Похожие презентации:
Revision on benzene, amines and amino acids 3
1. Benzene Amines and Amino Acids
REVISION2. Assessment Criteria
• Describe, using thermochemical evidence, the additional stabilityconferred by the structure.
• Write a mechanism for electrophilic substitution of benzene.
• Recognise initial step in the Friedel-Crafts acylation.
• Write equations for reactions of amino acids with either acid or alkali.
• Draw the correct zwitterions for a given amino acid.
• Understand the formation of peptide links and of proteins from amino
acids.
• Write the conditions and products of hydrolysis of proteins.
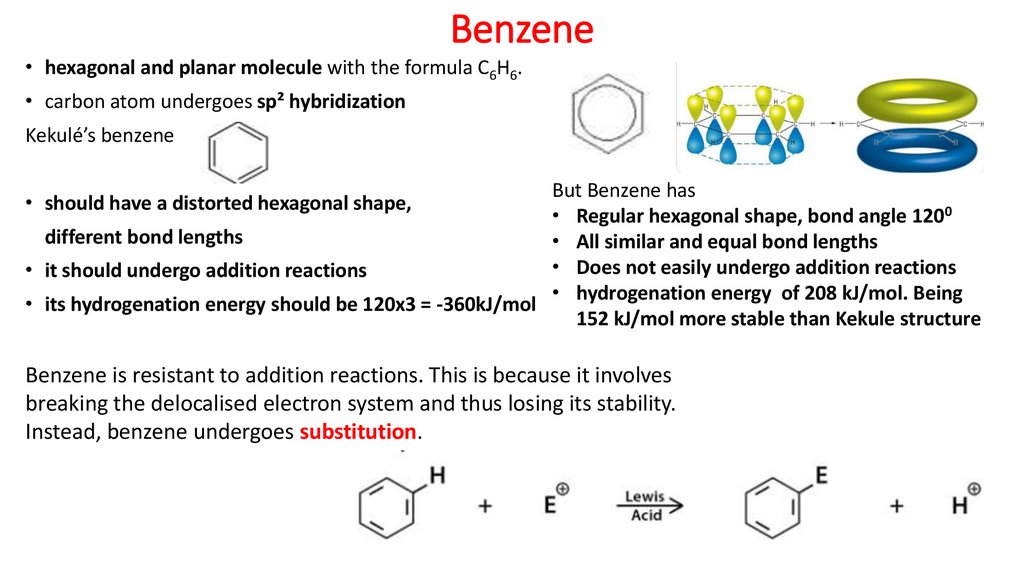
3. Benzene
• hexagonal and planar molecule with the formula C6H6.• carbon atom undergoes sp² hybridization
Kekulé’s benzene
But Benzene has
• Regular hexagonal shape, bond angle 1200
different bond lengths
• All similar and equal bond lengths
• Does not easily undergo addition reactions
• it should undergo addition reactions
• hydrogenation energy of 208 kJ/mol. Being
• its hydrogenation energy should be 120x3 = -360kJ/mol
152 kJ/mol more stable than Kekule structure
• should have a distorted hexagonal shape,
Benzene is resistant to addition reactions. This is because it involves
breaking the delocalised electron system and thus losing its stability.
Instead, benzene undergoes substitution.
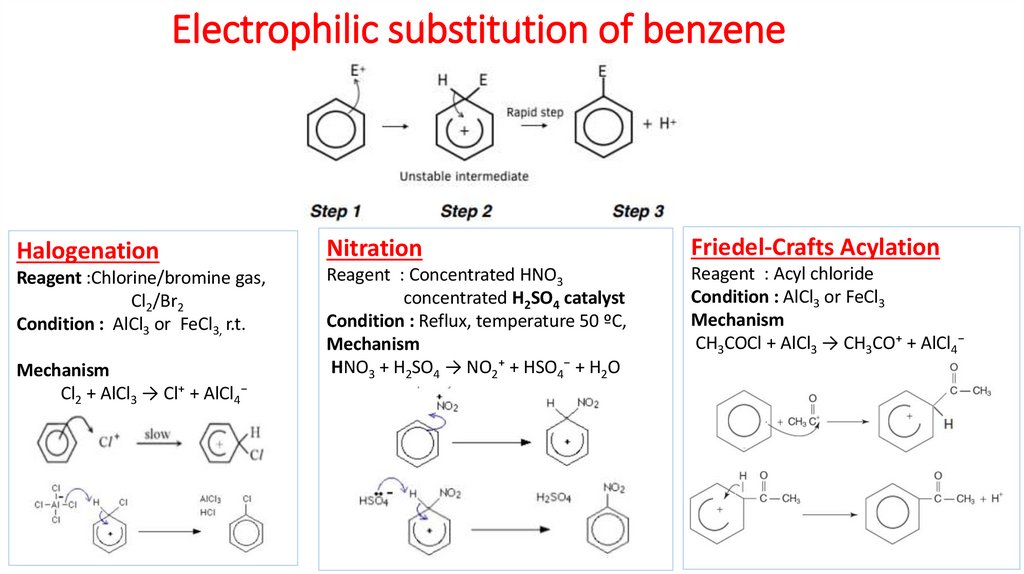
4. Electrophilic substitution of benzene
HalogenationNitration
Friedel-Crafts Acylation
Reagent :Chlorine/bromine gas,
Cl2/Br2
Condition : AlCl3 or FeCl3, r.t.
Reagent : Concentrated HNO3
concentrated H2SO4 catalyst
Condition : Reflux, temperature 50 ºC,
Mechanism
HNO3 + H2SO4 → NO2⁺ + HSO4⁻ + H2O
Reagent : Acyl chloride
Condition : AlCl3 or FeCl3
Mechanism
CH3COCl + AlCl3 → CH3CO⁺ + AlCl4⁻
Mechanism
Cl2 + AlCl3 → Cl⁺ + AlCl4⁻
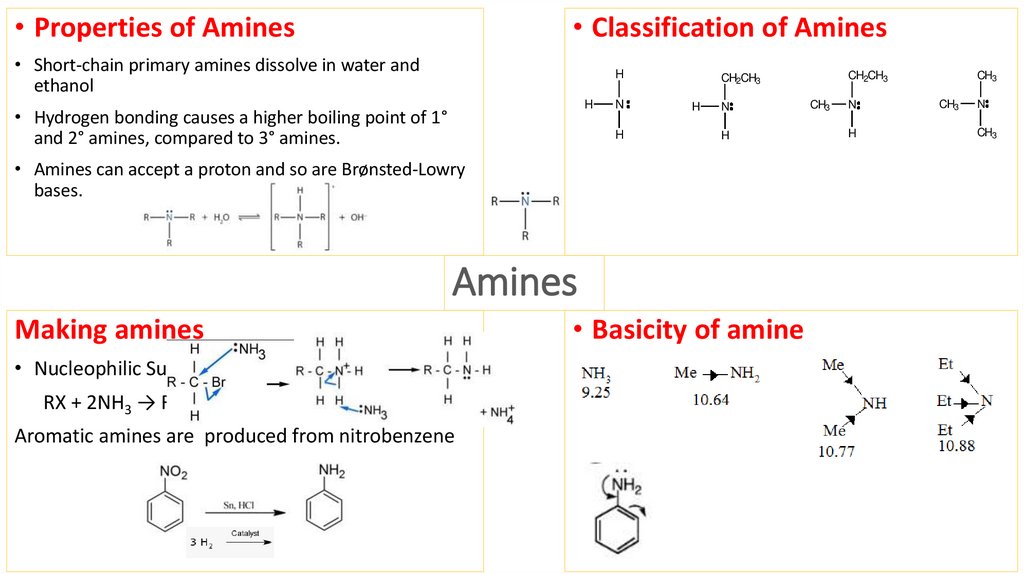
5. Amines
• Properties of Amines• Classification of Amines
• Short-chain primary amines dissolve in water and
ethanol
H
H
• Hydrogen bonding causes a higher boiling point of 1°
and 2° amines, compared to 3° amines.
N
H
H
N
H
• Amines can accept a proton and so are Brønsted-Lowry
bases.
Amines
Making amines
• Nucleophilic Substitution with Ammonia
RX + 2NH3 → RNH2 + NH4X.
Aromatic amines are produced from nitrobenzene
• Basicity of amine
CH3
CH2CH3
CH2CH3
CH3
N
H
CH3
N
CH3
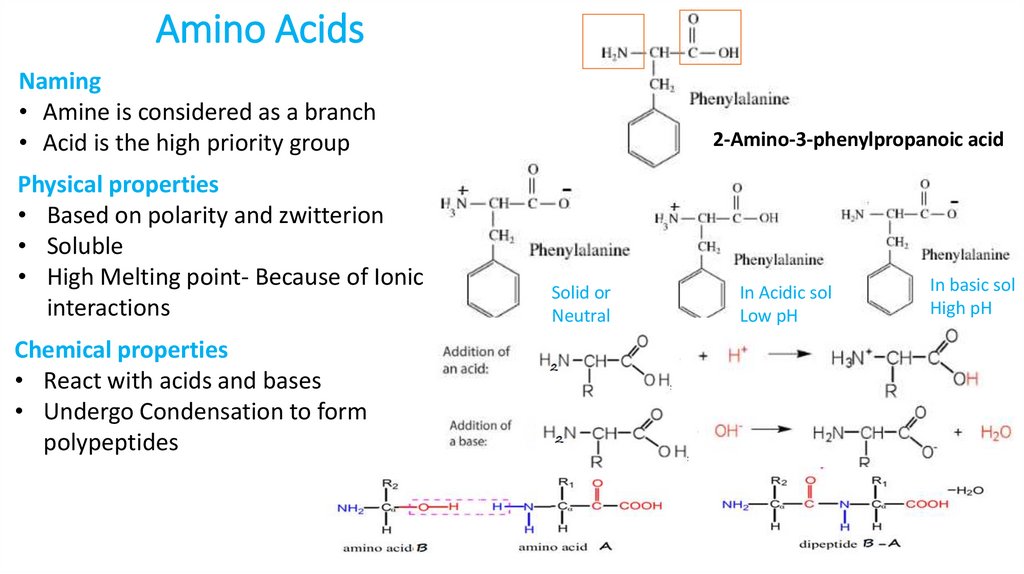
6. Amino Acids
Naming• Amine is considered as a branch
• Acid is the high priority group
Physical properties
• Based on polarity and zwitterion
• Soluble
• High Melting point- Because of Ionic
interactions
Chemical properties
• React with acids and bases
• Undergo Condensation to form
polypeptides
2-Amino-3-phenylpropanoic acid
Solid or
Neutral
In Acidic sol
Low pH
In basic sol
High pH
7.
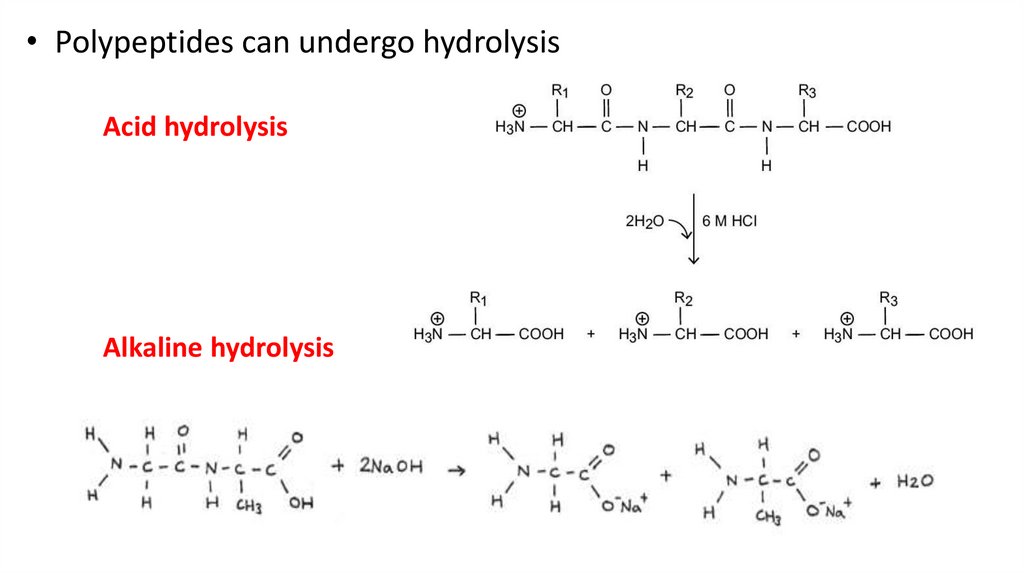
• Polypeptides can undergo hydrolysisAcid hydrolysis
Alkaline hydrolysis

 Химия
Химия