Похожие презентации:
CHE1226 Physical Chemistry School of Chemical Engineering Lecture 2 – Properties of gases
1. CHE1226 Physical Chemistry
School of Chemical EngineeringLecture 2 – Properties of gases
2. Table of contents
1. Kinetic Model of Gases• Theory
• Ideal Gas Law
• Dalton’s Law
2. Real Gases
• Collisions
• Van del Waals Equation of State
Learning Objective: Understand some of the theories that predict the properties of gases and describe
when they fail.
References:
P. Atkins and J. de Paula, Elements of Physical Chemistry, Chapter 1
3. Kinetic Model of Gases
• The kinetic model of gases is based on three assumptions:1. A gas consists of molecules in ceaseless random motion
2. The size of molecules is negligible in the sense that their diameters are much smaller
than the average distance travelled between collisions
3. The molecules don’t interact, except during collisions
• In terms of energy:
Kinetic Model of Gases = Ideal Gas Law
• Assuming that molecules don’t interact unless in direct contact implies that Ep is
independent of their separation and can be set to zero
• The total energy is therefore based on gas’ Ek. Thus, the faster the molecules move, the
greater the total energy of the gas
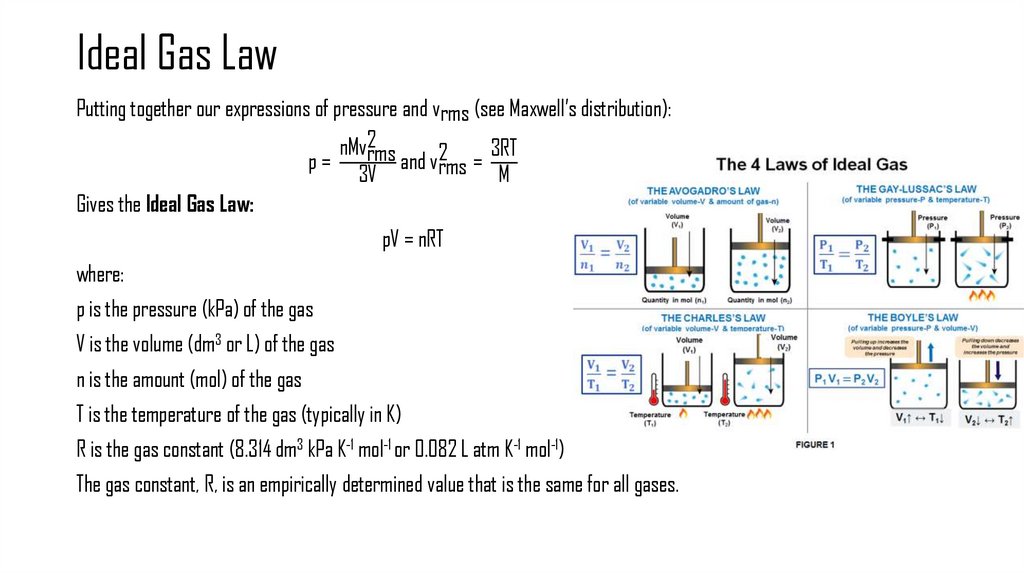
4. Ideal Gas Law
Putting together our expressions of pressure and vrms (see Maxwell’s distribution):nMv2rms
3RT
p=
and v2rms =
3V
M
Gives the Ideal Gas Law:
pV = nRT
where:
p is the pressure (kPa) of the gas
V is the volume (dm3 or L) of the gas
n is the amount (mol) of the gas
T is the temperature of the gas (typically in K)
R is the gas constant (8.314 dm3 kPa K-1 mol-1 or 0.082 L atm K-1 mol-1)
The gas constant, R, is an empirically determined value that is the same for all gases.
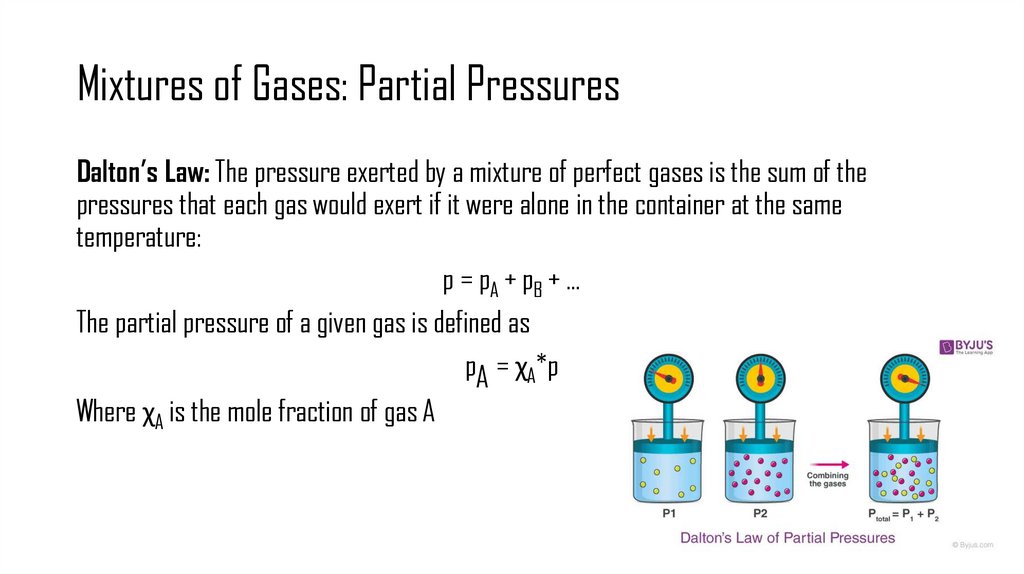
5. Mixtures of Gases: Partial Pressures
Dalton’s Law: The pressure exerted by a mixture of perfect gases is the sum of thepressures that each gas would exert if it were alone in the container at the same
temperature:
p = pA + pB + …
The partial pressure of a given gas is defined as
pA = χA*p
Where χA is the mole fraction of gas A
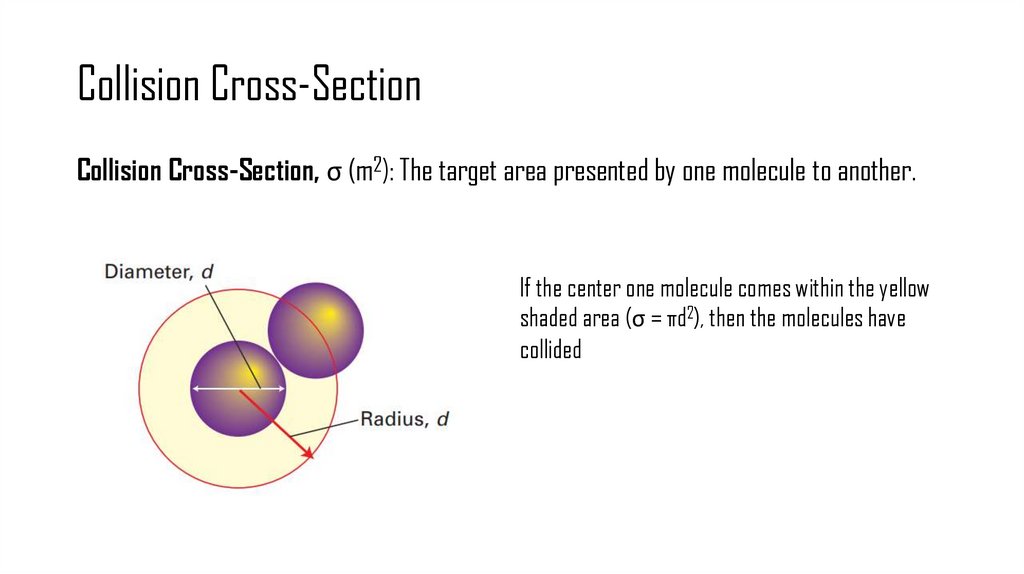
6. Collision Cross-Section
Collision Cross-Section, σ (m2): The target area presented by one molecule to another.If the center one molecule comes within the yellow
shaded area (σ = πd2), then the molecules have
collided
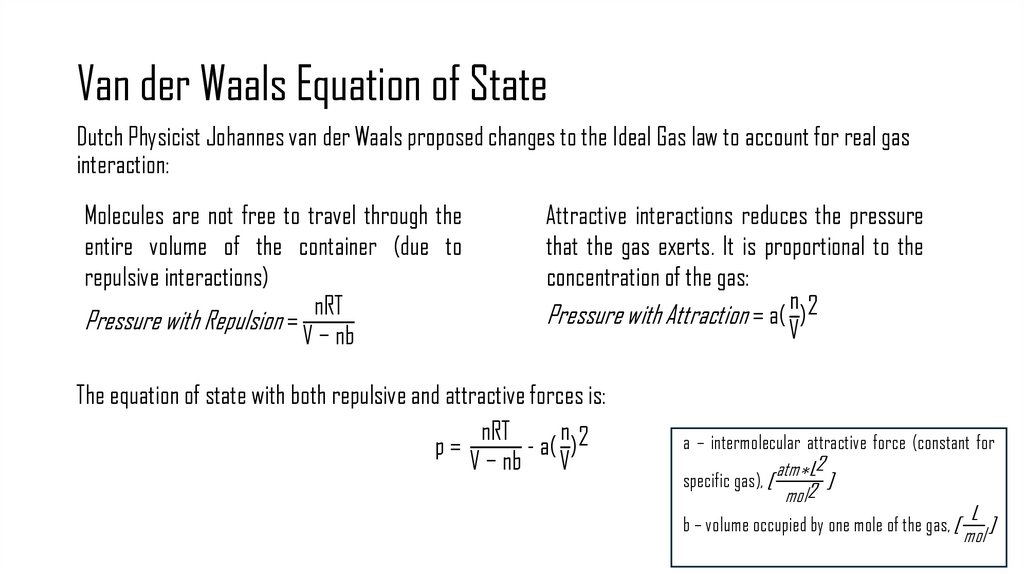
7. Van der Waals Equation of State
Dutch Physicist Johannes van der Waals proposed changes to the Ideal Gas law to account for real gasinteraction:
Molecules are not free to travel through the
entire volume of the container (due to
repulsive interactions)
nRT
Pressure with Repulsion =
V − nb
Attractive interactions reduces the pressure
that the gas exerts. It is proportional to the
concentration of the gas:
n2
Pressure with Attraction = a( )
V
The equation of state with both repulsive and attractive forces is:
nRT
n
p=
- a( ) 2
V − nb
V
a – intermolecular attractive force (constant for
atm∗L2
specific gas), [
]
mol2
b – volume occupied by one mole of the gas, [
L
]
mol
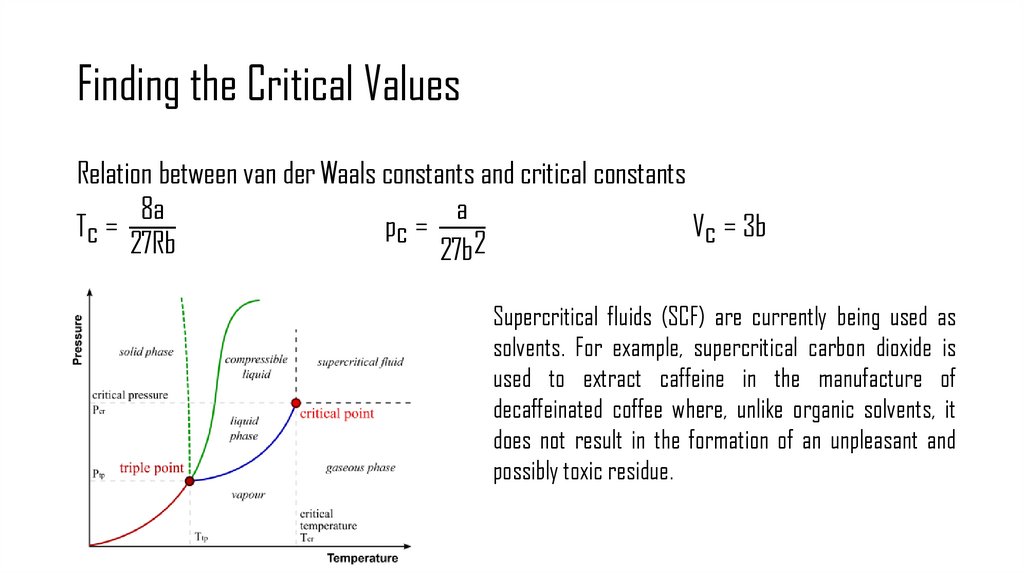
8. Finding the Critical Values
Relation between van der Waals constants and critical constants8a
a
Tc =
pc =
Vc = 3b
27Rb
27b2
Supercritical fluids (SCF) are currently being used as
solvents. For example, supercritical carbon dioxide is
used to extract caffeine in the manufacture of
decaffeinated coffee where, unlike organic solvents, it
does not result in the formation of an unpleasant and
possibly toxic residue.
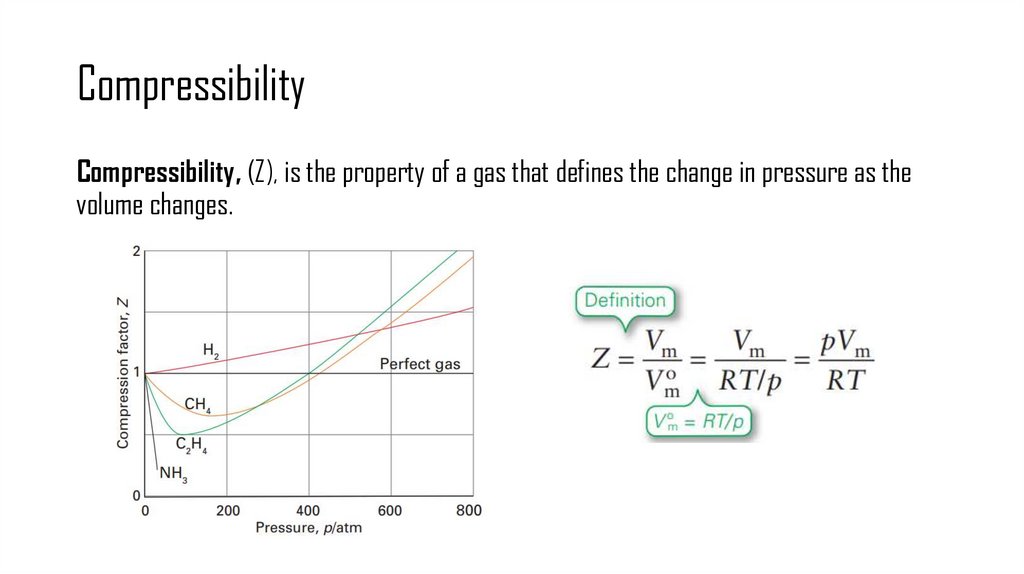
9. Compressibility
Compressibility, (Z), is the property of a gas that defines the change in pressure as thevolume changes.


 Физика
Физика








