Похожие презентации:
Revision on carbonyl compounds 4 (1)
1.
2.
3.
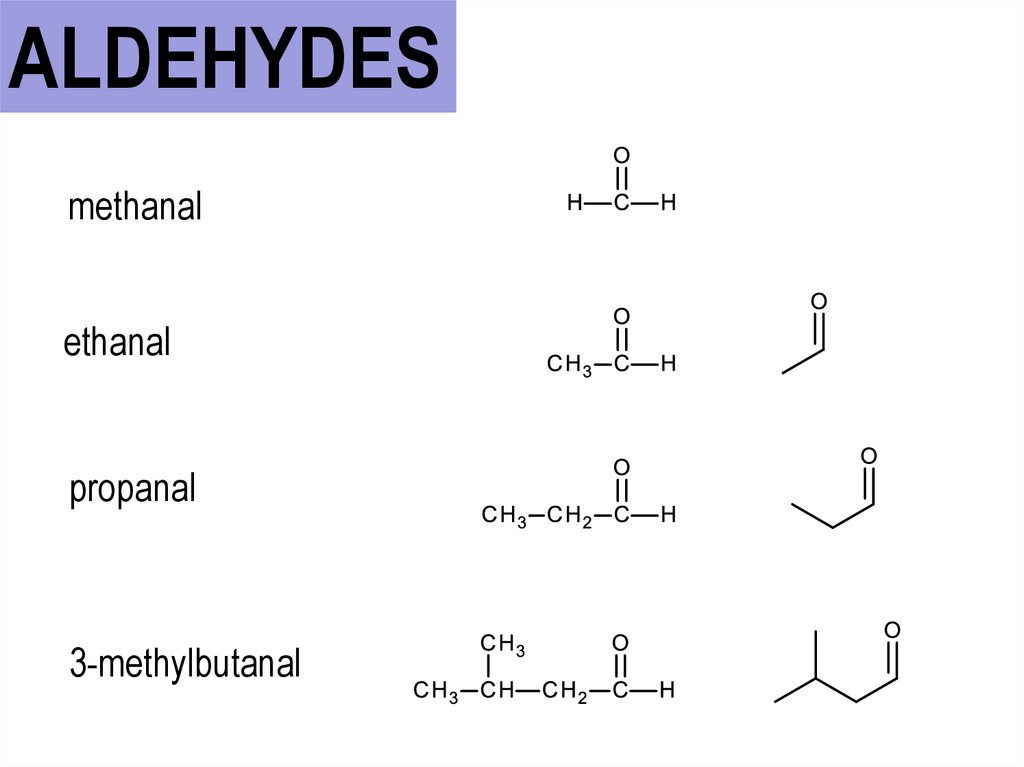
ALDEHYDESmethanal
ethanal
propanal
3-methylbutanal
4.
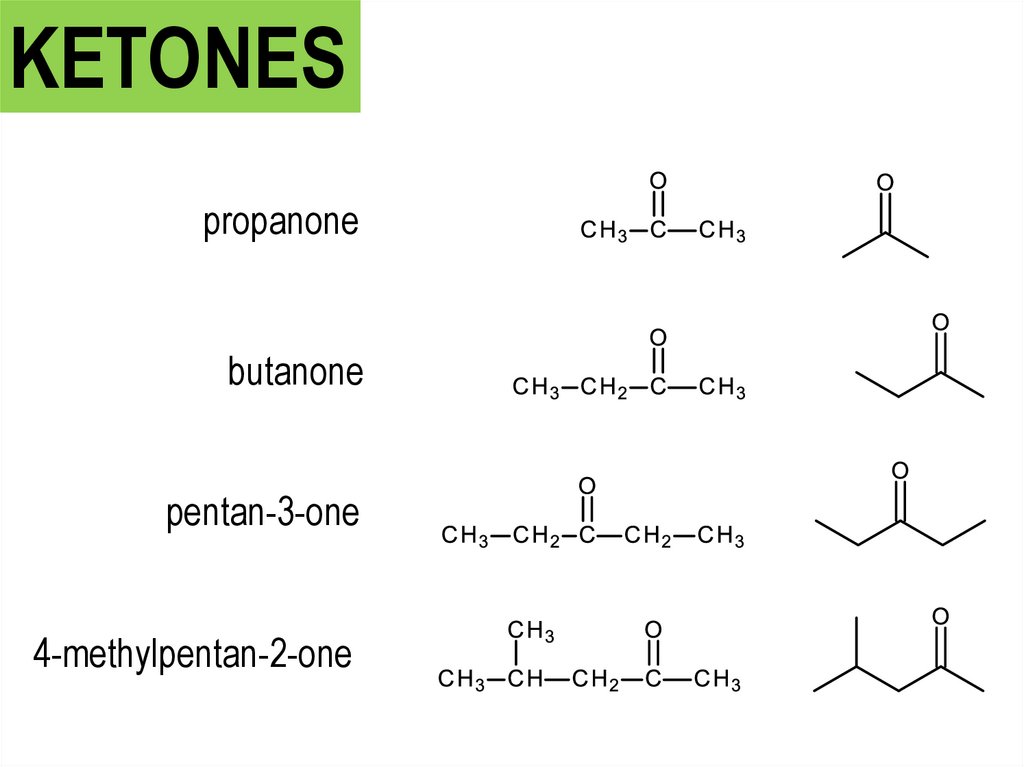
KETONESpropanone
butanone
pentan-3-one
4-methylpentan-2-one
5.
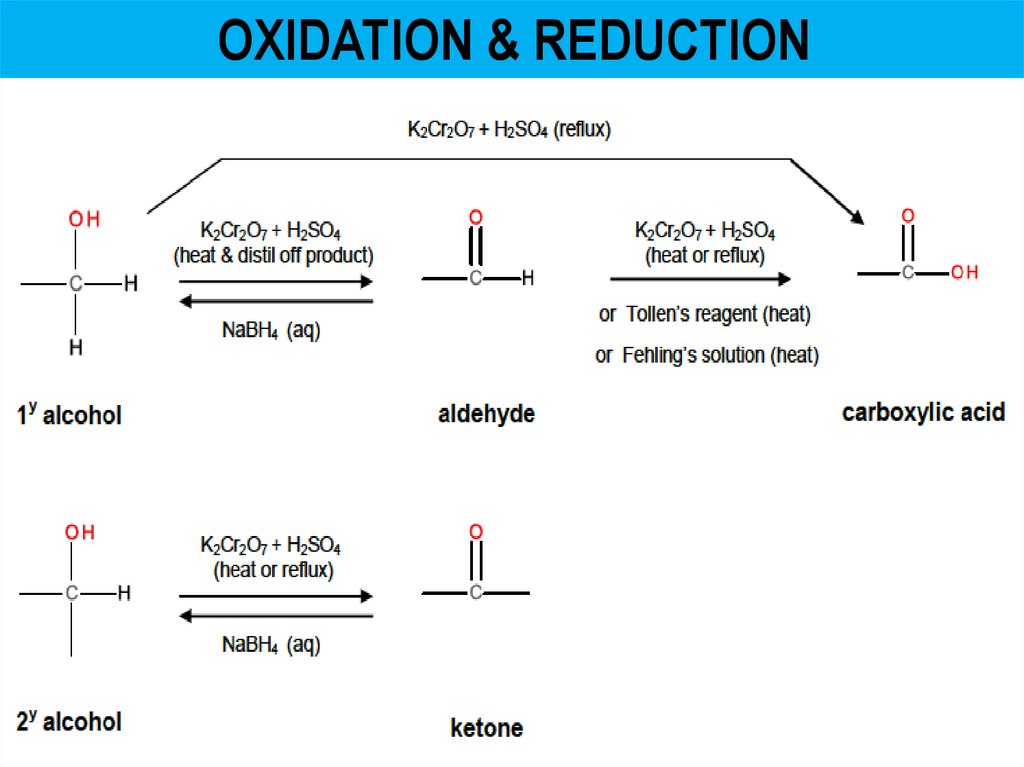
OXIDATION & REDUCTION6.
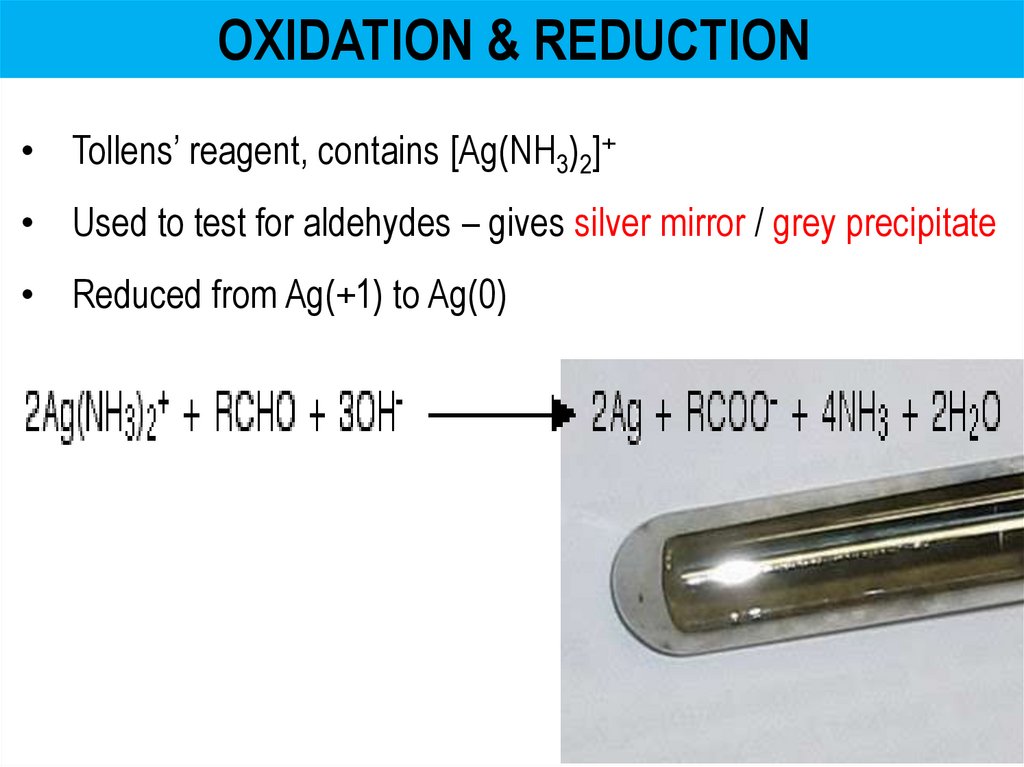
OXIDATION & REDUCTION• Tollens’ reagent, contains [Ag(NH3)2]+
• Used to test for aldehydes – gives silver mirror / grey precipitate
• Reduced from Ag(+1) to Ag(0)
7.
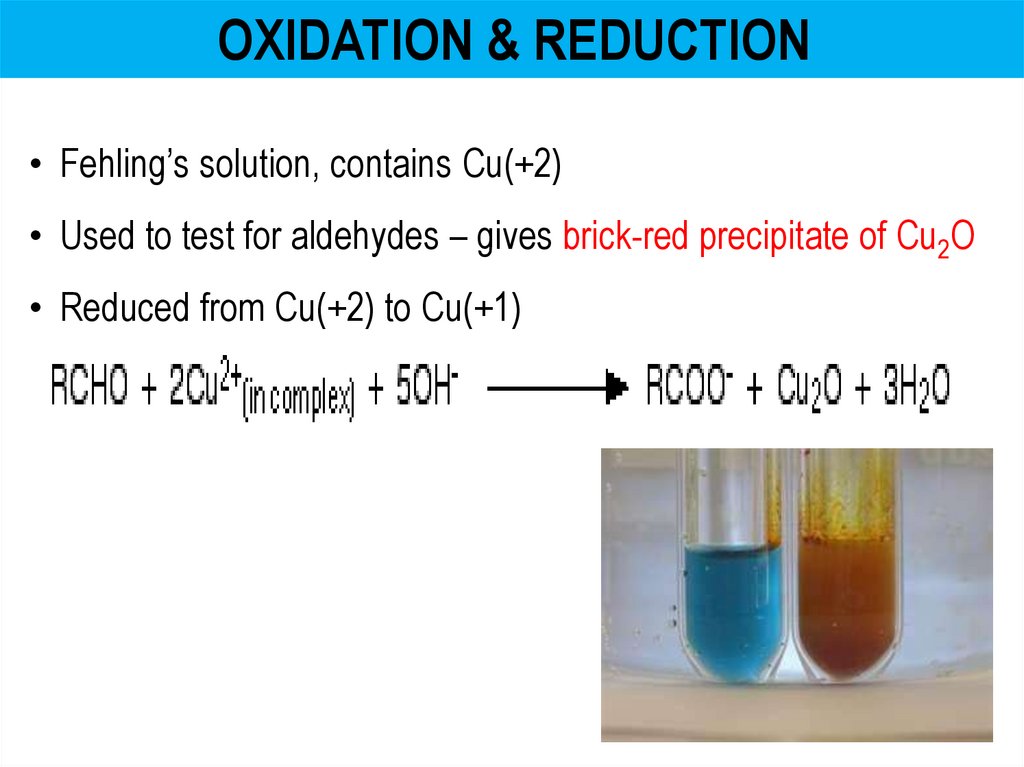
OXIDATION & REDUCTION• Fehling’s solution, contains Cu(+2)
• Used to test for aldehydes – gives brick-red precipitate of Cu2O
• Reduced from Cu(+2) to Cu(+1)
8.
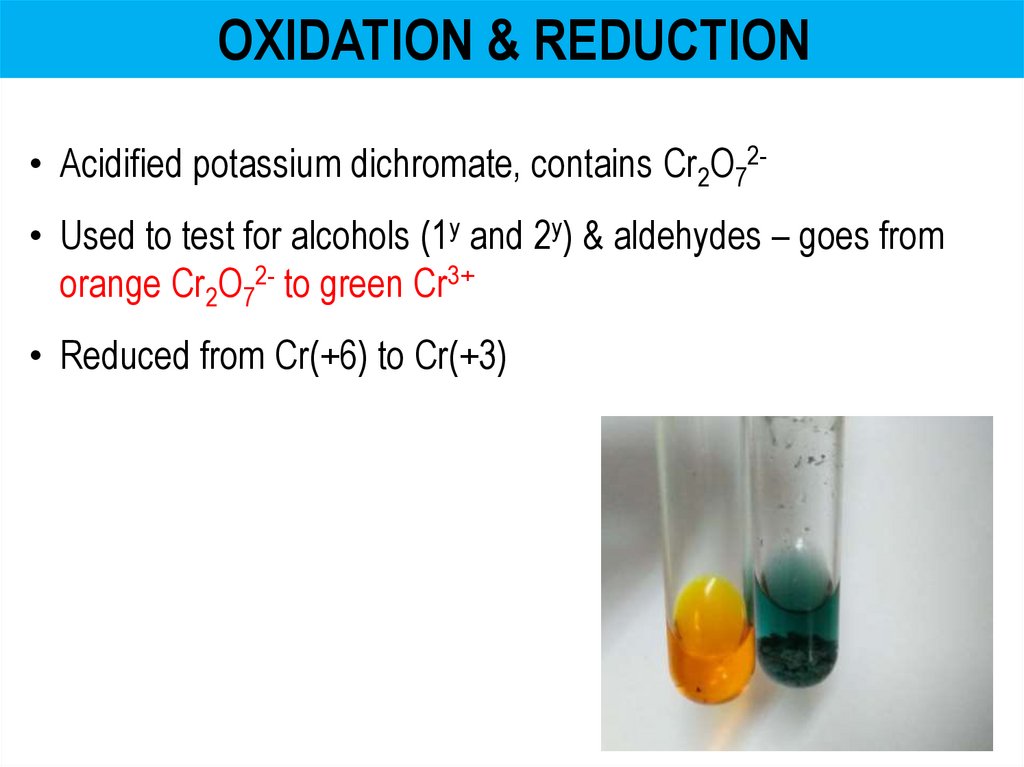
OXIDATION & REDUCTION• Acidified potassium dichromate, contains Cr2O72• Used to test for alcohols (1y and 2y) & aldehydes – goes from
orange Cr2O72- to green Cr3+
• Reduced from Cr(+6) to Cr(+3)
9.
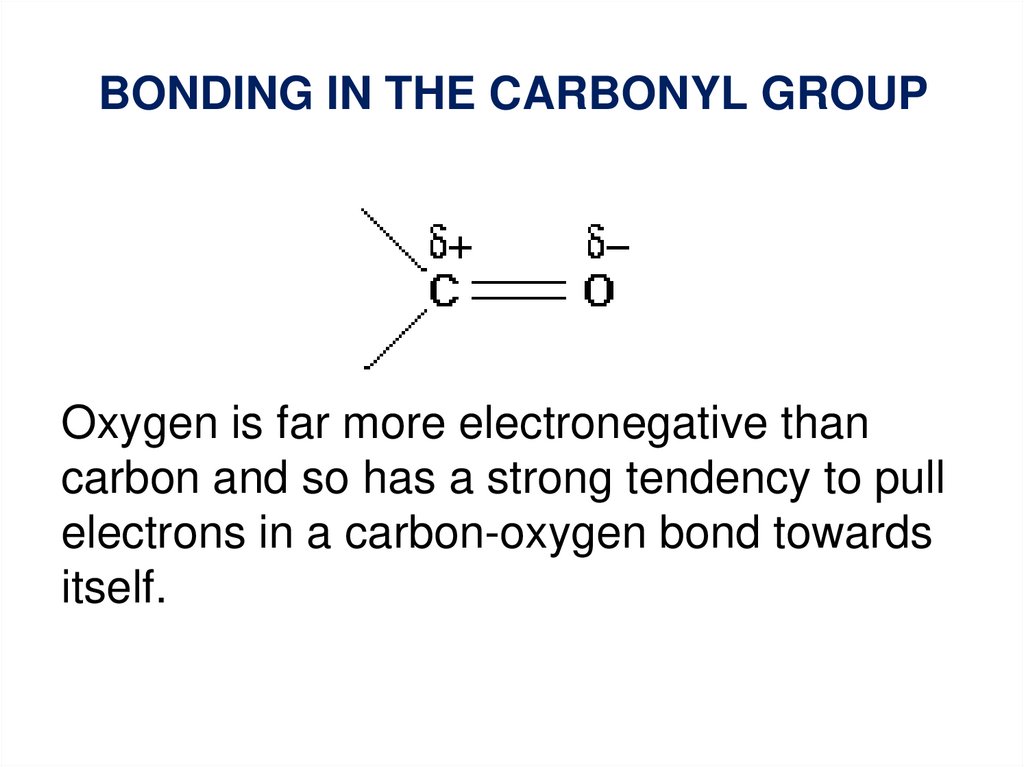
BONDING IN THE CARBONYL GROUPOxygen is far more electronegative than
carbon and so has a strong tendency to pull
electrons in a carbon-oxygen bond towards
itself.
10.
NUCLEOPHILIC ADDITION+
–
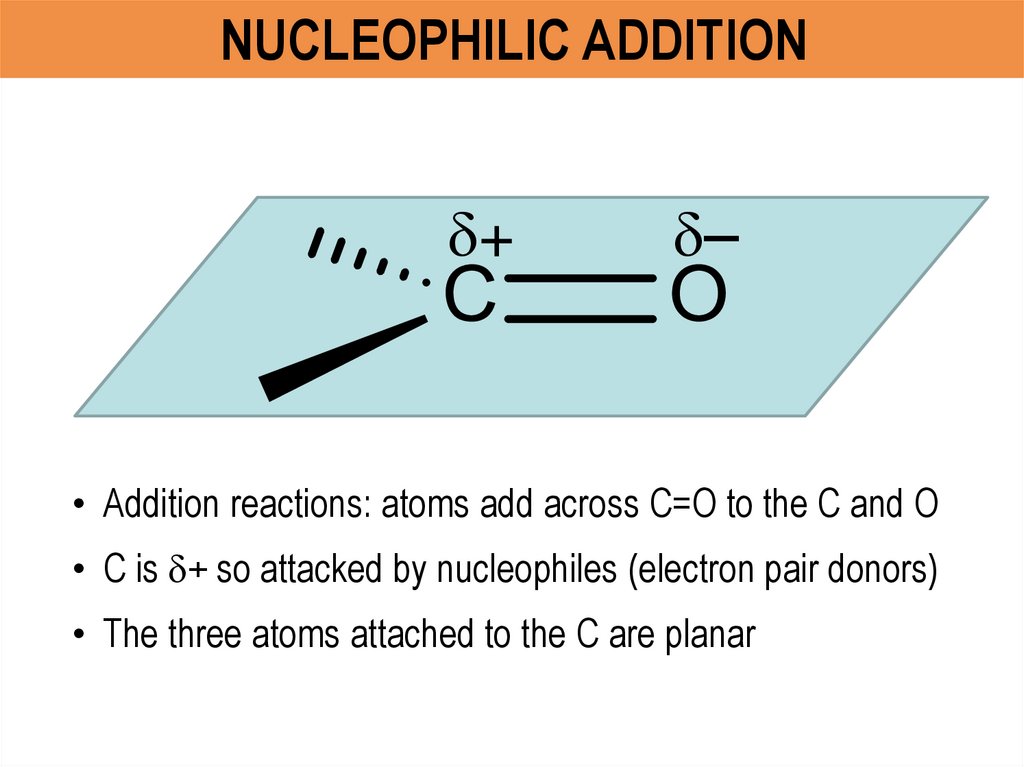
• Addition reactions: atoms add across C=O to the C and O
• C is + so attacked by nucleophiles (electron pair donors)
• The three atoms attached to the C are planar
11.
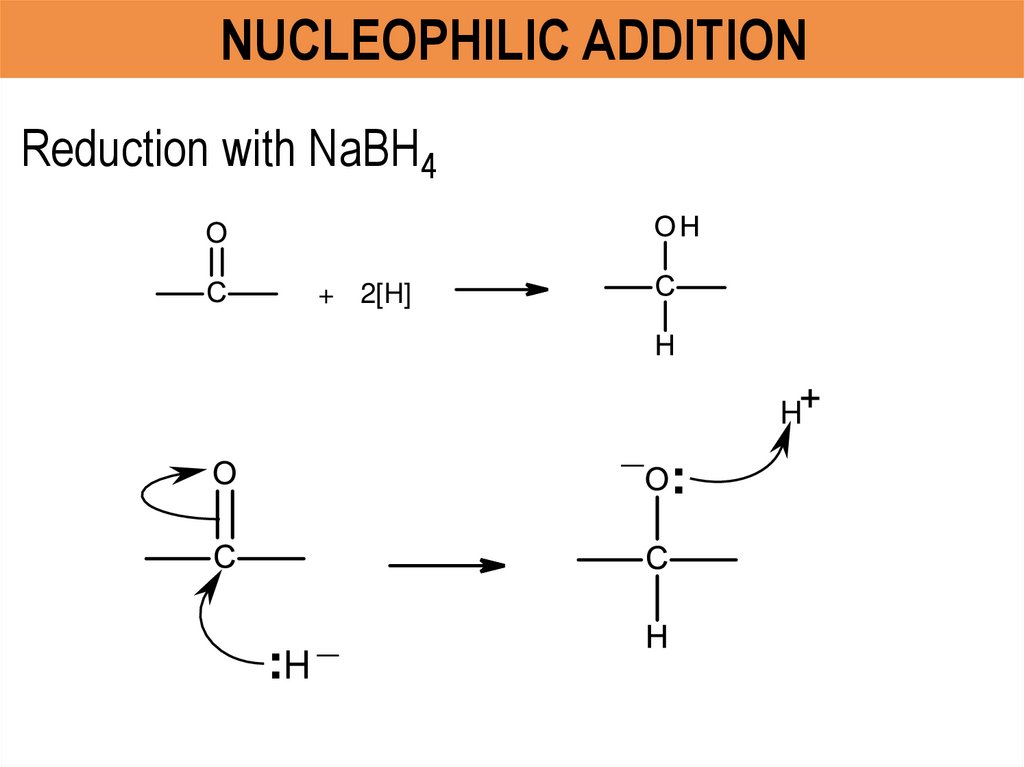
NUCLEOPHILIC ADDITIONReduction with NaBH4
+ 2[H]
H
_
:H
_
:
12.
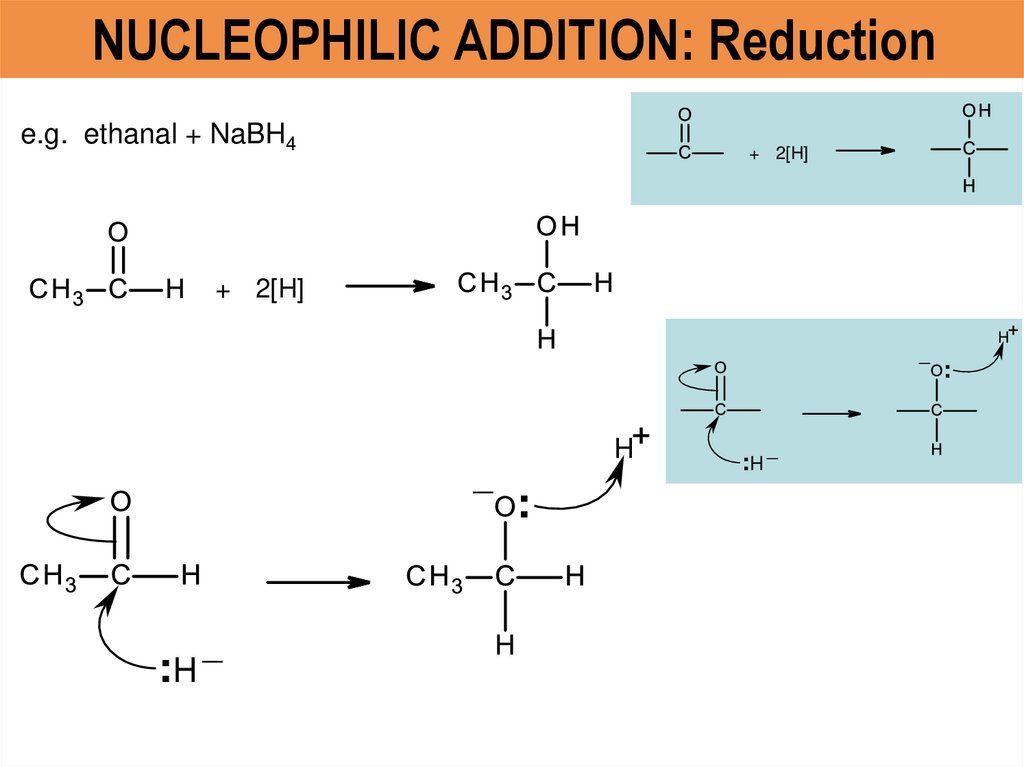
NUCLEOPHILIC ADDITION: Reductione.g. ethanal + NaBH4
+ 2[H]
+ 2[H]
H
_
_
:H
_
H
:
:H
_
:
13.
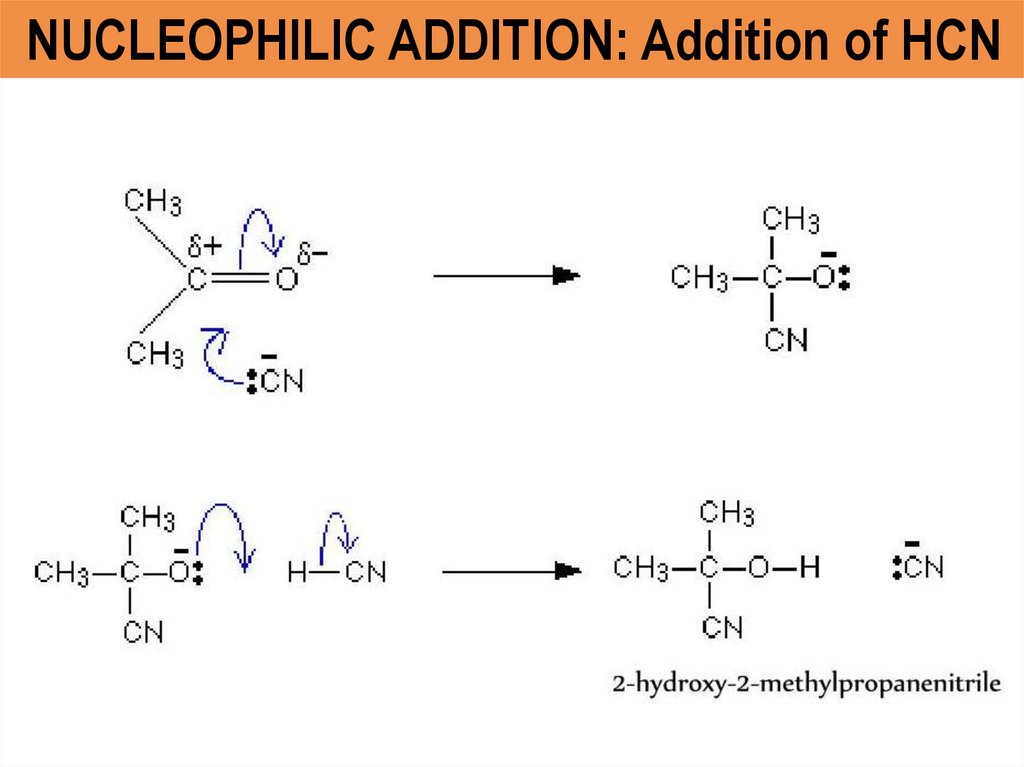
NUCLEOPHILIC ADDITION: Addition of HCN14. Esterification – CA and alcohol
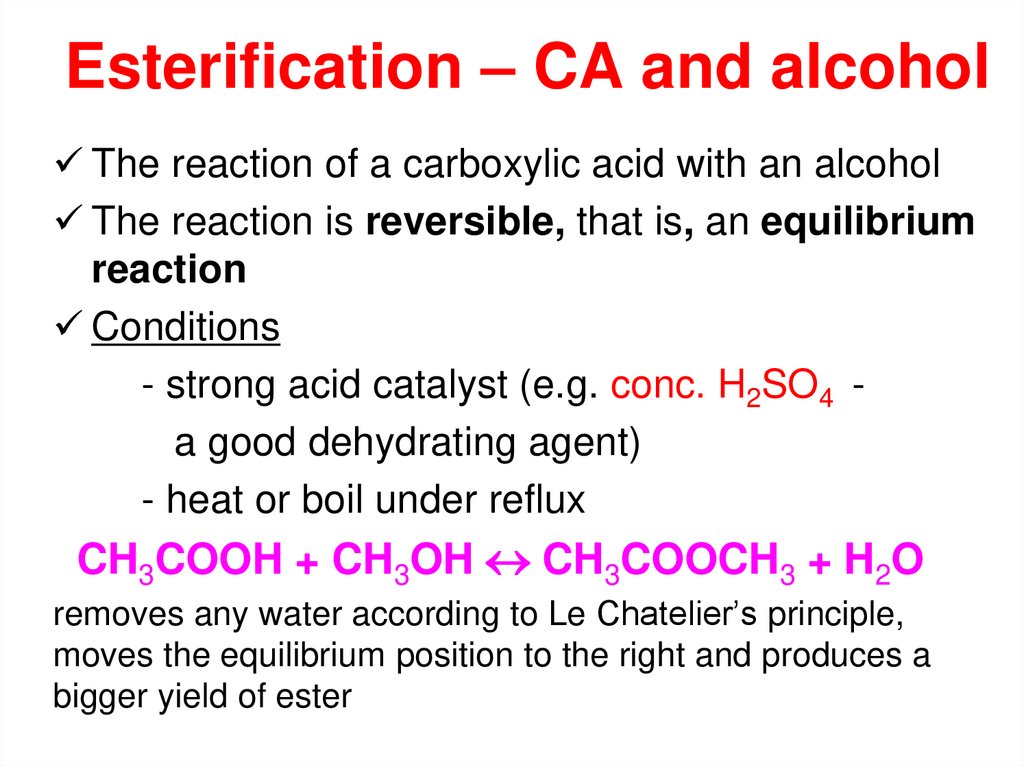
The reaction of a carboxylic acid with an alcoholThe reaction is reversible, that is, an equilibrium
reaction
Conditions
- strong acid catalyst (e.g. conc. H2SO4 a good dehydrating agent)
- heat or boil under reflux
CH3COOH + CH3OH CH3COOCH3 + H2O
removes any water according to Le Chatelier’s principle,
moves the equilibrium position to the right and produces a
bigger yield of ester
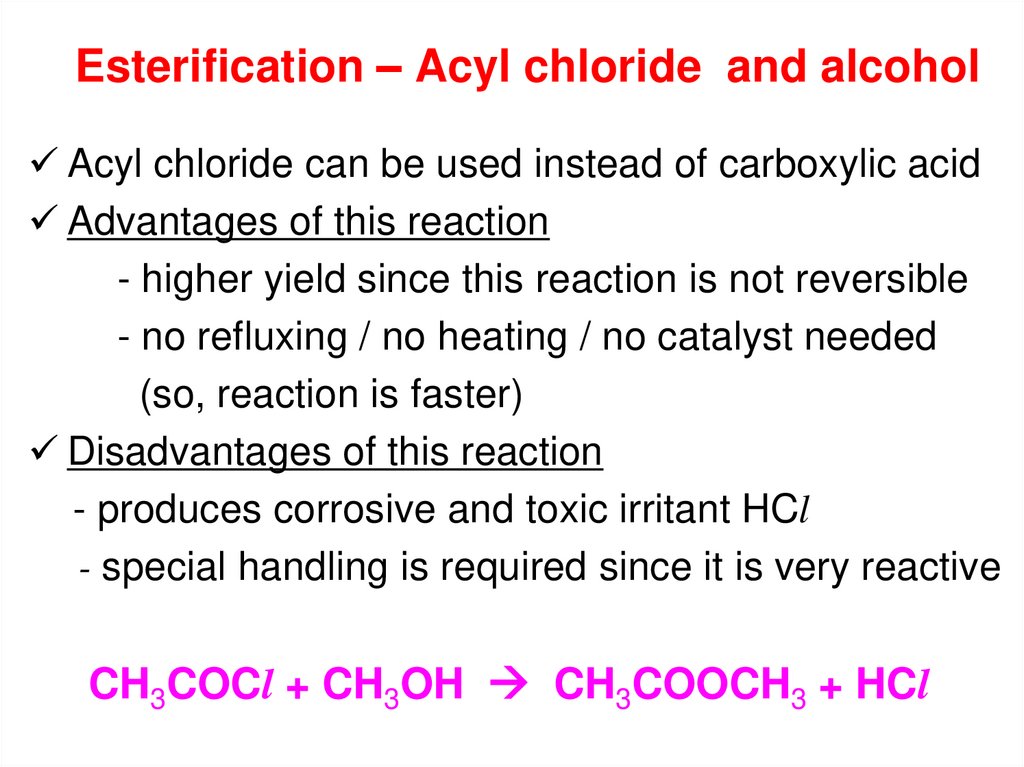
15. Esterification – Acyl chloride and alcohol
Acyl chloride can be used instead of carboxylic acidAdvantages of this reaction
- higher yield since this reaction is not reversible
- no refluxing / no heating / no catalyst needed
(so, reaction is faster)
Disadvantages of this reaction
- produces corrosive and toxic irritant HCl
- special handling is required since it is very reactive
CH3COCl + CH3OH CH3COOCH3 + HCl
16. Acidic Hydrolysis of Esters
In acidic hydrolysis:• An ester reacts with water to produce a
carboxylic acid and an alcohol.
• A strong acid catalyst is required and should
be heated under reflux.
O
O
H+
H—C—O—CH2—CH3 + H2O H—C—OH
+ H—O—CH2—CH3
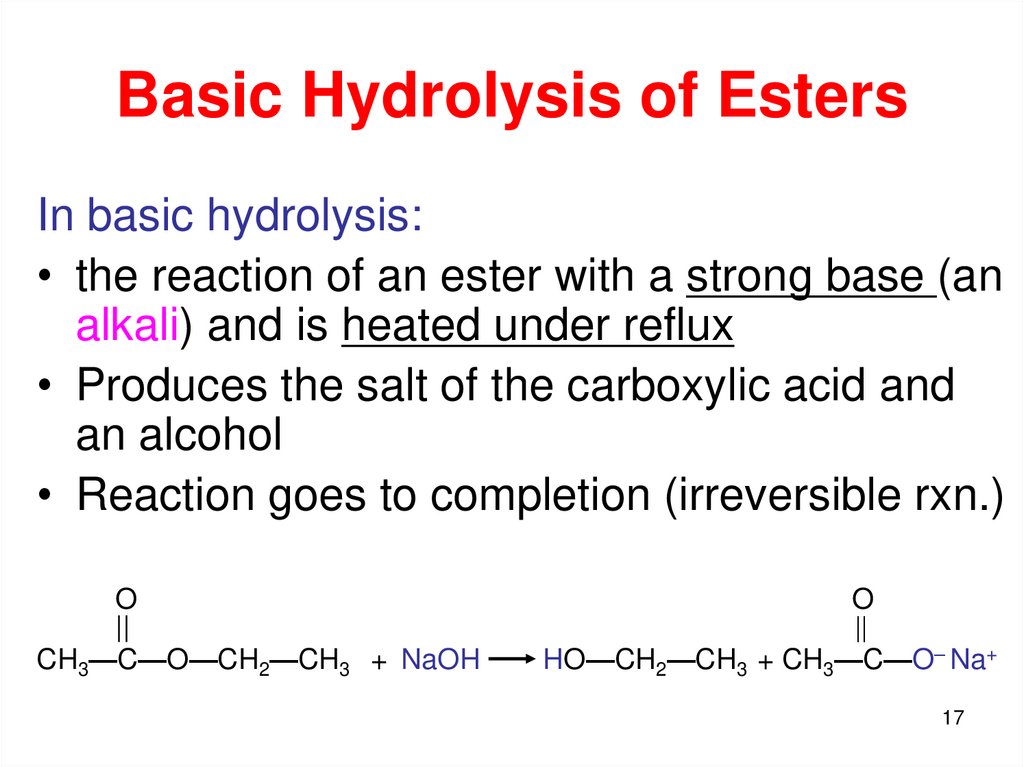
17. Basic Hydrolysis of Esters
In basic hydrolysis:• the reaction of an ester with a strong base (an
alkali) and is heated under reflux
• Produces the salt of the carboxylic acid and
an alcohol
• Reaction goes to completion (irreversible rxn.)
O
||
CH3—C—O—CH2—CH3 + NaOH
O
HO—CH2—CH3 + CH3—C—O– Na+
17
18.
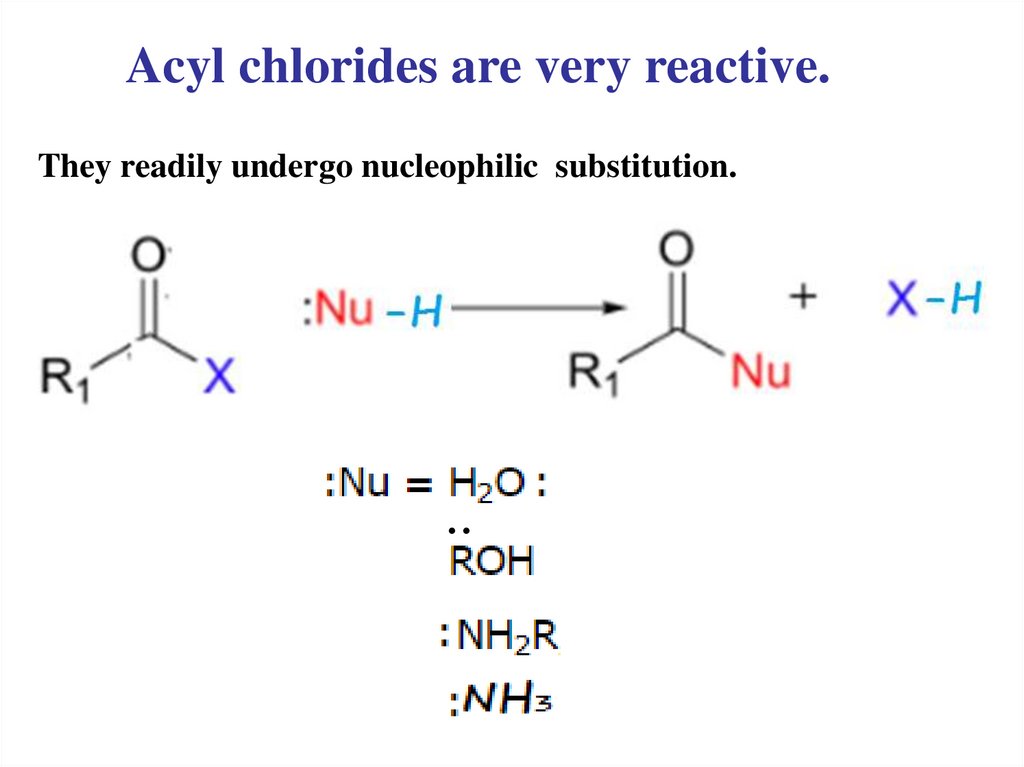
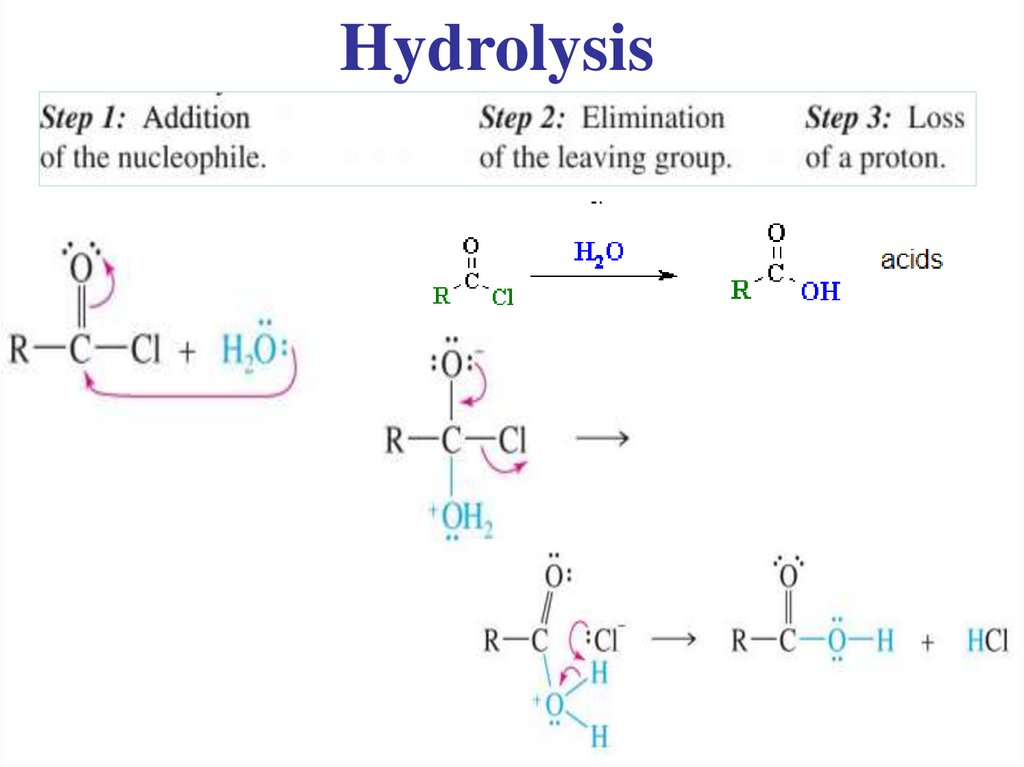
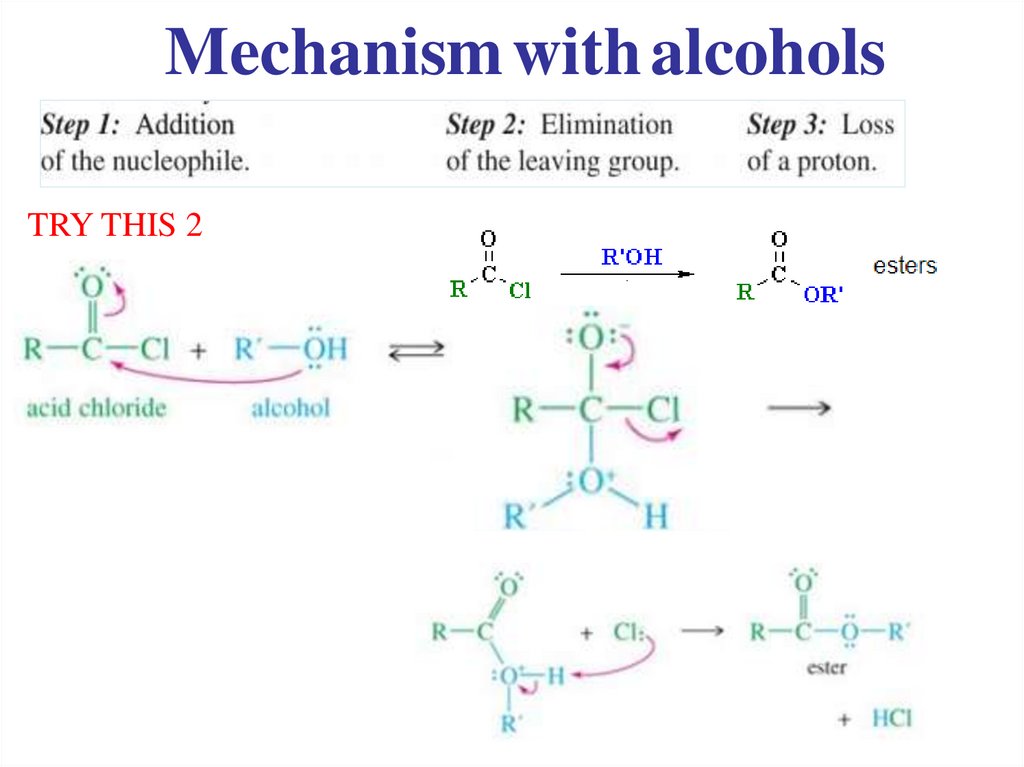
Acyl chlorides are very reactive.They readily undergo nucleophilic substitution.
..
19.
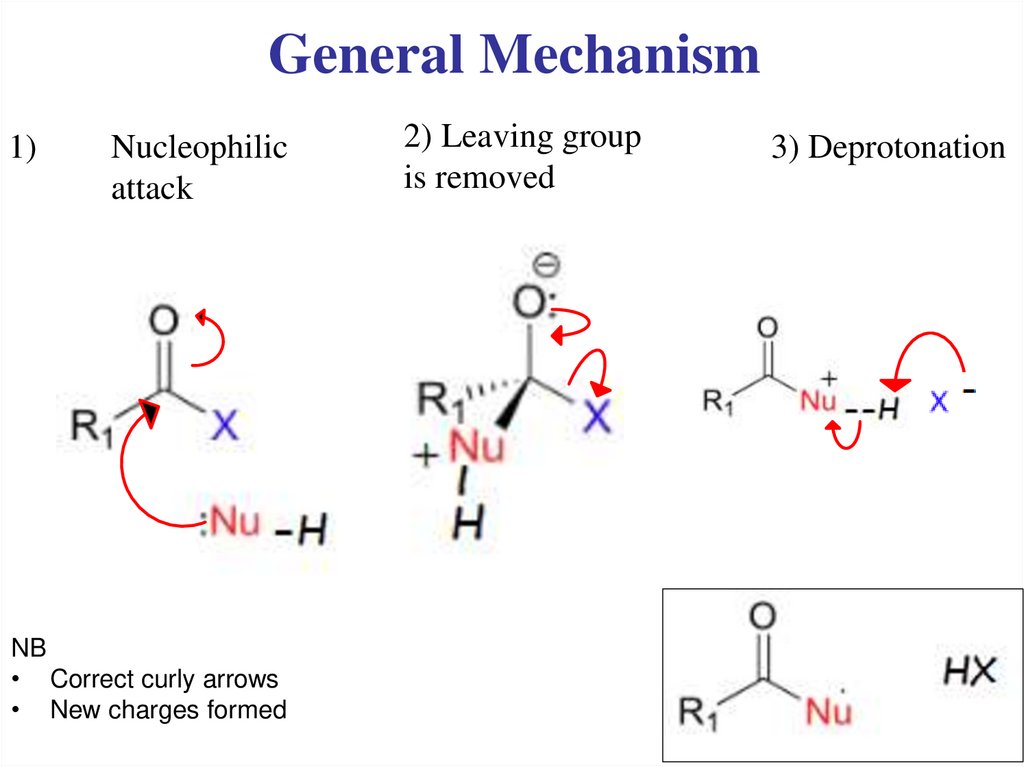
General Mechanism1)
Nucleophilic
attack
NB
• Correct curly arrows
• New charges formed
2) Leaving group
is removed
3) Deprotonation
20.
Hydrolysis21.
Mechanism with alcoholsTRY THIS 2



 Химия
Химия








