Похожие презентации:
Chem L3 The structure of the atom
1. Lecture #3 The structure of atom. Quantum numbers.
Chemistry by Assoc. Prof., PhD Matveyeva I.V.Lecture #3 The structure of atom.
Quantum numbers.
2.
Chemistry by Assoc. Prof., PhD Matveyeva I.V.The history of atomic model
2 (41)
3.
Chemistry by Assoc. Prof., PhD Matveyeva I.V.…1808
Solid Sphere Model or
Billiard Ball Model
proposed
by
John
Dalton
The Solid Sphere Model was
the first atomic model and was
developed by John Dalton in
the early 19th century. He
hypothesized that an atom is a
solid sphere that could not be
divided into smaller particles.
He came up with his theory as
a result of his research into
gases. He realized that certain
gases
only combined in
specific proportions.
3
4.
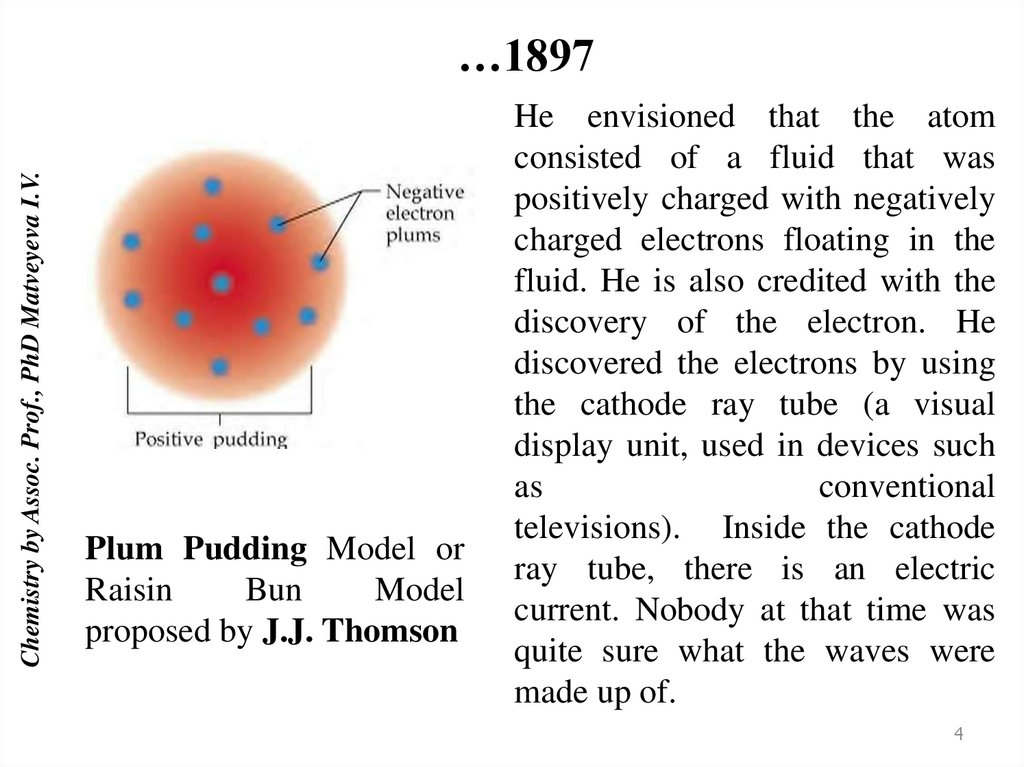
Chemistry by Assoc. Prof., PhD Matveyeva I.V.…1897
Plum Pudding Model or
Raisin
Bun
Model
proposed by J.J. Thomson
He envisioned that the atom
consisted of a fluid that was
positively charged with negatively
charged electrons floating in the
fluid. He is also credited with the
discovery of the electron. He
discovered the electrons by using
the cathode ray tube (a visual
display unit, used in devices such
as
conventional
televisions). Inside the cathode
ray tube, there is an electric
current. Nobody at that time was
quite sure what the waves were
made up of.
4
5.
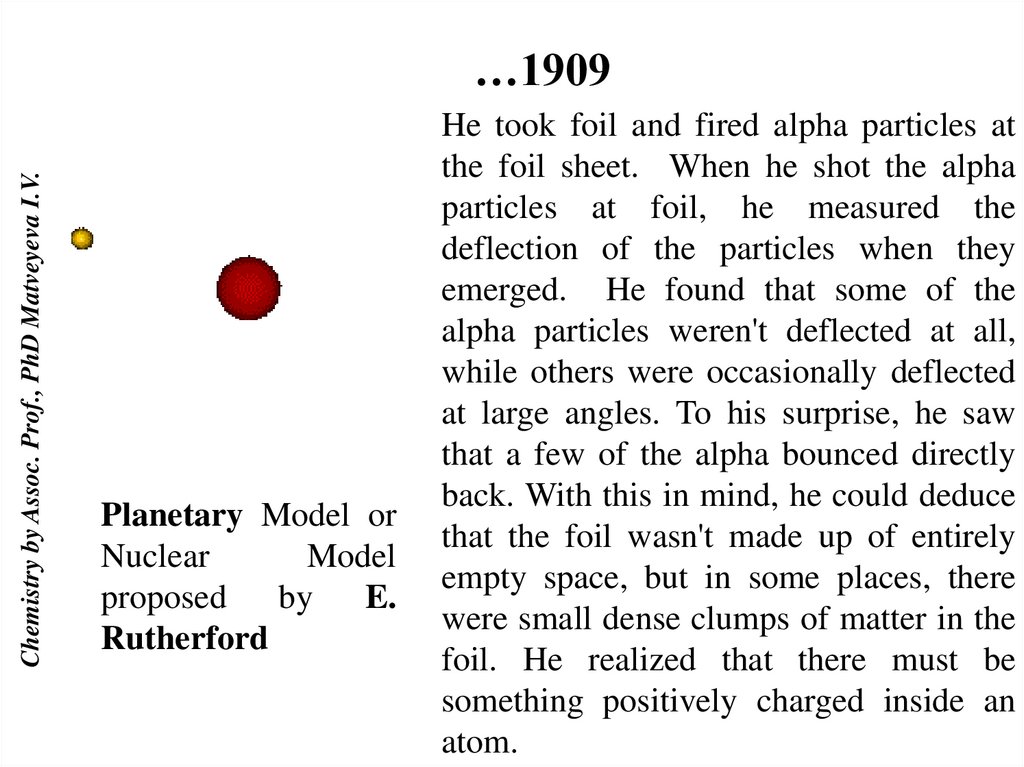
Chemistry by Assoc. Prof., PhD Matveyeva I.V.…1909
Planetary Model or
Nuclear
Model
proposed
by
E.
Rutherford
He took foil and fired alpha particles at
the foil sheet. When he shot the alpha
particles at foil, he measured the
deflection of the particles when they
emerged. He found that some of the
alpha particles weren't deflected at all,
while others were occasionally deflected
at large angles. To his surprise, he saw
that a few of the alpha bounced directly
back. With this in mind, he could deduce
that the foil wasn't made up of entirely
empty space, but in some places, there
were small dense clumps of matter in the
foil. He realized that there must be
something positively charged inside an
atom.
6.
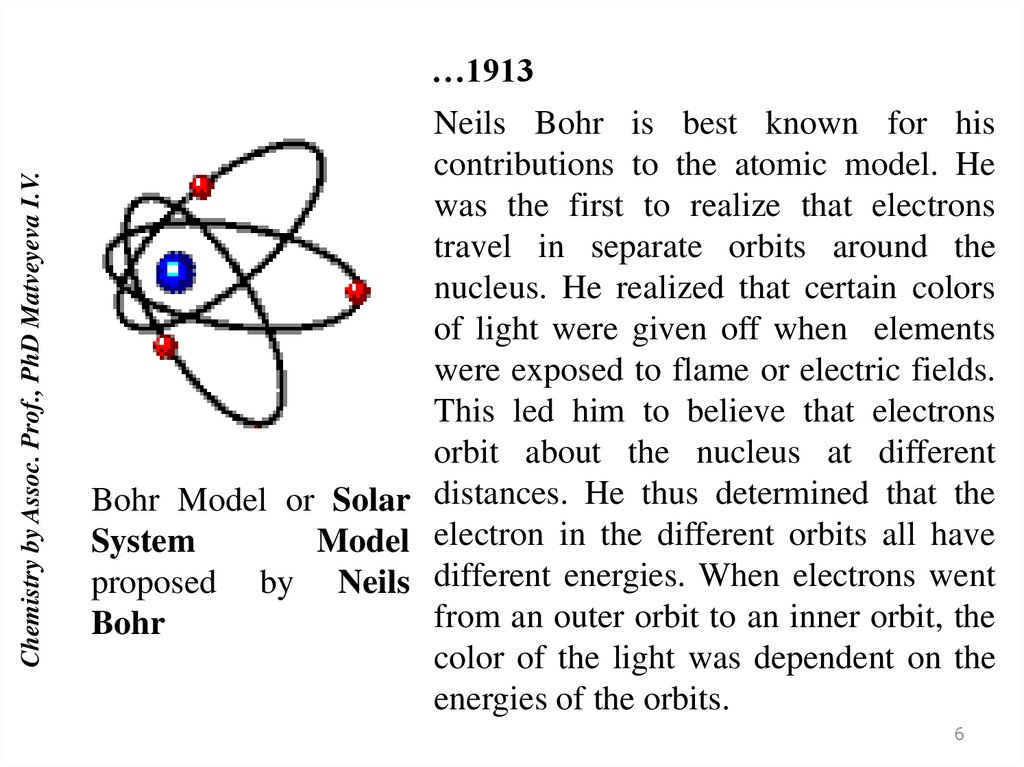
Chemistry by Assoc. Prof., PhD Matveyeva I.V.…1913
Neils Bohr is best known for his
contributions to the atomic model. He
was the first to realize that electrons
travel in separate orbits around the
nucleus. He realized that certain colors
of light were given off when elements
were exposed to flame or electric fields.
This led him to believe that electrons
orbit about the nucleus at different
Bohr Model or Solar distances. He thus determined that the
System
Model electron in the different orbits all have
proposed by Neils different energies. When electrons went
from an outer orbit to an inner orbit, the
Bohr
color of the light was dependent on the
energies of the orbits.
6
7.
Chemistry by Assoc. Prof., PhD Matveyeva I.V.FINALLY!!!
Electron Cloud Model or
Quantum
Mechanical
Model proposed by Louis
de Broglie & Erwin
Schrodinger
…1924
It is the most recent model of
the atom. Notice that instead
of showing the paths of the
orbit of the electrons it just
shows a "cloud" around the
nucleus.
The
cloud
represents where any one
electron could be at any
time. This electron cloud
model is based on quantum
mechanics.
7
8.
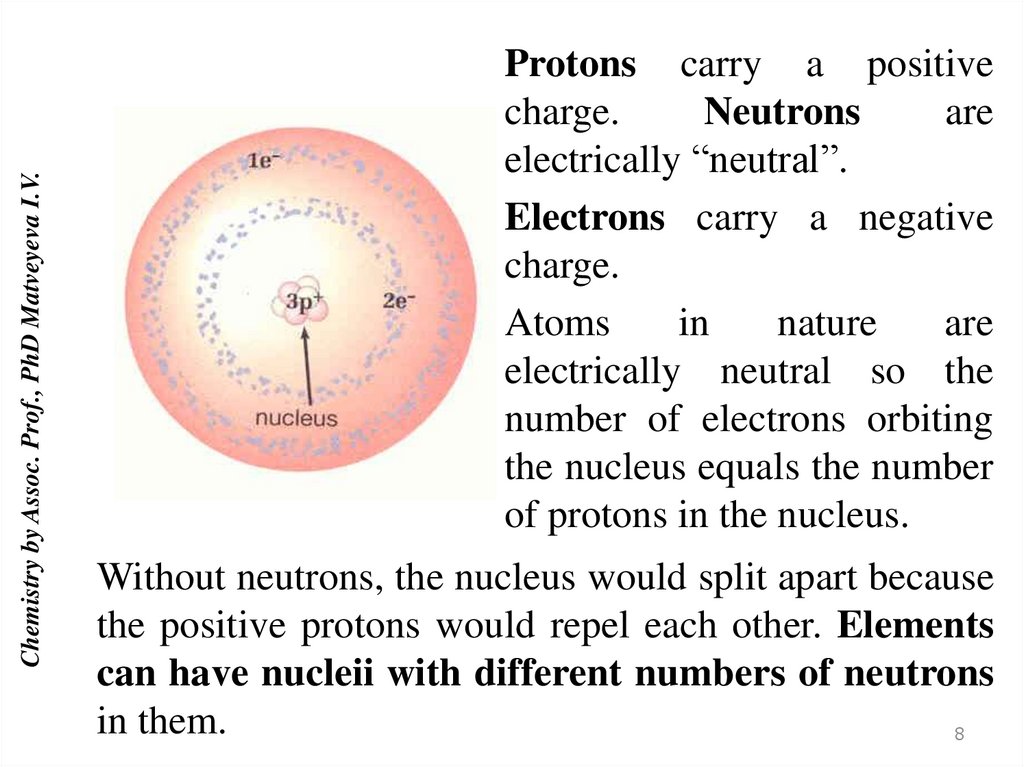
Chemistry by Assoc. Prof., PhD Matveyeva I.V.Protons carry a positive
charge.
Neutrons
are
electrically “neutral”.
Electrons carry a negative
charge.
Atoms
in
nature
are
electrically neutral so the
number of electrons orbiting
the nucleus equals the number
of protons in the nucleus.
Without neutrons, the nucleus would split apart because
the positive protons would repel each other. Elements
can have nucleii with different numbers of neutrons
in them.
8
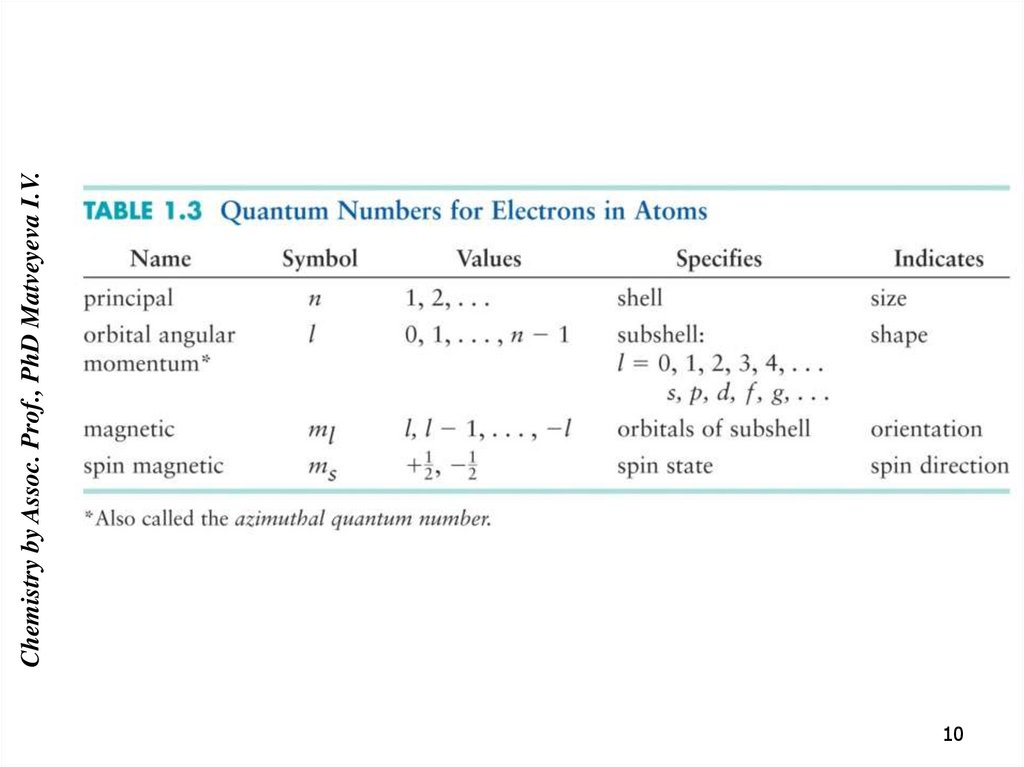
9. The laws that determine the position of the electrons in the atom.
Chemistry by Assoc. Prof., PhD Matveyeva I.V.The laws that determine the position of
the electrons in the atom.
1. Any particle tends to minimum reserve of potential
energy.
2. Pauli exclusion principle: Two electrons with the same
values of all four quantum numbers cannot be in the atom.
3. Hund principle: At this energy sublevel electrons are
arranged in such a way that their total spin was maximal.
4. The first Aufbau principle: First electrons fill orbitals
with a smaller sum of principal and orbital numbers, and
then orbitals with a larger value of sum of these two
numbers.
5. The second Aufbau principle: If two orbitals have the
same value of the sum of principal and orbital numbers, first
electrons fill orbital, whose principal quantum number has
9 (41)
less value.
10.
10Chemistry by Assoc. Prof., PhD Matveyeva I.V.
11.
Chemistry by Assoc. Prof., PhD Matveyeva I.V.Thank you for your attention!!!
11




 Физика
Физика








