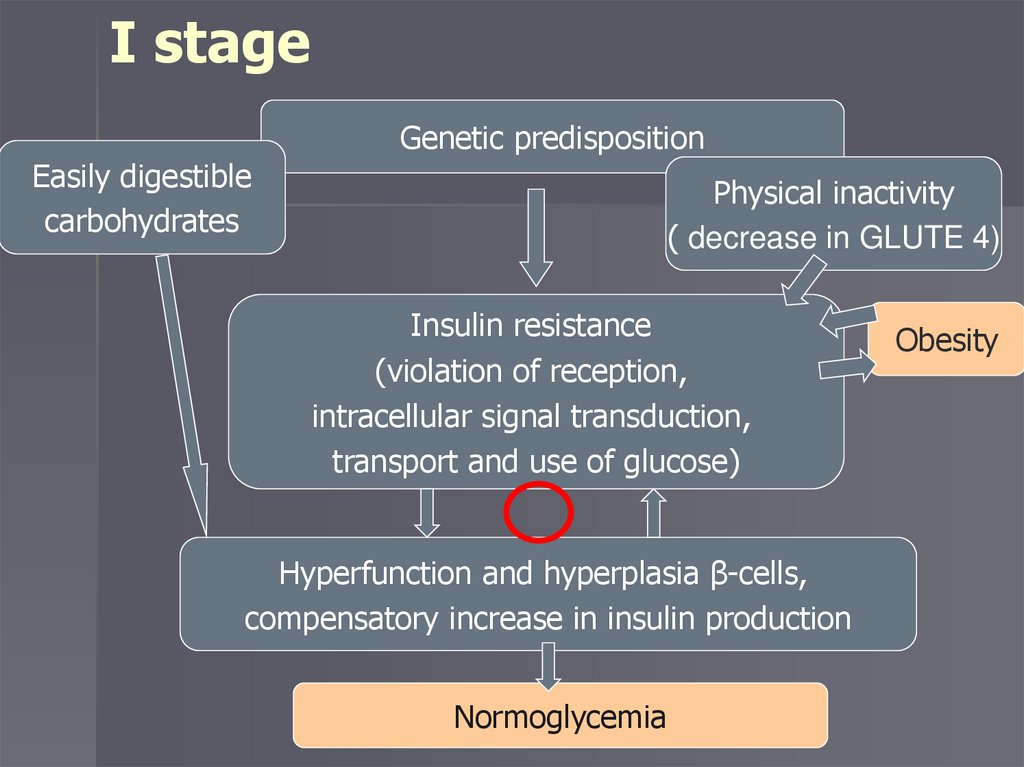
I stageGenetic predisposition
Easily digestible
carbohydrates
Physical inactivity
( decrease in GLUTE 4)
Insulin resistance
(violation of reception,
intracellular signal transduction,
transport and use of glucose)
Hyperfunction and hyperplasia β-cells,
compensatory increase in insulin production
Normoglycemia
Obesity
13.
II stageProgression of insulin resistance
Formation and / or progression of obesity
Reduced insulin receptor density;
↑ TNF α generation →
reduced kinase activity of IR
Relative insulin deficiency
Impaired glucose tolerance
14.
Insulin deficiency in the«impaired glucose
tolerance (IGT)» stage
Manifestations :
Violation of the secretion rhythm Ins: reduced capacity βcells respond with undulating peaks of insulin secretion to
fluctuations in glucose levels during the day
Mechanisms (?):
Genetic defects β- cells (defect of glucokinase and / or
glucose transporter GLUT-2 responsible for insulin
secretion in response to glucose stimulation)
The phenomenon of lipotoxicity: increased FFA
concentration → inhibition of glycolysis by inhibiting
pyruvate dehydrogenase → reduction of ATP formation →
violation insulin secretion
Violation of incretin production in the gastrointestinal tract
15.
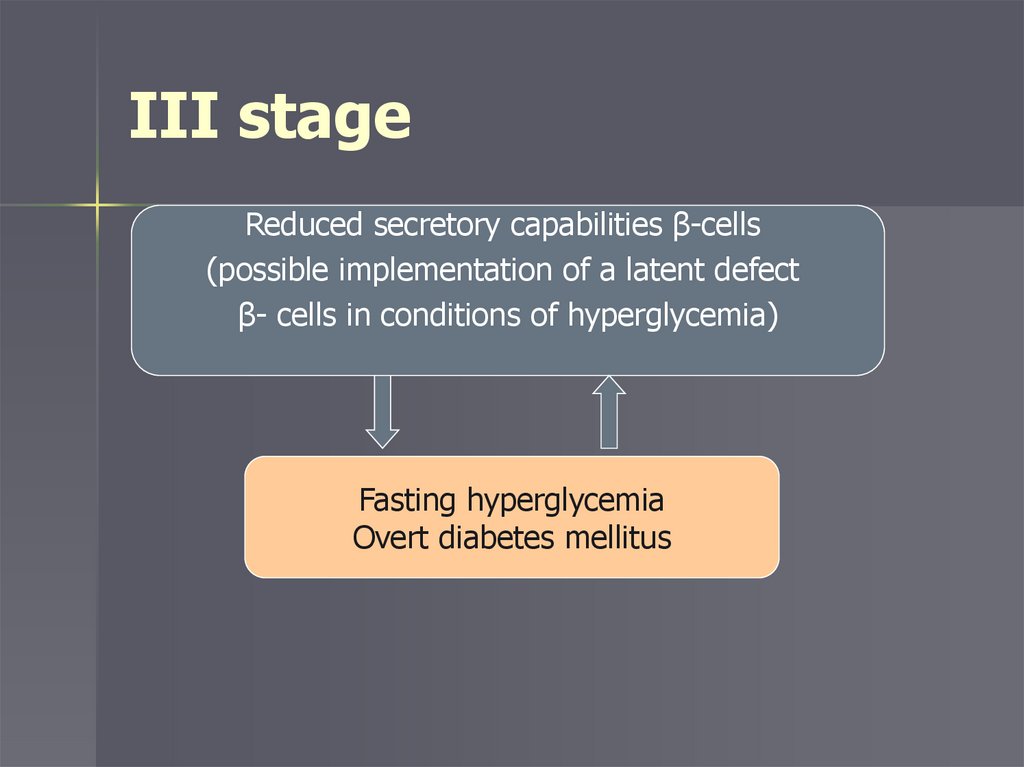
III stageReduced secretory capabilities β-cells
(possible implementation of a latent defect
β- cells in conditions of hyperglycemia)
Fasting hyperglycemia
Overt diabetes mellitus
16.
Insulin deficiency in DM 2Phase 1 of the secretory response to intravenous
glucose loading is reduced, the secretory response
to mixed food intake is delayed and reduced, the
concentration of proinsulin and its metabolic
products is increased, and the rhythm of
fluctuations in insulin secretion is disturbed
The phenomenon of glucose toxicity is important
17.
Significance of hyperglycemiaThe phenomenon of glucose toxicity biomolecular processes that cause the damaging
effect of long-term excess blood glucose on insulin
secretion and tissue sensitivity to insulin
Hyperglycemia leads to the development of oxidative
stress in many tissues; increased damage to betacells is associated with low levels of antioxidants in
them.
Closes the vicious circle in the pathogenesis of DM2.
Goal: to achieve normoglycemia in DM patients
18.
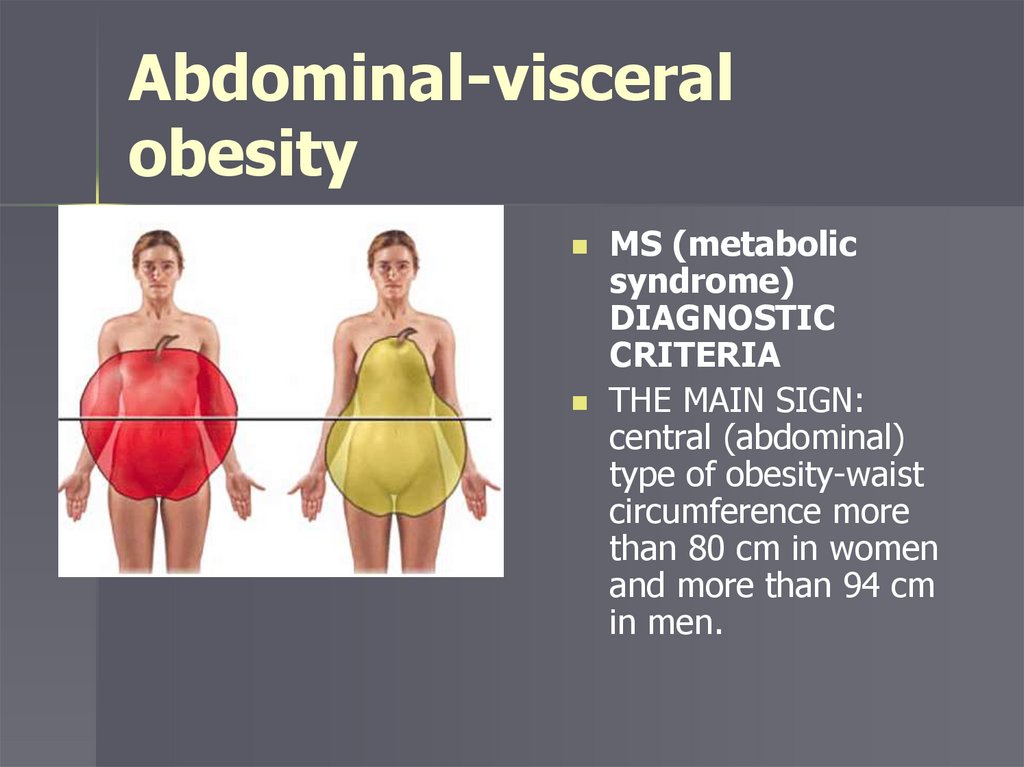
Abdominal-visceralobesity
MS (metabolic
syndrome)
DIAGNOSTIC
CRITERIA
THE MAIN SIGN:
central (abdominal)
type of obesity-waist
circumference more
than 80 cm in women
and more than 94 cm
in men.
19.
Abdominal obesity is one of the maincomponents of MS with a predominant
deposition of fat mass in the large omentum
and retroperitoneal space
An increase in the ideal body weight by 3540% leads to a decrease in the sensitivity of
tissues to insulin by more than 40%
20.
The role of obesity in thepathogenesis of MS
Features of visceral adipocytes:
↓ sensitivity to the anti-lipolytic action of insulin and ↑
sensitivity to lipolytic action of catecholamines
Activation of lipolysis → intake of a large amount of
free fatty acids (FFA) into portal circulation, and then into systemic circulation
Subcutaneous adipose tissue is more sensitive to the
inhibitory effect of insulin, which contributes to the reesterification of FFA to triglicerids and the progression
of obesity
21.
The role of obesity in thepathogenesis of MS
TNF-α - cytokine synthesized by adipocytes
Reduces the sensitivity of insulin receptors
promotes an increase in FFA by activating lipolysis
processes in adipose tissue (FFA inhibits the expression
of the gene responsible for the synthesis of GLUT-4)
In hepatocytes: inhibits the expression of genes involved
in glucose metabolism and FFA oxidation
- increases the expression of genes that regulate the
synthesis of cholesterol and fatty acids
22.
The role of obesity in thepathogenesis of MS
On enlarged lipocytes, the density decreases
and the conformation of insulin receptors is
disturbed
Inactivity also worsens the existing IR, as
translocation of glucose transporters (GLUT4) in muscle tissue at rest is sharply reduced
23.
Dyslipidemia andatherosclerosis
24.
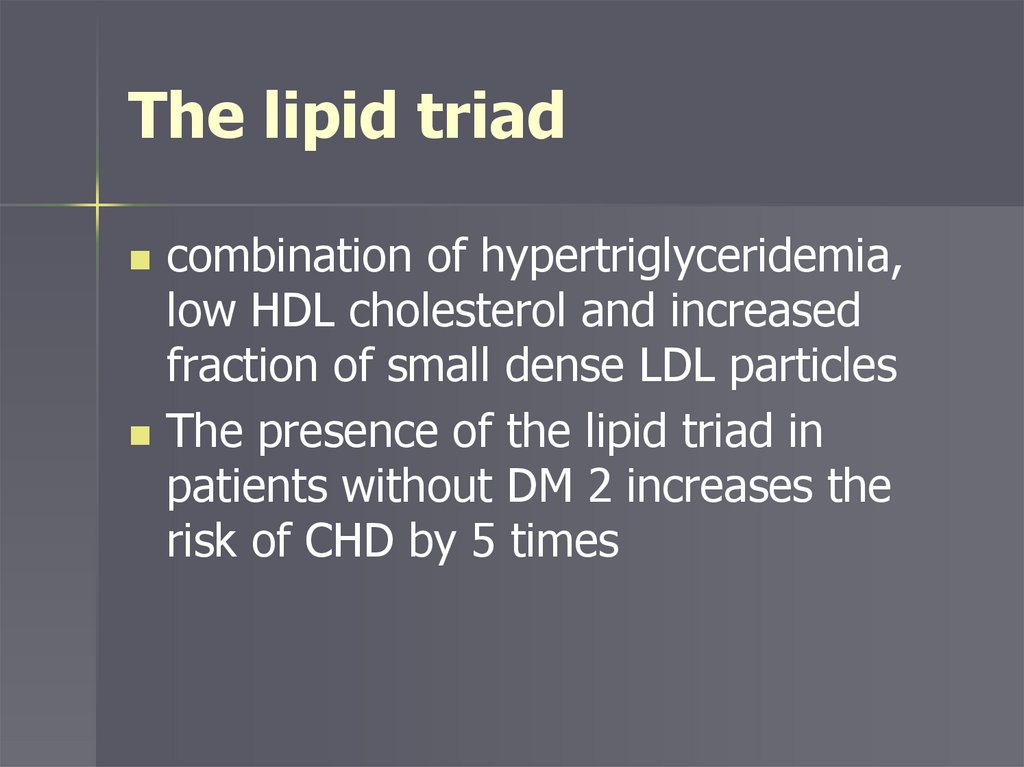
The lipid triadcombination of hypertriglyceridemia,
low HDL cholesterol and increased
fraction of small dense LDL particles
The presence of the lipid triad in
patients without DM 2 increases the
risk of CHD by 5 times
25.
Atherogenic changes in theblood lipid spectrum
increased levels of triglycerides, LDL cholesterol
Excessive release of FFA (substrate for TH synthesis)
→ increased production of VLDL (the main
transporters of TG)
persistent increase in Apo-B secretion and reduction of
its degradation → increase in VLDL production
↓ lipoprotein lipase and hepatic triglyceride lipase
activity → decline elimination of VLDL and LDL →
increases duration of circulation of atherogenic
lipoproteins in the blood
26.
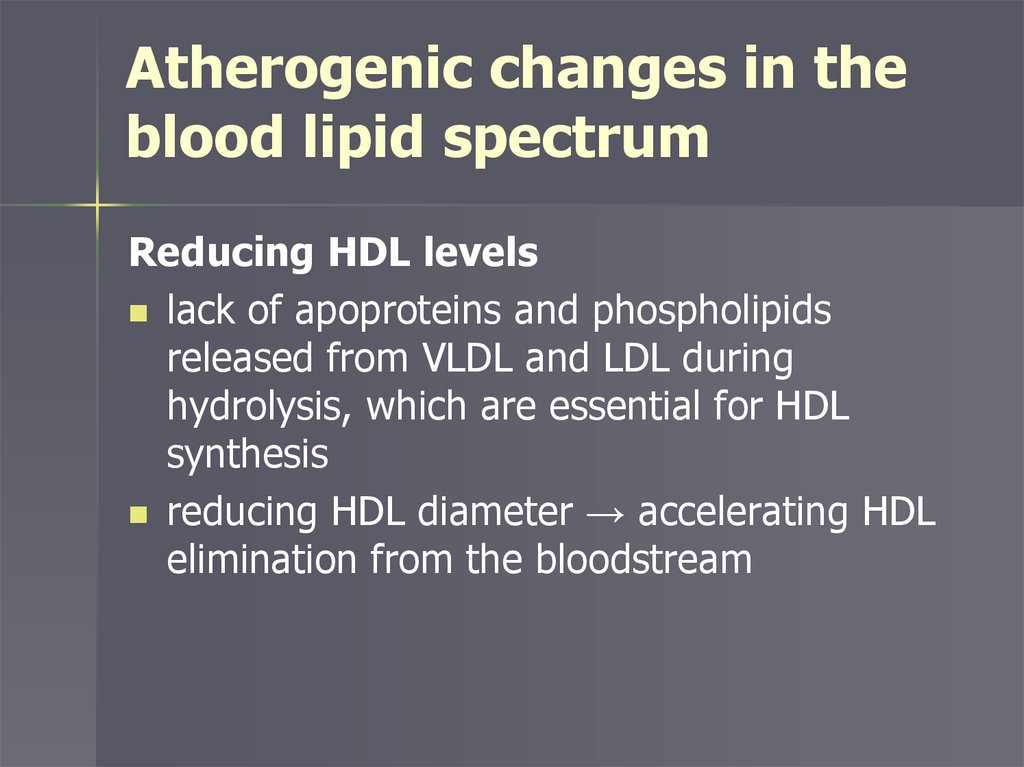
Atherogenic changes in theblood lipid spectrum
Reducing HDL levels
lack of apoproteins and phospholipids
released from VLDL and LDL during
hydrolysis, which are essential for HDL
synthesis
reducing HDL diameter → accelerating HDL
elimination from the bloodstream
27.
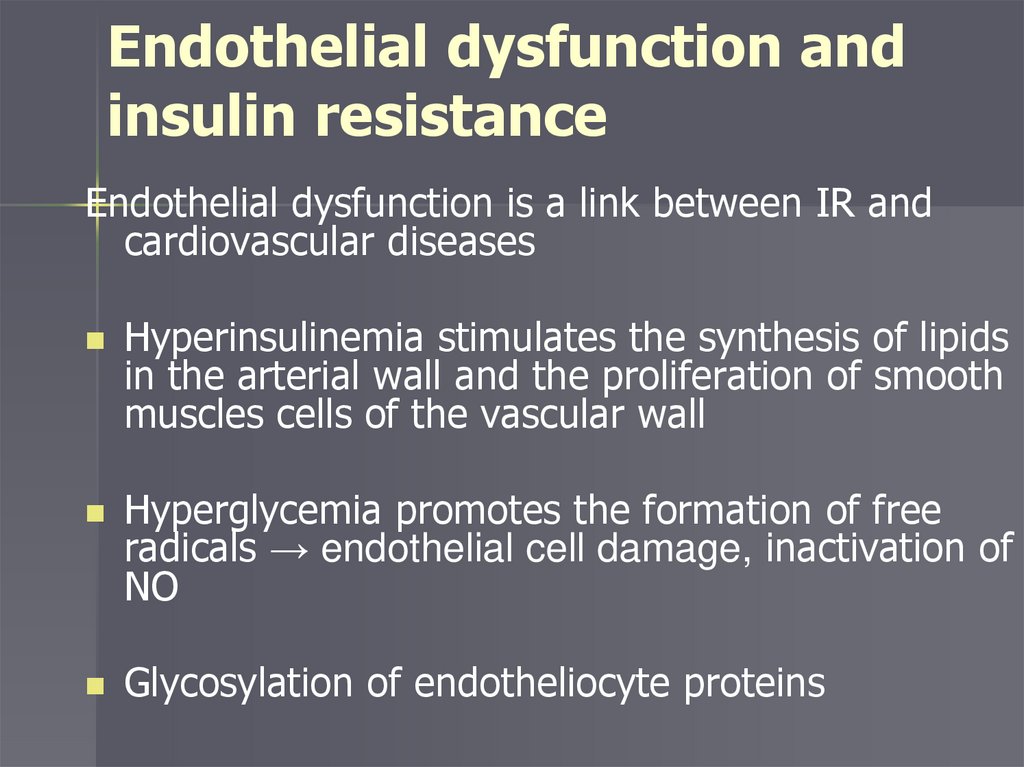
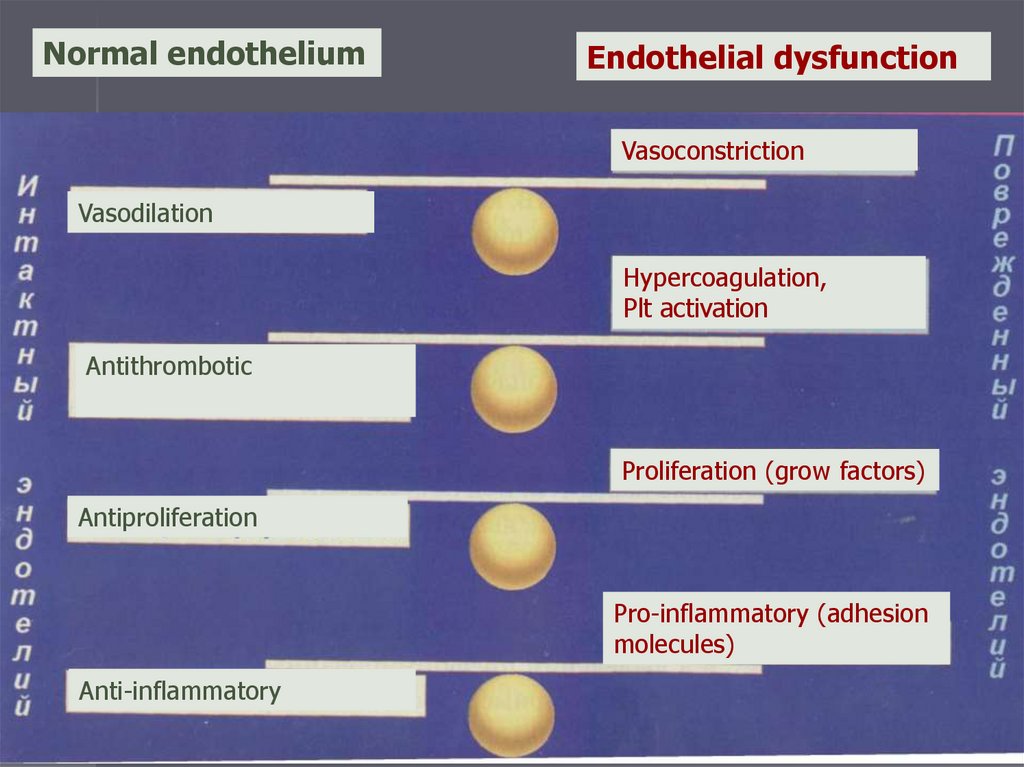
Endothelial dysfunction andinsulin resistance
Endothelial dysfunction is a link between IR and
cardiovascular diseases
Hyperinsulinemia stimulates the synthesis of lipids
in the arterial wall and the proliferation of smooth
muscles cells of the vascular wall
Hyperglycemia promotes the formation of free
radicals → endothelial cell damage, inactivation of
NO
Glycosylation of endotheliocyte proteins
28.
Normal endotheliumEndothelial dysfunction
Vasoconstriction
Vasodilation
Hypercoagulation,
Plt activation
Antithrombotic
Proliferation (grow factors)
Antiproliferation
Pro-inflammatory (adhesion
molecules)
Anti-inflammatory
29.
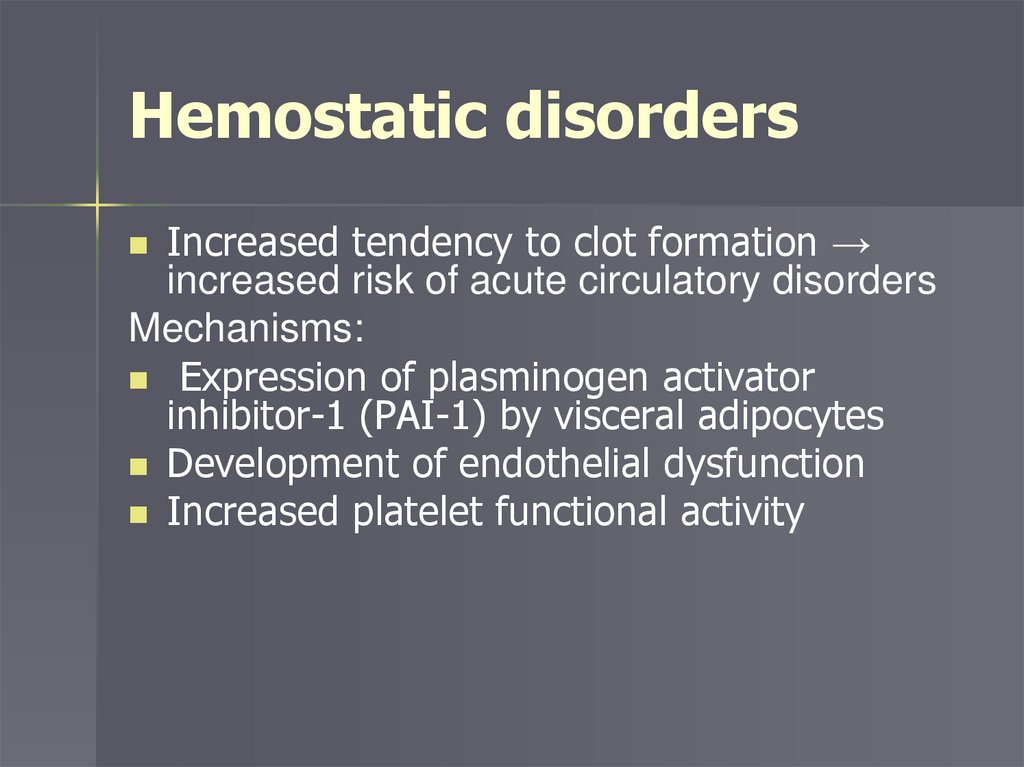
Hemostatic disordersIncreased tendency to clot formation →
increased risk of acute circulatory disorders
Mechanisms:
Expression of plasminogen activator
inhibitor-1 (PAI-1) by visceral adipocytes
Development of endothelial dysfunction
Increased platelet functional activity
30.
Early atherosclerosis and diabeticmacroangiopathies
■ Coronary (ischemic) heart disease (CHD)
■ Cerebrovascular disease (CVD)
■ Chronic obliterating diseases of the
peripheral arteries
31.
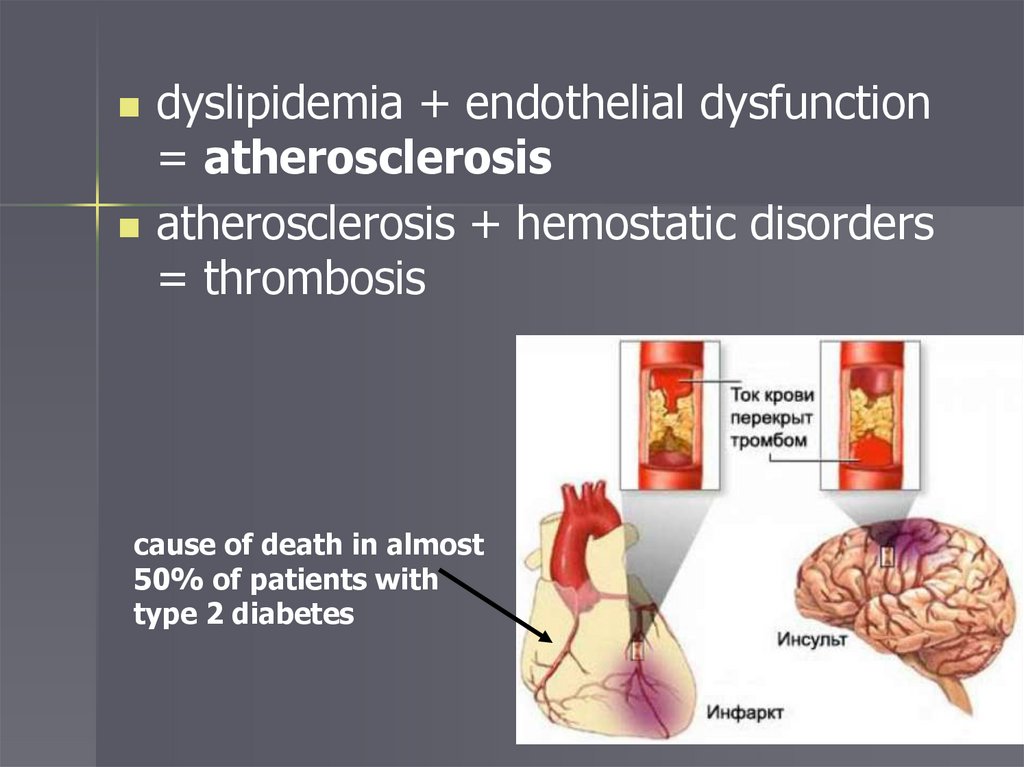
dyslipidemia + endothelial dysfunction= atherosclerosis
atherosclerosis + hemostatic disorders
= thrombosis
cause of death in almost
50% of patients with
type 2 diabetes
32.
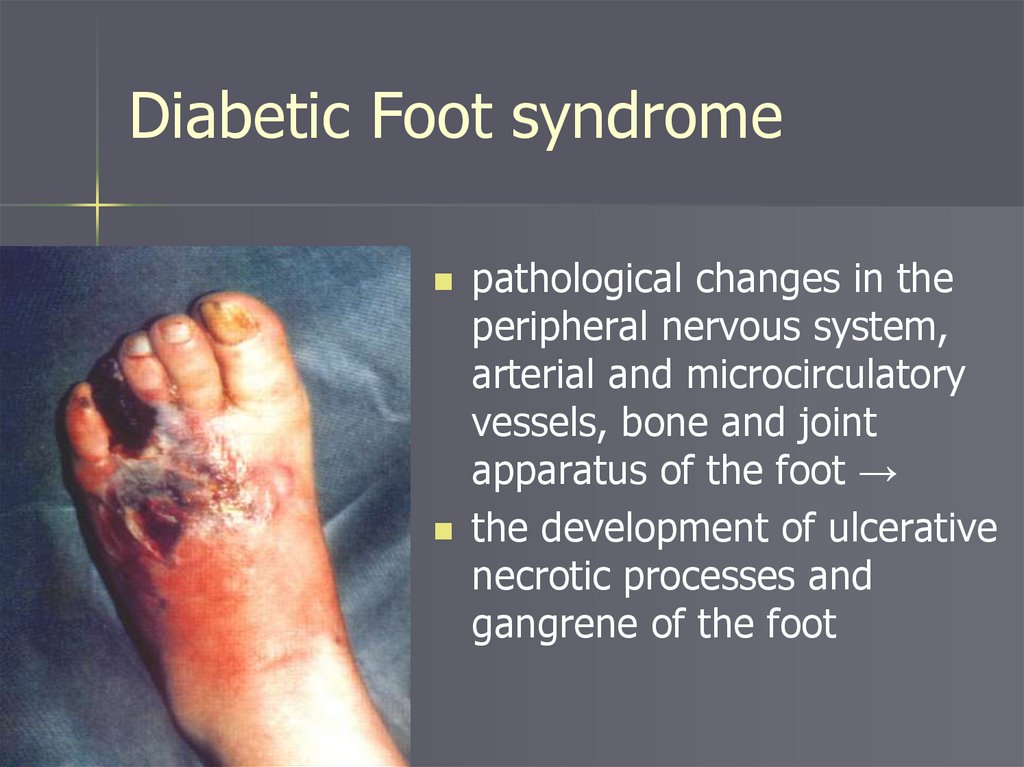
Diabetic Foot syndromepathological changes in the
peripheral nervous system,
arterial and microcirculatory
vessels, bone and joint
apparatus of the foot →
the development of ulcerative
necrotic processes and
gangrene of the foot
33.
Arterial hypertension34.
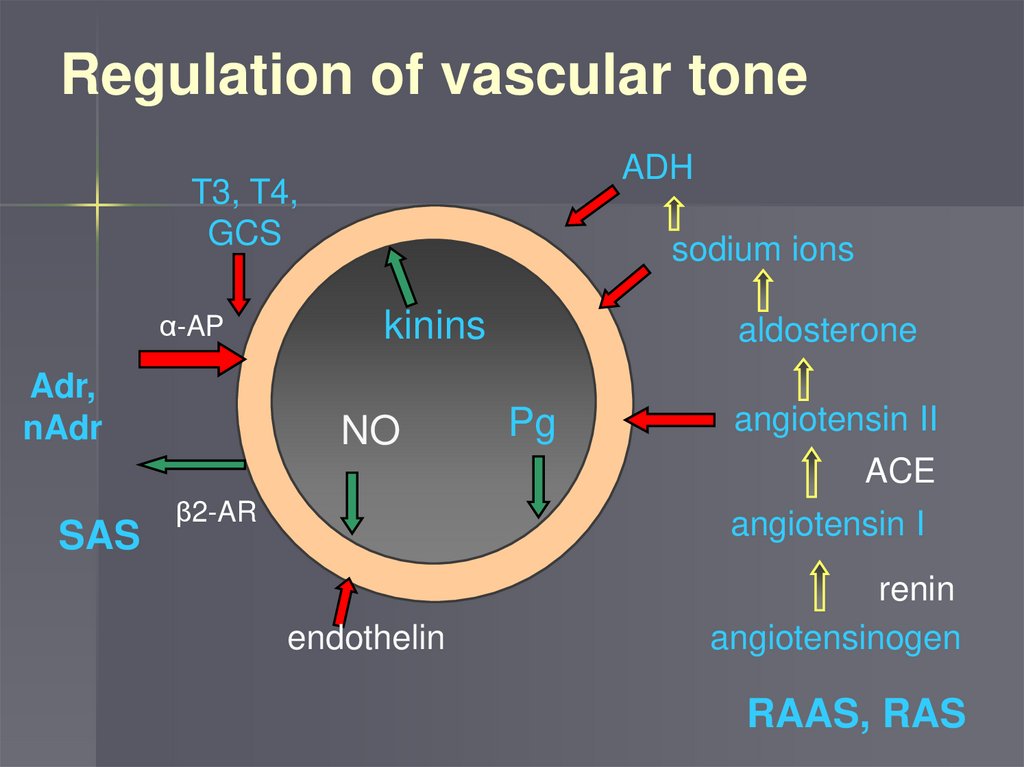
Regulation of vascular toneADH
T3, T4,
GCS
α-AP
Adr,
nAdr
sodium ions
kinins
NO
aldosterone
Pg
angiotensin II
ACE
SAS
β2-AR
angiotensin I
endothelin
renin
angiotensinogen
RAAS, RAS
35.
Mechanisms of action ofhyperinsulinemia on blood
pressure
it stimulates the activity of the sympathetic nervous
system
it stimulates the activity of RAAS
increases Na+ reabsorption in the proximal and distal
tubules of the nephron
blocks transmembrane ion exchange mechanisms (Na+,
K+ , and Ca2+ - dependent ATPase), increasing the
content of intracellular Na+ and Ca2+ in vascular MMCs
promotes the development of endothelial dysfunction,
reducing the production of NO
stimulates proliferation of smooth muscle cells of the
vascular wall
36.
Hyperuricemia37.
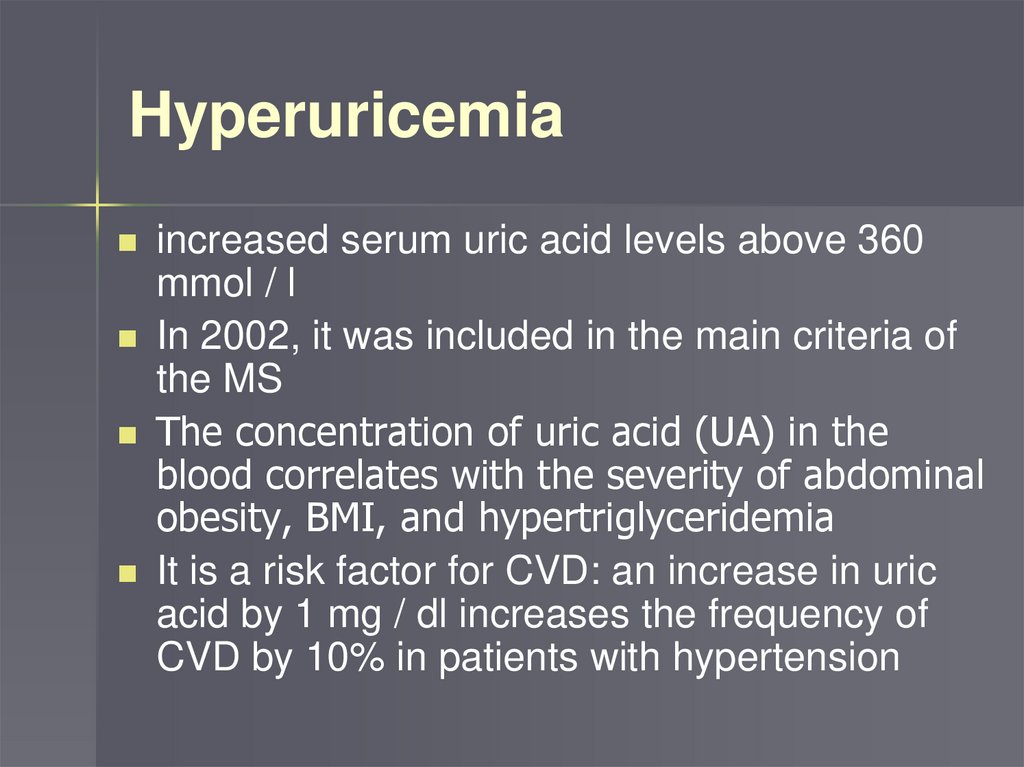
Hyperuricemiaincreased serum uric acid levels above 360
mmol / l
In 2002, it was included in the main criteria of
the MS
The concentration of uric acid (UA) in the
blood correlates with the severity of abdominal
obesity, BMI, and hypertriglyceridemia
It is a risk factor for CVD: an increase in uric
acid by 1 mg / dl increases the frequency of
CVD by 10% in patients with hypertension
38.
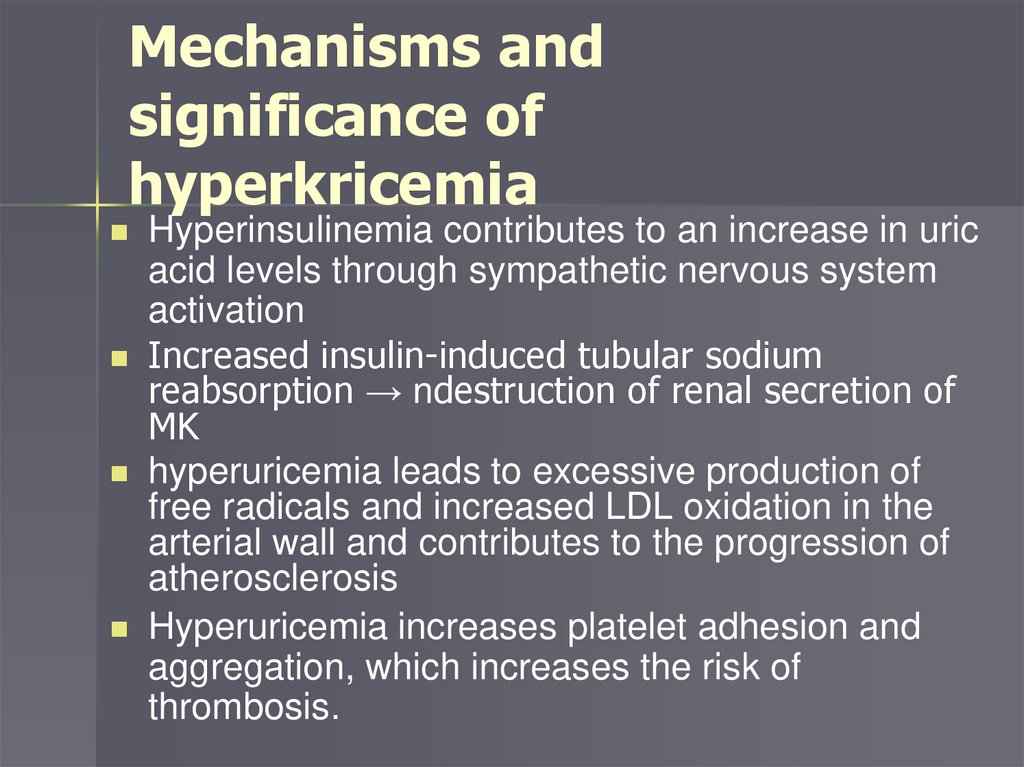
Mechanisms andsignificance of
hyperkricemia
Hyperinsulinemia contributes to an increase in uric
acid levels through sympathetic nervous system
activation
Increased insulin-induced tubular sodium
reabsorption → ndestruction of renal secretion of
MK
hyperuricemia leads to excessive production of
free radicals and increased LDL oxidation in the
arterial wall and contributes to the progression of
atherosclerosis
Hyperuricemia increases platelet adhesion and
aggregation, which increases the risk of
thrombosis.
39.
MicroalbuminuriaIt is associated with the development
of endothelial dysfunction and arterial
hypertension
With the progression of kidney
damage, it leads to the development
of CRF
40.
Principles of therapy ofthe
metabolic syndrome
41.
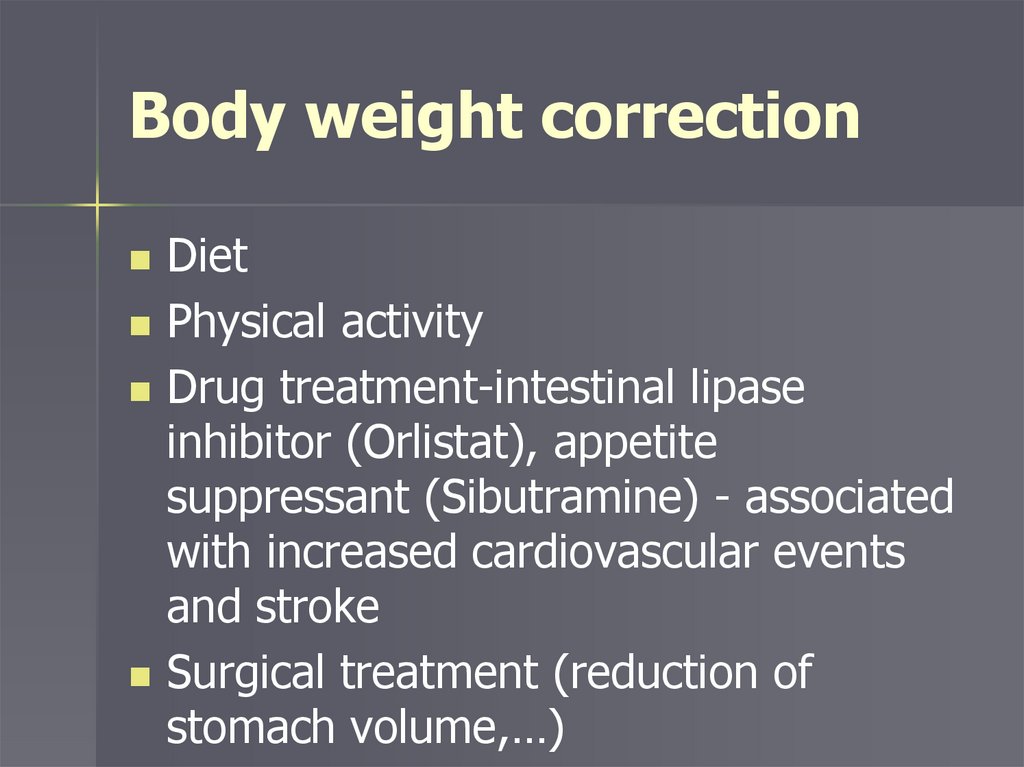
Body weight correctionDiet
Physical activity
Drug treatment-intestinal lipase
inhibitor (Orlistat), appetite
suppressant (Sibutramine) - associated
with increased cardiovascular events
and stroke
Surgical treatment (reduction of
stomach volume,…)
42.
Correction of insulinresistance
Diet
Physical activity
Biguanides (metformin)
Thiazolidinediones (Pioglitazone,
Rosiglitazone) - agonists PPAR γ - reduce
insulin resistance, increase the utilization of
FA, contribute to the correction of
dyslipidemia
43.
Correction of dyslipidemiaDiet
Body weight correction
Statins (reduce cholesterol synthesis in
the liver)
44.
Correction of hyperglycemiaand insufficiency of Ins
Reduced intestinal glucose absorption-alpha-
glycosidase (acarbose) inhibitors
Insulin production stimulants:
- Preparations of sulfonylureas (maninil)
- Meglitinids (repaglinid=novonorm)
- Incretin analogues of GLP 1 and DPP 4
inhibitors
Insulin
45.
Incretine systemGlucagon-like peptide-1 (GLP1) is produced in
intestine and stimulate beta cells
Dipeptidyl peptidase 4 (DPP4) is enzyme breaks
down the incretins
Glucagon-like
peptide-1
receptor agonists
– Liraglutide,…
Sitagliptin
(Januvia)
46.
Blood pressure correctionACE inhibitors
angiotensin receptor blockers
Long-acting calcium channels blockers
If necessary – other groups
Correction of the hemostatic
system
Antiplatelet agents - ASK
47.
Thank you for yourattention
























 Медицина
Медицина








